Abstract
Objective
Explore the possible protective effect of Sargentodoxa cuneata total phenolic acids on cerebral ischemia reperfusion injury rats.
Methods
Focal cerebral ischemia reperfusion rats model were established by linear thrombus. Nimodipine group, Naoluotong group, the high, middle and low dose of Sargentodoxa cuneata total phenolic acids groups were given related drugs via intragastric administration before operation for seven days, once a day. At the same time sham operation group, and ischemia reperfusion group were given the same volume of physiological saline. One hour after the last administration, establish focal cerebral ischemia- reperfusion model in rats by thread method, and the thread was taken out after 2 h ischemia to achieve cerebral ischemia reperfusion injury in rats. After reperfusion for 24 h, the rats were given neurologic deficit score. The brain tissue was taken to measure the levels of IL-6, IL-1β, TNF-α, Bcl-2, Bax, Casp-3 and ICAM-1; HE staining observed histopathological changes in the hippocampus and cortical areas of the brain; Immunohistochemistry was used to observe the expression of NGF and NF-KBp65.
Result
Focal cerebral ischemia reperfusion rats model was copyed successed. Compared with model group, each dose group of Sargentodoxa cuneata total phenolic acids could decreased the neurologic deficit score (P < 0.05 or P < 0.01), decreased the levels of IL-6, IL-1β, ICAM-1, TNF-α, Bax and Caspase-3 in brain tissue (P < 0.05 or P < 0.01), increased the levels of IL-10, Bcl-2, NGF in brain tissue (P < 0.05 or P < 0.01), decreased the express of NF-KBp65 in brain (P < 0.05 or P < 0.01).
Conclusion
Sargentodoxa cuneata total phenolic acids can improve focal cerebral ischemia reperfusion injury rats tissue inflammation, apoptosis pathway, increase nutrition factor to protect the neurons, reduce the apoptosis of nerve cells, activate brain cells self-protect, improve the histopathological changes in the hippocampus and cortical areas of the brain, reduce cerebral ischemia reperfusion injury.
Keywords: Sargentodoxa cuneata total phenolic acids, Anti-inflammation, Rat model, Reperfusion injury, Cerebral ischemia reperfusion
1. Introduction
Cerebrovascular disease is one of the common diseases that endanger the health of the elderly. It has the characteristics of high morbidity, high disability rate and high mortality. It is a serious threat to human health and should be focused on prevention and control (Piironen et al., 2014). Studies have shown that the reperfusion of blood flow in ischemic areas can cause severe brain injury and related dysfunction, namely cerebral ischemia reperfusion injury (Culman et al., 2012, Riverol et al., 2015). Therefore, it is necessary to prevent and treat focal cerebral ischemia reperfusion injury. Traditional Chinese medicine (TCM) has the characteristics of the overall concept, its medicine has the characteristics of the multiple targets, multiple paths to intervene cerebral ischemia reperfusion injury pathological process and with no or only low toxic side effects. The prevention and treatment of cerebral ischemia reperfusion injury in TCM gradually attracts more and more attention (Li et al., 2017). In the pathogenesis theory basis of “Toxic heat stroke” and “Poison damage brain collaterals”, and add the deepen understanding of traditional toxin factor, from the “poison” to treat stoke has become a research hotspot in the clinical and mechanism of stroke. Poison has the characteristic of heat, the shape of phlegm and blood stasis. The treatment of phlegm and blood stasis can help to dissipate and clear toxify. Therefore, it is the best combination of two theories of Chinese and western medicine in clinical practice to treat apoplexy diseases with activating blood stasis, clearing heat and detoxifying detoxification (Liu et al., 2016). Chinese pharmacopoeia records Sargentodoxa cuneata tastes bitter, flat in nature, which has the effect of clearing heat and detoxifying, activating blood and removing wind and relieving pain (National Pharmacopoeia Committee, 2015). The early stage of the laboratory studies have shown that Sargentodoxa cuneata water decoction has a good effect on cerebral ischemia, while the chemical composition of Sargentodoxa cuneata is mainly phenolic acid, but it has not been found to be related to cerebral ischemia reperfusion. Pharmacological studies have shown that Chinese medicine phenolic acids have specific effects on the cardiovascular system (Ju et al., 2017). This research mainly observe the effect of Sargentodoxa cuneata total phenolic acids on cerebral ischemia reperfusion injury rats, providing the basis and ideas for the clinical application and experimental research on the prevention and treatment of ischemic cerebrovascular diseases.
2. Materials and methods
2.1. Drugs and reagents
Sargentodoxa cuneata total phenolic acids, provided by the chemistry room of henan university of traditional Chinese medicine, Content was 70.04% determined by forint phenol method. Nimodipine tablet, Yabao pharma-ceutical group Co., Ltd., approval number: 140861; Naoluotong capsule, Jilin jinbao pharmaceutical Co., Ltd., approval number: 150401; Red tetrazazole (TTC), East China normal university chemical plant (Shanghai), approval number: 04102711; Intercellular adhesion molecule 1 (ICAM-1) ELISA detection kit, Interleukin-1 (IL-1) ELISA detection kit, Interleukin-6 (Il-6) ELISA detection kit, Tumor necrosis factor alpha (TNF- alpha) ELISA detection kit, Bcl-2 related X protein (Bax) ELISA detection kit, B cell lymphoma factor 2 (bcl-2) ELISA detection kit and Cysteine proteinase-3 (caspase-3) ELISA detection kit were provided by R&Dcompany, approval number: 20150901A; Coomassie bright blue kit, Nanjing institute of biological engineering, approval number: 20150821.
