Abstract
We have aimed to investigate the expression of genes related to rosmarinic acid (RA) synthesis and rosmarinic acid content in 2 Ocimum basilicum cultivars, green (cinnamon) and purple (red rubin) basil. Specifically, genes related to rosmarinic acid biosynthesis were cloned and characterized for O. basilicum. We obtained partial cDNAs of tyrosine aminotransferase (TAT) and 4-hydroxyphenylpyruvate reductase (HPPR), which were of 323 bp and 616 bp in size, respectively. The transcription levels of most genes related to rosmarinic acid synthesis were higher in green basil compared to purple basil, except for ObPAL and Ob4CL in the root. The highest expression was obtained in the leaves of green basil for all genes and the roots of purple basil for all genes, except for TAT. The highest rosmarinic acid content was obtained in the leaves of both cultivars, with higher RA accumulating in green basil compared to purple basil. The leaves had the highest RA content out of all plant organs, with the RA accumulation in the leaves of green basil being 1.64 times higher compared to purple basil. Further study is required to investigate whether a similar trend is observed across O. basilicum cultivars of different color types.
Keywords: Rosmarinic acid, Ocimum basilicum, Gene expression, Different organs
1. Introduction
basilicum Ocimum L. belongs to the Lamiaceae family. It is an aromatic annual herb that is an important economic crop with wide level of biological and pharmacological applications (Siddiqui et al., 2012, Sarahroodi et al., 2012, Al-Dhabi et al., 2014). Basil grows in mountain regions, including Africa, Asia, and South America. In Iran, India, and other tropical countries of Asia, this plant is a major essential-oil crop that is widely used in the food, perfume, pharmaceutical, cosmetic, and aromatherapy industries (Pirmoradi et al., 2013, Radulovic et al., 2013, Suh et al., 2015). In the past, basil was consumed for preventing the cardiovascular related diseases, along with acting as an antispasmodic, carminative, digestive, stomachic, and tonic agent (Umar et al., 2014, Fathiazad et al., 2012). Previous studies have shown that extracts of basil have various biological activities, such as being a potent antioxidant, having antihypertensive effects, along with anti-aging, anticancer, antiviral, antifungal, nematocidal, insect repellent, and antimicrobial properties (Saha et al., 2012, Sakr and Nooh, 2013). Basil contains phenolic compounds including rosmarinic acid (Tada et al., 1996).
Rosmarinic acid (RA) is a major polyphenolic compound in the Lamiaceae and Boraginaceae families. It is an ester of caffeic acid and 3,4-dihydroxyphenyl lactic acid (Saltas et al., 2013, Doring et al., 2014, Nie et al., 2014). RA has various biological properties, such as antioxidant, anti-mutagenic, anti-bacterial, and anti-viral capabilities, along with anti-allergic and anti-inflammatory effects (Petersen and Simmonds, 2003, Zhang et al., 2013, Kim et al., 2013, Zhang et al., 2014, Campos et al., 2014). This study aimed to compare the gene expression and rosmarinic acid content in the different organs of 2 O. basilicum cultivars, green (cinnamon) and purple (red rubin) basil.
2. Materials and methods
2.1. Seed germination
Green (cultivar cinnamon) and purple (cultivar red rubin) basil seeds were purchased from a flower seed mall in Korea and cultivated in Chungnam National University (Daejeon, Korea). When the flowers were in full bloom, the different organs (the flowers, stems, leaves, and roots) were harvested and immediately frozen dried at −20 °C for the gene expression and chemical analysis studies.
2.2. Cloning of cDNA tyrosine aminotransferase (TAT) and hydroxyphenylpyruvate reductase (HPPR)
To clone the TAT and HPPR genes, degenerate primers were designed using the conserved regions of the TAT and HPPR genes from other higher plants, respectively. PCR was performed in a BIO-RAD MyGenie32 (Bio-Rad Laboratories, California, USA) using the cDNA of cinnamon basil flowers under the following conditions: 95 °C for 30 s over 30 thermal cycles (denaturing at 95 °C for 2 min, primer annealing at Tm for 30 s, and primer extension at 72 °C for 1 min) and final extension at 72 °C for 10 min using the primers. The primer sequences and annealing Tm information is presented in Table 1. After amplification, 3 μl of PCR product was mixed with 1 μl of loading dye and analyzed on 1% agarose gel in 1X TAE (Tris base/acetic acid/EDTA) buffer. The gel documentation system was used to determine the respective obTAT and obHPPR genes fragment sizes. Gel Extraction Kit and T-Blunt vector (Solgent, Korea) was used for the purification of the amplicon and cloninn, further the cloned product was sequenced for the checking the similarity of the TAT and HPPR from O. basilicum using BLAST. RT-PCR was performed using the partial sequences that had been obtained.
