Abstract
Investigations were conducted to determine the insect pollinators visiting strawberry blossoms and their impact on fruit production. Various pollinators observed during the blooming period of strawberry were viz. Apis mellifera, Apis cerana, Apis florea. Apis dorsata, soil nesting solitary bees such as Andrena leana and A. ilerda, butterflies, houseflies, syrphid flies and some beetles. The percentage of fruit set was much higher in open pollinated plants than control. There was 11.20 per cent malformed fruit in open pollinated plots as compared to 17.44 per cent in controlled one. Further the fruits obtained from the open pollinated plants were of good quality and large as compared to the controlled plants. Growers are recommended to take advantage of the several pollinators, either the honey bee or the native pollinators (Syrphidae and native bees). The importance of diversifying pollination sources, avoiding the dependence on a single specific group is stressed. This study also suggests measures which envisage the conservation, establishment and increase of native pollinators’ populations in the typical agro-ecosystem of region.
Keywords: Pollinators, Honeybee, Strawberry, Fruit set, Conservation
1. Introduction
Flowers of all the current commercial strawberry cultivars (Fragaria x ananassa Duch.) are hermaphrodite and self-fertile. However, these flowers may not be completely self-fertilizing (McGregor, 1976). Indeed, the stamens are positioned in such a way within the flower that, when anthers dehisce, pollen drops on many, but not necessarily all of the pistils (McGregor, 1976). The fertilized ovules (achenes), through auxin release, promote receptacle development (Nitsch, 1950). The achenes, resulting from fertilized ovules, are surrounded by a well-developed fleshy tissue, while receptacle zones containing non-fertilized ovules will not develop, originating a misshapen and smaller berry (Vincent et al., 1990). If there is no insect-transported pollen, the combined action of gravity and wind assures most of the pollination, even though the pollination rate of the achenes rarely surpasses 60% (Pion et al., 1980). There is a relationship between the number of fertilized ovules (achenes) and berry weight (Nitsch, 1950, Oliveira et al., 1983, Chagnon et al., 1989, Albano et al., 2005a).
Honey bees (Apis mellifera L.) are recognized as the main pollinator of the strawberry crop (Nye and Anderson, 1974, Goodman and Oldroyd, 1988, Chagnon et al., 1989, De Oliveira et al., 1990, Svensson, 1991, Free, 1993). Nonetheless, several recent studies have been carried out with the aim of extending the range of appropriate pollinators for this crop. For greenhouse conditions Bombus spp. is another widely used pollinator group (Paydas et al., 2000, Zaitoun and AL-Ghzawi, A.A., Shannag, H.K., Rahman, A., AL-Tawahaa, R.M., 2006). Several species of stingless bees have also been the subject of many studies. In Japan, Nannotrigona testaceicornis Lepeletier and Trigona minangkabau have been successful tested for strawberry pollination inside greenhouses (Maeta et al., 1992, Kakutani et al., 1993) and Malagodi-Braga and Kleinert (2004) in Brazil have shown that Tetragonisca angustula Latreille is an effective strawberry pollinator that can promote a significant increase in overall strawberry production. Some Megachilidae, such as Osmia rufa L., were also found to be effective pollinators of this crop, applicable in plastic tunnels or greenhouses (Vincent et al., 1990).
The use of managed species (A. mellifera, Bombus spp. and others) as pollinators may be vital in: i) large monocultural crops, where a great pollination effort is required; ii) ecosystems where populations of natural pollinators are reduced due to lack of adequate habitat and the use of pesticides; iii) enclosed crops such as in greenhouses; and iv) seasonal crops that precede the annual activity of pollinator insects (Teixeira and Branco, 2006). However, the problems that beehives have been facing in recent years (parasites, africanisation and others) and the consequent decline of their number, have resulted in the decrease of the available colonies for pollination and the rise of rental prices (Delaplane and Mayer, 2000). This, together with threats to native bee abundance and diversity, has contributed to the increase in research efforts on the role of native bee species in pollination of agricultural crops (Stubbs and Drummond, 2001). Furthermore “Colony Collapse Disorder” (CCD) has recently created a very serious problem for beekeepers and could threaten the pollination industry (Johnson, 2008). Native pollinators are especially appealing because they are more adapted to regional conditions and may assure pollination of strawberry flowers even when climatic conditions do not favour honey bee activity (De Oliveira et al., 1990). On certain crops, some native bee species were shown to possess a pollination efficiency that is equivalent, or higher, to that of A. mellifera (Freitas and Paxton, 1998, Canto-Aguilar and Parra-Tabla, 2000). In the particular case of strawberry crops, there is evidence for a complementary effect of native bees and honey bees visits on flower pollination (Chagnon et al., 1993, Malagodi-Braga and Kleinert, 2007). Chagnon et al. (1993) also suggest that the introduction of beehives may be questionable in sites where population densities of natural pollinators are high. Several methods have been used to compare the efficiency and effectiveness of flower-visiting insects as pollinators in strawberry crop. Nye and Anderson (1974) estimated pollination efficiency of different visiting- insect categories by attributing scores based on factors such as the amount of loose pollen carried on the body of the insect, body size, hairiness, and degree of activity. Abrol (1989) compared the efficiency of different insect pollinators on the basis of their field behavior, nectar-pollen carrying capacity and ability to pollinate flowers per unit of time. Chang et al., 2001, Zaitoun and AL-Ghzawi, A.A., Shannag, H.K., Rahman, A., AL-Tawahaa, R.M., 2006 performed comparative studies with A. mellifera and A. cerana Fabr., and with bumble bees and honey bees, respectively, analyzing several pollination effects in factors like fruit weight and percentage of malformed fruits. In view of the above, the present investigations were undertaken with the objective to determine pollinator complex, their population dynamics in relation to weather factors and the impact of pollinator visitation on qualitative and quantitative fruit production.