2.2. Experimental apparatus
2838-A4 MCAO thread, Beijing xiongan biotechnology co. Ltd; HWS12 electric thermostatic water bath, Shanghai constant Scientific Instrument co. Ltd; FA2204B Electronic balance, Shanghai Precision Scientific Instrument co. Ltd; TDL-40B table low speed automatic balancing centrifuge, Changsha Xiangzhi centrifuge instrument co. Ltd; BIORAD-680 enzyme marker, BIO-RAD co. Ltd.
2.3. Animals and grouping
Wistar rats, Male, SPF level, weighing 250–280 g, purchased by Shandong Lukang Pharmaceutical company limited by shares, certificate number: 37005400000023; Animal experiment certificate number: SYXK (Yu) 2015–0005, ethical batch number: 15010017.
2.4. Model making and drug delivery (Zhu et al., 2015, Tian et al., 2015)
A total of 98 rats, weighing 250–280 g, were randomly divided into 7 groups, Sham operation group, Ischemia reperfusion group, Nimodipine group, Naoluotong group, the high, middle and low dose of Sargentodoxa cuneata total phenolic acids group. Nimodipine was given by intragastric administration of 20 mg/kg and Naoluotong was given by intragastric administration of 500 mg/kg. The high, middle and low dose of Sargentodoxa cuneata total phenolic acids were given by intragastric administration of 300 mg/kg, 150 mg/kg and 75 mg/kg. At the same time, sham operation group and ischemia reperfusion group were given the same volume of physiological saline for seven days, once a day. On the sixth day of the evening at 8 o'clock, ban feeding but does not prohibit feeding water. On the seventh day of the morning at 8 o'clock weighing weight in batches, after administration of 1 h, rats were anesthetized with 10% chloral hydrate (0.3 ml/100 g) intraperitoneal injection, on left neck make a median operation, separated the left carotid artery (CCA) and wear two lines; Separate the external carotid artery (ECA) and wear a line, ligated ECA. The proximal end of the common carotid artery was Ligated and the distal end was tied to make thread throught. A small opening of about 0.2 mm wide was cut at about 5 mm of the total carotid artery distance, and inserted the line plug into the internal carotid artery by the common carotid bifurcation. That is, to block the entrance of the central artery in the brain, and to tie up the proximal end of the common carotid artery. After 2 h, gently draw the suture line to a slight resistance, and achieve reperfusion, establish blocked arteries in the brain-reperfusion (MCAO) animal model. The sham operation group only exposure to the left of the blood vessels, do not plug wire processing.
2.5. Detection indicators
After the reperfusion of 22 h, neurological deficit score was scored in all rats: Using Longa standard score (Rei and Higdon, 2003). Grading standard: (0) points, no neurological deficit symptoms and normal activity; (1) points, the forepaw cannot be fully extended; (2) points, make the circuit of the hemiplegic side; (3) points, dump the body to the hemiplegic side when walking; (4) points, can not walk spontaneously and losing consciousness; (5) points, death. Scoring of 0 points and 5 points were eliminated.
Rats were killed and brain tissue was quickly removed, then put it in the −20 °C low temperature refrigerator cooling 15 min, excluding the cerebellum, the olfactory bulb, and the rest of the lower brainstem, cut into 2 parts along the coronal plane. The first part is the anterior pole of the brain and the 1 mm fromer of visual cross crown, sagittal excision of left brain tissue, using saline made 10% brain homogenate. Then 4 °C, 3500 r/min centrifuged 10 min. Take supernatant fluid to determine the levels of IL-6, IL-1β, TNF-α, Bcl-2, Bax, Casp-3 and ICAM-1 in brain homogenate. The second part is the 1 mm later of visual cross crownto the end of brain, conventional paraffin embedding, HE staining was used to observe the morphological changes, and immunohistochemical staining was used to determine the content of NGF and NFκBp65.
3. Statistics processing method
The data were analyzed by SPSS 17.0 for windows statistical software, all data are expressed by mean ± standard ( ± s) deviation. A single factor variance analysis was used for each group, among the groups, the least significant difference (LSD) method was used to test the variance homogeneity and the Games-Howell method was used to test the heterogeneity of variance. Ranked datas was test by Ridit.
4. Results
4.1. Comparison of mortality and neural function deficits score of rat in each group
As we can see from Table 1: Model group had the highest mortality, Nimodipine group, Naoluotong group, the high, middle and low dose of Sargentodoxa cuneata total phenolic acids group had lower mortality rates. The results showed that each group which is given drugs could reduce the mortality rate of cerebral ischemia reperfusion rats, reduce brain tissue damage and protect brain tissue. Compared with the sham operation group, the neurological deficit score of the model group was significantly increased (P < 0.01), which indicated that the model was successful. Compared with the model group, the neurological deficit score decreased significantly in Nimodipine group, Naoluotong group, the high dose of Sargentodoxa cuneata total phenolic acids group (P < 0.01), decreased obviously in middle dose of Sargentodoxa cuneata total phenolic acids group (P < 0.05), and decreased slightly in low dose of Sargentodoxa cuneata total phenolic acids group (P > 0.05). The results showed that each group which is given drugs have the different degree of improvement of brain nerve function of rats with focal cerebral ischemia reperfusion injury.
Table 1.
Effects of mortality and neurological deficit score on focal cerebral ischemia reperfusion injury rat model ( ± s).
| Group | Number | Dose (mg/kg) | Mortality (%) | Neurological deficit score |
|---|---|---|---|---|
| Sham operation group | 14 | – | 0 | 0.0 ± 0.0** |
| Ischemia reperfusion group | 9 | – | 35.71 | 2.89 ± 0.78 |
| Naoluotong group | 10 | 20 | 28.57 | 1.60 ± 0.97** |
| Nimodipine group | 11 | 500 | 21.43 | 1.91 ± 0.70** |
| High dose group | 11 | 300 | 21.43 | 1.64 ± 0.50** |
| Middle dose group | 11 | 150 | 21.43 | 2.18 ± 0.87* |
| Low dose group | 10 | 75 | 28.57 | 2.40 ± 0.97 |
Compared with model group P < 0.05.