Table 1.
Primers used for cloning ObTAT and ObHPPR.
| Primer name | Primer sequence (5′-3′) | Annealing Tm (°C) |
|---|---|---|
| ObTAT forward | RTHCCTGGHTGGMGBYTWGGTTGG | 55 |
| ObTAT reverse | CCTGGAAGRAKKATAACRGATTCCTCY | 55 |
| ObHPPR forward | GGATTAGGGTTACCAACACGCC | 45.7 |
| ObHPPR reverse | AACAAGGTCAGCCATGGCTTTAC | 45.7 |
2.3. Total RNA isolation and cDNA synthesis
Plant Total Mini Kit (Geneaid, Taiwan) was used for the extraction of the total RNA. A 5 μl volume of DNase I (264 μg/ml) was added to the center of the RB Column matrix after step 2 (RNA binding), and incubated for 10 min at room temperature (RT), before proceeding to Step 3(washing). The quality of the total RNA was checked on 1% agarose gel, and was then used in spectrophotometer analysis to determine the concentration of total RNA. The kits procured from ReverTra Ace-α kit (Toyobo, Japan) was used for the preparation cDNA.
2.4. Gene expression analysis by qRT-PCR
Gene-specific primers (Table 2) were designed using the Primer3 Website (http://frodo.wi.mit.edu/priemr3/), based on the sequences of ObPAL, ObC4H, Ob4CL, ObTAT, and ObHPPR (GenBank accession numbers: AB436791.1, HM990150.1, KC576841.1, KJ004760, and KJ004761). The GADPH gene was used as the reference gene. 10 μl of 2X SYBR Green Real-Time PCR Smart mix (Solgent, Korea), 1 μl (10pmole/μl) of each of the specific primers, 5 μl of the cDNA template, and 3 μl distilled water (DW) was used for RT-PCR experiment. Thermal cycling conditions were: 95 °C for 15 s, 40 cycles at 95 °C for 20 s, 55 °C for 40 s, 72 °C for 20 s, 10 s collection fluorescence at 95 °C, 5 s at 65 °C, and 0.5 s at 95 °C. The amplicons were analyzed using Bio-Rad CFX Manager 2.0 software. The experiments were performed in triplicate, and the results were represented by presenting the means and standard deviations.
Table 2.
Primers used for gene expression analysis.
| Primer name | Primer sequence (5′-3′) | Size (bp) |
|---|---|---|
| ObPAL forward | CAGGCGACCTGGTCCCACTAT | |
| ObPAL reverse | ACGCCCGCTAGAGTGAGTGC | 121 |
| ObC4H forward | AACCACCGGAATCTCACCGA | |
| ObC4H reverse | GAGGACCTCCTTCGCGTGGT | 114 |
| Ob4CL forward | TGTCTCCGGTGTGAAGCCAA | |
| Ob4CL reverse | ATATGCATCCGCGGCCACTA | 100 |
| ObTAT forward | CTGGATGGCGCTTAGGTTGG | |
| ObTAT reverse | TCTGGGACTGCAGCCTGTATGA | 136 |
| ObHPPR forward | CTCCACAAGAGCAGACACCA | |
| ObHPPR reverse | TTGCCCCATAAGCTACTGCT | 241 |
| ObGAPDH forward | AACATTATCCCCAGCAGCAC | |
| ObGAPDH reverse | TAGGAACTCGGAATGCCATC | 172 |
2.5. High performance liquid chromatography (HPLC) analysis of rosmarinic acid
Samples of the different O. basilicum organs from the 2 cultivars were dried in a freeze-dryer at −80 °C for about 2 days (about 48 h). The dried samples (10 mg) of the different organs (flowers, leaves, stems, and roots) were extracted with 3 ml of 80% MeOH and 0.1% acetic acid under sonication for 40 min at RT. After centrifugation, the supernatant was filtered before the HPLC analysis. C18 colum was used for the separation of the compounds further, detected at 330 nm. Acetic acid and methanol was used as the mobile phase with different proportions. The compound was quantified by comparing the peak area of standard RA.
