Abstract
One of the most common deadliest parasitic diseases is Malaria. The biology and the pathogenesis of this fascinating parasite are not yet fully understood which make discovering effective alternative drugs a challenging task. Moreover, the emergence of resistant strains added an additional burden in the journey of malaria elimination. Traditional medicine used to be an alternative therapy choice owing to the presence of potent natural products. Ziziphus spina-christi (L.) considered being one of the common potent natural plant in gulf region and other nations. Therefore, this study designed to evaluate the ameliorative role of Z. spina-christi leaf extracts (ZSCLE) against Plasmodium chabaudi-induced hepatic injury. The study involved three groups were as follows; a vehicle control group, infected with 106P. chabaudi-parasitized erythrocytes group and ZSCLE treated-infected mice with 106P. chabaudi-parasitized erythrocytes group. The results showed a remarkable reduction of parasitemia level and notable reverse of the anemic picture among ZSCLE treated-infected mice. The effects of ZSCLE on the liver functions enzymes and on the histopathological pictures of liver were significant. It could be concluded that Z. spina-christi leaf extracts have a protective role against Plasmodium infection that also marked through significant restoration of hepatic oxidative markers.
Keywords: Malaria, Ziziphus spina-christi, Liver, Histology, Oxidative stress, Mice
1. Introduction
Malaria is a life-threatening blood disease that still causing high mortality rate regardless to all the efforts that have been made in preventing the disease. In fact, three main integrated actions are considered the power in preventing malaria which is vector control, effective vaccination and potent antimalarial drug. Application of Vector control targets reduction of malaria transmission (Benelli, 2015) whereas malaria vaccine, currently in clinical phase III trials, aims toward malaria elimination (Mian-McCarthy et al., 2012).
For antimalarial drugs, emerging resistance against effective drugs like artemisinin-based combination therapies as well as the previous first-line therapies has complicated malaria treatment (Kumar et al., 2015). Therefore as continuing to all the efforts in fighting malaria, scientist explored different potential therapy in the folkloric medicine. Zizyphus spina-cristi (L.) Willd (ZSC), common names Sidr in Arabic and Christ’s thorn or Jerusalem-thorn in English, is a member of Rhamnaceae family. The genus Zizyphus has medicinal importance and has been used to maintain a healthy life style by the local Arab people. Z. spina-christi has been reported to have antimicrobial activity against pathogens that are normally quite resistant to modern medications (Nazif, 2002). Recent studies showed a significant effect of Z. spina-christi on Plasmodium berghei parasite induced hepatic and spleen tissue damage (Hafiz and Mubaraki, 2016). Therefore, examining the liver during malaria is informative as liver pathology usually varies from slight discrepancies in liver function tests to severe liver failure (Anand and Puri, 2005, Whitten et al., 2011). Indeed, the fascinating life cycle of Plasmodium parasite explains the importance of liver where the clinically silent pre-erythrocytic stages of the parasite took place (Cowman and Crabb, 2006).
Regarding the study model, previous studies have used Plasmodium chabaudi rodent malaria parasite as an appropriate model to study the role of liver during malaria infection since it shared many of the pathological and immunological features with the human malaria parasite, P. falciparum (Hall et al., 2005). Therefore, the current work proposed to study the Ameliorative role of Z. spina-christi leaf extract against hepatic injury induced by P. chabaudi-infected erythrocytes.
2. Materials and methods
2.1. Leaf extracts preparation
Ziziphus spina-christi leaf extracts (ZSCLE) was prepared with minor modifications according to Hafiz and Mubaraki (2016). A defined amount of ZSCLE (100 g) in air dried powder was extracted using 70% methanol at 27 °C. Then it was reserved at 4 °C for 24 h. Reduced pressure (bath temperature 50 °C) was used to concentrate the extracts before being dried in a vacuum evaporator. For experimental use, distilled water was used to dissolve the residue.
2.2. Mice and P. chabaudi infection
Using the animal house facility at King Faisal Hospital, Riyadh, adult C57BL/6 malemice were obtained. Animals were maintained in a specific pathogen-free condition upon strict agreement with the institutional and national official guideline at the department of Zoology animal housing facilities. P. chabaudi waspassaged in Swiss albino mice and once parasitemia reached 30%, infecting mice with parasitized blood was conducted. An intraperitonial injection of 1 × 106 P. chabaudi-infected erythrocytes was inoculated to the disignated groups of mice. Giemsa stain was used for blood smears staining and parasitemia were calculated. To estimate the cell number per ml, the Neubauer chamber was used.