Compared with model group P < 0.01.
4.2. Comparison of IL-6, IL-1β, TNF-α levels of rat in each group
As we can see from Table 2. Compared with the Sham operation group, the level of IL-6, IL-1β, and TNF-α in the brain tissues of the model group increased significantly (P < 0.01), indicating that the model was successful. Compared with the model group, the level of IL-6, IL-1β and TNF-α in the brain tissues of Nimodipine group, Naoluotong group and the high dose of Sargentodoxa cuneata total phenolic acids group significantly decreased (P < 0.01); The IL-1β level significantly decreased (P < 0.01), IL-6 and TNF-α level obviously decreased in the brain tissues of middle dose of Sargentodoxa cuneata total phenolic acids group (P < 0.05); the level of IL-6, IL-1β, and TNF-α in the brain tissues of low dose of Sargentodoxa cuneata total phenolic acids group obviously decreased (P < 0.01). The results showed that each group which is given drugs has the effect of reducing the injury of inflammatory response to brain tissue of rats with focal cerebral ischemia reperfusion injury.
Table 2.
Effects of IL-6, IL-1β, TNF-α levels on focal cerebral ischemia reperfusion injury rat model brain ( ± s).
| Group | Number | Dose (mg/kg) | IL-6 (pg/ml) | IL-1β (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|---|---|
| Sham operation group | 14 | – | 22.79 ± 3.32** | 4.42 ± 0.59** | 40.32 ± 7.72** |
| Model group | 9 | – | 28.74 ± 3.18 | 5.97 ± 0.78 | 54.91 ± 8.49 |
| Naoluotong group | 10 | 20 | 23.31 ± 1.94** | 4.45 ± 0.82** | 40.62 ± 7.46** |
| Nimodipine group | 11 | 500 | 23.83 ± 2.77** | 4.64 ± 0.63** | 44.75 ± 7.27* |
| High dose group | 11 | 300 | 24.16 ± 3.39** | 4.49 ± 0.74** | 42.54 ± 5.29** |
| Middle dose group | 11 | 150 | 24.40 ± 2.90* | 4.74 ± 0.50** | 43.35 ± 8.00* |
| Low dose group | 10 | 75 | 25.82 ± 2.65* | 4.92 ± 0.74* | 45.37 ± 8.72* |
Compared with model group P < 0.05.
Compared with model group P < 0.01.
4.3. Comparison of Bax, Bcl-2, Casp-3 levels of rat in each group
As we can see from Table 3. Compared with the Sham operation group, the level of Bax and Casp-3 significantly increased and Bcl-2 significantly decreased in the brain tissues of the model group (P < 0.01), indicating that the model was successful. Compared with the model group, the level of Bax and Casp-3 significantly decreased and Bcl-2 significantly increased in the brain tissues of Nimodipine group, Naoluotong group and the high dose of Sargentodoxa cuneata total phenolic acids group (P < 0.01); The Bax level significantly decreased (P < 0.01), Casp-3 level obviously decreased and Bcl-2 level obviously increased in the brain tissues of middle dose of Sargentodoxa cuneata total phenolic acids group (P < 0.05); The level of Bax and Casp-3 obviously decreased and Bcl-2 obviously increased in the brain tissues of the low dose of Sargentodoxa cuneata total phenolic acids group (P < 0.01). The results showed that each group which is given drugs could increase inhibiting apoptosis gene expression, reduce promoting apoptosis gene expression, inhibit cell apoptosis of focal cerebral ischemia reperfusion model of rats brain tissue, thus protecting brain tissue, alleviate symptoms of brain ischemia.
Table 3.
Effects of Bax, Bcl-2, Casp-3 levels on focal cerebral ischemia reperfusion injury rat model brain tissues ( ± s).
| Group | Number | Dose (mg/kg) | Bax (ng/ml) | Bcl-2 (ng/ml) | Casp-3 (pmol/L) |
|---|---|---|---|---|---|
| Sham operation group | 14 | – | 1.39 ± 0.19** | 25.20 ± 3.12** | 20.84 ± 3.69** |
| Model group | 9 | – | 1.75 ± 0.17 | 20.21 ± 2.93 | 26.80 ± 2.07 |
| Naoluotong group | 10 | 20 | 1.43 ± 0.09** | 23.89 ± 3.15** | 21.30 ± 3.93** |
| Nimodipine group | 11 | 500 | 1.45 ± 0.07** | 24.46 ± 1.09** | 21.35 ± 2.89** |
| High dose group | 11 | 300 | 1.46 ± 0.15** | 24.10 ± 2.68** | 21.52 ± 2.67** |
| Middle dose group | 11 | 150 | 1.50 ± 0.09** | 23.37 ± 2.45* | 22.68 ± 1.80* |
| Low dose group | 10 | 75 | 1.58 ± 0.15* | 22.84 ± 3.03* | 22.98 ± 3.27* |
Compared with model group P < 0.05.
Compared with model group P < 0.01.