2. Materials and methods
2.1. Field work
The work was carried out in a strawberry field laid out at University Campus Udheywala Jammu, during Rabi 2014–2015. Strawberry (Variety Chandler) runners were transplanted in the second week of October in 3 m × 2 m plots with a plant density of approximately 60,000 plants/ha; plants were planted, in double-rows, at a distance of 30 cm from each other, growing on black plastic mulching; a drip irrigation system was used. The row to row and plant to plant distance was kept as 45 cm × 30 cm. In this field, Integrated Pest Management (IPM) procedures were adopted for crop protection.
The field observations were made during the 2014 blooming period (from late March until late May). Observations on bee/insect count were made on the number of bees and other pollinators right from commencement of the flowering till its complete cessation. Bee counts were made per 100 flowers per minute and mean population was calculated accordingly. Every day, several closed flower buds were enclosed in small synthetic nylon netting bags in order to exclude all insect visits. For this purpose, 60 flower buds chosen at random were marked and isolated from insect visits by enclosing them in synthetic nylon netting (self pollination) and equal number of buds left for open pollination (open pollination). After the harvest, fruit set was compared in both the treatments. The exclusion bags permitted airflow around the flowers and allowed wind to move the flower. The bags were removed when the flowers senesced. At the moment of ripeness normally considered for commercial purposes, the fruits were picked and their weight recorded. Then, in the laboratory, physico-chemical parameters were recorded as given below.
2.2. Color and shape of the fruit
Color and shape of the fruit was observed with the naked eye by comparing the fruits from a unit sample of 10 fruits with three replications.
2.3. Size
A unit sample of 10 fruits with three replication was drawn and length and diameter was taken with the help of digital clipper.
2.4. Average weight
A unit sample of 10 fruits with three replications with their respective weights was taken with the help of top pan balance and expressed as gms (g). The average weight was calculated of five fruits by dividing total weight by the number of fruits.
2.5. Volume
Volume of fruit was measured by water displacement method. Subsequently, the average of five fruits from each replication was calculated and expressed in cubic centimeter (cc).
2.6. Specific gravity
Specific gravity of fruit was calculated by dividing the fruit weight in air by volume of water displaced.
2.7. Total soluble solids
Total soluble solids (TSS) were measured by refractometer and the results were expressed as degree brix (0Brix). The readings were corrected by incorporating the appropriate correction factor for temperature variation (Anonymous, 1985).
2.8. Titratable acidity
Titratable acidity was estimated by titrating a known aliquot of the sample against 0.1 N sodium hydroxide solution to a faint pink color using Phenolphthalein as an indicator. The total titratable acidity was calculated and expressed as per cent malic acid.
2.9. Statistical analysis of data
The recorded data were analyzed for their variation between different treatments using Sokal and Rholf (1981).
3. Results and discussion
3.1. Diversity of insect pollinators visiting strawberry bloom
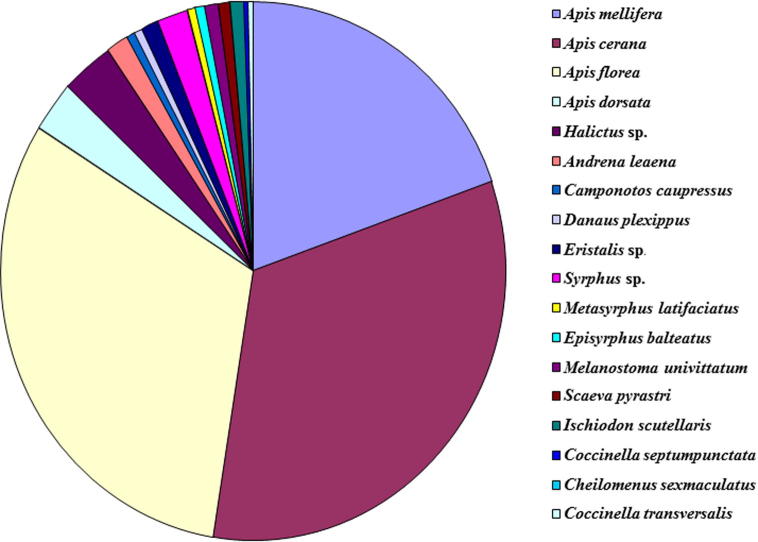
The data presented in Table 1 and Fig. 1 showed that strawberry blossoms attracted insects belonging to 4 orders, 7 families and 12 species. Of all these insects, honey bees viz. A. cerana, A. mellifera, A. florea, A. dorsata were the dominant flower visitors. Their abundance was in the order: A. melliferra > A. cerana > A. florea > A. dorsata. The other insect visitors included soil nesting solitary bees such as Andrena leana and A. ilerda, butterflies, houseflies, syrphid flies and some beetles. The latter group of insects visited the strawberry bloom at interrupted intervals and was not considered as the reliable pollinators. Evidently, the honey bees, which frequently visited the strawberry bloom in large numbers throughout the day, constitute the most important pollinators. Bees are by far the most effective strawberry flower pollinators (Antonelli et al., 1988). Singh (1979) in India reported honey bees and some other insects as the most important flower visitors of strawberry. Woo et al. (1986) in South Korea reported that, hoverflies (syrphidae) and solitary bees, especially Andrenidae, were common visitors to strawberry flowers. Abrol (1989) found A. cerana as an important pollinator of strawberries and comprised more than 80 per cent flower visitors. The other pollinators included Lasioglossum species, Xylocopa spp. ants and flies. It was previously reported that honeybees (A. mellifera and A. cerana) are worthy pollinators (McGregor, 1976). Kabayashi, 1970, Maeta, 1978a recorded some other pollinators such as Eristalis cerealis (Diptera) and Osmia cornifrons frequenting flowers of strawberry. Several investigators reported that among the various insects (honeybees, flies, beetles, thrips, and butterflies) visiting strawberries honeybees accounted for about 63 percent (Nye and Anderson, 1974, Singh, 1979, Pion et al., 1980). Anderson (1964) collected 108 species of insects belonging to 35 families visiting strawberry flowers in Utah and USA. He categorized one species of honeybees, 2 species of Osmia, 1 species of Halictus and 2 species of Eristalis as the most important pollinators. Free (1968) reported that very few bumble bees were visiting strawberry flowers and relatively cold weather deferred honey bees from doing so. Hooper (1932) reported that pollination is carried mainly by insects other than bees and especially by Diptera when it is cold. Insect pollinators are also reported to vary from crop to crop from one location to another and during different parts of the year. In South Korea, hoverflies (syrphidae) and solitary bees, especially Andrenidae, were common visitors to strawberry flowers (Woo et al., 1986).