2.3. Experimental design
Three groups were as follows; a vehicle control group, infected with 106 P. chabaudi-parasitized erythrocytes group and ZSCLE treated-infected mice with 106 P. chabaudi-parasitized erythrocytes group. The last group was gavage with 100 μl of 300 mg/kg ZSCLE for 8 days (Alzahrani et al., 2016). At day 8 post-infection (p.i.), scarification of all mice was performed.
2.4. Liver function tests
The blood plasma was separated and kept at −20 °C until use. The plasma was then analyzed using commercial kits according to the instructions of the manufacturer (BioMérieux, Marcy-l’Étoile, France) for alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP).
2.5. Histopathology of liver
Liver was aseptically extracted and fixed in neutral buffered formalin. Following fixation, dehydrating, embedding specimens in wax, 5 microns thickness sections were prepared. Haematoxylin and eosin stains were used to stain the sections for histological examinations.
2.6. Oxidant/antioxidant status
Phosphate buffer was used for homogenization of known weights of liver tissues.50 mMTris–HCl ice-cold medium containing 300 mM sucrose at pH 7.4 was used for homogenization according to (ADZU et al., 2007). Liver homogenates were used for biochemical studies.
2.6.1. Malondialdehyde (MDA) level
MDA level in liver homogenate of mice was measured according to the method of (Ohkawa et al., 1979) where the formed thiobarbituric acid reactive substances absorbance was determined at 535 nm.
2.6.2. Catalase
The activity of catalase in the liver homogenate was determined following the method of (Aebi, 1984). In this assay, a known quantity of H2O2 was added, after which the reaction was stopped with a CAT inhibitor. The remaining H2O2 reacts with 3,5 dichloro-2-hydroxybenzene sulfonic acid and 4-aminophenazone in the presence of horseradish peroxidase, resulting in a chromophore color. The absorbance was measured at 240 nm.
2.7. Statistical analysis
A statistical package program (SPSS version 17.0) was used to perform one-way ANOVA and statistical comparisons between the groups with Duncan’s test. P ≤ .05 was considered as significant for all the statistical analyses.
3. Results
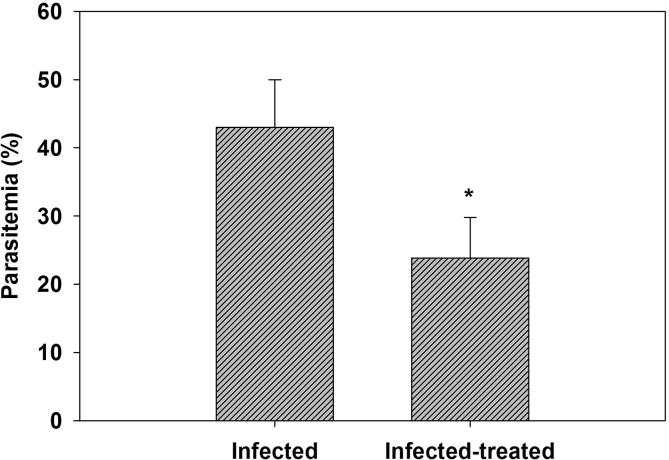
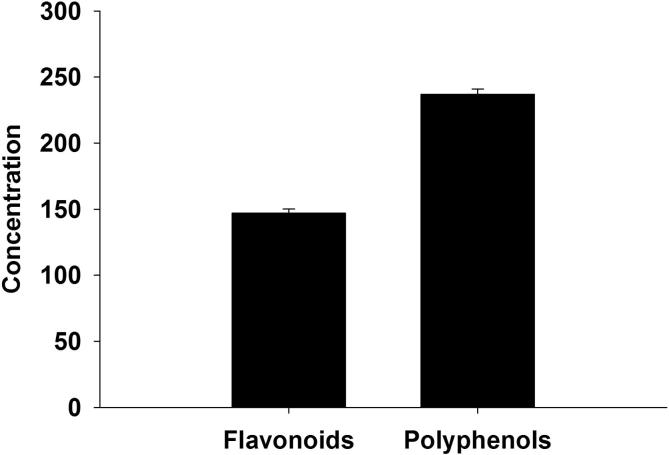
Aiming toward evaluating the antiplasmodial and antioxidant role of ZSCLE, parasitemia level, hematological and biochemical liver analysis have been conducted. Firstly, observing the parasitemia level provide an initial indication of the ZSCLE effectiveness against P. chabaudi-induced hepatic injury. In comparison with untreated infected group, parasitemia level was reduced significantly in ZSCLE treated infected mice (Fig. 1). This reduction may highlight the possible antimalarial activity of ZSCLE. This effect might be referred to data indicated that the methanolic leaf extract of Z. spina-christi contains high content of both of phenolic and flavonoid compounds (Fig. 2).
