Abstract
Arbuscular mycorrhizal fungi (AMF) inoculation and biochar amendment has been reported to improve growth of several crop plants however their role in stress amelioration individually as well as in combination has not been worked out. This experiment was conducted to evaluate the application of AMF and biochar on the performance of chickpea under drought stress. The treatments included the individual as well as combined treatment of AMF and biochar to drought stressed and normal chickpea plants. Plants inoculation improved growth in terms of shoot and root length, leaf area and number of branches which was observed to show a steep decline due to drought stress. Drought declined the AMF colonization potential though biochar amendment ameliorated the negative effects of drought significantly by improving the spore population, number of mycelium, vesicle and arbuscules and the percentage of colonization as well. Increased chlorophyll synthesis in biochar and AMF treated plants was obvious, which lead to significant enhancement in the net photosynthetic efficiency. Drought stress also declined the relative water content (RWC) and membrane stability index (MSI), while treatment of biochar and AMF either individually or in combination mitigated the deleterious effects to considerable extent and caused a significant enhancement in RWC and MSI under normal conditions. Amendments with biochar and AMF inoculation increased the nitrogen fixation attributes including the number and weight of nodules, leghemoglobin content and activity of nitrate reductase enzyme leading to greater uptake and assimilation of nitrogen in them when compared to drought stressed plants. Drought stressed chickpea plants exhibited considerable reduction in uptake of nitrogen and phosphorous which was ameliorated by biochar and AMF treatments. It could be suggested that increase in growth and physiological attributes in chickpea due to biochar amendments and AMF inoculation under drought stress were plausibly due to their involvement in nitrogen and phosphorous uptake, chlorophyll synthesis and photosynthesis.
Keywords: Biochar, Arbuscular mycorrhizal fungi, Membrane stability index, Nitrogen fixation, Photosynthesis, Drought, Cicer arietinum
1. Introduction
Drought is one of the major threats to chickpea (Cicer arietinum L.) production worldwide. The problem is more severe in arid regions, which had been home to grain legumes. While more frequent droughts are expected in years to come, which may have devastating impacts on chickpea production worldwide (Ahmad et al., 2014, Fang and Xiong, 2015, Dikilitas et al., 2016, Alwhibi et al., 2017, Dubey et al., 2018).
Biochar is generated when solid biomass is subjected to high temperatures or gasification under limited air availability (Lehmann, 2007). Mostly prepared from the residues like manure, wood chips etc and is usually visible as simple charcoal-like product. Nowadays biochar has been employed for scientific and commercial usage as a soil amendment for improving the productivity of crops. For example, it has been accepted that biochar mediates carbon sequestration, assists in management and amendment of agricultural soils, waste management, environmental remediation and generation of renewable energy (Iijima et al., 2015, Afshar et al., 2016, Farhangi-Abriz and Torabian, 2018). Biochar as an achievable input and amendment for agricultural management can be a better integrated step for achieving the food security (Biederman and Harpole, 2013). Rich in carbon, biochar is having enough quantities of plant nutrients therefore can make nutrient deficient soils fertile for sustaining the crop and hence increasing the crop productivity (Ippolito et al., 2012). Treatment of acidic soils with biochar leads to maintenance of pH, cation exchange enhancement and nutrient retention leading to promotion of growth promoting soil microorganisms like PGPR and AMF (Changxun et al., 2016, Soudek et al., 2017). Improved growth due to treatment of biochar has been reported in several crop plants (Harel et al., 2012, Zhao et al., 2016). Treatment of biochar to Poncirus trifoliate increased growth by improving the root growth and the characteristics of soil like pH, fertility etc. (Changxun et al., 2016). Beneficial impact of biochar in regulation of growth through direct or indirect mechanisms can vary with plant species. Biochar promotes growth by enhancing the uptake and assimilation of nutrients, growth of beneficial soil microbes and improving the synthesis of phytohormones (Mehari et al., 2015).
Arbuscular mycorrhizal fungi (AMF) form symbiotic association with several plant species. AMF enhances growth of host plants by improving the soil structure and regulating the mineral nutrition of the host plant (Kumar et al., 2013). In addition, AMF has been well reported to stimulate growth under stressful growth conditions (Navarro et al., 2013, Hashem et al., 2016, Kumar et al., 2016). For example, under salt stress conditions AMF acts as an essential bio-ameliorator of stress leading to increased stress withstanding potential (Hameed et al., 2014). AMF colonization initiates morphological, nutritional and physiological changes in host plants to counter the stresses and also enhances plant growth and vigour (Ahanger et al., 2014, Alqarawi et al., 2014). More importantly AMF brings modification of the root architecture for improving access of water and nutrients (Wu et al., 2010, Hameed et al., 2014). By modifying the physiological status of host plants through enhancement in the leaf area and phosphorous content, AMF optimizes the rate of photosynthesis in host plants (Zhu et al., 2014, Boyer et al., 2015, Wahid et al., 2016).
Although the combined impacts of AMF and biochar are stronger than the single effect of each, most of the studies have so far focused on a single non-chemical mean to alleviate the adverse impact of drought (Afshar et al., 2016, Razaq et al., 2017). Only limited scientific investigations have studied the chickpea responses, particularly oxidative stress, to the combined effects of these two non-chemical agents (Ahmad et al., 2014, Alwhibi et al., 2017).
Therefore, this study was conducted to evaluate the potential effect of biochar and AMF application in improving performance of chickpea plants under drought and well-watered conditions.
2. Material and methods
2.1. Plant material
Seeds of chickpea (Cicer arietinum L., cv Giza 531) were obtained from Field Crops Research Institute, Agriculture research Center, Giza, Egypt. The seeds were disinfected in % aqueous ethanol (5:95, v/v) for five min, washed with sterile H2O2 and subsequently germinated on wet blotter at 25 °C for 5 days. Healthy germinated seeds with uniform growth were planted (one seed/pot) in the plastic pots (uniform size, 5 kg capacity) containing mixture of peat, perlite, and sand (1:1:1, v/v/v). The pots were arranged in a completely randomized block design with three replications.
2.2. Biochar
Woody branches of button mangrove (Conocarpus erectus L.) were collected from the Agricultural Research Station of Derab, Riyadh, Saudi Arabia. The branches were cut into 10–15 cm pieces and pyrolysed at 450 °C for 2.5 h in an Oven-Electric Furnace (Heraeus MR 170, Germany). The biochar produced was grinded and garbled (0.5 mm) to obtain fine particles. Organic carbon (C) of the farm residue and biochar samples was measured after oxidisation with potassium dichromate (K2Cr2O7) following Nelson and Sommers (1996). The total K contents were determined with a flame photometer (Jenway Flame Photometer, Bibby Scientific Ltd-Stone-Staffs-St15 0SA–UK.), P was estimated by a spectrophotometer as described by Sparks (1996), and N was determined by the micro-Kjeldahl method (AOAC, 1965). The biochar had 3.9, 2.8, 7.2, 761.8 and 9.5 (mg g−1) nitrogen, phosphorus, potassium, pH and EC (dS m−1), respecticely. Before sowing, the biochar (size < 3 mm) was mixed carefully with the seed cradle in pots of biochar treatments at 3% (w/w).