Table 1.
Insect pollinators visiting strawberry bloom.
| Order | Family | Insect visitors | Period of activity | Percentage proportion | Status (%) |
|---|---|---|---|---|---|
| Hymenoptera | Apidae | Apis mellifera | 4th week of Jan to May | 19.95 | 89.17 |
| Apidae | Apis cerana | 4th week of Jan to May | 33.00 | ||
| Apidae | Apis florea | 4th week of Jan to May | 31.36 | ||
| Apidae | Apis dorsata | 4th week of Jan to May | 4.86 | ||
| Halictidae | Halictus sp. | 4th week of Jan to May | 2.95 | 11.83 | |
| Andrenidae | Andrena leaena | 4th week of Jan to May | 1.30 | ||
| Hymenoptera | Formicidae | Camponotos compressus | 4th week of Jan to May | 0.60 | |
| Lepidoptera | Danaidae | Danaus plexippus | 4th week of Jan to May | 0.53 | |
| Diptera | Syrphidae | Eristalis sp. | 4th week of Jan to May | 0.85 | |
| Syrphidae | Syrphus sp. | 4th week of Jan to May | 1.80 | ||
| Syrphidae | Metasyrphus latifaciatus | 4th week of Jan to May | 0.60 | ||
| Syrphidae | Episyrphus balteatus | 4th week of Jan to May | 0.55 | ||
| Syrphidae | Melanostoma univittatum | 4th week of Jan to May | 0.55 | ||
| Syrphidae | Scaeva pyrastri | 4th week of Jan to May | 0.90 | ||
| Ischiodon scutellaris | 4th week of Jan to May | 0.40 | |||
| Coleoptera | Coccinellidae | Coccinella septumpunctata | 2nd week of Feb-May | 0.25 | |
| Coccinellidae | Cheilomenus sexmaculatus | 2nd week of Feb-May | 0.20 | ||
| Coccinellidae | Coccinella transversalis | 2nd week of Feb-May | 0.15 | ||
| Coccinellidae | Coccinella trifasciata | 2nd week of Feb-May | 0.11 | ||
| Coccinellidae | Illeis cincta | 2nd week of Feb-May | 0.09 |
Fig. 1.
Percentage proportion of insect visitors on strawberry bloom.
3.2. Foraging behavior
Differences were found between the behavior of A. mellifera, Acerana, A. florea and A. dorsata and native bees during their visits to strawberry flowers. While A. mellifera always landed on the top of flowers, native bees landed on the stamen zone, and occasionally even on the petal zone. During visits, both A. mellifera and native bees performed a circular movement around the flower, allowing contact with basal pistils (which are close to the stamens), but while A. mellifera ensured a contact with the head as its proboscis was inserted into nectaries, native bees made that contact using the whole body, amid the rows of stamens, alternating between pollen and nectar collection. While foraging for nectar, the body size of A. mellifera allowed permanent contact of its thorax and abdomen with the apical pistils, promoting the transport and deposit of pollen within this flower region. However native bees, due to their smaller body size, rarely contacted the apical region, restricting most of their action to the basal zone. It should be noted that this description referred to the whole sample of observed individual bees of the Halictidae family. In post study observations, other native bees, such as those belonging to the Andrenidae and Megachilidae families, with larger body sizes, were occasionally observed on flowers, showing a similar behavior to that of A. mellifera. Eristalis spp. (Diptera, Syrphidae) were mostly observed performing a circular movement around flower stamen rows, reaching the nectaries with their proboscis and probing the anthers, one by one. During their visits, these indigenous syrphid flies allowed permanent contact of their bodies not only with the basal but also with the apical pistils, due to their large body sizes. A. dorsata lay across the flowers rubbing pollen against the stigma whereas A. florea being of smaller size restricted movements in the basal zone. A. cerana commenced activities much earlier than A. mellifera, A. dorsata and A. florea and activities ceased later than other bees. Interestingly populations of A. florea were observed in large numbers throughout the day. In earlier studies, Abrol (1989) compared the efficiency of different insect pollinators on the basis of their field behavior, nectar-pollen carrying capacity and ability to pollinate flowers per unit of time.