4.4. Effects of ICAM-1 level on focal cerebral ischemia reperfusion injury rat model brain tissues
As we can see from Table 4: compared with the Sham operation group, the level of ICAM-1 significantly increased in the brain tissues of the model group (P < 0.01), indicating that the model was successful. Compared with the model group, the level of ICAM-1 significantly decreased in the brain tissues of Nimodipine group, Naoluotong group, the high and middle dose of Sargentodoxa cuneata total phenolic acids group (P < 0.01); And the level of ICAM-1 obviously decreased in the brain tissues of low dose of Sargentodoxa cuneata total phenolic acids group (P < 0.05). The results showed that each group which is given drugs could reduce the level of ICAM-1 and cerebral infarction area focal cerebral ischemia reperfusion model.
Table 4.
Effects of ICAM-1 level on focal cerebral ischemia reperfusion injury rat model brain tissues ( ± s).
| Group | Number | Dose (mg/kg) | ICAM-1 (ng/ml) |
|---|---|---|---|
| Sham operation group | 14 | – | 21.79 ± 4.33** |
| Model group | 9 | – | 27.32 ± 2.93 |
| Naoluotong group | 10 | 20 | 23.39 ± 4.55** |
| Nimodipine group | 11 | 500 | 23.17 ± 3.46** |
| High dose group | 11 | 300 | 23.92 ± 3.66** |
| Middle dose group | 11 | 150 | 24.40 ± 3.47** |
| Low dose group | 10 | 75 | 25.70 ± 2.31* |
Compared with model group P < 0.05.
Compared with model group P < 0.01.
4.5. Effects of NGF and NF-KBp65 immuno-positive expression levels on focal cerebral ischemia reperfusion injury rat model brain tissues
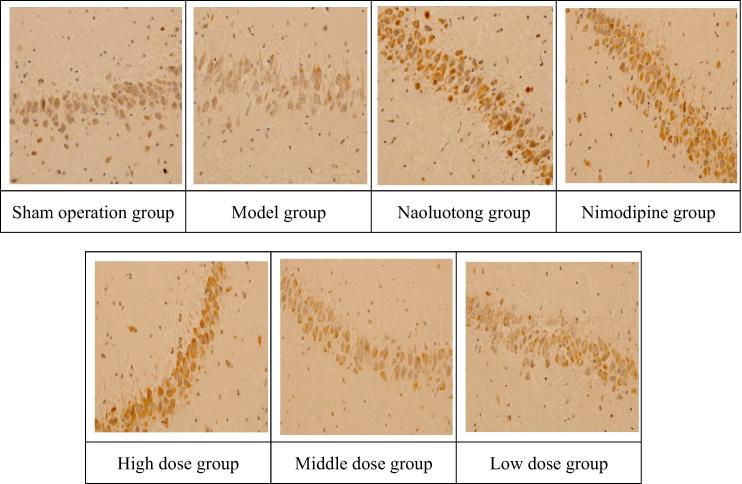
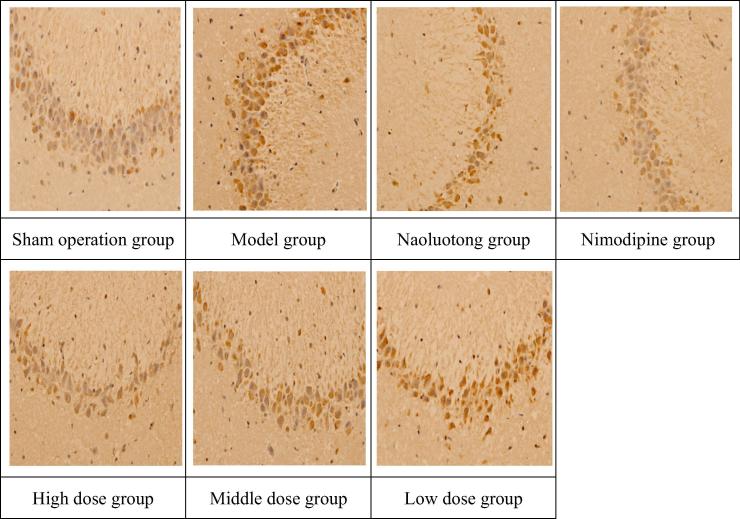
As we can see from Table 5 combined Appendix A, Appendix B. Compared with the Sham operation group, the immuno-positive expression levels of NGF and NF-KBp65 significantly increased in the brain tissues of the model group (P < 0.01), indicating that the model was successful. Compared with the model group, the immuno-positive expression level of NGF significantly increased and NF-KBp65 significantly decreased in the brain tissues of Nimodipine group, Naoluotong group, the high dose of Sargentodoxa cuneata total phenolic acids group (P < 0.01); The immuno-positive expression level of NGF significantly increased (P < 0.01) and NF-KBp65 obviously decreased (P < 0.05) in the brain tissues of middle dose of Sargentodoxa cuneata total phenolic acids group; The immuno-positive expression level of NGF obviously increased (P < 0.05) in the brain tissues of low dose of Sargentodoxa cuneata total phenolic acids group. The results showed that each group which is given drugs could promote the expression of NGF in brain nerve cells, inhibit the expression of NF-KBp65, and prevent degeneration or death of brain nerve cells in rats, thus maintaining normal function of brain nerve and alleviating the damage caused by cerebral ischemia and reperfusion.
Table 5.
Effects of NGF and NF-KBp65 immuno-positive expression levels on focal cerebral ischemia reperfusion injury rat model brain tissues ( ± s).
| Group | Number | Dose (mg/kg) | NGF | NF-KBp65 |
|---|---|---|---|---|
| Sham operation group | 14 | – | 18.57 ± 3.63** | 29.33 ± 2.80** |
| Model group | 9 | – | 23.27 ± 3.67 | 36.71 ± 3.31 |
| Naoluotong group | 10 | 20 | 31.19 ± 2.16** | 32.39 ± 1.31** |
| Nimodipine group | 11 | 500 | 32.84 ± 1.73** | 30.79 ± 1.59** |
| High dose group | 11 | 300 | 31.98 ± 5.82** | 31.95 ± 4.95** |
| Middle dose group | 11 | 150 | 29.35 ± 5.27** | 33.45 ± 3.03* |
| Low dose group | 10 | 75 | 27.03 ± 2.72* | 34.14 ± 2.96 |
Compared with model group P < 0.05.
