Abstract
In order to find effective measures to control a Moroccan shallow reservoir (Sidi Abderrahmane), a better understanding of phytoplankton composition, abundance, spatial and temporal distribution it is necessary. Trophic level and the stability status were assessed upon the basis of Shannon diversity index (H′), species richness (S), and evenness (J) index. Statistical tests were used to evaluate the different relationships between phytoplankton and the concentrations of several physico-chemical parameters, and the main soluble nutrients. In surveys, the samples were taken fortnightly from May 2011 to December 2012. 64 taxa belonging to seven groups of phytoplankton were identified, including Bacillariophyceae (25 taxa), Chlorophyceae (22 taxa), and Cyanophyceae (9 taxa). Aulacoseira granulata, Nitzschia palea, Scenedesmus acuminatus, and Oscillatoria sp, were the main contributors to the dissimilarity in temporal distribution. Phytoplankton population never reached a monospecificity situation. Shannon and evenness indices were between (0.0001 < H′ < 0.15; 0.003 < J < 0.085) and manifested a young phytoplankton community with high multiplying power. There were significant correlations between total phytoplankton (r = 0.015, p < .01) and water temperature. Significant negative correlations were observed between transparency and Cyanophyceae (r = −0.208, p < .05) and between the number of species and transparency (r = −0.206, p < .05), orthophosphorus (r = −0.377, p < .01), and nitrates (r = −0.301, p < .01). A negative correlation was found between Orthophosphorus and Chlorophyceae (r = −0.377, p < .01). Similar correlations were also observed with nitrates and Chlorophyceae (r = −0.297, p < .01), Silica and Bacillariophyceae (r = 0, p < .01) and total phytoplankton (r = −0.372, p < .07). The underwater light condition, as indicated by Secchi depth fluctuations, hydraulic process conditions (short residence time, short outflow/inflow ratio) were shown to be the limiting factors in regulating the density of phytoplankton. With reference to Palmer pollution index, test results indicated an oligotrophic or mesotrophic reservoir. The data presented provide the first contemporary account of the level of algal diversity present, the prominent environmental conditions and trophic status of Sidi Abderrahmane reservoir waters.
Keywords: Phytoplankton, Diversity, Trophic status, Shallow reservoir, Morocco
1. Introduction
Phytoplankton interacts with the aquatic environment where it lives and is affected by many biotic and abiotic factors (Thakur et al., 2013). Their composition and dynamics play a crucial role in biodiversity, energy flow through the aquatic ecosystem (Jindal et al., 2014), and water for human uses (Fisher et al., 2009, Sharma et al., 2013). Therfore, phytoplankton can be used to predict the effect of change in the aquatic ecosystem (Perkins et al., 2010, Yvon-Durocher, 2010). Accordingly, the use of phytoplankton assemblages for monitoring the ecological status of drinking reservoirs has been widely recommended (Fetahi et al., 2014). Furthermore, access to safe drinking-water is essential to human health (WHO, 2011). The dynamics of reservoir lakes has been changed worldwide, considering the impact of both climate change and anthropogenic pollution (Shuai et al., 2013, Wagner et al., 2016). This situation becomes more precarious in the mediteranean area known by an acute aridity, such as Morocco (El ghachtoul, Y., Alaoui Mhamidi, M., Gabi, H., 2005, Douma et al., 2009, Gartet, 2009). Shallow reservoirs differ markedly in comparison to deep reservoirs, especially with respect to matter exchange and plankton dynamics (Petaloti et al., 2004). Sidi Abderrahmane a Moroccan shallow reservoir, located in the extreme downstream of Oum Rbia basin at the end of other reservoirs series, and is surrounded by agglomerations and agricultural fields. This reservoir was considered the main supplier of drinking freshwater, industries, crafts, irrigation in this semi-arid region. However, in our knowledge, there are no studies on phytoplankton community structure and trophic status in Sidi Abderrahmane reservoir.
This study was based on a monitoring network conducted fortnightly between May 2011 and December 2012. The main objectives of this work are to investigate (1) the qualitative and quantitative phytoplankton, and (2) temporal and spatial phytoplankton organization. (3) The influence of some abiotic parameters was on and (4) the assessment of the trophic status was determined. The obtained results will help to assess and predict the growth and development of phytoplankton blooms, and it well be a reference for further studies in similar Moroccan reservoirs.