3.3. Seasonal activity of strawberry pollinators in relation to weather parameters
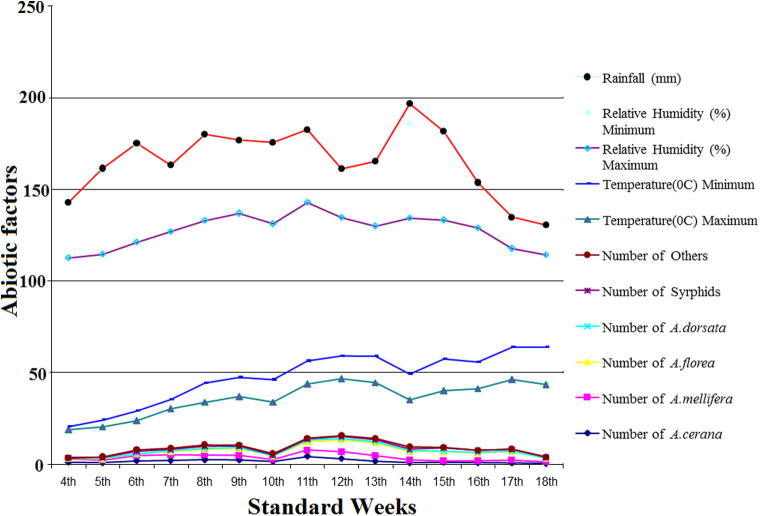
Observations made during the blooming period of strawberry revealed that the activity all the four honeybee species A. cerana, A. mellifera, A. dorsata and A. florea started during the 4th standard week when the maximum and minimum temperature, morning and evening relative humidity and rainfall were 15.40 and 1.70 °C, 92.10 and 30.10 and 0.00 respectively (Table 2, Fig. 2). In case of A. cerana peak population was observed during 11th standard week when the maximum and minimum temperature, morning and evening relative humidity and rainfall were 29.50 and 12.60 °C, 86.60 and 39.70 and 0.00, respectively. The activity of A. mellifera peaked during the 12th standard week of 2014 when the maximum and minimum temperature, morning and evening relative humidity and rainfall were 31.10 and 12.40 °C, 75.60 and 26.40 and 0.00 respectively whereas the activity in A. florea peaked during the 13th standard week of 2014 when maximum and minimum temperature, morning and evening relative humidity and rainfall were 30.40 and 14.40 °C, 71.00 and 35.40 and 0.00, respectively. The population of A. dorsata peaked during 11th standard week when the maximum and minimum temperature, morning and evening relative humidity and rainfall were 29.50 and 12.60 °C, 86.60 and 39.70 and 0.00, respectively The population of syrphids peaked during the 15th standard week of 2014 when the maximum and minimum temperature, morning and evening relative humidity and rainfall were 30.80 and 17.40 °C, 75.90 and 47.70 and 0.50, respectively. Other pollinators which include Lepidopterans, ants, flies and other Dipterans were found peak during the 14th standard week of 2014 when the maximum and minimum temperature, morning and evening relative humidity and rainfall were 25.60 and 14.20 °C, 85.10 and 51.40 and 10.90, respectively. The data clearly revealed that each bee species/insect pollinators had specific ecological threshold for its field activities.
Table 2.
Seasonal abundance of insect pollinators on strawberry bloom during January 2014 to April 2014 (where, A.c = Apis cerana, A.m = Apis mellifera, A.f = Apis florea and A.d = Apis dorsata).
| Standard week | Number of insect pollinators/100 flowers/minute |
Temperature (°C) |
Relative humidity (%) |
Rainfall (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. cerana | A. mellifera | A. florea | A. dorsata | Syrphids | Others | Maximum | Minimum | Maximum | Minimum | ||
| 4th | 0.875 | 2.000 | 0.250 | 0.250 | 0.000 | 0.000 | 15.4 | 1.7 | 92.1 | 30.1 | 0.0 |
| 5th | 0.750 | 1.375 | 0.625 | 0.500 | 0.125 | 0.625 | 16.3 | 3.8 | 90.4 | 45.6 | 1.2 |
| 6th | 1.625 | 3.000 | 0.625 | 0.125 | 1.375 | 1.000 | 15.8 | 5.5 | 92.0 | 52.6 | 1.4 |
| 7th | 2.000 | 3.000 | 1.875 | 0.375 | 0.625 | 0.750 | 21.5 | 5.1 | 91.7 | 36.1 | 0.0 |
| 8th | 2.375 | 2.500 | 3.125 | 0.375 | 1.250 | 0.875 | 23.1 | 10.6 | 88.7 | 46.1 | 0.9 |
| 9th | 2.250 | 2.500 | 3.750 | 0.500 | 0.750 | 0.625 | 26.5 | 10.4 | 89.6 | 40.0 | 0.0 |
| 10th | 1.375 | 1.000 | 2.000 | 0.000 | 0.375 | 1.000 | 28.0 | 12.4 | 85.1 | 44.3 | 0.0 |
| 11th | 4.125 | 3.500 | 4.500 | 0.875 | 0.625 | 0.500 | 29.5 | 12.6 | 86.6 | 39.7 | 0.0 |
| 12th | 2.875 | 3.875 | 6.375 | 0.625 | 1.250 | 0.500 | 31.1 | 12.4 | 75.6 | 26.4 | 0.0 |
| 13th | 1.500 | 3.125 | 6.875 | 0.500 | 1.250 | 0.750 | 30.4 | 14.4 | 71.0 | 35.4 | 0.0 |
| 14th | 0.750 | 1.500 | 4.625 | 0.375 | 0.750 | 1.375 | 25.6 | 14.2 | 85.1 | 51.4 | 10.9 |
| 15th | 0.875 | 0.750 | 5.250 | 0.125 | 1.625 | 0.500 | 30.8 | 17.4 | 75.9 | 47.7 | 0.5 |
| 16th | 0.750 | 1.125 | 4.250 | 0.125 | 1.000 | 0.375 | 33.4 | 14.6 | 73.4 | 24.3 | 0.2 |
| 17th | 0.625 | 1.500 | 4.750 | 0.125 | 0.875 | 0.375 | 37.8 | 17.7 | 54.0 | 17.0 | 0.0 |
| 18th | 0.375 | 0.875 | 2.000 | 0.000 | 0.375 | 0.125 | 39.6 | 20.4 | 50.4 | 16.1 | 0.0 |
Fig. 2.