Fig. 1.
Parasitemia of infected and infected-treated mice with ZSCLE at day 8 postinfection with P. chabaudi. *: Significant change at P < .01 between infected mice and infected-treated mice.
Fig. 2.
Total flavonoid and phenolic compounds present in ZSCLE. Phenolic was measures as mg gallic acid equivalents per gram of the sample. Flavonoid was measured as mg quercetin equivalents per gram of the sample.
Expectedly, in accordance with high parasitemia level during P. chabaudi infection, anemic signs were observed as haemoglobin level and erythrocytes counts showed a notable reduction in comparison with control group. Significantly, ZSCLE treated infected mice record a restoration of haemoglobin level and erythrocytes counts as well as a reduction in the inflammatory cells count (Table 1). These results are reinforced through signifying the level of mean corpuscular hemoglobin (MCH) that showed significant reduction after treatment with ZSCLE.
Table 1.
Changes in leucocyte count, erythrocyte count and haemoglobin due to ZLE treatment of mice infected with Plasmodium chabaudi infected erythrocytes.
| Parameter | Control | Infected | Infected-treated |
|---|---|---|---|
| Leucocytes × 103/mm3 | 10.2 ± 2 | 14.6 ± 2a | 12.8 ± 2ab |
| Erythrocytes × 106/mm3 | 8.6 ± 0.7 | 5.6 ± 0.6a | 7.8 ± 1.3ab |
| Haemoglobin (gldL) | 14.7 ± 1.6 | 11.1 ± 1.1a | 13.3 ± 1.4ab |
| MCH (pg) | 17.09 ± 1.2 | 19.82 ± 1.1 | 17.1 ± 1.2 |
Values are means ± SD. a: Significant change at P ≤ 0.05 with respect to control group. b: Significant change at P ≤ 0.05 between infected mice and infected-treated mice.
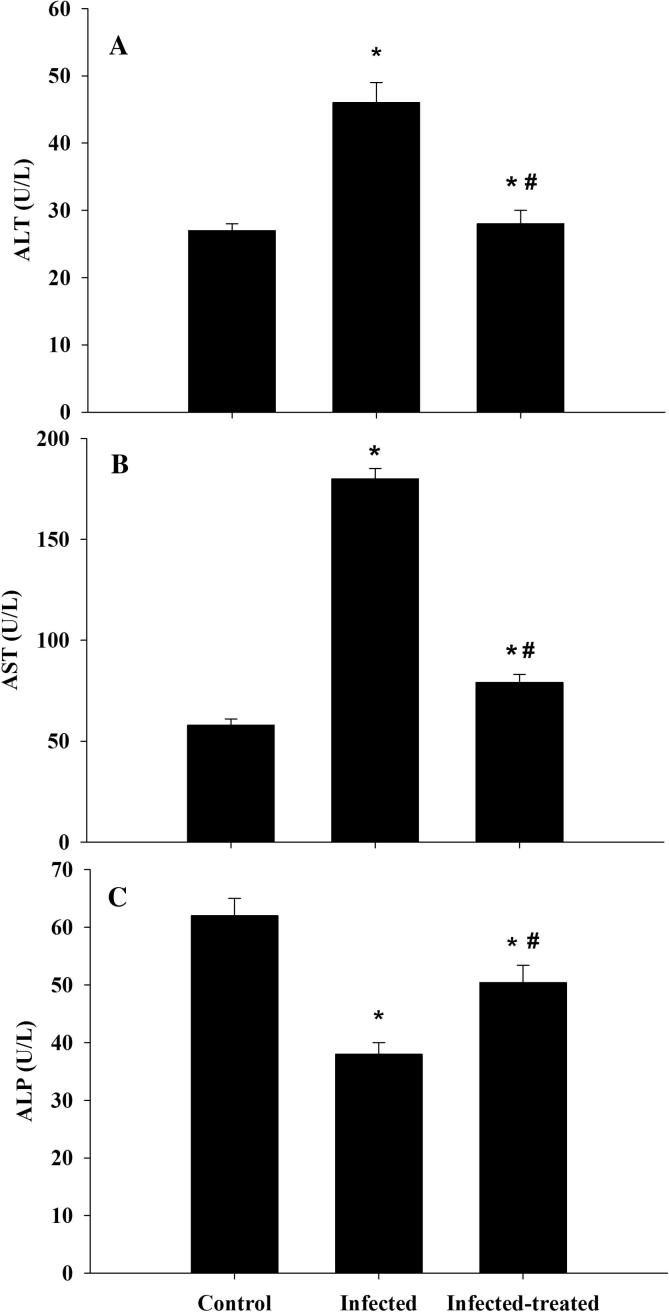
Regarding liver function tests, the levels of ALT and AST enzymes, in comparison with control group, increased significantly among mice infected with P. chabaudi infected erythrocytes while ALP level decreased significantly. These observations were reversed upon ZSCLE treatment, highlighting the ability of ZSCLE in relieving the burden of malaria pathogenesis (Fig. 3).