3. Results and discussion
3.1. Cloning and sequence analysis of TAT and HPPR from the O. basilicum cultivars
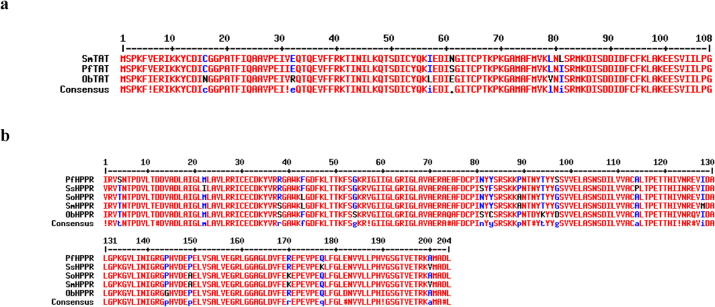
ObTAT and ObHPPR were cloned from the flowers of O. basilicum by RT-PCR. The partial length of the cDNA sequence of TAT (KJ004760) and HPPR (KJ004761) fragments was 323 bp and 616 bp, respectively. ObHPPR shared 89% identity with P. frutescens HPPR, 88% identity with Solenostemon scutellarioides HPPR, 87% identity with Salvia officinalis HPPR, and 86% identity with S. miltiorrhiza HPPR (Fig. 1).
Fig. 1.
Multiple alignment of amino acids with other amino acids. (a) ObTAT amino acids with other TATs. SmTAT (DQ334606.1) S. miltiorrhiza, PfTAT (HQ221576.1) P. frutescens. (b) Amino acids of ObHPPR with other HPPRs. PfHPPR (HM587131.1) P. frutescens, SsHPPR (AJ507733.2) S. scutellarioides, SoHPPR (EU924744.1) S. officinalis, SmHPPR (DQ099741.1) S. miltiorrhiza.
3.2. Expression levels of ObPAL, ObC4H, Ob4CL, ObTAT, and ObHPPR in different organs of O. basilicum
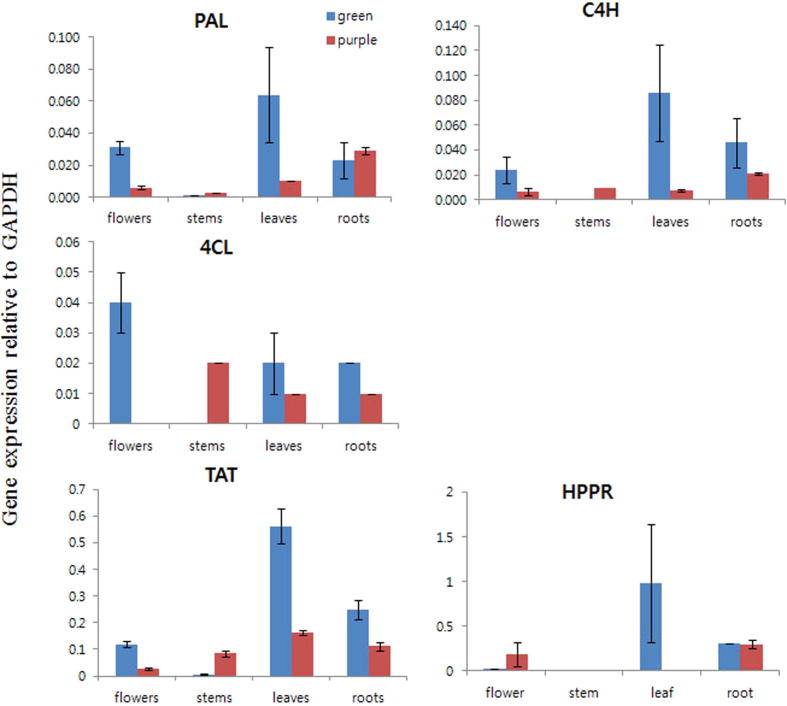
The expression levels of ObPAL, ObC4H, Ob4CL, ObTAT, and ObHPPR are shown in Fig. 2. The relative expression of green ObPAL (PAL of green basil) to GAPDH (RG) was highest in the leaf (0.064). The highest expression of green ob4CL was detected in the flowers (0.044) and the lowest in the stems (0.003). ObTAT and ObHPPR showed similar patterns of expression, with the highest expression being in the leaf (0.562 and 0.988, respectively) and the lowest expression in the stem (0.006 and 0.003, respectively). In particular, the ObHPPR expression in the leaf was about 329-fold higher compared to the stem. However, purple basil had the highest ObPAL, ObC4H, and ObHPPR (RG of 0.029, 0.021, and 0.302, respectively) in the root, while the RG of Ob4CL was 0.022 in the stem and ObTAT was 0.162 in the leaf.