2.3. Mycorrhizal inoculum
The mycorrhizal inoculum, was including Claroideoglomus etunicatum (syn. Glomus etunicatum), Rhizophagus irregularis (syn. Glomus intraradices), and Funneliformis mosseae (syn. Glomus mosseae], was applied at 25 g of trap soil culture (approx. 750 spores/g trap soil)/pot as detailed elsewhere (Hashem et al., 2016). The trap culture method was used following Stutz and Morton (1996). Maize was used as trap plant.
2.4. Drought treatment
The drought stress (DS) was imposed by regulating the amount of Jensen’s nutrient solution (withholding water supply) added to each pot as described elsewhere (Jemo et al., 2017). The mixture of peat, perlite, and sand in each pot was saturated with Jensen’s nutrient solution and left overnight to equilibrate. The mixture samples were weighed, placed in an oven at 105 °C for 2 h and weighed again, consequently the volume of water to be added was determined as the percentage of added water. All pots were watered daily with Jensen’s nutrient solution maintaining 60% water holding capacity for two weeks after germination, and then half of the pots were exposed to DS by withholding water supply (50%) for another 6 weeks.
2.5. Treatments, growth conditions and harvest
The pots were divided into eight groups separately, each in three replicates, four groups were irrigated normally without any drought stress and four groups were under drought stress. Under both groups, pots were receiving arbuscular mycorrhizal fungi (AMF) and biochar (BC) or not. The germinating seeds were sown in the pots and grown under greenhouse conditions (day/night temperature 24 °C/16 °C; humidity 50–60%; day/night length each of 12 h) for 6 weeks after stress. The plants were, then, harvested, and roots were separated from shoots, the third top leaf (from the top) of each plant was used for biochemical analysis. At harvest, the plants were removed from the pots carefully and shoot and root lengths were measured with a ruler. Leaf area was recorded with a leaf area meter (DT Area Meter, Model MK2; Delta-T Devices, Cambridge, UK). Number of primary and secondary branches were counted.
2.6. Determination of relative leaf relative water contents (RWC)
Top fully expanded leaves were separated and were weighed to record the fresh weight, the leaf samples were then put in water for 30 min, removed, cleaned with tissue paper and weighed to record turgid weight. The same leaf samples were put into the oven (at 70 °C) till constant weight to record the dry weight. The relative leaf relative water contents was determined using the following formula of Smart and Bingham (1974):
2.7. Determination of membrane stability index (MSI)
For estimation of MSI, fresh leaf samples (100 mg) were taken in test tubes containing 10 mL double distilled water in two sets. One set was kept in water bath for half an hour at 40 °C and the electric conductivity was recorded as C1. Another set was kept in water bath at boiling temperature (100 °C) and EC was recorded as C2. MSI was calculated using the following formula of Sairam et al. (1997)
2.8. Photosynthetic pigments
The photosynthetic pigments were extracted from hypocotyl leaf samples (0.5 g) in 80% acetone as described by Arnon (1949). The optical densities of the supernatant were recorded at 480, 645 and 663 nm.
2.9. Photosynthetic rate and stomatal morphology
Photosynthetic rate was determined at the upper canopy on two mature leaflets per plant from 11:00 to 14:00 h with a portable photosynthesis system (Li-Cor model 6400, Lincoln, Nebraska, USA). Imprints abaxial surface of leaf samples were photographed through a Leitz DMRD light micro-scope (Leica Mikroskopie & Systeme GmbH, Wetzlar, Germany) with an associated camera (Leica DFC 420). Stomatal aperture size and stomatal density were measured (with the images using UTHSCSA Image-Tool software (UTHSCSA Image Tool for Windows version 3.00) and were calculated following Doheny-Adams et al. (2012).
2.10. Determination of arbuscular mycorrhizal colonization
At end of the experiment, mycorrhizal spores were extracted from the pot mixture (peat, perlite, sand) of each treated pot by wet sieving and decanting method following method of Daniels and Skipper (1982) modified by Utobo et al. (2011). Roots were washed carefully using ice-cold water to remove the adhered soil. Thereafter, roots were cleaned with KOH (10%) and subsequently stained with trypan blue in lactophenol (Phillips and Hayman, 1970). The stained root segments were examined under light microscope at 400× magnification. The intensity of fungal infection (mycelium, vesicles and arbuscules) and development within the infected regions of the roots were calculated according to the following formula:
2.11. Number of nodules, nodule weight, leghemoglobin content and nitrate reductase activity
Fresh roots were separated very carefully, and nodules were speared from roots and number was recorded with help of Leica MZF LIII stereomicroscope and were weighed instantly to record nodule fresh weight. For estimation of leghemoglobin, fresh nodules (2 g) were macerated in liquid N2 and 50 mM KPO4 (pH 7.4) buffer containing 1 mM EDTA was added. Thereafter, mixture was stirred until it thawed to a homogenate at 2 °C and was transferred to centrifuge tubes. After centrifugation at 10,000g for 10 min at 4 °C supernatant was collected and mixed with 50 mM KPO4 (pH 7.4) buffer containing 1 mM EDTA. The color intensity was recorded spectrophotometrically at 710 nm (Keilin and Wang, 1945). Nitrate reductase activity of the nodules was assayed according to the methods of Finka et al. (1977). The activity of nitrate reductases was expressed as μmNO2 released/h/g FW fresh. A standard curve of potassium nitrate was used as a reference.
2.12. Estimation of nitrogen and phosphorous
The phosphorus was extracted by nitric-perchloric acid digestion and measured using the Vanado-molybdophosphoric colorimetric method. Standard curve of each mineral (10–100 µg−1 mL) used as reference.
2.13. Statistical analysis
The recorded data were analyzed by analysis of variance technique using statistical software SPSS-21. Least significant difference test was used for mean separation at p < 0.05). The correlation coefficients, among various attributes, were calculated by Microsoft Excel.
3. Results
Drought stress caused significant reduction in shoot and root lengths, leaf area, and number of primary and secondary branches in chickpea by 7.42, 19.04, 35.35, 10.20 and 33.33%, 22.09, 38.82, 18.01 and 44.35% and 25.90, 35.54, 43.95, 27.47 and 51.97% respectively relative to control (Table 1). However, application of biochar and AMF mitigated the drought-induced reduction in all morphological traits. In this regard, combined application of biochar and AMF was more effective, except for leaf area where there was no difference between biochar alone and combined application of biochar and AMF (Table 1). Application of both biochar and AMF also improved the shoot and root lengths, leaf area, and number of primary and secondary branches of chickpea under well-watered conditions. Like under drought, combined application of biochar and AMF was more effective in improving the morphological traits of chickpea under well-watered conditions (Table 1).