Fig. 3. C.
hanges in ALT, AST and ALP due to ZSCLE treatment of mice infected with P. chabaudi infected erythrocytes. *: Significant change at P < .01 with respect to control group. #: Significant change at P < .01 between infected mice and infected-treated mice.
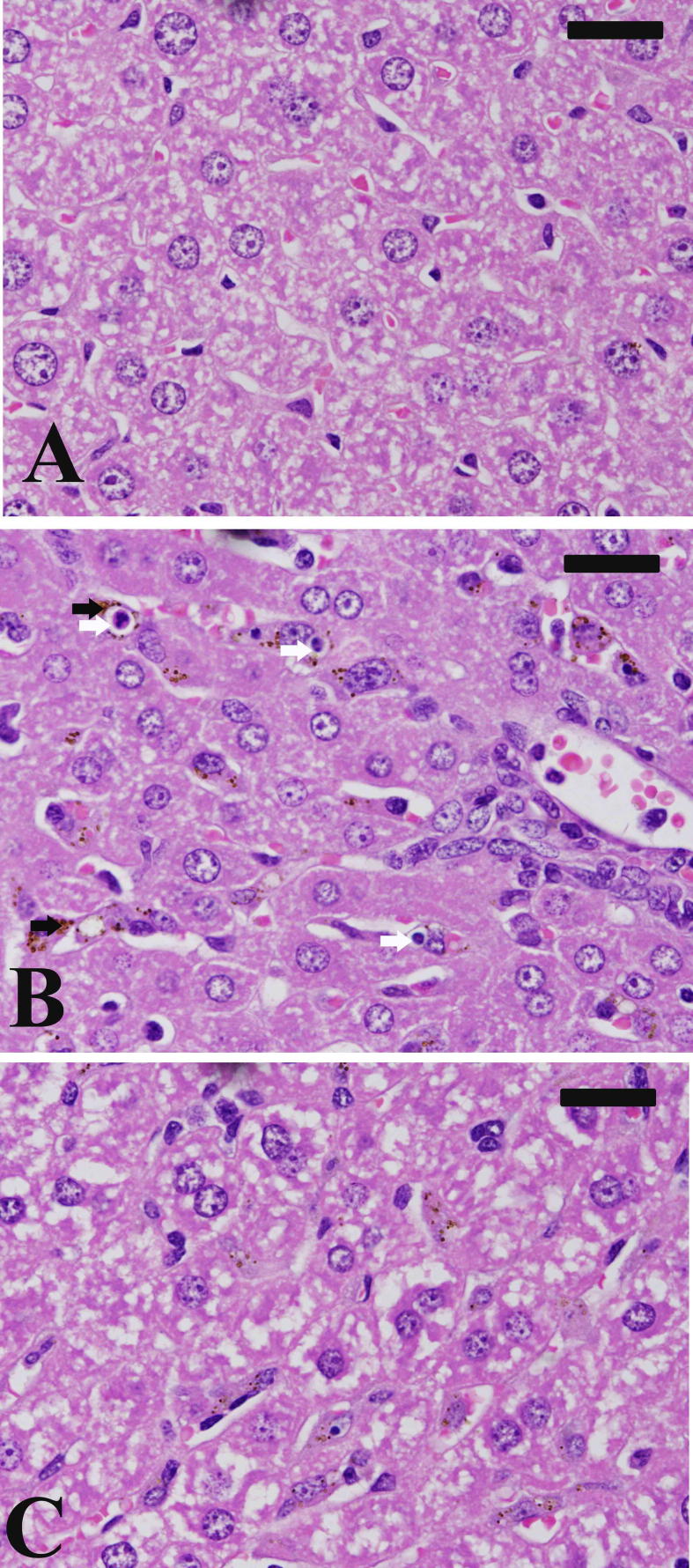
Examination of the liver sections of control (Fig. 4A) and both infected (Fig. 4B) and infected treated (Fig. 4C) animals showed the prominent inflammatory cell infiltration, increased number of van Kupffer cells and hepatocyte vacuolation in mice infected with P. chabaudi-infected erythrocytes. Remarkably, the liver sections of infected-ZSCLE treated mice group showed a preservation and improvement of the hepatocellular structure in comparison with that of infected mice group (Fig. 4C).