2. Material and methods
2.1. Study area
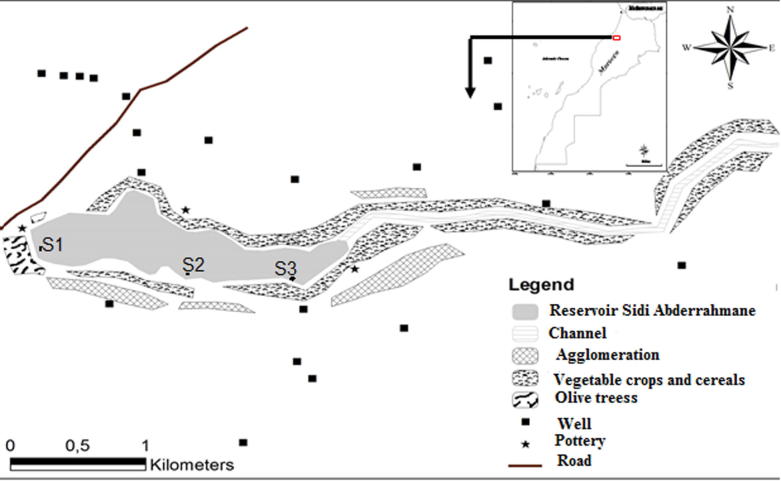
Sidi Abderrahmane reservoir was built in 1965 at the northwest of Safi city (32° 19′29 0.38 N 9°10′34.77 W). It has a maximum depth of 6 m, a surface area of 1 km2, a total capacity of 3.2 million m3, and is localized at the extreme downstream part of Oum Rbia basin. Agricultural lands and agglomerations surround it. Water of this lake was used for drinking, industries and irrigation purposes (Fig. 1).
Fig. 1.
Samples of the location in Sidi Abderrahmane lake-reservoir (Safi, Morocco), (S1: downstream, S2: Middle, S3: Upstream).
2.2. Sampling and analytical methods
The Monitoring network started from May 2011 to December 2012. Sampling frequencies were fortnightly collected and three sampling stations were designated (Fig. 1). All samples were taken horizontally from surface water.
2.2.1. Physicochemical analyses
Water temperature, pH, and dissolved oxygen concentration were measured in situ using a multiparameter water quality meter (WTW pH 330i and HANNA HI 9142). Transparency was measured via Secchi disc method. Samples were transported with 1–5 L bottles in cold storage to the laboratory. Nutrients (orthophosphorus, nitrites, nitrates, ammonium, silica) and chlorophyll-a, were analyzed by colorimetric method (Rodier, 2009).
2.2.2. Biological parameters
For the taxonomic study, samples were taken by a very low horizontal speed lines by towing 20 µm mesh size phytoplankton net against the current, at surface level, for ten minutes. samples were collected in 1 liter labeled plastic containers by filtering 50 L of water, and immediately preserved with 4% formalin and fixed with Lugol’s iodine for quantitative and qualitative analysis. Enumerations were carried out using an Olympus light microscopy (Olympus BX 40). The species classification was done with standard and specific literature (Bourrelly, 1966, Bourrelly, 1968, Bourrelly, 1970). Phytoplankton density was determined via fixed subsamples and settled in 5 and 10 ml chambers at least 6 h according to Uthermöhl (1958). These samples were examined in laboratory using Epson inverted microscope. Chlorophyll-a (µg L−1) were measured spectrophotometrically by the convetional method described by Rodier, (2009).
2.2.3. Statistical analysis
Phytoplankton structure was analyzed regarding abundance, total of species, density, diversity index (H′) (Shannon, 1948) and evenness index (Pielou, 1966) (J').
Shannon-Weiner Index
| (1) |
where:
pi = the proportion of individuals of genus (Relative density)
ln = natural logarithms of individual species i
Evenness index
| (2) |
The indices (S) as log2, (H') and (J') of each sampling station were plotted together on a two-dimensional graphic representation in a diversity model called DIMO model (Qinghong, 1994) and regarded as a synthetic tool.
The variables were calculated for each station and each month. The Data were statistically analyzed using Anova -one way and Pearson tests. All analyses were taken with SPSS software, version 17. Palmer (1969) carried out an extensive literature survey to assess the tolerance of algal species to organic pollution and developed an “Algal Species Pollution Index” for rating water quality. The top 20 algal species are rated on a scale from 1 to 6 (intolerant to tolerant), and the index is simply calculated by summing up the scores of all relevant species present within the sample. Pollution score with values > 20 are consistent with high organic pollution, scores between 15 and 19 are taken as probable evidence of organic pollution, and < 10 Pollution score with value is consistent with unpolluted water body (Jindal et al., 2014).
3. Results
3.1. Species composition
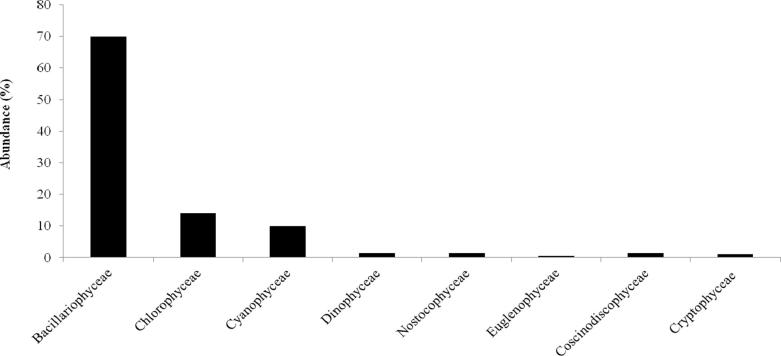
The identified phytoplankton comprised a total of 64 taxa belongin to the following families: Bacillariophyceae (25), chlorophyceae (22), Cyanophyceae (9), Dinophyceae (3), Euglenophyceae (2), Coscinodiscophyceae (1), Nostocophyceae (1), and Cryptophyceae (1) (Table 1). Bacillariophyceae formed the most dominant taxa. They represent over 70% of total species. Chlorophyceae represent 14% for total phytoplankton species with 20 specises. Cyanophyceae constituted the third dominant family representing 10% of the total phytoplankton composition identified in this investigation (9 species). Nostocophyceae, Coscinodiscophyceae and Dinophyceae represent all of them 4.5%, Cryptophyceae 1%, and Euglenophyceae is represented by only 0.5% (Fig. 2).