Seasonal abundance of insect pollinators visiting strawberry blossoms per 100 flowers per 10 min.
3.4. Correlation between insect pollinators population and weather parameters on strawberry
Analysis of data on seasonal abundance of pollinators on strawberry bloom in relation to different environmental variables such as maximum and minimum temperature, morning and evening relative humidity and rainfall revealed that changes in maximum and minimum temperature, morning and evening relative humidity did not significantly influence the field activities of A. cerana, A. mellifera, A. dorsata, syrphids and other insect visitors (Table 3). However, foraging populations of A. florea responded significantly to variations in maximum and minimum temperature. The relationship between A. florea population at maximum and minimum temperature was found to be highly significant. Foraging populations of A. florea were significantly and negatively influenced by morning relative humidity. However, the evening relative humidity though negatively influenced the population but the relationship was not significant. Rainfall had a negative effect on the foraging activity of all the insect pollinators except A. dorsata but the relationship was not significant with foraging populations of any of the pollinators.
Table 3.
Correlation coefficient between population dynamics of insect pollinators and weather parameters on strawberry.
| Insect pollinators | Temperature (°C) |
Relative humidity (%) |
Rainfall (mm) | ||
|---|---|---|---|---|---|
| Maximum | Minimum | Morning | Evening | ||
| Apis cerana | 0.016 | −0.051 | 0.227 | 0.058 | −0.115 |
| Apis mellifera | −0.032 | −0.220 | 0.147 | −0.164 | −0.121 |
| Apis florea | 0.597 | 0.596 | −0.401 | −0.246 | −0.251 |
| Apis dorsata | −0.067 | −0.010 | 0.174 | 0.176 | 0.144 |
| Syrphids | 0.291 | 0.228 | −0.088 | −0.204 | −0.198 |
| Others | −0.91 | −0.070 | 0.276 | 0.168 | −0.032 |
Each bee pollinator has specific ecological threshold for foraging activity which differ inter and intra specifically depending upon the level of adaptation of a given species in an environment (Burill and Dietz, 1981, Abrol and Kapil, 1986). Burill and Dietz (1981) reported that the bee activity increased with temperature but was not effected by relative humidity and vapour pressure. Nunez (1977) found that in case of A. mellifera, morning activity was related to nectar flow and in the evening it was correlated with the photoperiod. Iwama (1977) found that the interaction between temperature and light intensity was responsible for the flight activity of Tetragonisca angustica. Abrol and Kapil (1986) found that light intensity and solar radiations were important factors controlling flight activity of Megachile lanata. In India, honey bees and some other insects were recorded as the most important flower visitors (Singh, 1979).
3.5. Impact of pollination treatments on physico-chemical characters of fruit
The data in Table 5 shows that of the fruits obtained, the various parameters were analyzed and found that the color of the fruits was red in open pollinated and partial red in control treatments respectively. The fruits obtained from open pollinated plants were well formed and in control a sizeable number of the fruits were misshapen. The size of the fruits was medium to large in open pollinated plants whereas small size was recorded in control plants. The average weight of the fruit in case of open pollinated plants was 15.25 (g) per fruit whereas 12.24 (g) in case of control. Similarly, the volume of the fruit recorded was found to be 16.15 (c.c) in open pollinated plot whereas in case of control it was 15.20 (c.c). The specific gravity of the fruit in open pollinated treatment was 0.94 whereas 0.91 in case of fruits obtained from control was recorded. The TSS (0brix) of fruits was analyzed and found that it was 6.83 and 6.72 in open pollinated and control treatment respectively. It was further found that the fruits obtained from open pollinated crop were less acidic with 0.69 percent and 0.82 in control treatment. Further, the fruits obtained from open pollinated plants were possessing good and superior fruit contents over control which is a clear cut indication that insects play a very vital role in fruit quality and quantity.
Table 5.
Physico-chemical characters of fruits obtained from different pollination treatments.
| Treatment | Fruit parameters |
|||||||
|---|---|---|---|---|---|---|---|---|
| Color | Shape | Size | Av. weight (g) | Volume | Sp. gravity | TSS (0Brix) | Acidity (%) | |
| Open pollinated | Red | Well formed | Medium | 15.25 | 16.15 | 0.94 | 6.83 | 0.69 |
| No Pollination (control) | Partial red | Misshapen | Small | 12.24 | 15.20 | 0.91 | 6.72 | 0.82 |
3.6. Impact of pollination treatments on qualitative and quantitative yield parameters
Strawberry crop is a highly cross-pollinated and depends heavily upon pollinating insects for fruit production. The data in Table 4 shows that the percentage of fruit set in open pollinated and control plots was 70 percent and 45 percent, respectively. The data further shows that there was 11.20 percent malformed fruiting in open pollinated treatment as compared to 17.44 percent in controlled one. In open pollinated plots 1.55 folds increase in yield was recorded. The average weight of the fruit in case of open pollinated plants was 15.25 (g) per fruit whereas 12.24 (g) in case of control.
Table 4.
Qualitative and quantitative effect of pollination on strawberry attributes.
| Treatment | Parameters |
|||
|---|---|---|---|---|
| No. of buds | Fruit set | Malformed fruits | Percentage of malformed fruits | |
| Open pollination | 191.66 + 10.40 | 134.00 + 1.52 | 15.00 + 1.00 | 11.20 |
| Control | 191.66 + 10.40 | 86.00 + 1.52 | 15.00 + 1.00 | 17.44 |
Values are mean + S.D of n = 5.