Table 1.
Influence of biochar and arbuscular mycorrhizal fungi on morphological characteristics of chickpea under drought stress.
| Treatments | Shoot length (cm) | Root length (cm) | Leaf area (cm2) | Number of primary branches/plant | Number of secondary branches/plant |
|---|---|---|---|---|---|
| Control | 34.9d | 27.2d | 5.01d | 1.32d | 3.40d |
| AMF | 44.8b | 38.0b | 8.19ab | 1.61b | 6.11b |
| Biochar | 37.7c | 33.6c | 7.75c | 1.47c | 5.10c |
| Biochar + AMF | 47.1a | 42.2a | 8.94a | 1.82a | 7.08a |
| Drought | 22.0g | 15.7h | 2.59g | 1.04g | 2.22f |
| Drought + AMF | 29.7e | 23.3f | 4.07e | 1.17f | 3.07e |
| Drought + Biochar | 27.0f | 20.9g | 3.63f | 1.12f | 2.78f |
| Drought + Biochar + AMF | 30.9e | 25.9e | 3.88f | 1.20e | 3.08e |
| LSD (p 0.05) | 0.8001 | 1.2495 | 0.1666 | 0.0295 | 0.1229 |
AMF = An arbuscular mycorrhizal fungi.
Means sharing the same letter, for a parameter, do not differ significantly at P ≤ 0.05.
Drought stress imparted significant decline in mycorrhizal colonization, number of spores, mycelium, vesicles and arbuscules hence caused decline in intensity of colonization (Table 2). However, biochar treatment protected AMF from the deleterious effects of drought by improving the number of spores (36.73%), mycelium (79.68%), vesicles (28.65%) and arbuscules (28.55%) over drought stressed plants. Relative to control plants, number of spores, mycelium, vesicles and arbuscules declined by 59.56, 86.91, 51.21 and 41.07% due to drought stress. However, the application of biochar enhances an increase in number of spores, mycelium, vesicles and arbuscules by 5.34%, 11.79%, 41.80% and 29.72% over the control plants (Table 2).
Table 2.
Influence of biochar and drought on total colonization and Intensity of mycorrhizal colonization in chickpea under drought stress.
| Treatments | Total colonization (%) |
Intensity of mycorrhizal colonization (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Spores | Mycelium | Vesicles | Arbuscules | Mycelium |
Vesicles |
Arbuscules |
|||||||
| P | M | A | P | M | A | P | M | A | |||||
| Control | 873.0b | 76.63b | 38.54b | 36.81b | 8.2 | 36.0 | 55.7 | 67.9 | 25.0 | 7.04 | 14.0 | 19.1 | 66.7 |
| Drought | 353.6d | 10.03d | 18.80d | 21.69d | 76.9 | 15.1 | 7.89 | 19.7 | 33.7 | 46.5 | 59.4 | 18.5 | 21.9 |
| Biochar | 922.3a | 86.88a | 66.23a | 52.38a | 2.8 | 24.1 | 72.9 | 47.8 | 27.1 | 25.0 | 3.88 | 10.6 | 85.4 |
| Drought + Biochar | 558.6c | 49.37c | 26.35c | 30.36c | 20.9 | 60.5 | 18.5 | 34.6 | 29.8 | 35.5 | 37.8 | 16.0 | 46.1 |
| LSD (p 0.05) | 21.437 | 5.6926 | 5.319 | 4.8778 | 3.072 | 6.114 | 8.342 | 12.436 | 1.923 | 5.912 | 8.217 | 0.725 | 12.718 |
P: Poor, M: Moderate, A: Abundant. Means sharing the same letter, for a parameter, do not differ significantly at P ≤ 0.05.
The Pearson correlation coefficients of mycelium, vesicles, arbuscules, shoot height, number of primary branches, number of secondary branches, leaf area, root depth and total spores number was presented in Table 3. The vesicles showed positive and significant correlation with mycelium growth. Arbuscules also showed positive and significant correlation with mycelium growth and vesicles. Shoot height was increase with the increase of the mycelium growth. Number of primary branches was also increased with the increase of mycelium growth. The number of secondary branches improved with mycelium growth as correlation value showed positive and significant relation. The leaf area (cm2) was increase as the mycelium, positive and significant correlation was recorded. As the mycelium application increases, the root depth was also increased and finally the uptake of the nutrients micro-nutrients was increased. Total spores/100 g soil was increase as the mycelium growth was increased in the soil. The mycelium showed positive and significant correlation with total spores in the soil. The vesicles showed positive and significant correlation with arbuscules, shoot height (cm), number of primary branches, number of secondary branches, leaf area (cm2) root depth (cm) and total spores/100 g soil. The arbuscules showed positive and significant correlation with shoot height (cm), number of secondary branches, leaf area (cm2) root depth (cm) and total spores/100 g soil. Shoot height (cm) was increase and showed positive correlation with number or primary branches, number of secondary branches, leaf area (cm2), root depth (cm) and total spores/100 g soil. The number of secondary branches increases and showed positive and significant correlation with leaf area (cm2), root depth (cm) and total spores/100 g soil. Root depth (cm) was increases as the leaf area (cm2) was increase. Leaf area (cm2) showed positive and significant correlation. Root depth showed positive and significant correlation with total spores/100 g soil. As total spores/100 g increase the root depth was also improved.
Table 3.
Pearson correlation coefficients of mycelium; vesicules; arbuscules; shoot length; number of primary branches; number of secondary branches; leaf area; root depth and total spores number.
| V | A | ShL | NPB | NSB | LA | RD | TS | |
|---|---|---|---|---|---|---|---|---|
| M | 0.83140 | 0.84962 | 0.90243 | 0.87676 | 0.87698 | 0.85765 | 0.91284 | 0.97662 |
| V | 1.00000 | 0.96636 | 0.86573 | 0.93092 | 0.89390 | 0.86608 | 0.88740 | 0.85666 |
| A | 1.00000 | 0.84461 | 0.91280 | 0.86749 | 0.84503 | 0.88150 | 0.86917 | |
| ShL | 1.00000 | 0.97797 | 0.98853 | 0.99172 | 0.97564 | 0.96021 | ||
| NPB | 1.00000 | 0.98811 | 0.98081 | 0.96911 | 0.93517 | |||
| NSB | 1.00000 | 0.99202 | 0.97858 | 0.94096 | ||||
| LA | 1.00000 | 0.96369 | 0.93521 | |||||
| RD | 1.00000 | 0.95076 | ||||||
| TS | 1.00000 |
M: Mycelium; V: Vesicules; A: Arbuscules; ShL: shoot length (cm); NPB: Number of primary branches; NSB: Number of secondary branches; LA: Leaf area (cm2); RD: Root depth (cm); TS: Total spores (Spores/100 g soil).