Compared with model group P < 0.01.
4.6. Effect of pathological changes in cerebral cortex on focal cerebral ischemia reperfusion injury rat model
As we can see from Table 6, Appendix A, Appendix B. By the Ridit test, compared with the sham operation group, the model group had significantly statistical significantly (P < 0.01), indicating that there were significant pathological changes in the cerebral cortex region of the model group, and the model was successful. Compared with the model group, the pathological changes of the cerebral cortex were significantly improved in Nimodipine group, Naoluotong group, the high and middle dose of Sargentodoxa cuneata total phenolic acids group (P < 0.01) and obviously improved in low dose of Sargentodoxa cuneata total phenolic acids group (P < 0.05). The results showed that each group which is given drugs could improve the pathological damage in cerebral cortex of rats with focal cerebral ischemia reperfusion model in different degrees and to protect brain tissue.
Table 6.
Effect of pathological changes in cerebral cortex on focal cerebral ischemia reperfusion injury rat model ( ± s).
| Group | Number | Dose (mg/kg) | — | + | ++ | +++ |
|---|---|---|---|---|---|---|
| Sham operation group | 14 | – | 14 | 0 | 0 | 0 |
| Model group | 9 | – | 0 | 0 | 2 | 7 |
| Naoluotong group | 10 | 20 | 2 | 5 | 2 | 1 |
| Nimodipine group | 11 | 500 | 3 | 5 | 2 | 1 |
| High dose group | 11 | 300 | 3 | 5 | 2 | 1 |
| Middle dose group | 11 | 150 | 2 | 4 | 4 | 1 |
| Low dose group | 10 | 75 | 1 | 3 | 4 | 2 |
From the regression analysis of the count data (Hu et al., 2018): *Compared with model group P < 0.05; **Compared with model group P < 0.01.
“–” Cerebral cortex is not edema, and the nerve cells are normal; “+” Cerebral cortex nerve cell edema, a small number of neuronal necrosis, the infarct area occupies less than 1/3 of cortex; “++” Cerebral cortex nerve cell edema, most neuronal necrosis, the infarct area occupies 1/3 ∼ 2/3 of cortex; “+++” Cerebral cortex nerve cell edema, most of the neuron necrosis, the infarct area occupies more than2/3 of cortex.
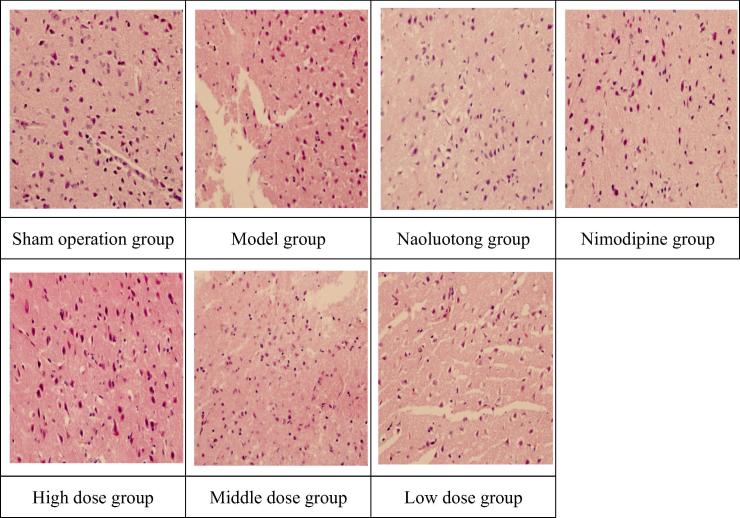
Histopathological observation of cerebral cortex in rats with focal cerebral ischemia reperfusion model (Appendix C). There was no edema in the cerebral cortex and the nerve cells were normal in the sham operation group; In the model group, cerebral cortex nerve cell edema severely, neuron necrosis severely and infarct area occupies more than 2/3 of cortex; In Nimodipine group, Naoluotong group, high dose of Sargentodoxa cuneata total phenolic acids group, cerebral cortex nerve cell edema improved significantly, neuron necrosis decreased significantly and infarct area occupies less than 1/3 of cortex; In middle dose of Sargentodoxa cuneata total phenolic acids group, cerebral cortex nerve cell edema improved, neuron necrosis decreased and infarct area occupies 1/3–2/3 of cortex; In low dose of Sargentodoxa cuneata total phenolic acids group, cerebral cortex nerve cell edema obviously, neuron necrosis severely and infarct area occupies more than 2/3 of cortex.
4.7. Effect of pathological changes in hippocampus on focal cerebral ischemia reperfusion injury rat model
As we can see from Table 7. By the Ridit test, compared with the sham operation group, the model group had significant statistical significance (P < 0.01), indicating that there were significant pathological changes in the hippocampus region of rats in the model group, and the model was successful. Compared with the model group, the pathological changes of the hippocampus were significantly improved in Nimodipine group, Naoluotong group, the high dose of Sargentodoxa cuneata total phenolic acids group had significant statistical significance (P < 0.01) and obviously improved in the middle and low dose of Sargentodoxa cuneata total phenolic acids group (P < 0.05). The results showed that each group which is given drugs could improve the pathological damage in hippocampus of rats with focal cerebral ischemia reperfusion model in different degrees and to protect brain tissue.