Fig. 2.
Expression levels of rosmarinic acid biosynthetic genes.
3.3. Analysis of rosmarinic acid content in different organs of O. basilicum cultivars
Rosmarinic acid was measured in the flowers, stems, leaves, and roots of the 2 O. basilicum cultivars (green and purple) (Fig. 3). The highest RA accumulated in the leaves of the 2 basil cultivars, with a dry weight concentration of 69.38 μg/g (green colored cultivar) and 42.52 μg/g (purple colored cultivar). In particular, the RA content of green basil leaves was about 1.5-fold higher compared to that of purple basil. Very low RA concentration obtained in the stems of both cultivars, with a concentration of 13.52 μg/g (green basil) and 7.86 μg/g (purple basil). Higher RA accumulated in the leaves, stems, and roots of green basil compared to purple basil. However, RA accumulation was higher in the flowers of purple basil compared to green basil. Green basil had markedly higher RA content in the roots compared to purple basil.
Fig. 3.
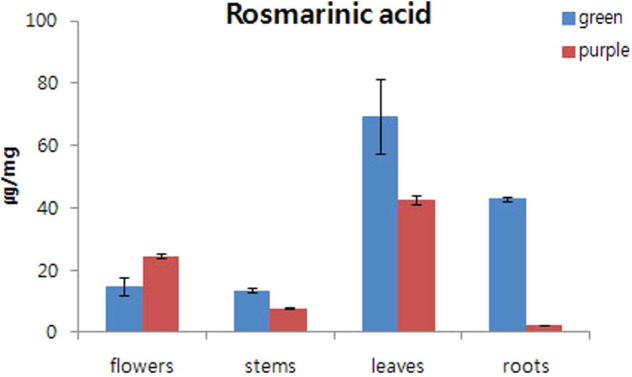
Accumulation of rosmarinic acid in different organs of green and purple basil cultivars. Data represent the mean ± SD values of 3 replicates. “green” refers to green basil and “purple” refers to purple basil.
The RA content of Rosmarinus officinalis was the highest of all polyphenols in all plant organs (del Bano et al., 2003). Of note, O. basilicum has high RA levels compared to Salvia officinalis L. (Zgorka and Glowniak, 2001). The RA of O. basilicum is induced in leaves rather than the flowers, with the results of the present study showing that higher levels of RA accumulate in the leaves the 2 studied of O. basilicum cultivars compared to the other organs. In particular, the RA content of the leaves of green basil was significantly high. Using Real-time PCR, the gene related RA expression levels of different organs of the 2 O. basilicum cultivars seemed similar. Almost all genes related to the RA pathway had higher expression in green basil compared to purple basil, except for PAL and 4CL in the root. The expression levels of genes related RA biosynthesis differs among plants. For example, the gene expression of PAL is highest in the root of Salvia miltiorrhiza and the leaves of Agastache rugosa. The expression of the C4H gene is highest in the flower of Agastache rugosa, while that of 4CL is highest in the leaves of Agastache rugosa (Tuan et al., 2012, Hou et al., 2013). In green basil (cinnamon basil), the highest transcription of PAL was obtained in the leaves. Previous studies have found that more RA accumulates in the leaves compared to the stem in Holy basil (Ocimum sanctum L.) and R. officinalis; however, once the flower develops, the highest RA accumulates in the flower compared to the leaf (Hakkim et al., 2007). In contrast, the current study showed that the highest RA content of the leaves occurred after the flowers bloomed in both cultivars, with a dry weight concentration of 69.38 μg/g and 42.52 μg/g in the green and purple colored cultivars, respectively.