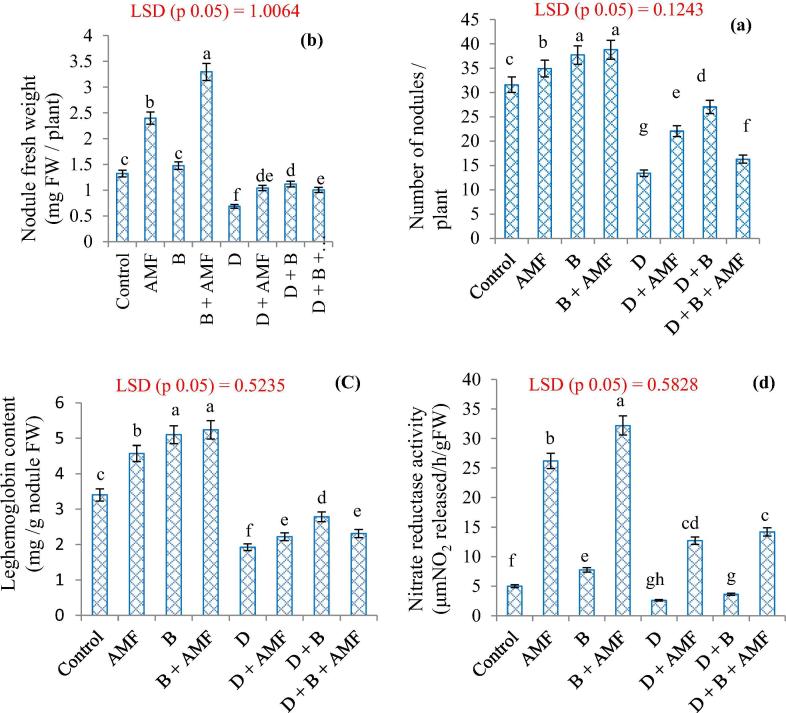
Drought stress adversely affected the number of nodules, nodule fresh weight, leghemoglobin content and nitrate reductase activity were reduced by 61.63, 48.10, 44.11 and 48.30% respectively. However, AMF inoculation in addition to biochar enhanced these parameters and also assuaged the negative effects of drought stress to significant level. Relative to drought stressed plants, negative effect of drought on number of nodules, nodule fresh weight, leghemoglobin content and nitrate reductase activity was mitigated by 40.90, 34.75, 13.51 and 79.60% in drought + AMF, by 38.21, 38.83, 36.13 and 28.65% in drought + biochar and by 17.76, 36.31, 16.88 and 81.72% in drought + AMF + biochar. Additionally, under normal conditions, number of nodules, nodule fresh weight, leghemoglobin content and nitrate reductase activity was more in biochar + AMF by 18.55, 59.87, 35.11 and 84.44% respectively (Fig. 1A–D).
Fig. 1.
Influence of biochar and arbuscular mycorrhizal fungi on (a) number of nodules, (b) nodule fresh weight, (c) leghemoglobin content and (d) nitrate reductase activity in chickpea under drought stress. D: Drought, B: Biochar, AMF: Arbuscular Mycorrhizal Fungi. Data presented is mean of three replicates and mean values designated by different letters are significantly different at P < 0.05.
Pearson’s correlation coefficients of mycelium, vesicles, arbuscules, nodule number, nodule fresh weight, leghemoglobin and nitrate reductase were presented in Table 4. The mycelium growth showed positive and significant correlation for vesicles, arbuscules, nodule number, nodule fresh weight, leghemoglobin and nitrate reductase. The nodule fresh weight showed positive and significant correlation with vesicles. The nodule number also showed positive and significant correlation with vesicles. The leghemoglobin content was improved with the production of vesicles and showed significant correlation. The nitrate reductase content was also showed positive and significant correlation with vesicles. Arbuscules showed positive and significant correlation with nodule number, nodule fresh weight. Both the nodule number and nodule fresh weight was increased with the increase of arbuscules. As the nodule number increase, the nodule fresh weight also increases. The nodule number showed positive and significant correlation with nodule fresh weight. The leghemoglobin content was also showed positive and significant correlation with nodule number. The nitrate reductase contents were increase and showed positive correlation with nodule formation. Data regarding nodule fresh weight was presented in Table 2. Nodule fresh weight was showed positive and significant correlation with leghemoglobin contents as well as nitrate reductase content was also improved. Leghemoglobin and nitrate reductase content showed positive and significant correlation with nitrate reductase. Fig. 2, shows positive influence of biochar and AMF inoculation on plant under drought stress.
Table 4.
Pearson correlation coefficients of mycelium; vesicules; arbuscules; nodule number; nodule fresh weight; leghemoglobin and nitrate reductase.
| M | V | A | Noduleno | Nodulefwt | LG | Nitratered | |
|---|---|---|---|---|---|---|---|
| M | 1.00000 | 0.83140 | 0.84962 | 0.90516 | 0.89288 | 0.91758 | 0.90155 |
| V | 1.00000 | 0.96636 | 0.93116 | 0.95194 | 0.90063 | 0.92573 | |
| A | 1.00000 | 0.92623 | 0.94589 | 0.89176 | 0.90180 | ||
| Noduleno | 1.00000 | 0.99609 | 0.99451 | 0.99264 | |||
| Nodulefwt | 1.00000 | 0.98340 | 0.98448 | ||||
| LG | 1.00000 | 0.99302 | |||||
| Nitratered | 1.00000 |
M: Mycelium; V: Vesicules; A: Arbuscules; Noduleno: Nodule number; Nodulefwt: Nodule fresh weight; LG: Leghemoglobin; Nitratered: Nitrate reductase.
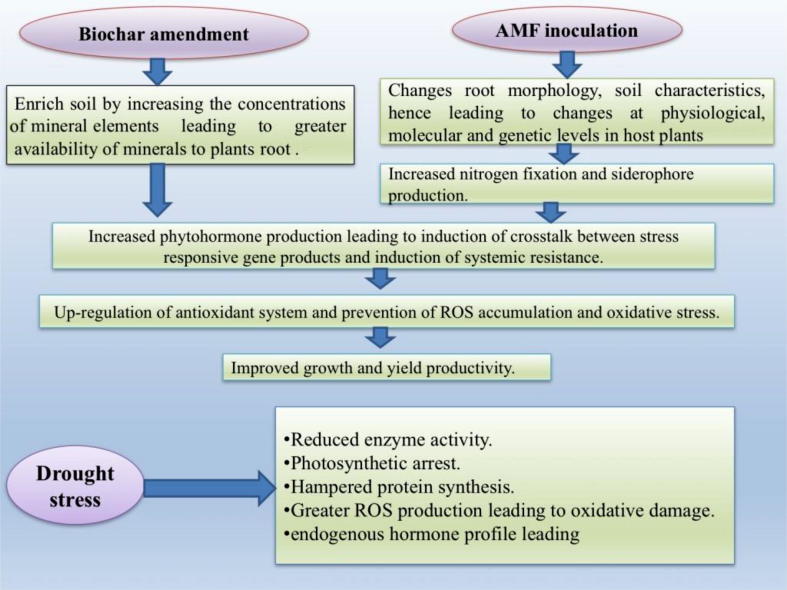
Fig. 2.