Table 7.
Effect of pathological changes in hippocampus on focal cerebral ischemia reperfusion injury rat model ( ± s).
| Group | Number | Dose (mg/kg) | – | + | ++ | +++ |
|---|---|---|---|---|---|---|
| Sham operation group | 14 | – | 14 | 0 | 0 | 0 |
| Model group | 9 | – | 0 | 0 | 3 | 6 |
| Naoluotong group | 10 | 20 | 4 | 2 | 3 | 1 |
| Nimodipine group | 11 | 500 | 3 | 5 | 2 | 1 |
| High dose group | 11 | 300 | 3 | 4 | 3 | 1 |
| Middle dose group | 11 | 150 | 2 | 4 | 3 | 2 |
| Low dose group | 10 | 75 | 2 | 3 | 3 | 2 |
From the regression analysis of the count data (Hu et al., 2018): *Compared with model group P < 0.05; **Compared with model group P < 0.01.
“–” Hippocampus tissue is not edema, and the nerve cells are normal; “+” Hippocampus tissue nerve cell edema, a small number of neuronal necrosis, the infarct area occupies less than 1/3 of hippocampal tissue; “++” Hippocampus tissue nerve cell edema, most neuronal necrosis, the infarct area occupies 1/3–2/3 of hippocampal tissue; “+++” Hippocampus tissue nerve cell edema, most of the neuron necrosis, the infarct area occupies more than 2/3 of hippocampal tissue.
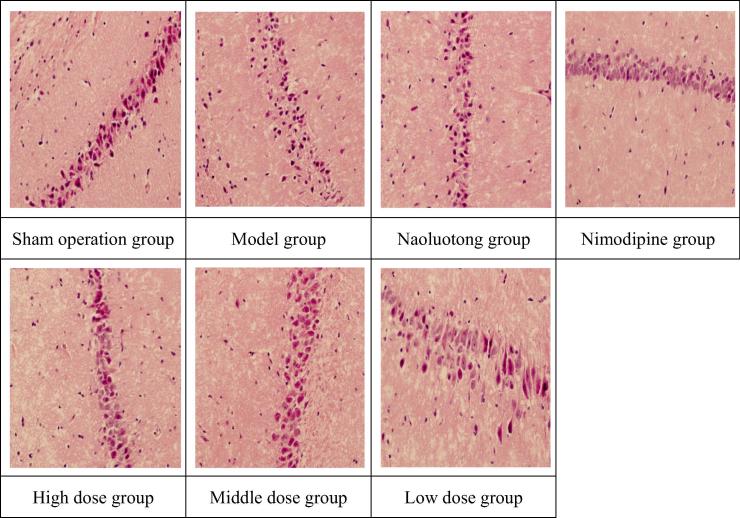
Histopathological observation of hippocampus in rats with focal cerebral ischemia reperfusion model (Appendix D): There was no edema in the hippocampal tissue and the nerve cells were normal in the sham operation group; In the model group, hippocampal tissue nerve cell edema severely, neuron necrosis severely and infarct area occupies more than 2/3 of hippocampal tissue; In Nimodipine group, Naoluotong group, high dose of Sargentodoxa cuneata total phenolic acids group, hippocampal tissue nerve cell edema improved significantly, neuron necrosis decreased significantly and infarct area occupies less than 1/3 of hippocampal tissue; In middle dose of Sargentodoxa cuneata total phenolic acids group, hippocampal tissue nerve cell edema improved, neuron necrosis decreased and infarct area occupies 1/3–2/3 of hippocampal tissue; In low dose of Sargentodoxa cuneata total phenolic acids group, hippocampal tissue nerve cell edema obviously, most of neuron necrosis and infarct area occupies more than 2/3 of hippocampal tissue.
5. Discussion
The pathologic mechanism of brain tissue damage caused by cerebral ischemia is complicated, including inflammatory reaction, oxidative stress, apoptosis, energy metabolism, calcium overload and other factors (Ge et al., 2016). With the increase of age, the function of the body gradually declines, and the five organs are all qi deficiency, causing the blood to run smoothly and even stasis. Therefore, TCM believed that qi deficiency, blood stasis and heat toxicity are the main factors in the development of cerebral ischemic diseases. In view of the above pathogenesis, making “detoxification and dredge meridian” as the general treatment. Our laboratory through summarizing the literature, break the long-standing principle of activating blood stasis, and put forward the view of using the drugs with the effects of activating blood stasis, clearing heat and detoxifying detoxification for the prevention and treatment of cerebral ischemia (Cheng et al., 2012). This view provides a new method for the prevention and treatment of ischemic cerebrovascular diseases and provides a new way for the study of cerebral ischemic diseases. Pharmacological studies have shown that Sargentodoxa cuneata has a good protective effect on the cardiovascular system, and effects of hypoxia, anti-inflammatory, anti-oxidation, analgesia, immunosuppression anti-virus. The chemical composition of Sargentodoxa cuneata is mainly phenolic acid, phenolic acids as a kind of natural products with unique physiological and pharmacological activities has strong antioxidant effect. It also has good efficacy in anti-tumor, anti-aging, anti-inflammation and immunity (Ma et al., 2013).