4. Conclusions
This study investigates the accumulation of RA contents in different organs of 2 O. basilicum cultivars. All genes related to the RA biosynthetic pathway had considerably higher expression in the leaves of green basil compared to purple basil and the other plant organs. Low expression of the genes connected to the RA pathway in the stem was obtained, along with low RA accumulation. Significantly higher expression was obtained for genes related to the tyrosine-derived pathway in green basil compared to organs of purple basil. ObTAT and ObHPPR were shown the highest expression levels in leaves and the highest RA content was accumulated in leaves. ObTAT and ObHPPR, downstream genes in rosmarinic acid biosynthetic pathway expressed higher transcript levels compare to upstream genes related to rosmarinic acid biosynthetic pathway. Therefore RA contents will be more products if genes related downstream in rosmarinic acid biosynthetic pathway are overexpression. In conclusion, our study may be helpful for understanding the role of RA biosynthetic genes in O. basilicum.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Jae Kwang Kim, Email: kjkpj@inu.ac.kr.
Sang Un Park, Email: supark@cnu.ac.kr.
References
- Al-Dhabi N.A., Arasu M.V., Park C.H., Park S.U. Recent studies on rosmarinic acid and its biological and pharmacological activities. EXCLI J. 2014;13 [PMC free article] [PubMed] [Google Scholar]
- Campos D.A., Madureira A.R., Gomes A.M., Sarmento B., Pintado M.M. Optimization of the production of solid Witepsol nanoparticles loaded with rosmarinic acid. Colloids Surf., B. 2014;115:109–117. doi: 10.1016/j.colsurfb.2013.10.035. [DOI] [PubMed] [Google Scholar]
- del Bano M.J., Lorente J., Castillo J., Benavente-Garcia O., del Rio J.A., Ortuno A., Quirin K.W., Gerard D. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxidant activity. J. Agri. Food Chem. 2003;51:4247–4253. doi: 10.1021/jf0300745. [DOI] [PubMed] [Google Scholar]
- Doring A.S., Pellegrini E., Della Batola., Nali M., Lorenzini C., Petersen M. How do background ozone concentrations affect the biosynthesis of rosmarinic acid in Melissa officinalis? J. Plant. Phys. 2014;171:35–41. doi: 10.1016/j.jplph.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Fathiazad F., Matlobi A., Khorrami A., Hamedeyazdan S., Soraya H., Hammami M., Maleki-Dizaji N., Garjani A. Phytochemical screening and evaluation of cardioprotective activity of ethanolic extract of Ocimum basilicum L. (basil) against isoproterenol induced myocardial infarction in rats. DARU J. Pharm. Sci. 2012;20:87. doi: 10.1186/2008-2231-20-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkim F.L., Shankar C.G., Girija S. Chemical composition and antioxidant property of holy basil (Ocimum sanctum L.) leaves, stems, and inflorescence and their in vitro callus cultures. J. Agric. Food Chem. 2007;55:9109–9117. doi: 10.1021/jf071509h. [DOI] [PubMed] [Google Scholar]
- Hou X., Shao F., Ma Y., Lu S. The phenylalanine ammonia-lyase gene family in Salvia miltiorrhiza: genome-wide characterization, molecular cloning and expression analysis. Mol. Biol. Rep. 2013;40:4301–4310. doi: 10.1007/s11033-013-2517-3. [DOI] [PubMed] [Google Scholar]
- Kim Y.B., Kim J.K., Uddin M.R., Xu H., Park W.T., Tuan P.A., Li X., Chung E., Lee J.H., Park S.U. Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS ONE. 2013;8:e64199. doi: 10.1371/journal.pone.0064199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H., Peng Z., Lao N., Wang H., Chen Y., Fang Z., Hou W., Gao F., Li X., Xiong L., Tan Q. Rosmarinic acid ameliorates PTSD-like symptoms in a rat model and promotes cell proliferation in the hippocampus. Prog. Neuro-psychoph. Biol. Psychiatr. 2014;51:16–22. doi: 10.1016/j.pnpbp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Petersen M., Simmonds M.S. Rosmarinic acid. Phytochemistry. 2003;62:121–125. doi: 10.1016/s0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- Pirmoradi M.R., Moghaddam M., Farhadi N. Chemotaxonomic analysis of the aroma compounds in essential oils of two different Ocimum basilicum L. varieties from Iran. Chem. Biodivers. 2013;10:1361–1371. doi: 10.1002/cbdv.201200413. [DOI] [PubMed] [Google Scholar]