Possible mechanism of biochar and arbuscular mycorrhizal fungi-induced drought tolerance in C. arietinum by modulating dynamics tolerance expression.
Results depicting the impact of biochar, AMF and drought individually as well as combined on chlorophylls (Chls) pigments are depicted in Table 5. Chl a, Chl b and total chls were reduced by 54.61, 46.81 and 39.84% due to drought stress, however amelioration of 58.36, 27.30 and 41.24% due to biochar and 60.08, 40.87 and 45.87% due to inoculation of AMF was observed. Relative to control, under normal growth conditions observed enhancement in chl a, chl b, carotenoids and total chls due to biochar was 16.23, 12.11, 1.55 and 13.44%, due to AMF was 21.39, 19.09, 8.58 and 19.29%, and due to biochar + AMF was 30.33, 29.62, 1.30 and 27.03% respectively. Significant amelioration of drought induced decline in chl a, chl b. Carotenoids and total chls was observed due to addition of biochar and AMF with impact being much pronounced in biochar + AMF. Relative to drought stressed plants decline in chl a, chl band total chls was ameliorated by 61.77, 48.09 and 49.49% respectively in drought + biochar + AMF treated C. arietinum plants (Table 5).
Table 5.
Influence of biochar and arbuscular mycorrhizal fungi on chlorophyll a, chlorophyll b, carotenoids and total chlorophylls (mg/g FW) of chickpea under drought stress.
| Treatments | Photosynthetic pigment (mg/g fresh weight) |
|||
|---|---|---|---|---|
| Chl a | Chl b | Carotenoid | Total Chls | |
| Control | 1.532e | 0.6073d | 0.3790g | 2.51e |
| AMF | 1.949b | 0.7506b | 0.4146e | 3.11b |
| Biochar | 1.829c | 0.6910c | 0.3850f | 2.90c |
| Biochar + AMF | 2.199a | 0.8630a | 0.3840f | 3.44a |
| Drought | 0.6953f | 0.3230g | 0.4953c | 1.51f |
| Drought + AMF | 1.742c | 0.5463f | 0.5096b | 2.79d |
| Drought + Biochar | 1.670d | 0.4443e | 0.4553d | 2.57e |
| Drought + Biochar + AMF | 1.819c | 0.6223d | 0.5530a | 2.99c |
| LSD at: 0.05% | 0.0273 | 0.0197 | 0.0152 | 0.0379 |
Chl a: Chlorophyll a; Chl b: Chlorophyll b; Total Chls: Total Chlorophylls; AMF = An arbuscular mycorrhizal fungi.
Means sharing the same letter, for a parameter, do not differ significantly at P ≤ 0.05.
The Pearson’s correlation coefficients of mycelium, vesicles, arbuscules, chlorophyll a, chlorophyll b, carotenoid pigment and total photosynthetic pigments were presented in Table 6. The mycelium showed positive and significant correlation with vesicles, arbuscules, chlorophyll a, chlorophyll b and total photosynthetic pigments while negative correlation was recorded for carotenoid. Chlorophyll a and chlorophyll b contents recorded positive and significant correlation with mycelium. Carotenoid pigment showed negative and significant correlation with vesicles while positive recorded for arbuscules. Chlorophyll contents (a & b) recorded positive and significant correlation with vesicles. Total photosynthetic contents were increased as the vesicles increase as vesicles showed positive and significant correlation with total photosynthetic contents. Chlorophyll content increased as the concentration of arbuscules increase. Chlorophyll a showed positive and significant correlation with arbuscules, also the chlorophyll b contents showed positive and significant correlation. The total photosynthetic pigments increase as the concentration of arbuscules increases. However, the total photosynthetic pigments showed positive and significant correlation with arbuscules, while negative and significant correlation was recorded for carotenoid pigment. As the chlorophyll a concentration increase the concentration of chlorophyll b also increase and showed significant correlation with each other. However, the total photosynthetic pigments also increase with chlorophyll a concentration and showed significant correlation with each other. While negative and significant correlation was recorded for carotenoid and chlorophyll a. The carotenoid pigment showed negative and significant correlation with chlorophyll b while significant correlation recorded for total photosynthetic pigments. The carotenoid pigments decrease as the concentration of total photosynthetic pigments was increase. The carotenoid pigment showed negative and significant correlation was recorded for total photosynthetic pigments.
Table 6.
Pearson correlation coefficients of mycelium; vesicules; arbuscules; chlorophyll a; chlorophyll b; carotenoid pigment and total photosynthetic pigments.
| M | V | A | Cha | Chb | Carot | Totapp | |
|---|---|---|---|---|---|---|---|
| M | 1.00000 | 0.83140 | 0.84962 | 0.86294 | 0.92353 | −0.73493 | 0.89594 |
| V | 1.00000 | 0.96636 | 0.98463 | 0.94705 | −0.82365 | 0.97134 | |
| A | 1.00000 | 0.96324 | 0.94663 | −0.78238 | 0.96757 | ||
| Cha | 1.00000 | 0.95501 | −0.83245 | 0.98416 | |||
| Chb | 1.00000 | −0.85654 | 0.96738 | ||||
| Carot | 1.00000 | −0.76087 | |||||
| Totapp | 1.00000 |
M: Mycelium; V: Vesicules; A: Arbuscules; Cha: Chlorophyll a; Chb: Chlorophyll b; Carot: carotenoid pigment; Totapp: total photosynthetic pigments.
The application of both biochar and AMF caused significant stimulation in photosynthetic attributes like stomatal pore aperture, stomatal density and photosynthetic rate in control chickpea seedlings and mitigated the adversely effect of drought significantly (Table 7). Relative to control plants, stomatal pore aperture, stomatal density and photosynthetic rate increased by 13.28, 26.35 and 8.00% due to treatment of biochar, by 29.03, 39.34 and 21.04% due to AMF inoculation and by 39.47, 45.58 and 30.09% due to combined treatment of biochar + AMF. Drought declined stomatal pore aperture, stomatal density and photosynthetic rate by 50.59, 33.94 and 50.43% respectively. Treatment of biochar and AMF ameliorated the negative effects of drought on stomatal pore aperture, stomatal density and photosynthetic rate significantly over the drought stressed plants. Relative to drought stress plants maximal amelioration of 44.87, 30.64 and 39.36% was observed in drought + biochar + AMF treated plants (Table 7). Addition of AMF and biochar induced increase in RWC and strengthened the membranes under normal conditions and also mitigated the negative effects of drought significantly. Relative to control, RWC and MSI increased by 8.02 and 8.10% in biochar + AMF treated plants with AMF being much affective individually. Under drought condition RWC and MSI was declined by 31.54 and 37.93% respectively (Table 7).