Fig. 4.
ZSCLE ameliorates hepatic tissue damage induced by P. chabaudi-parasitized erythrocytes. Stained paraffin sections of mouse liver. (A) non-infected liver with normal architecture. (B) Infected liver with some inflammatory cells around the central vein. Also, malaria pigments (black arrow) and apoptotic bodies (white arrow) are prominent. (C) infected-treated liver with less induced inflammation and malaria pigments. Sections are stained with hematoxylin and eosin. Bar = 25 µm.
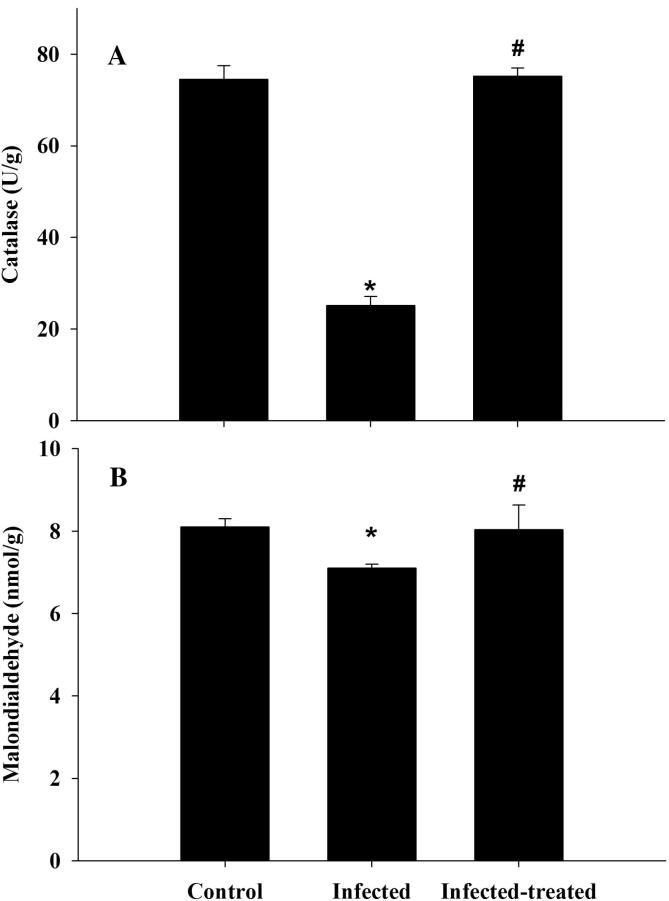
Regarding the hepatic oxidative markers, the liver oxidative damage in mice infected with P. chabaudi was detected through significant (P ≤ .05) alteration of malondialdehyde (MDA) and catalase compared to the control group. Considerably, the levels of hepatic MDA and catalase were restored among ZSCLE treated mice (Fig. 5).
Fig. 5.
Induced changes in catalase and malondialdehyde after treatment of P. chabaudi-infected mice with ZSCLE. *: Significant change at P < .01 with respect to control group. #: Significant change at P < .01 between infected mice and infected-treated mice.