Table 1.
List of identified phytoplankton species in Sidi Abderrahmane reservoir (May 2011-December 2012).
| Sites |
||||
|---|---|---|---|---|
| Taxa | S1 | S2 | S3 | Seasonality |
| Cl. Chlorophyceae | ||||
| O. Chlorococcales | ||||
| Ankistrodesmus gelifactum (Chodat) Bourrelly | + | − | − | S-SP |
| Ankistiodesmus falcatus (corda) Ralfs | + | − | − | S |
| Coelastrum microporum Nägeli | + | − | − | S |
| Coelastrum reticulatum (Dangeard) Senn | + | − | − | S-A |
| Dictyospharium pulchellum Wood | + | − | − | A-W |
| Monoraphidium sp. | + | + | − | S |
| Ankyra sp. | + | + | − | SP |
| Elakalothris gelatinosa wille | + | + | + | W |
| O. Desmidiale | ||||
| Staurastrum sp. | + | + | + | S-W-A |
| Cosmarium laeve Rabenhorst | + | − | − | S-A |
| O. Trebouxiophyceae ordo incertae sedis | ||||
| Crucigenia quadrata Morren | + | − | − | A-S-SP |
| Oocystis borgei f. nivicola Kol | + | − | − | A-W |
| Oocystis lacustris Chodat | + | + | + | W |
| O. Sphaeropleale | ||||
| Scenedesmus acuminatus (Lagerh) Chodat | + | + | + | S-A-SP |
| Pediastrum simplex (Meyen) Corda | + | − | + | S-SP |
| Monoraphidium sp. | + | + | + | SP |
| Tetraedron caudatum (Corda)Hansg | + | − | − | A |
| O. Desmidiale | ||||
| Closterium sp. | − | − | + | W |
| O. Chlorellales | ||||
| Chlorella vulgaris Beijerinck | + | + | + | P-S |
| O. Clamydomonadales | ||||
| Clamydomonas sp. | + | + | + | A |
| Phacotus sp. | + | + | + | A |
| Cl. Bacillariophyceae (Bacillariophyceaeophyceae) | ||||
| O. Achnanthale | ||||
| Achnanthes lanceolata (Brébisson) Grunow | + | + | + | S-A-W |
| Achnanthes minitussima Kützing | + | + | + | S-A |
| Achnanthes lanceolata (Brébisson) Grunow | + | + | + | S-A |
| Cocconeis placentula(Ehrenberg) var. euglypta (Ehrenberg) | + | + | + | S−A |
| O. Coscinodiscale | ||||
| Cyclotella ocellata Pantocsek | + | + | + | SP |
| O. Bacillariophyceaele | ||||
| Fragilaria ulna (Nitzsch.)Lange Bertalot var. ulna | − | + | + | P |
| Fragilaria vaucheriae(Kützing) Lange Bertalot | − | + | + | P |
| Fragilaria brevistriata Grunow | − | + | + | P |
| Synedra ulna (Nitzsch) Ehrenberg 1832 | − | + | + | P |
| O. Naviculale | ||||
| Pleurosigma angulatum (Queckett) W.Smith | + | + | + | A-S |
| Amphora sp. | − | + | + | P |
| Cymbella affinis Kützing | − | + | + | P |
| Cymbella microcephala Grunow | − | + | + | P |
| Gyrosigma acumunatum (Kützing) Rabenhorst | − | + | + | P |
| Gyrosigma sp. encerii (W. Smith) var.modifera (Grunow) Clev | − | + | + | P |
| Navicula cryptocephala Kützing | + | + | + | S-W |
| Navicula capitatoradiata Germain | + | + | + | S-W |
| Navicula recens Lange-Bertalot | + | + | + | S |
| Navicula cryptocephala Kûtzing | + | + | + | P |
| Navicula viridula (Kützing) Ehrenberg | + | + | + | A-W |
| Diploneis ovalis (Hilse) Cleve | + | + | + | A |
| Pinnularia polyonca (Brébisson) W.Smith | + | − | − | P |
| O. Bacillariale | ||||
| Nitzshia palea (Kützing) W.Smith | + | + | + | SP |
| O. Centrale | ||||
| Aulacosiera grannulata var angustissima | + | + | + | P |
| O. Pennale | ||||
| Surerella sp. | − | + | + | P |
| Cl. Coscinodiscophyceae | ||||
| O. Coscinodiscale | ||||
| Cyclotella ocellata Pantocsek | − | + | + | W |
| O. Biddulphiale | ||||
| Hydrosera sp. | + | − | − | W |
| Cl. Dinophyceas | ||||
| O. Peridinilale | ||||
| Ceratium hirundinella (O.F.Müller) Schrank | + | + | + | W-A |
| Gymnodinium sp. | + | − | − | A |
| Peridinium sp. | + | + | − | W-A |