Table 7.
Influence of biochar and arbuscular mycorrhizal fungi on stomatal pore aperture, stomatal density, photosynthetic rate, RWC and membrane stability index of chickpea under drought stress.
| Treatments | Stomatal pore aperture (μm2) | Stomatal density (Spore mm−2) | Photosynthetic rate (μmol m−2 S−1) | Relative water content (%) | Membrane stability index (%) |
|---|---|---|---|---|---|
| Control | 101.2d | 92.5d | 23.0d | 85.9c | 86.2c |
| AMF | 142.6b | 152.5b | 29.13b | 87.1b | 92.3a |
| Biochar | 116.7c | 125.6c | 25.0c | 86.8b | 90.5b |
| Biochar + AMF | 167.2a | 170.0a | 32.9a | 93.4a | 93.8a |
| Drought | 50.0h | 61.1g | 11.4g | 58.8g | 53.5g |
| Drought + AMF | 87.9f | 84.8f | 16.7f | 77.4e | 77.3de |
| Drought + Biochar | 78.8g | 82.3f | 15.9f | 72.8f | 69.4f |
| Drought + Biochar + AMF | 90.7e | 88.1e | 18.8e | 81.8d | 79.7d |
| LSD at: 0.05% | 2.6311 | 0.8704 | 0.3932 | 0.8279 | 0.6193 |
AMF = An arbuscular mycorrhizal fungi.
Means sharing the same letter, for a parameter, do not differ significantly at P ≤ 0.05.
Treatment of chickpea with biochar and AMF proved beneficial in enhancing the uptake of nitrogen (N) and phosphorous (P) under normal growth condition and were also effective in mitigating the negative effects of drought to considerable extent, with effect being much obvious when added combined together. In shoot percent increase in N and P was 12.95 and 21.90% due to AMF, 2.83 and 11.00% due to biochar and 22.94 and 27.41% due to biochar + AMF over control. Relative to control, drought reduced N and P by 60.19 and 81.17% in shoot and 50.25 and 49.21% in root. However, in shoot this reduction in N and P was mitigated by 37.56 and 32.62% in drought + biochar and by 51.57 and 29.01% in drought + AMF and by 55.91 and 37.22% in drought + AMF + biochar over the drought stressed plants. Similar ameliorative results were observed in root tissue with the treatment of biochar and AMF (Table 8).
Table 8.
Influence of biochar and arbuscular mycorrhizal fungi on total nitrogen and total phosphorous contents in hoot and root of chickpea under drought stress.
| Treatments | Shoot |
Root |
||
|---|---|---|---|---|
| Total nitrogen (mg g−1 dwt) | Total phosphorus (mg g−1 dwt) | Total nitrogen (mg g−1 dwt) | Total phosphorus (mg g−1 dwt) | |
| Control | 3.09c | 0.4553d | 1.56d | 0.3773c |
| AMF | 3.55b | 0.5830b | 2.14b | 0.422b |
| Biochar | 3.18c | 0.5116c | 1.72c | 0.419b |
| Biochar + AMF | 4.01a | 0.6273a | 2.57a | 0.473a |
| Drought | 1.23g | 0.2513h | 0.776h | 0.1916g |
| Drought + AMF | 2.54e | 0.3540g | 1.176f | 0.236f |
| Drought + Biochar | 1.97f | 0.3730f | 0.993g | 0.269e |
| Drought + Biochar + AMF | 2.79d | 0.4003e | 1.28e | 0.302d |
| LSD at: 0.05% | 0.11 | 0.0226 | 0.0727 | 0.0121 |
AMF = An arbuscular mycorrhizal fungi.
Means sharing the same letter, for a parameter, do not differ significantly at P ≤ 0.05.
4. Discussion
With the increasing intensification of climate change drought stress outbreaks have become frequent in most areas of the globe. With such adverse conditions prevailing in the agricultural areas the growth of existing crops is hampered to considerable extent resulting in considerable decline in their yield. Nevertheless, there are other related factors that get involved to further aggravate the drought situation. Cumulative activities of these factors mostly bring negative impacts on the physiological and biochemical basis of plant metabolism. Biochar amendments (Ippolito et al., 2012, Mehari et al., 2015) and AMF inoculation (Ahanger et al., 2014, Abd_Allah et al., 2015, Hashem et al., 2016) have been reported to impart certain complex beneficial changes in plants varying from morphology to physiology to biochemistry and these changes are often believed to occur through their involvement in the modifications of soil solution (Ahmad et al., 2014, Rao and Chaitanya, 2016). Therefore, the utilization of biochar and AMF can be better alternatives for enhancing the growth and productivity of plants under extreme conditions like drought stress. In the present study, biochar amendment and AMF inoculation ameliorated the growth inhibitory effects of drought significantly on the length of shoot and root, leaf area and number of branches in C. arietinum. Improved growth in biochar and AMF treated plants may be due to their effect on the activity of transport proteins located in membranes involved in controlling the cellular division, wall extensions and cell elongation (Ahmad et al., 2014, Hameed et al., 2014, Trupiano et al., 2017). In drought stressed strawberry it has been demonstrated that decline in shoot and root length was ameliorated by the inoculation of AMF through its impact on the water use efficiency (Boyer et al., 2015). Biochar is rich in several mineral elements including Mg, K, N and Ca, and maintains the soil pH (Biederman and Harpole, 2013, Razaq et al., 2017). In rice, biochar amendment increased the leaf area index, biomass and yield by increasing the uptake of essential mineral elements (de MeloCarvalho et al., 2013). Reports discussing the combined effect of AMF and biochar are very scanty and it should be pointed here that combined treatment mitigated the negative impact more than their individual treatment. Treatment of biochar has been reported to improve growth by altering the root chemistry, respiration, the nitrogen and carbon content reflecting in overall better growth performance (Xu et al., 2015, Razaq et al., 2017, Paetsch et al., 2018). Similar to our study drought induced decline in the colonization potential of AMF has been reported in sainfoin (Kong et al., 2014) and strawberry (Boyer et al., 2015). Greater colonization measured interms of number of mycelium, vesicles and arbuscules in biochar treated plants depicts its beneficial role in protection of AMF and the host plant as well. By protecting AMF spore formation and its subsequent invasion into host root tissues from the drought stress, present study provides strong evidence in favor of use of biochar in plant protection through modulation of AMF growth. Biochar modulates the physical and chemical properties of soil suitable for improved AMF colonization (Yusif and Dare, 2017).