4. Discussion
Z. spina-christi was studied to further confirm its antiplasmodial and antioxidant activities against malaria. The importance of exploring traditionally used herbal medicines is attributed to different factors. The emergence resistance to anti-malaria drugs, the absence of vaccination and the existing remedy effects of some plants such as Artemisia annua (the origin of successful anti-malarial drug, artemisinin) (Lee et al., 1989, Dua et al., 2004)), all of which are factors for initiate examination of herbal medicines validity.
This study was conducted for further validation of Z. spina-christi effectiveness against malaria. Measuring the parasitemia level considers being an initial validation of ZSCLE among mice infected with P. chabaudi. In accordance with Mishra and Bhatia study (2014), ZSCLE was able to significantly reduce the parasitemia level among ZSCLE treated infected mice. Moreover, the result of this study was in a line with recent finding of Hafiz and Mubaraki (2016) that showed a remarkable reduction of parasitemia level upon P. berghei-induced infection. Expectedly, high parasitemic level caused discrepancies among hematological parameters that record signs of anemia, low haemoglobin level and erythrocytes counts. ZSCLE, notably, was able to reverse the anemic picture and reinstate the normal levels.
The action of ZSCLE is due to the presence of several active components like essential oils, alkaloids, flavonoids and phenolic compounds (Kadioglu et al., 2016).
With regards to liver, malaria-associated liver pathogenesis has varied from elevated liver enzymes, jaundice to hepatomegaly and liver failure (Anand and Puri, 2005, Whitten et al., 2011). The levels of liver function enzymes, ALT and AST, increased substantially among mice infected with P. chabaudi infected erythrocytes while ALP level decreased significantly. A study by (Dahiru and Obidoa, 2008) on the effect of an aqueous leaf extract of Z. mauritiana on chronic ethanol-induced hepatotoxicity showed significant reduction of ALT, ALP and AST enzymes among Z. mauritiana treated group of rat. This effect on the levels of injured tissue marker and lipid peroxidation was attributed to the presence of tannins, saponins and phenolic compounds in Ziziphus extract. Concomitantly, the present study demonstrated similar effect of ZSCLE on the liver functions enzymes. Furthermore, in accordance with the liver function enzymes, the histological appearance of the infected-ZSCLE treated mice group evidently verify the ability of ZSCLE to reserve the hepatocellular membrane integrity and restore the hepatic architecture. Similarly, the effectiveness of Z. spina-christi in treating and inhibiting hepatic fibrosis caused by carbon tetrachloride was reported by (Amin and Mahmoud-Ghoneim, 2009). Moreover, (Hafiz and Mubaraki, 2016) found that upon ZSCLE treatment of liver of infected mice with P. berghei, the signs of inflammatory cell infiltration and hepatocyte vacuolation were significantly ameliorated. Former study by Ali and Hamed (2006) examined Z. spina-christi extracts role on the histopathological pictures of liver, kidney and spleen that been affected by Schistosoma infection and found that Z. spina-christi extracts have pronounce ameliorating effects on hepatic tissues.
The effects of ZSCLE on the oxidative marker of infected liver tissues reinforced the ameliorative role of ZSCLE against hepatic injury induced by Plasmodium chabaudi infected erythrocytes. This effect evident through significant restoration of hepatic MDA and catalase levels among ZSCLE treated mice. Correspondingly, Yossef et al. (2011) study recorded the protective effect of ZSCLE among mice intoxicated with carbon tetrachloride (CCl4) (Yossef et al., 2011). Moreover, a previous study showed the amelioration effect of ZSCLE on hepatic disorders through restoring the normal levels of MDA in Schistosoma mansoni infected mice (El-Rigal et al., 2006). Importantly, recent study showed the protective effect of ZSCLE on hepatic and splenic tissues through reinstatement the normal levels of the oxidative markers and impeding the progression of hepatic and splenic fibrosis upon P. berghei induced hepatic and splenic inflammation (Hafiz and Mubaraki, 2016).