| Cl. Euglenophyceae | ||||
| O. Euglenale | ||||
| Euglena sp | + | + | + | W |
| Trachelomonas volvocinopsis Svirenko | + | − | + | W-A |
| Cl. Cyanophyceae | ||||
| O. Nostocale | ||||
| Anabaena sp. | − | + | + | S-A |
| Lyngbya sp. | − | + | + | A-W |
| Limnotrix redekeï HUB | − | + | + | A |
| Merismopedia sp. | − | + | + | A-W |
| Aphanocapsa elachista | − | + | + | A |
| Spirulina sp. | − | + | + | W |
| Oscillatoria sp. | − | + | + | W |
| Aphanizomenon morren ex bornet et flahault 1888 | − | + | + | A |
| Dictyosphaerium pulchellum Wood | + | + | + | W |
| Cl. Nostocophyceae | ||||
| O. Chroococcale | ||||
| Snowella lacustris (Chodat) Komárek et Hindák | + | + | + | S |
| Cl. Cryptophyceae | ||||
| O. Cryptomonadales | ||||
| Cryptomonas ovata Ehrenberg | + | + | + | A-W |
S: Summer, W: Winter, A: Autumn, SP: Spring, P: Perennial, +: Present, −: Absent.
Fig. 2.
Species abundance of phytoplankton class in Sidi Abderrahmane reservoir (May 2011-December 2012).
3.2. Spatial and temporal distribution of the population density,
Marked monthly variations were observed in population densities of phytoplankton (Table 2). The highest density was observed in September 2012 with 718⋅103 Cells L−1. The lowest value was registered in January with 25⋅103 Cells L−1.
Table 2.
Total numbers of phytoplankton (103 Cell L−1) in Sidi Abderrahmane reservoir (May 2011-December 2012).
| 2011 |
2012 |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | May | Jun | Jul | Aug | Sept | Oct | Dec | Jan | Feb | Mar | Abr | May | Jun | Jul | Aug | Sept | Oct | Nov | Dec | |
| Total Numbers (103 Cells L−1) | S1 | 116 | 258 | 320 | 432 | 101 | 135 | 174 | 62 | 65 | 137 | 108 | 168 | 650 | 345 | 126 | 698 | 407 | 322 | 454 |
| S2 | 124 | 259 | 175 | 568 | 107 | 119 | 109 | 34 | 55 | 157 | 112 | 122 | 701 | 394 | 166 | 717 | 402 | 306 | 378 | |
| S3 | 128 | 211 | 185 | 467 | 105 | 189 | 167 | 25 | 69 | 191 | 190 | 196 | 720 | 286 | 144 | 718 | 367 | 389 | 386 | |
| Number of species (S) | S1 | 24 | 27 | 13 | 28 | 15 | 16 | 12 | 10 | 11 | 24 | 19 | 22 | 19 | 29 | 19 | 61 | 10 | 22 | 10 |
| S2 | 23 | 27 | 13 | 34 | 17 | 10 | 12 | 8 | 11 | 28 | 18 | 24 | 31 | 27 | 16 | 56 | 16 | 29 | 11 | |
| S3 | 25 | 25 | 18 | 20 | 13 | 12 | 12 | 11 | 11 | 24 | 17 | 23 | 30 | 25 | 19 | 56 | 13 | 23 | 11 | |
In the present study, the water of Sidi Abderrahmane reservoir is rich of Bacillariophyceae flora, with 25 species. In winter, Aulacoseira granulata var angustissima was a dominant Bacillariophyceae species at both sites during our investigation with 46% of the total species. The decrease in density was more pronounced in winter. Cyclotella ocellata and Nitzschia palea were found to be predominant in spring for both sites, and represented 50.3% of the total density. Pediastrum simplex was the dominant chlorophyceae specie in spring (51%). Scenedesmus acuminatus and staurastrum sp. were the predominant species in summer with the domination of Scenedesmus acuminatus at both sites (45%). A Cyanohyceae lyngbya sp. appeared in autumn, winter and in the end of summer. Chlorella vulgaris was the dominant Chlorophyceae specie in autumn for both sites (63%). Oocystis borgei followed them and appeared in the end of December. Cymbella sp. and Achnanthes sp. were perennial in this investigation. Only one species of Cryptophyceae, Cryptomonas ovata, was recorded during the present investigation.