The pathogenesis of cerebral ischemia reperfusion injury is still inconclusive. At present, it is widely believed that oxidative stress injury, inflammatory response and energy metabolism disorder play an important role in brain tissue damage caused by cerebral ischemia and reperfusion. When cerebral ischemia injury occurs, the activation of transcription factor such as NF-κB, making inflammatory cytokines activated in brain tissues. Inflammatory cytokines such as IL-6, IL-1 and TNF-α were highly expressed. At the same time, the positive expression of NF-KBp65 was increased and played an important role in the pathogenesis of ischemic brain injury. TNF-α is an anti-tumor activity factor, which is considered to be the initiating medium of inflammatory response, and IL-1 and IL-6 are the main inflammatory factors that mediate tissue damage (Ge et al., 2017). ICAM-1 is a cell adhesion molecule, which promotes with inflammatory factors and forms a vicious cycle, aggravating inflammatory response and brain tissue injury after cerebral ischemia reperfusion (CIRI) (Song et al., 2015). This study found that Sargentodoxa cuneata total phenolic acids in each dose group can decreased the levels of IL-6, IL-1, TNF-α, NF-KBp65 and ICAM-1, indicating that the total phenolic acid of Sargentodoxa cuneata has the effect of inhibiting the inflammatory response of cerebral ischemia reperfusion injury rat brain tissue. After cerebral infarction, free radical, excitatory amino acid, neurotrophic factor, nitric oxide, calcium overload and other factors participate in the pathological process of injury after ischemia. Studies have shown that neurotrophic factors play a significant role in promoting the recovery of neurons, neurogenesis, new synaptic formation and neural function recovery around the cerebral ischemia (Yang et al., 2011, Li et al., 2016). Ca2+ overload produces energy metabolism disorder, aggravates brain tissue damage and thus forms a vicious circle, leading to the death of neurons. Neurotrophic factor NGF has anti-free radical effect and can maintain the cell Ca2+ homeostasis. It is helpful to repair damaged neurons, and NGF has protective effect on CIRI after focal cerebral ischemia (Fang et al., 2016, Al-Enazi et al., 2018, Gao et al., 2017, Ge et al., 2017). This study found that Sargentodoxa cuneata total phenolic acids in each dose group can increased the positive expression of NGF in different degrees showed that it can promote regeneration and repair after neuron injury in rat brain tissue. Bcl-2 high expression can inhibit neuronal apoptosis induced by ischemia, and Bax is the most important apoptosis gene in Bcl-2. Bax overexpression can induce apoptosis by inhibiting Bcl-2. Caspase-3 is a protease that promotes cell apoptosis, which can promote the apoptosis of neurons in the lesion area during acute cerebral infarction (Zhang and Wang, 2014, Khan et al., 2018, Narkhede et al., 2017, Yilmaz et al., 2017). This study found that Sargentodoxa cuneata total phenolic acids in each dose group can decreased the levels of Bax and Caspase-3 and increased the level of Bcl-2, which showed that it has a regulation on the express of Bcl-2, Bax and Caspase-3. Apoptosis is the main form of delayed neuronal death in ischemic area after ischemic injury. Although the blood supply can be restored after a short period of cerebral ischemia, it can cause the death of selective neurons, and the cortex and hippocampus are the most susceptible parts. Therefore, after transient cerebral ischemia reperfusion, specific pathological changes occurred in the hippocampus and cortex of rats. And targeted observation of cerebral ischemia reperfusion injury in cortical and hippocampal areas of rats can better understand the effects of gametophyllin.
To sum up, the protective effects of Sargentodoxa cuneata total phenolic acids can be achieved by improving the inflammatory response and cell apoptosis pathway of cerebral ischemia reperfusion injury rats. At the same time, the mortality was decreased, the score of neurological impairment was reduced on rats with cerebral ischemia reperfusion injury. The experimental results further validate the view of using the drugs with the effects of activating blood stasis, clearing heat and detoxifying detoxification for the prevention and treatment of cerebral ischemia proposed by our laboratory. And it provides theoretical basis and experimental thinking for the clinical application and experimental study of the prevention and treatment of ischemic cerebrovascular diseases of Sargentodoxa cuneata total phenolic acids.
Acknowledgement
This research was financially supported by the National International Cooperation base (2016-151); Science And Technology Research In Henan Province (17210231004); Country's Major New Drug Initiative (2009ZX09103-324).
Footnotes
Peer review under responsibility of King Saud University.
Appendix A. NGF immunohistochemical images of focal cerebral ischemia reperfusion model in rats (HEx400).
Appendix B. NF-KBp65 immunohistochemical images of focal cerebral ischemia reperfusion model in rats (HEx400).
Appendix C. Pathological images of brain tissue in cerebral cortex area of focal cerebral ischemia reperfusion model in rats (HEx400).
Appendix D. Pathological images of brain tissue in hippocampus of focal cerebral ischemia reperfusion model in rats (HEx400).