The data presented in Table 4 shows that the fruits obtained from open pollinated plants were well formed and in control a sizeable number of the fruits misshapen. The size of the fruits was medium to large in open pollinated plants compared to control plants. Fruits obtained from open pollinated plots were superior in physic-chemical characteristics such as the volume of the fruit, specific gravity, the TSS and the acidity. Evidently, insect pollination must be encouraged in strawberry cultivation to boost the quality and quantity of fruit. In general, strawberries depend on pollinating insects for quality and quantity of fruit production. Allen and Gaede (1963) reported that fruit setting of Shasta strawberries in caged and undisturbed plants by man, insects or breeze set no fruits, while those uncaged and undisturbed set 20 percent, uncaged but receiving wind from the fan set 77 percent, whereas those caged but brush pollinated daily set 97 percent flowers. Skrebtsova (1957) reported the influence of visits of honeybees to the strawberry flowers that more visits resulted in heavier berries: 16–20 visits resulted in berries weighing average 5.36 g and 21–25 visits produced berries that averaged 8.13 g. Muttoo (1952) reported that location of an apiary near a strawberry plot increased the average per acre production of berries 840–1225 pounds. Petkov (1963) reported that 31–39 percent of flowers isolated from bees developed fruits compared to 55–60 percent of those freely visited by pollinating insects, the isolated flowers developed 60–65 percent culls compared to 14–17 percent from bee visited flowers and the average weight of fruit from the isolated flowers was only one third of that from the bee visited flowers.
The comparison of results between the “Unpollinated” treatment and others revealed a strong contribution of insect visits for the pollination of strawberry crops, in accordance with other studies (Chagnon et al., 1989, Chagnon et al., 1993, López-Medina, 2002, Malagodi-Braga, 2002, Albano et al., 2005a, Albano and Salvado, 2005b, López-Medina et al., 2006). Cirnu et al., (1978) in Romania, reported that when plants from greenhouses were isolated from honeybees there was 50–59% fruit set compared to more than 80% fruit set when honeybees were present, and the final yield of plants was 107 percent greater. Skrebtsova (1957) reported that the percentage of flowers that set fruit increased with the number of bee visits per flower up to 15–20 visits. She also reported that until about 60 visits had been made the mean weight of the berries continued to increase. Hughes (1962) reported that in caged plants there was a decrease in yield and malformed fruits were produced, some of which were quite unsaleable. Ahn et al. (1989) recorded a 9 times increase in fruit weight when bees were used in strawberry fields. Strawberry flowers produced 11.9 g of fruit on the first cluster without honeybee A. mellifera while with honeybees fruit weight increased by 4 times to 65.9 g, however, with use of A. cerana produced 64.9 g of fruit weight (Chang et al., 2001). Houbaert et al. (1992) studied the effect of pollination by honey bees on yield and fruit quality of strawberries. They obtained 18 per cent higher yield in bee pollinated plots as compared to those with open pollination. Insufficient pollination and fertilization due to caging led to inferior fruit quality. Chagnon et al. (1989) found that honey bee pollination is essential for commercial fruit production in strawberries, whereas, Gundia (1995) recommended the use of bumble bees for pollination of strawberry in green houses. Evidently, exploration of insect pollinators and management of pests is essential for commercial fruit production in strawberry. The multitude of various factors affecting pollinator attractiveness and their role in fruit production constitutes a problem for investigation.
3.7. Selection and management of potential pollinators
The results presented in this study indicate the existence of a set of potentially useful pollinators, with equivalent effectiveness levels, which includes both native pollinators (Syrphidae and native bees) and domesticated honey bees. However, results regarding the Syrphidae group should be interpreted with caution since they were based on a small sample. However, the information gathered in this study, supplemented with the data made available in Albano et al. (2009), may provide useful information for further discussion on management issues concerning these insects, seeking to maximise pollination in strawberry crops. During the early blooming phases, when the number of indigenous insects (native bees and Syrphidae) are relatively low (Albano et al., 2009), it is important to advise growers to install beehives of A. mellifera, in order to increase the probability of honey bee visits to compensate the lack of native pollinators. As stated in Albano et al. (2009), the success of using honey bee colonies may require the use of certain management practices that seek to maintain and enhance the number of foragers throughout the blooming season (Currie, 1997, Ohishi, 1999). It is important to recognize the competition faced by strawberry flowers, either by other crops or natural vegetation, since this type of crop is not especially attractive to pollinators (Currie, 1997, McGregor, 1976). Furthermore, strawberry cultivars vary in their attractiveness to pollinators (Vincent et al., 1990, Abrol, 1992).
The best option for growers may be to increase diversification of pollination sources, avoiding the dependence of a single specific group. The installation of beehives needs to conform to certain recommendations in order to increase the success of their use in this crop in the long term. The monitoring of these beehives by professional apiculture technicians should be considered.
Acknowledgement
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
Acknowledgments
Declaration of interest
The authors confirm that there is no conflict of interests and are also liable for the content and writing of this article.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abrol D.P. Studies on ecology and behaviour of insect pollinators frequenting strawberry blossoms and their impact on yield and fruit quality. Trop. Ecol. 1989;30(1):96–100. [Google Scholar]
- Abrol D.P. Energetics of nectar production in some strawberry cultivars as a predictor of floral choice by honey bees. J. Biosci. 1992;17(1):41–44. [Google Scholar]
- Abrol D.P., Kapil R.P. Factors affecting pollination activity of Megachile lanata. P Indian AS-Anim Sci. 1986;95:757–769. [Google Scholar]
- Ahn S.B., Kim I.S., Cho W.S., Choi K.M. Survey on the use of honeybee pollination of strawberries grown in plastic green house. Korean J. Apic. 1989;4(1):1–8. [Google Scholar]
- Albano, S., De Oliveira, D., Salvado, E., Quaresma, M., Borges, P., Mexia, A., 2005. Avaliação da importância dos insectos polinizadores na melhoria da produção na cultura do morango. Actas da Associação Portuguesa de Horticultura, II Colóquio Nacional da Produção de Morango e Pequenos Frutos, São Teotónio, Portugal, pp. 121–129.