A positive correlation between colonization of AMF and morphological criteria was recorded in this paper, which was reported in previous studies as well (Kong et al., 2014, He et al., 2017) under drought stress. The synergetic induction of plant growth by AM fungi are attributed to consequent increase in growth hormones in the plant under mycorrhizal colonization that further initiates morphological criteria (Hameed et al., 2014, He et al., 2017)
Drought stress declined the nodule number and weight, leghemoglobin content and activity of nitrate reductase considerably leading to declined N content. However, AMF and biochar improved the nitrogen fixing attributes and the also ameliorated the negative effects of drought significantly. Iijima et al. (2015) have also demonstrated increased nodule growth and nitrogenase activity leading to greater N uptake in soybean. In Sesbania sesban inoculation of AMF increased the number and fresh weight of nodules, leghemoglobin content and activity of nitrogenase resulting in greater N uptake and assimilation (Abd_Allah et al., 2015). It is imperative to say that cumulative interaction between biochar and AMF in regulation of N fixation has not been worked therefore further studies are required to unravel the underlying mechanisms. Recently, Farhangi-Abriz and Torabian (2018) have also demonstrated greater nitrogen assimilation in biochar treated soybean plants due to increased nodulation, leghemoglobin content and up-regulation in the activities of nitrate reductase and GS-GOGAT. Increased N fixation and subsequent assimilation in biochar + AMF treated plants may have assisted greater photosynthetic protection and the production of N-containing metabolites for greater drought stress tolerance through maintenance of tissue osmolarity (Ahanger et al., 2014). Our finding of the correlation between AM fungi and nodulation reported significant and positive correlation are in agreement with the findings of Zarea et al., 2011, Ahanger et al., 2014, Liu et al., 2016, Hashem et al., 2016. The increase in phosphorus availability to plants because AM fungi improves quantity and quality of the nodulation (Abd_Allah et al., 2015, Liu et al., 2016) which also has been reported with PSB (phosphate-solubilizing bacteria) in different crops (Othman and Panhwar, 2014, Wahid et al., 2016).
Treatment of biochar and AMF resulted in significant enhancement in the photosynthetic attributes like chlorophyll pigment synthesis, stomatal features including stomatal pore size and density reflecting in significant enhancement in the net photosynthetic rate. Similarly, Xu et al. (2015) have also demonstrated improved photosynthetic rate in peanut due to increased C and N fixation following amendment of biochar (Rao and Chaitanya, 2016). In Silybum marianum (Afshar et al., 2016) treatment of biochar prevented the drought induced decline in photosynthesis by improving the synthesis of chlorophyll pigments and enhancing the water holding capacity of soil for prevent of drought triggered oxidative damage (Rao and Chaitanya, 2016). Zhu et al. (2014) has demonstrated greater chlorophyll synthesis and photosynthetic efficiency in AMF inoculated black locust seedlings. However combined effect of biochar and AMF on photosynthetic attributes has not been worked out. Therefore, it could be suggested that presence of Mg due to biochar amendment and subsequent enhancement in its uptake due to AMF inoculation could be possible reasons for greater photosynthetic efficiency (Rao and Chaitanya, 2016). In addition, biochar (Wang et al., 2014) and AMF (Hashem et al., 2016) treated have been shown to exhibit greater antioxidant activity leading to improved protection of photosynthetic apparatus from the drought induced oxidative damage (Rao and Chaitanya, 2016). He et al. (2017) is of the opinion that AMF mediated alteration of the endogenous hormone profile leads to photosynthetic regulation under changing environment. Younis et al. (2015) have demonstrated improved the growth and yield in Spinacia oleracea and amelioration of nickel induced decline in chlorophyll synthesis, stomatal conductance and CO2 concentration resulting in enhanced biochemical performance. The presence of sufficient quantities of mineral elements in biochar has been confirmed as key reason for the enhanced chlorophyll content (Milla et al., 2013). Stresses intensify the degradation of chlorophylls through excessive generation of free radicals affecting the electron transport and hence the formation of photoassimilates. In the present study biochar and AMF treatment to drought stressed plants lessened the generation of free radicals possibly by increased uptake of N resulting in allocation of significant amounts to Rubisco protein.
We reported here significant positive correlations between AM fungal colonization and chlorophylls (A&B) contents which corroborates the findings of Aroca et al. (2013) in L. sativa and Hashem et al. (2016) in A. gerrardii. The mechanism behind the positive correlations between increased chlorophyll content and AM fungal colonization, was shown by Sheng et al. (2008) that enhanced mineral uptake especially magnesium (Mg) could be one of the reasons. In this context, our previous reports AM fungi increased Mg content in colonized plant tissues (Abd_Allah et al., 2015, Hashem et al., 2016) and acting as an allosteric enzyme regulator or cofactor of chlorophyll synthase (Ahanger et al., 2014) and play an important role in the structural stabilization of macromolecules, such as proteins and cell tissues (Cowan, 2002, Gransee and Führs, 2013, Niu et al., 2015). Conversely, the deficiency of Mg caused significant reduction in chlorophylls content and reduced net CO2 assimilation (Cakmak and Yazici, 2010).
In the present study, AMF and biochar treatment protected the Cicer arietinum plants from the drought induced membrane dysfunction. Stress induced membrane dysfunctioning results from the increased radical generations leading to lipid peroxidation. Similar results of declined MSI has been reported by Mirzaee et al. (2013) in B. napus and Alwhibi et al. (2017) in Solanum lycopersicum. Radicals have the potential to diffuse through membrane aquaporin for affecting the cells over longer distance (Ahmad et al., 2014, Sofo et al., 2016). Biochar and AMF treatment may have restricted the diffusion of radicals by eliminating them at the sites of production. AMF inoculation improves the polyunsaturated fatty acid concentrations in cellular membranes for maintaining their structural and functional integrity (Alqarawi et al., 2014).
In the present study, we observed significant enhancement in the uptake of N and P in biochar and AMF treated plants resulting in greater production of assimilatory products for better drought adaptation (Razaq et al., 2017, Paetsch et al., 2018). Biochar amendment increases soil carbon and water holding capacity resulting in improved growth rate under drought conditions (Paetsch et al., 2018). Growth retardations in plants subjected to drought result from the cumulative effect of several factors including temperature increment and availability of soil water which reflect in non-availability of minerals for efficient uptake impeding the mineral use efficiency (Jatav et al., 2014). Reduced N and P in drought stressed C. arietinum plants directly affected the photosynthetic efficiency of plants. As N forms an important component of Rubisco protein and P has a critical role in maintenance of cellular redox homeostasis by forming part of several metabolic compounds (Ahanger et al., 2014). Therefore, in present study the increased N and P due to biochar and AMF treatment justifies their importance in improving the photosynthetic efficiency. Similar to our results the combined treatment of biochar and AMF to maize plants improved the availability as well uptake of P resulting in enhancement in yield (Mau and Utami, 2014). However present study depicts the interactive role of AMF and biochar in enhancing the drought tolerance by maintaining the P uptake and assimilation in C. arietinum. Drought induces decline in photosynthesis by declining the synthesis of Rubisco protein and mineral uptake (Vurukonda et al., 2016). In addition to this increased leaf area due to treatment of biochar and AMF may have possibly contributed to the greater photosynthetic rate in them.