References
- Al-Enazi N.M., Awaad A.S., Zain M.E., Alqasoumi S.I. Antimicrobial, antioxidant and anticancer activities of laurencia catarinensis, laurencia majuscula and padina pavonica extracts. Saudi Pharm. J. 2018;26(1):44–52. doi: 10.1016/j.jsps.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Zhang X.L., Bai M., Miao M.S. Prevention characteristic and analysis of promoting blood circulation by removing blood stasis, clearing heat and detoxicating on cerebral ischemia. J. Tradit. Chin. Med. 2012;27(5):615–619. [Google Scholar]
- Culman J., Nguyen-Ngoc M., Glatz T., Gohlke P., Herdegen T., Zhao Y. Treatment of rats with pioglita-zone in the reperfusion phase of focal cerebral ischemia: a preclinicalstroke trial. Exp. Neurol. 2012;238(2):243–253. doi: 10.1016/j.expneurol.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Fang X.Y., Wang L.L., Miao M.S. Neuroprotective effects of cerebral ischemic preconditioning. J. Experimental Chin. Med. 2016;22(18):112–117. [Google Scholar]
- Gao W., Wang Y., Basavanagoud B., Jamil M.K. Characteristics studies of molecular structures in drugs. Saudi Pharm. J. 2017;25(4):580–586. doi: 10.1016/j.jsps.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S., Liu Z., Furuta Y., Peng W. Characteristics of activated carbon remove sulfur particles against smog. Saudi J. Biol. Sci. 2017;24(6):1370–1374. doi: 10.1016/j.sjbs.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J.S., Lu H.J., Song X.J. Neuroprotection of LBP on a mouse model of transient focal cerebral ischemia and its protective mechanisms of inhibiting oxidative stress. J. Stroke Neurol. Dis. 2016;33(9):790–794. [Google Scholar]
- Ge J.B., Lu H.J., Xj Song. Protective effects of LBP on cerebral ischemia reperfusion injury in mice and mechanism of inhibiting NF-κB, TNF-α, IL-6 and IL-1β. Chin. Med. J. 2017;42(2):326–331. doi: 10.19540/j.cnki.cjcmm.20161222.016. [DOI] [PubMed] [Google Scholar]
- Hu L.P. The fundamental knowledge for the regression analysis of the count data. Chin. Sichuan Mental Health. 2018;5:385–393. [Google Scholar]
- Ju A.C., Geng S.H., Yang X.P. Effect of salvianolic acid B by intranasal administration on cognitive function and neurogenesis of cerebral ischemia rats. Chin. Tradition. Patent Med. 2017;48(12):2481–2485. [Google Scholar]
- Khan A., Jan G., Khan A., Jan F.G., Danish M. Evaluation of antioxidant and antimicrobial activities of bergenia ciliata sternb (rhizome) crude extract and fractions. Pakistan J. Pharm. Sci. 2018;31(1):31–35. [PubMed] [Google Scholar]
- Li Y.C., Wang J.X., Liu K. Effects of Shennao Fuyuan Decoction combined with implantation of UC-MSCs on expression of neurotrophic factors in cerebral ischemia-reperfusion rats. Chin. Herbal Med. 2016;47(5):781–787. [Google Scholar]
- Li X.Q., Zan J., Feng Z.J. Dendrobium nobile polysaccharides reduce cerebral ischemia reperfusion injury in rats. Chin. Tradition. Patent Med. 2017;39(04):677–683. [Google Scholar]
- Liu C., Liu J.X., Ren F.F. The research progress of traditional Chinese medicine to protect cerebral ischemia reperfusion injury. Chin. J. Gerontol. 2016;36(2):481–484. [Google Scholar]
- Ma R.J., Zhang R., Xu X.Q. Response surface analysis method optimize the ultrasonic extraction process of total phenol in large blood vine. Tradition. Chin. Med. Patent Prescription. 2013;35(2):404–407. [Google Scholar]
- Narkhede A.N., Nirmal P.S., Nagarkar B.E., Singh E.A., Harsulkar A.M., Jagtap S.D. Validation of the immunomodultory potential of amarkand species. Indian J. Pharm. Sci. 2017;79(6):965–973. [Google Scholar]
- National Pharmacopoeia Committee . China medical Science and Technology Press; Beijing, China: 2015. Pharmacopoeia of People's Republic of China. [Google Scholar]
- Piironen K., Tiainen M., Mustanoja S., Kaukonen K.M., Meretoja A., Tatlisumak T., Kaste M. Mild hypothermia after intravenous thrombolysis in patients with acute stroke: a randomized controlled trial. Stroke. 2014;45(2):486–491. doi: 10.1161/STROKEAHA.113.003180. [DOI] [PubMed] [Google Scholar]
- Rei B., Higdon J.V. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J. Nutr. 2003;133(10):3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- Riverol M., Becker J.T., López O.L., Raji C.A., Thompson P.M., Carmichael O.T., Gach H.M., Longstreth W.T., Fried L., Tracy R.P., Kuller L.H. Relationship between systemic and cerebral vascular disease and brain structure integrity in normal elderly individuals. J. Alzheimers Disease Jad. 2015;44(1):319–328. doi: 10.3233/JAD-141077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.Z., Sun L.L., Ren Y.Z. Effects of electroacupuncture on the expressions of IL-1β and ICAM-1 in bilateral brain tissues of rats with cerebral ischemia/reperfusion injury. J. Chin. Experimental Animals. 2015;23(3):278–284. [Google Scholar]
- Tian C.Y., Zhang H.N., He J.B. Effect of Gridi-13 on expression of NSE, TNF-α, and IL-6 after focal cerebral ischemia in rats. Chin. Tradition. Herbal Drugs. 2015;46(5):716–720. [Google Scholar]
- Yang J.P., Liu H.J., Yang H., Feng P.Y. Therapeutic time window for the neuroprotective effects of NGF when administered after focal cerebral ischemia. Neurol. Sci. Off. J. Italian Neurol. Soc. Italian Soc. Clin. Neurophysiol. 2011;32(3):433–441. doi: 10.1007/s10072-011-0512-9. [DOI] [PubMed] [Google Scholar]
- Yilmaz B., Cevik H., Bildaci T.B., Ozdogan S. Renal stiffness on patients with gestational diabetes. Acta Medica Mediterranea. 2017;33(4):663–666. [Google Scholar]
- Zhang M., Wang G.M. The effects of dodder flavone on apoptosis and expression of Bcl-2, Bax and Caspase-3 in rats with cerebral ischemia reperfusion injury. Chin. Med. Pharmacol. Clin. 2014;30(5):78–81. [Google Scholar]
- Zhu F.P., Zhou Y.L., Li X.J. The study on effect of taohongsiwu liquid on SOD and MDA in rats with limb ischemia reperfusion injury. Chin. Med. Guide. 2015;21(21):17–19. [Google Scholar]