3.3. Species diversity and trophic level
The Range of diversity indices and the number of species (S) in Sidi Abderrahmane were demonstrated in Table 2 and Table 3. A variation was noticed in species diversity in the present investigation. The maximum number of species (61) was observed in September. The lowest number was detected in January (8).
Table 3.
Shannon (H′) and evenness (J) Indices in Sidi Abderrahmane reservoir (May 2011-December 2012).
| Month Intervals | Species Diversity H′ |
Species Equitability J |
|||||
|---|---|---|---|---|---|---|---|
| Sites |
Sites |
||||||
| S1 | S2 | S3 | S1 | S2 | S3 | ||
| 2011 | May | 0.028 | 0.031 | 0.033 | 0.015 | 0.017 | 0.018 |
| Jun | 0.035 | 0.051 | 0.061 | 0.019 | 0.028 | 0.033 | |
| Jul | 0.035 | 0.02 | 0.01 | 0.019 | 0.011 | 0.01 | |
| Aug | 0.076 | 0.069 | 0.074 | 0.042 | 0.038 | 0.05 | |
| Sept | 0.013 | 0.083 | 0.072 | 0.007 | 0.046 | 0.039 | |
| Oct | 0.095 | 0.031 | 0.029 | 0.052 | 0.017 | 0.016 | |
| Dec | 0.062 | 0.05 | 0.049 | 0.034 | 0.027 | 0.027 | |
| 2012 | Jan | 0.001 | 0.003 | 0.002 | 0.003 | 0.007 | 0.005 |
| Feb | 0.017 | 0.014 | 0.015 | 0.011 | 0.014 | 0.01 | |
| Mar | 0.031 | 0.048 | 0.065 | 0.017 | 0.026 | 0.035 | |
| Abr | 0.008 | 0.008 | 0.007 | 0.01 | 0.032 | 0.042 | |
| May | 0.028 | 0.064 | 0.041 | 0.015 | 0.035 | 0.022 | |
| Jun | 0.032 | 0.072 | 0.094 | 0.017 | 0.040 | 0.051 | |
| Jul | 0.048 | 0.044 | 0.031 | 0.026 | 0.024 | 0.017 | |
| Aug | 0.036 | 0.010 | 0.017 | 0.020 | 0.006 | 0.009 | |
| Sept | 0.113 | 0.155 | 0.101 | 0.062 | 0.085 | 0.055 | |
| Oct | 0.006 | 0.037 | 0.037 | 0.004 | 0.020 | 0.020 | |
| Nov | 0.008 | 0.104 | 0.058 | 0.004 | 0.057 | 0.032 | |
| Dec | 0.042 | 0.031 | 0.039 | 0.023 | 0.017 | 0.021 | |
The highest diversity (H′ = 0.1500 bits; J = 0.0850) was observed in September 2012. The lowest value (H′ = 0.001 bits; J = 0.003) was detected in January. Shannon index demonstrate a positive correlation with Bacillariophyceae (r = 0.211, p < .05), Cyanophyceae (r = 0.301, p < .01), total phytoplankton (r = 0.366, p < .01) and number of species (r = 0.200, p < .01).
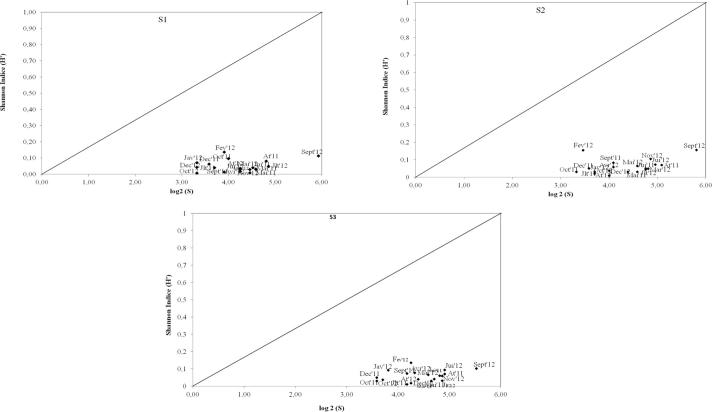
The results of DIMO model application was illustrated in Fig. 3. The graphs show that the spatial evolution was type 1 “diversity-type” Which was characterized by a variable richness and regularity, while the diversity is constant. By the application of Palmer’s Algal Species Pollution Index (Table 4), Sidi Abderrahmane showed a score of 5 and it can be classified as an “unpolluted” water body.
Fig. 3.
DIMO model application by simultaneous representation of the (Log2 (S)), diversity index (H') and equitability (line Diagonal = maximum equitability (J' = 1)).
Table 4.