- Albano, S., Salvado, E., Borge,s P., Mexia, A., 2005. Análise comparativa dos vários agentes de polinização na cultura do morangueiro.Actas do V Congresso Ibérico de Ciências Hortícolas, Porto, Portugal, pp. 20–25.
- Albano, S., Salvado, E., Borges, P.A.V., Mexia A., 2009. Floral visitors, their frequency, activity rate and Index of Visitation Rate in the strawberry fields of Ribatejo, Portugal: selection of potential pollinators. Part 1. Adv. Hort. Sci. 23(4), 238–245.
- Allen W.W., Gaede S.E. Strawberry pollination. J Econ Entomol. 1963;56:823–825. [Google Scholar]
- Anderson L.D. The effect of sevin on honey bees. Gleanings in Bee Cult. 1964;92(6):358–364. [Google Scholar]
- Antonelli A.L., Mayer D.F., Burgett D.M., Sjulin T. Pollinating insects and strawberry yields in the Pacific Northwest. Am. Bee. J. 1988;128:618–620. [Google Scholar]
- Burill R.M., Dietz A. The response of honeybees to variations in solar radiation and temperature. Apidologie. 1981;12:319–328. [Google Scholar]
- Canto-Aguilar M.A., Parra-Tabla V. Importance of conserving alternative pollinators: assessing the pollination efficiency of the squash bee, Peponapis limitaris in Cucurbita moschata (Cucurbitaceae) J. Insect. Conserv. 2000;4(3):203–210. [Google Scholar]
- Chagnon M., Gingras J., De Oliveira D. Effect of honey bee (Hymenoptera: Apidae) visits on the pollination rate of strawberries. J. Econ. Entomol. 1989;82(5):1350–1353. [Google Scholar]
- Chagnon M., Gingras J., De Oliveira D. Complementary aspects of strawberry pollination by honey and indigenous bees (Hymenoptera) J. Econ. Entomol. 1993;86(2):416–420. [Google Scholar]
- Chang Y.D., Lee M.Y., Mah Y. Pollination on strawberry in the vinyl house by Apis mellifera L. and Apis cerana Fab. Acta Hort. 2001;561:257–262. [Google Scholar]
- Cirnu I., Fota G., Grosu E. Rezultate privind polenizarea cu ajutorul albinelor a culturilor de capsuni (Fragaria sp) in solarii. Apicultura in Romania. 1978;53:12–14. [Google Scholar]
- Currie, R.W., 1997. Pollination constraints and management of pollinating insects for crop production, pp. 121–151. In: Darrow, G.M., 1966 - The strawberry: History, breeding and physiology. Holt, Rinehart and Winston, New York, USA, pp. 447.
- Oliveira D.D., Pion S., Paradis R.O. Entomogamie et production du fraisier'Redcoat', Fragaria X ananassa Duch., au Quebec. Bull. Soc. Entomol. Fr. 1983;88:356–359. [Google Scholar]
- De Oliveira, D., Savoie, L., Vincent, C., 1990. August. Pollinators of cultivated strawberry in Québec. In: VI International Symposium on Pollination. 288, pp. 420–424.
- Delaplane K.S., Mayer D.F. CABI Publishing, Oxon; UK: 2000. Crop pollination by bees; p. 344. [Google Scholar]
- Free J.B. second ed. Academic Press; London, UK: 1993. Insect pollination of crops; p. 768. [Google Scholar]
- Free J.B. The behaviour of bees visiting runner beans (Phaseolus multiflorus) J. Appl. Entomol. 1968;5:631–638. [Google Scholar]
- Freitas S.B.M., Paxton R.J. A comparison of two pollinators: the introduced honey bee Apis mellifera and an indigenous bee Centris tarsata on cashew Anacardium occidentale in its native range of NE Brazil. J. Appl. Ecol. 1998;35(1):109–121. [Google Scholar]
- Goodman R.D., Oldroyd B.P. Honeybee pollination of strawberries (Fragaria x ananassa Duchesne) Aust. J. Exp. Agric. 1988;28(3):435–438. [Google Scholar]
- Gundia M. The use of bumble bees, Bombus terrestirs, for pollination of greenhouse strawberry. Hassadeh. 1995;75(9):49–50. [Google Scholar]
- Hooper C.H. The insect visitor of fruit blossoms. J. R. Soc. Arts. 1932;81:86–105. [Google Scholar]
- Houbaert D., Borremans G., Baetc W., Jacobs F.J. Quality improvement of strawberries through optimal pollination. Fruit Lts. 1992;5(20):26–29. [Google Scholar]
- Hughes H.M. Progress Report MAAF, NAAS; Rep Efford Exp Hort Stn: 1962. Pollination studies; p. 1961. [Google Scholar]
- Iwama S. Influence of climatic factors on external flight activity of Tetragonisca Angustica: Boletim de zoologia São Paulo. 1977;2:189–201. [Google Scholar]
- Johnson, R., 2008. Recent honey bee colony declines. CRS Report for Congress, 28 May, pp. 19. Available in http://www.fas.org/sgp/crs/misc/RL33938.pdf.