- Radulovic N.S., Blagojevic P.D., Miltojevic A.B. Alpha-Linalool – a marker compound of forged/synthetic sweet basil (Ocimum basilicum L.) essential oils. J. Sci. Food Agric. 2013;93:3292–3303. doi: 10.1002/jsfa.6175. [DOI] [PubMed] [Google Scholar]
- Saha S., Mukhopadhyay M.K., Ghosh P.D., Nath D. Effect of methanolic leaf extract of Ocimum basilicum L. on benzene-induced hematotoxicity in mice. Evid. Complement. Altern. Med. 2012;2012:176385. doi: 10.1155/2012/176385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr S.A., Nooh H.Z. Effect of Ocimum basilicum extract on cadmium-induced testicular histomorphometric and immunohistochemical alterations in albino rats. Anatomy Cell Biol. 2013;46:122–130. doi: 10.5115/acb.2013.46.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltas D., Pappas C.S., Daferera D., Tarantilis P.A., Polissiou M.G. Direct determination of rosmarinic acid in Lamiaceae herbs using diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and chemometrics. J. Agric. Food Chem. 2013;61:3235–3241. doi: 10.1021/jf305520m. [DOI] [PubMed] [Google Scholar]
- Sarahroodi S., Esmaeili S., Mikaili P., Hemmati Z., Saberi Y. The effects of green Ocimum basilicum hydroalcoholic extract on retention and retrieval of memory in mice. Ancient Sci. Life. 2012;31:185–189. doi: 10.4103/0257-7941.107354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui B.S., Bhatti H.A., Begum S., Perwaiz S. Evaluation of the antimycobacterium activity of the constituents from Ocimum basilicum against Mycobacterium tuberculosis. J. Ethnopharmacol. 2012;144:220–222. doi: 10.1016/j.jep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Suh S.Y., Baskar T.B., Kim H.H., Al-Dhabi N.A., Park S.U. Ethylene inhibitors promote shoot organogenesis of Aloe arborescens Miller. South Ind. J. Biol. Sci. 2015;1:43–46. [Google Scholar]
- Tada H., Murakami Y., Omoto T., Shimomura K., Ishimaru K. Rosmarinic acid and related phenolics in hairy root cultures of Ocimum basilicum. Phytochemistry. 1996;42:431–434. [Google Scholar]
- Tuan P.A., Park W.T., Xu H., Park N.I., Park S.U. Accumulation of tilianin and rosmarinic acid and expression of phenylpropanoid biosynthetic genes in Agastache rugosa. J. Agric. Food Chem. 2012;60:5945–5951. doi: 10.1021/jf300833m. [DOI] [PubMed] [Google Scholar]
- Umar A., Zhou W., Abdusalam E., Tursun A., Reyim N., Tohti I., Moore N. Effect of Ocimum basilicum L. on cyclo-oxygenase isoforms and prostaglandins involved in thrombosis. J. Ethnopharmacol. 2014;152:151–155. doi: 10.1016/j.jep.2013.12.051. [DOI] [PubMed] [Google Scholar]
- Zgorka G., Glowniak K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001;26:79–87. doi: 10.1016/s0731-7085(01)00354-5. [DOI] [PubMed] [Google Scholar]
- Zhang S., Ma P., Yang D., Li W., Liang Z., Liu Y., Liu F. Cloning and characterization of a putative R2R3 MYB transcriptional repressor of the rosmarinic acid biosynthetic pathway from Salvia miltiorrhiza. PLoS ONE. 2013;8:e73259. doi: 10.1371/journal.pone.0073259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Yan Y., Wang B., Liang Z., Liu Y., Liu F., Qi Z. Selective responses of enzymes in the two parallel pathways of rosmarinic acid biosynthetic pathway to elicitors in Salvia miltiorrhiza hairy root cultures. J. Biosci. Bioeng. 2014;117:645–651. doi: 10.1016/j.jbiosc.2013.10.013. [DOI] [PubMed] [Google Scholar]