Algal species pollution index (Palmer, 1969) in Sidi Abderrahmane reservoir (May 2011-December 2012).
| Indicator species | Pollution index | Sidi Abderrahmane |
|---|---|---|
| Euglena viridis | 6 | − |
| Nitzschia palea | 5 | − |
| Oscillatoria limosa | 4 | + |
| Scenedesmus quadricauda | 4 | − |
| Oscillatoria tenuis | 4 | − |
| Stigeoclonium tenue | 3 | − |
| Synedra ulna | 3 | − |
| Ankistrodesmus falcatus | 3 | − |
| Pandorina morum | 3 | − |
| Oscillatoria chlorina | 2 | − |
| Chlorella vulgaris | 2 | − |
| Arthrospira jenneri | 2 | − |
| Cyclotella meneghiniana | 2 | − |
| Melosira varians | 2 | + |
| Euglena gracilis | 1 | − |
| Nitzschia acicularis | 1 | + |
| Navicula cryptocephala | 1 | − |
| Oscillatoria princeps | 1 | − |
| Oscillatoria putrida | 1 | − |
| Gomphonema parvulum | 1 | − |
| Pollution rating | 5 |
+ Present, − Absent (note that an alga is considered to be present if there are 50 or more individuals per liter.
4. Discussion
4.1. Phytoplankton composition
The present study is done to provide phytoplankton composition, abundance, spatial and temporal distribution in Sidi Abderrahmane water reservoir. During this investigation, Bacillariophyceae flora is the dominant taxa in Sidi Abderrahmane waters with 25 species. Barinova and Chekryzheva (2014) concluded that the dominance of Bacillariophyceae in a temperate lake is an indicator of its oligotrophic status. The algal population was mainly dominated by the Cyanophyceae Oscillatoria sp. which represents 50% of the algal population. Unlike some studies, Cyanophyceae was abundant at high temperature in summers and minimum at low temperature during winters (Jindal et al., 2014). Dinophyceae members were present during winter and in the end of autumn. Relatively low values of temperature and high values of dissolved oxygen were responsible for the winter maxima of Dinophyceae (Jindal et al., 2014). Vyas and Kumar, (1968) reported the presence of euglenoids during the rainy season. This is further confirmed by the appearance of Euglena sp. in winter, in all samples sites. Poor occurrence of euglenoids may be attributed to the absence of organic matter in Sidi Abderrahmane. The results indicated that the phytoplankton community never reached a monospecificity situation. Non-stressed waters were often characterized by high diversity, (Thakur et al., 2013).
DIMO model application shows that the changes affecting the phytoplankton structure were mostly due to changes in the number of species and individuals. Nevertheless, the community is homogeneous, stable and well structured over time.
Nonetheless, different phytoplankton taxa were identified as indicators of the level of trophic status (Hutchinson, 1967, Palmer, 1969). Atici and Obali (2010) concluded that several species like Cymbella, Nitzschia, Cyclotella genus were tolerant to eutrophic conditions environmental factors. However, during our study these species were dominant in Sidi Abderrahmane surface waters.
4.2. Relationship between Physico-chemical parameters and phytoplankton
The qualitative and quantitative monitoring of different groups of the phytoplankton, including bioindicator species and calculating biodiversity indices were used to explore the trophic status in the water body. This aspect was also supported by monitoring physico-chemical parameters.
Sidi Abderrahmane was a warm polymictic lake. A Positive correlation was observed between water temperature and Chlorophyceae (r = 0.362, p < .01), total phytoplankton (r = 0.015, p < .01) and number of the species (r = 0.234, p < .01). Narrow fluctuation of dissolved oxygen was observed during this investigation with the average value of 10.42 ± 2.40 mg L−1. Dissolved oxygen had a positive correlation with a number of species (r = 0.186, p < .05). The results exhibited the variations in transparency values with an average value 0.638 ± 0.193 m. the pH was alkaline. A transparency had a negative correlation with water temperature (r = −0.29, p < .01), Cyanophyceae (r = −0.208, p < .05) and Number of species (r = − 0.206, p < .05). It exposed a season effect (r = 0.001, p < .01) but no sites effect was observed during our study period.
In this investigation, the mean of Orthophosphates values was 0.64 ± 0.62 mg L−1. A negative correlation was found between Orthophosphorus and chlorophyceae (r = −0.377, p < .01), and between Orthophosphorus and a number of species (r = −0.377, p < .01). Ammonium had a negative correlation with Chlorophyceae (r = −0.176, p < .01). Also, a negative correlation was discovered between nitrates and Chlorophyceae (r = −0.297, p < .01), and number of the species (r = −0.301, p < .01). Nitrates was translated into a season effect by mean ANOVA test (r = 0; p < .001). Silica was a negative correlation with Bacillariophyceae (r = 0, p < .01) and total phytoplankton (r = −0.372, p < .07). However, in Sidi Abderrahmane water body, no algal bloom was detected at the same period.
The speed mixing water column increase was important and short residence time seemed to have a role on the stability of phytoplankton regeneration cycles.