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RG-198.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adzu B., Haruna A.K., Salawu O.A., Katsayal U.A., Njan A. In vivo antiplasmodial activity of ZS-2A: a fraction from chloroform extract of Zizyphus spina-christi root bark against Plasmodium berghei berghei in mice. Int. J. Biolog. Chem. Sci. 2007;1:281–286. [Google Scholar]
- Aebi H.U.E. Academic Press; New York: 1984. Methods in Enzymatic Analysised^eds; pp. 276–286. [Google Scholar]
- Ali S.A., Hamed M.A. Effect of Ailanthus altissima and Zizyphus spina-christi on Bilharzial Infestation in Mice: histological and histopathological studies. J. Appl. Sci. 2006;6:1437–1446. [Google Scholar]
- Alzahrani F., Al-Shaebi E.M., Dkhil M.A., Al-Quraishy S. In vivo Anti-eimeria and in vitro Anthelmintic activity of Ziziphus spina-christi leaf extracts. Pak. J. Zool. 2016;48:409–413. [Google Scholar]
- Amin A., Mahmoud-Ghoneim D. Zizyphus spina-christi protects against carbon tetrachloride-induced liver fibrosis in rats. Food Chem. Toxicol. 2009;47:2111–2119. doi: 10.1016/j.fct.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Anand A.C., Puri P. Jaundice in malaria. J. Gastroenterol. Hepatol. 2005;20:1322–1332. doi: 10.1111/j.1440-1746.2005.03884.x. [DOI] [PubMed] [Google Scholar]
- Benelli G. Research in mosquito control: current challenges for a brighter future. Parasitol. Res. 2015;114:2801–2805. doi: 10.1007/s00436-015-4586-9. [DOI] [PubMed] [Google Scholar]
- Cowman A.F., Crabb B.S. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Dahiru D., Obidoa O. Evaluation of the antioxidant effects of Ziziphus mauritiana lam. leaf extracts against chronic ethanol-induced hepatotoxicity in rat liver. Afr. J. Tradit. Complement. Altern. Med. 2008;5:39–45. doi: 10.4314/ajtcam.v5i1.31254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua V.K., Qjha V.P., Roy R. Anti-malarial activity of some xanthones isolated from the roots of Andrographis paniculata. J. Ethnopharmacol. 2004;95:247–251. doi: 10.1016/j.jep.2004.07.008. [DOI] [PubMed] [Google Scholar]
- El-Rigal N.S., Aly S.A., Rizk M., Said A. Use of Ailanthus altissima and Ziziphus spina christi extracts as folk medicine for treatment of some hepatic disorders in Schistosoma mansoni infected mice. Trends Med. Res. 2006;1:100–112. [Google Scholar]
- Hafiz T.A., Mubaraki M.A. The potential role of Ziziphus spina-christi leaf extracts against Plasmodium berghei-induced liver and spleen injury. Biomed. Res.-Ind. 2016;27:1027–1032. [Google Scholar]
- Hall N., Karras M., Raine J.D. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- Kadioglu O., Jacob S., Bohnert S., Nass J., Saeed M.E., Khalid H., Merfort I., Thines E., Pommerening T., Efferth T. Evaluating ancient Egyptian prescriptions today: anti-inflammatory activity of Ziziphus spina-christi. Phytomedicine. 2016;23:293–306. doi: 10.1016/j.phymed.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Kumar S., Kumari R., Pandey R. New insight-guided approaches to detect, cure, prevent and eliminate malaria. Protoplasma. 2015;252:717–753. doi: 10.1007/s00709-014-0697-x. [DOI] [PubMed] [Google Scholar]
- Lee I.S., Elsohly H.N., Croom E.M., Hufford C.D. Microbial-metabolism studies of the antimalarial sesquiterpene artemisinin. J. Nat. Prod. 1989;52:337–341. doi: 10.1021/np50062a020. [DOI] [PubMed] [Google Scholar]
- Mian-McCarthy S., Agnandji S.T., Lell B. A phase 3 trial of RTS, S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 2012;367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra T., Bhatia A. Antiplasmodial effects of the aqueous ethanolic seed extract of Ziziphus mauritiana against Plasmodium berghei in Swiss albino mice. International Journal of Pharmacol. Res. 2014;4:111–116. [Google Scholar]
- Nazif N.M. Phytoconstituents of Zizyphus spina-christi L. fruits and their antimicrobial activity. Food Chem. 2002;76:77–81. [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal-tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Whitten R., Milner D.A., Jr., Yeh M.M., Kamiza S., Molyneux M.E., Taylor T.E. Liver pathology in Malawian children with fatal encephalopathy. Hum. Pathol. 2011;42:1230–1239. doi: 10.1016/j.humpath.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yossef, H.E.-D., Khedr, A., Mahran, M.Z., 2011. Hepatoprotective activity and antioxidant effects of El Nabka (Zizyphus spina-christi) fruits on ratshepatotoxicity induced by carbon tetrachloride. Nat. Sci. 9, 1–7.