- Kabayashi M. Apple pollination by Eristatis cerealis and their proliferation method. Nougyou Obobi Engei. 1970;45:505–508. (In Japanese) [Google Scholar]
- Kakutani T., Inoue T., Tezuka T., Maeta Y. Pollination of strawberry by the stingless bee, Trigona minangkabau, and the honey bee, Apis mellifera: an experimental study of fertilization efficiency. Res. Popul. Ecol. 1993;35(1):95–111. [Google Scholar]
- López-Medina, J., 2002. Análisis y evaluación agronómica de las deformaciones de fruto en fresa (Fragaria x ananassa Duch.). Posibles soluciones. Resultados preliminaries. Work document for internal use. (In Spanish).
- López-Medina J., Palácio Villegas A., Vazquezortiz E. Misshaped fruit in strawberry, an agronomic evaluation. Acta Hort. 2006;708:77–78. [Google Scholar]
- Maeta Y. Comparative studies on the biology of the bees of the genus Osmia in Japan, with special reference to their management for pollination of crops (Hymenoptera, Megachilidae) Bull. Tohoku Nat. Agric. Exp. Stn. 1978;57:1–221. (In Japanese) [Google Scholar]
- Maeta Y., Tezuka H., Susuki K. Utilisation of the Brazillian stingless bee, Nannotrigona testaceicornis as a pollinator of strawberries. Honeybee Sci. 1992;13(2):71–78. [Google Scholar]
- Malagodi-Braga K.S. Thesis, Instituto de Biociências, Universidade de São Paulo, São Paulo, Brazil; 2002. Estudo de agentes polinizadores em cultura de morango (Fragaria x ananassa Duchesne- Rosaceae) (In Portuguese) [Google Scholar]
- Malagodi-Braga K.S., Kleinert A.M.P. Could Tetragonisca angustula Latreille (Apinae, Meliponini) be effective as strawberry pollinator in greenhouse? Aust. J. Agric. Res. 2004;55(7):771–773. [Google Scholar]
- Malagodi-Braga K.S., Kleinert A.M.P. How bee behavior on strawberry flower (Fragaria ananassa Duchesne) can influence fruit development? Biosci. J. Uberlândia. 2007;23(1):76–81. [Google Scholar]
- McGregor, S.E., 1976. Insect pollination of cultivated crop plants. Available in http://www.beeculture.com/content/pollination handbook/strawberry-1.html (9 Mar. 2009).
- Muttoo R.N. Bees produce more fruit. Indian Bee J. 1952;1(7):118–119. [Google Scholar]
- Nitsch J.P. Growth and morphogenesis of strawberries as related to auxin. Am. J. Bot. 1950;37:211–215. [Google Scholar]
- Nunez J.A. Nectar flow by melliferous flora and gathering flow by Apis mellifera ligustica. J. Insect Physiol. 1977;23:265–276. [Google Scholar]
- Nye W.P., Anderson J.L. Insect pollinators frequenting strawberry blossoms and the effect of honey bees on yield and fruit quality. J. Am. Soc. Hortic. Sci. 1974;99(1):40–44. [Google Scholar]
- Ohishi, T., 1999. Appropriate management of honeybee colonies for strawberry pollination. Honeybee Sci. 20(1), 9–16.
- Paydas S., Eti S., Kaftanoglu O., Yasa E., Derin K. Effects of pollination of strawberries grown in plastic greenhouses by honeybees and bumblebees on the yield and quality of the fruits. Acta Hort. 2000;513:443–451. [Google Scholar]
- Petkov V. Nectar and honey productivity of winter rape. Izv. Inst. Ovoshcharstvo. 1963;5:181–188. [Google Scholar]
- Pion S., De Oliveira D., Paradis R.O. Agents pollinisateurs et productivité du fraisier ‘Redcoat’. Phytoprotection. 1980;61:72–78. [Google Scholar]
- Singh Y. Pollination activity on strawberry at Jeolikote (District Nainital, India) Indian Bee J. 1979;541:117–119. [Google Scholar]
- Skrebtsova N.D. The role of bees in pollinating strawberries. Pchelovodstvo, Mosk. 1957;34:34–36. [Google Scholar]
- Sokal, R.R., Rholf, F.J., 1981. Biometry 2nd edition San Francisco.
- Stubbs, C.S., Drummond, F.A., (Eds.), 2001. Bees and crop pollination: crisis, crossroads, conservation. Lanham, MD: Entomological Society of America. Lanham, Mary Land., pp. 156.
- Svensson B. The importance of honeybee-pollination for the quality and quantity of strawberries (Fragaria x ananassa) in central Sweden. Acta Hort. 1991;288:260–264. [Google Scholar]
- Teixeira G., Branco M. ISA Press; Portugal: 2006. Pólen. Série Didáctica Botânica; p. 65. [Google Scholar]
- Vincent, C., De Oliveira, D., Bélanger, A., 1990. The management of insect pollinators and pests in Québec strawberry plantations, pp. 177–192. - In: Bostanian, N.J., I.T., Wilkaniec, Z., Radajewska, B., 1997. Solitary bee Osmia rufa L. (Apoidea, Megachilidae) as pollinator of strawberry cultivated in an unheated plastic tunnel. Acta Hort. 439, 489–493.
- Woo K.S., Choo H.Y., Choi K.R. Studies on the ecology and utilization of pollinating insects. The Korean J. Apic. 1986;1(1):54–61. [Google Scholar]
- Zaitoun S.T., AL-Ghzawi A.A., Shannag H.K., Rahman A., AL-Tawahaa R.M. Comparative study on the pollination of strawberry by bumble bees and honey bees under plastic house conditions in Jordan valley. J. Food Agr. Environ. 2006;4(2):237–240. [Google Scholar]