A few phytoplankton species composition in Sidi Abderrahmane waters was common with otherMoroccon reservoirs: 24 species are common with the retaining Bin Elouidane (Cherifi and Loudiki, 2002) (23 species), Allal El Fassi (Bouhadioui, 1997) (19 species), and Lalla Takerkouste (Cherifi, 1992) (25 species). Phytoplankton composition is also similar to those of some reservoirs in India: Rewalsar (32 species), Prashar (30 species), Kuntbhyog (30 species) (Thakur et al., 2013). The presence of inorganic phosphorus at 0.03 mg L−1 concentration is sufficient to cause algal blooms (Sheela et al. 2011). However, if the increase in nutrients promotes the appearance of efflorescence, other factors may intervene to prevent this occurrence (Sheela et al., 2011). In Comparaison to the same preceding reservoirs, the phytoplankton density in Sidi Abderrahmanee water column was generally reduced as indicated by the low concentration of chlorophll-a. The underwater light condition, as indicated by the Secchi depth, pH, and the level of hydrodynamic stability appeared to be a limiting factor in regulating the density of phytoplankton.
Biological characteristics of algae and zooplankton biomasses were also an important factor controlling the density and growth of phytoplankton. Similar results were confirmed in many studies (Wei et al., 2010, Wagner et al., 2016, Zhang et al., 2016).
5. Conclusions
The purpose of the study was to investigate mainly the qualitative and quantitative phytoplankton abundance, seasonal, and spatial variations in the organization of the phytoplankton community. This exploration was supported by physicochemical analysis. Pearson correlation revealed a significant relationship between physicochemical parameters and different phytoplankton groups. 64 species was identified.Palmer pollution index indicated an unpolluted water body. Hydrodynamic stability, turbidity, pH, temperature, and predation pressure seem to have repercussions on the phytoplankton regeneration stability. Many identified species of cyanobacteria can be considered as potentially toxic, which need a vigilance control, especially during summer. Our results suggested that management efforts should be focused accordingly to check the deteriorating water quality and trophic status of this shallow reservoir.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Wafae Belokda, Email: wafaebelokda@gmail.com.
Faissal Aziz, Email: faissalaziz@gmail.com, faziz@kth.se.
Khalid Elkalay, Email: elkalay_khalid@yahoo.fr.
References
- Atici T., Obali O. The Bacillariophyceaes of Asartepe Dam Lake (Ankara), with environmental and some physicochemical properties. Turkish J. Bot. 2010;34:541–548. [Google Scholar]
- Barinova S., Chekryzheva T. Phytoplankton dynamic and bioindication in the Kondopoga Bay, Lake Onego (Northern Russia) J. Limnol. 2014;73–2:282–297. [Google Scholar]
- Bouhadioui, A., 1997. Bilans biogéochimiques de l’azote et du phosphore et dynamique des populations phytoplanctoniues de la retenue de barrage Allal El Fassi. Thèse de 37me cycle, faculté des sciences et techniques, Fès-Saiss,Maroc, 187p.
- Bourrelly P. Edition Boubée, paris; TI-Algues vertes: 1966. Les algues d’eau douce; p. 511p. [Google Scholar]
- Bourrelly P. Edition Boubée, paris; TII-Algues Jaunes et brunes Chrysophycées, Phéophycées, Xantophycées et Diatomophycées: 1968. Les algues d’eau douce; p. 438p. [Google Scholar]
- Bourrelly P. Les Eugleniens, Péridiniens et Cryptomonadiens. Edition Boubée, paris; Algues Bleues et rouges: 1970. Les algues d’eau douce; p. 512p. [Google Scholar]
- Cherifi, O., 1992. Evolution et dynamique du phytoplancton en relation avec certains paramètres biotiques et abiotiques au niveau de la retenue Lalla Takerkoust (Maroc).Thèse 3ème cycle faculté des sciences semlalia, maarrakech, 155p.
- Cherifi O., Loudiki M. Variations de la structure trophique du lac-réservoir oligotrophe Bin El Ouidane (Maroc) R. sci. de l'eau. 2002;15(1):193–208. [Google Scholar]
- Douma, M., Loudiki, M., Oudra, B., Mouhri, K., Ouahid, Y., Francisca, F., del Campo, F., 2009. Taxonomic diversity and toxicological assessment of Cyanobacteria in Moroccan inland waters. R.sci. de l'eau. 22–3: pp. 435–449.
- El ghachtoul, Y., Alaoui Mhamidi, M., Gabi, H., 2005. Eutrophisation Des Eaux Des Retenues Des Barrages Smir Et Sehla (Maroc): Causes, Conséquences Et Consignes De Gestion. R.sci. de l'eau. 18: pp. 75–89.
- Fetahi T., Schagerl M., Mengistou S. Key drivers for phytoplankton composition and biomass in an Ethiopian highland lake. Limnol. 2014;46:77–83. [Google Scholar]
- Fisher M.M., Miller S.J., Chapman A.D., Keenan L.W. Phytoplankton dynamics in a chain of subtropical blackwater lakes: the Upper St. Johns River, Florida. USA. Lake Reservoir Manage. 2009;25:73–86. [Google Scholar]
- Gartet, A., El fengour, M., Gratet, J., Etconesa, G.C., 2009. Dégradation de la qualité des eaux du barrage sahla: traitement et gestion des risques de pollution. papeles de geografía. 0213-178, 49–50: pp. 41–54.
- Hutchinson G.E. Wiley; New York: 1967. A treatise on limnology. Introduction to lake biology and the limnoplankton. [Google Scholar]
- Jindal R., Thakur R.K., Singh U.B., Ahluwalia A.S. Phytoplankton dynamics and water quality of Prashar Lake, Himachal Pradesh. India. Sustain. Wat. qual. ecol. 2014;3–4:101–113. [Google Scholar]
- Palmer G. A composite rating of algae tolerating organic pollution. J. Phys. 1969;5:78–82. doi: 10.1111/j.1529-8817.1969.tb02581.x. [DOI] [PubMed] [Google Scholar]
- Perkins D.M., McKie B.G., Malmqvist B., Gilmour S.G., Reiss J., Woodward G. Environmental warming and biodiversity-ecosystem functioning in freshwater microcosms: partitioning the effects of species identity, richness and metabolism. Adv. Ecol. Res. 2010;43:177–209. [Google Scholar]
- Petaloti C., Voutsa D., Samara C., Sofoniou M., Stratis I., Kouimtzis T. Nutrient dynamics in shallow lakes of Northern Greece. Envir. Sci. Pollut. Res. 2004;11–1:11–17. doi: 10.1065/espr2003.06.156. [DOI] [PubMed] [Google Scholar]
- Pielou E.C. The measurement of diversity in different types of biological colledions. J. Theoret. Biol. 1966;13:131–144. [Google Scholar]
- Qinghong L. A model for species diversity monitoring at community level and its applications. Envir. Monit. Assess. 1995;34:271–284. doi: 10.1007/BF00554798. [DOI] [PubMed] [Google Scholar]
- Rodier, J., 2009. L’Analyse de l’Eau. Eaux naturelles, Eaux résiduaires, Eau de mer, Dunod, Paris, France, 9th édition.
- Shannon C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948;27 379–423 and 623-656. [Google Scholar]
- Sharma C., Jindal R., Singh U.B., Ahluwalia A.S., Thakur R.K. Population dynamics and species diversity of plankton in relation to hydrobiological characteristics of river Sutlej, Punjab India. Ecol. Envir. Conserv. 2013;19–3:717–724. [Google Scholar]
- Sheela A.M., Letha J., Joseph S. Environmental status of a tropical lake system. Envir. Monit. Assess. 2011;180:427–449. doi: 10.1007/s10661-010-1797-5. [DOI] [PubMed] [Google Scholar]
- Shuai X., Bin H., Zhong B.W., Jun L., Ai J.N., Liu Y.Y. Seasonal variation of phytoplankton nutrient limitation in Lake Taihu, China: A monthly study from Year 2011 to 2012. Ecotoxic. Envir. Saf. 2013;94:190–196. doi: 10.1016/j.ecoenv.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Thakur R.K., Jindal R., Uday B.S., Ahluwalia A.S. Plankton diversity and water quality assessment of three freshwater lakes of Mandi (Himachal Pradesh, India) with special reference to planktonic indicators. Envir. Monit. Assess. 2013;3(10):013–3178. doi: 10.1007/s10661-013-3178-3. [DOI] [PubMed] [Google Scholar]
- Utermöhl H. Zur Vervoll kommnung der quantitativen Phytoplankton Methodik Mitteilungen Internationale Vereinigung für Theoretische und Angewandte. Limnol. 1958;9:1–39. [Google Scholar]
- Vyas L.N., Kumar H.D. Studies on phytoplankton and other algae of Indra Sagar tank, Udaipur India. Hydrobiol. 1968;31:421–434. [Google Scholar]
- Wei Z., Lei W., Lianfang Z. Effect of nutrient level on phytoplankton community structure in different water bodies. J. Envir. Sci. 2010;22:32–39. doi: 10.1016/s1001-0742(09)60071-1. [DOI] [PubMed] [Google Scholar]
- WHO . fourth ed. WHO Press; World Health Organization, Geneva, Switzerland: 2011. Guidelines for Drinking Water Quality. [Google Scholar]
- Yvon-Durocher G. Ecological networks in a changing climate. Adv. Ecol. Res. 2010;42:72–138. [Google Scholar]
- Wagner H., Fanesi A., Wilhelm C. Freshwater phytoplankton responses to global warming. J. Plant Physiol. 2016 doi: 10.1016/j.jplph.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Zhang M., Yu Y., Yang Z., Kong F. Deterministic diversity changes in freshwater phytoplankton in the Yunnan-Guizhou Plateau lakes in China. Ecolo. Indic. 2016;63:273–281. [Google Scholar]