5. Conclusion
Drought stress induced decline in C. arietinum was mitigated by the treatment of AMF and biochar. AMF and biochar treatment improved the growth by positively affecting the synthesis of chlorophylls, rate of photosynthesis and the maintenance of relative water content. Additionally, the ability of AMF and biochar to improve nitrogen fixation ability of the C. arietinum plants may have significantly contributed to growth promotion and the greater drought tolerance by maintaining the optimal concentration of amino acids. However, the combined application of AMF and biochar was more affective as compared to individual treatments.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding to the Research Group number (RGP-271).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Abeer Hashem, Email: https://orcid.org/0000-0001-6541-347X, habeer@ksu.edu.sa.
Elsayed Fathi Abd_Allah, Email: https://orcid.org/0000-0002-8509-8953.
References
- Abd_Allah E.F., Hashem A., Alqarawi A.A., Bahkali A.H., Alwhibi M.S. Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 2015;22(3):274–283. doi: 10.1016/j.sjbs.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar R.K., Hashemi M., DaCosta M., Spargo J., Sadeghpour A. Biochar application and drought stress effects on physiological characteristics of Silybum marianum. Commun. Soil Sci. Plant Anal. 2016;47(6):743–752. [Google Scholar]
- Ahanger M.A., Hashem A., Abd_Allah E.F., Ahmad P. Arbuscular mycorrhiza in crop improvement under environmental stress. In: Ahmad P., Rasool S., editors. Emerging Technologies and Management of Crop Stress Tolerance. 2014. pp. 69–95. [Google Scholar]
- Ahmad P., Hameed A., Abd_Allah E.F., Sheikh S.A., Wani M.R., Rasool S., Jamsheed S., Kumar A. Biochemical and molecular approaches for drought tolerance in plants. In: Ahmad P., Wani M.R., editors. Physiological Mechanisms and Adaptation Strategies 1 in Plants Under Changing Environment: Volume 2. © Springer Science+Business Media; New York: 2014. [Google Scholar]
- Alqarawi A.A., Abd_Allah E.F., Hashem A. Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J. Plant Inter. 2014;9(1):802–810. [Google Scholar]
- Alwhibi M.S., Hashem A., Abd_Allah E.F., Alqarawi A.A., Soliman D.W.K., Wirth S., Egamberdieva D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agri. 2017;16(8):1751–1757. [Google Scholar]
- Arnon D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R., Ruiz-Lozano J.M., Zamarreño Á.M., Paz J.A., García-Mina J.M., Pozo M.J., López-Ráez J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013;170(1):47–55. doi: 10.1016/j.jplph.2012.08.020. [DOI] [PubMed] [Google Scholar]
- A.O.A.C. (ASSOCIATION OF OFFICIAL AGRICULTURAL CHEMISTS). 1965, Official methods of analysis (10th Ed.), Washington, D.C. 308, 764, 757.
- Biederman L.A., Harpole W.S. Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioener. 2013;5:202–214. [Google Scholar]
- Boyer L.R., Brain P., Xu X.M., Jeffries P. Inoculation of drought-stressed strawberry with a mixed inoculum of two arbuscular mycorrhizal fungi: effects on population dynamics of fungal species in roots and consequential plant tolerance to water deficiency. Mycorrhiza. 2015;25(3):215–227. doi: 10.1007/s00572-014-0603-6. [DOI] [PubMed] [Google Scholar]
- Cakmak I., Yazici A.M. Magnesium: a forgotten element in crop production. Better Crops. 2010;94:23–25. https://www.kali-gmbh.com/en/pdf-articles/article-201006-better-crops-magnesium.pdf [Google Scholar]
- Changxun C., Zhiyong P., Shuang P. Effect of biochar on the growth of Poncirus trifoliata (L.) Raf. seedlings in Gannan acidic red soil. Soil Sci. Plant Nut. 2016;62(2):194–200. [Google Scholar]
- Cowan J.A. Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals. 2002;15:225–235. doi: 10.1023/a:1016022730880. [DOI] [PubMed] [Google Scholar]
- Daniels B.A., Skipper H.D. Methods for the recovery and quantitative estimation of propagules from soil. In: Schenck N.C., editor. Methods and Principles of Mycorrhizal Research. Amer. Phytopathol. Soc.; 1982. pp. 29–36. [Google Scholar]
- de MeloCarvalho M.T., Madari B.E., Bastiaans L., van Oort P.A.J., Heinemann A.B., da Silva M.A.S., Maia A.H.N., Meinke H. Biochar improves fertility of a clay soil in the Brazilian Savannah: short term effects and impact on rice yield. J. Agri. Rural Devel. Trop. Subtrop. 2013;114(2):101–107. http://nbn-resolving.de/urn:nbn:de:hebis:34-2013081343330 [Google Scholar]
- Dikilitas M., Karakas, Hashem A., Abd_Allah E.F., Ahmad P. Oxidative stress and plant responses to pathogens under drought conditions. In: Ahmad Parvaiz., editor. Water Stress and Crop Plants: A Sustainable Approach. first ed. John Wiley & Sons, Ltd.; 2016. [Google Scholar]
- Doheny-Adams T., Hunt L., Franks P.J., Beerling D.J., Gray J.E. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2012;367:547–555. doi: 10.1098/rstb.2011.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey A., Kumar A., Abd_Allah E.F., Hashem A., Khan M.L. Growing more with less: breeding and developing drought resilient soybean to improve food security. Ecol Indic. 2018 (in press) [Google Scholar]
- Fang Y., Xiong L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life Sci. 2015;72(4):673–689. doi: 10.1007/s00018-014-1767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhangi-Abriz S., Torabian S. Biochar improved nodulation and nitrogen metabolism of soybean under salt stress. Symbiosis. 2018;74(3):215–223. [Google Scholar]
- Finka R.L., Warner R.L., Muzit T.J. Effect of herbicides on in vivo nitrate and nitrite reduction. Weed Sci. 1977;25:18–22. [Google Scholar]
- Gransee A., Führs H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil. 2013;368:5–21. [Google Scholar]
- Hameed A., Wu Q.S., Abd_Allah E.F., Hashem A., Kumar A., Lone H.A., Ahmad P. Role of AM fungi in alleviating drought stress in plants. In: Miransari M., editor. Use of Microbes for the Alleviation of Soil Stresses. Springer Science+Business Media; New York: 2014. [Google Scholar]
- Harel Y.M., Elad Y., Rav-David D., Borenstein M., Shulchani R., Lew B., Graber E.R. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil. 2012;357:245–257. [Google Scholar]
- Hashem A., Abd Allah E.F., Alqarawi A.A., Al-Huqail A.A., Shah M.A. Induction of osmoregulation and modulation of salt Stress in Acacia gerrardii Benth. by arbuscular mycorrhizal fungi and Bacillus subtilis (BERA 71). BioMed. Res. Int. 2016:6294098. doi: 10.1155/2016/6294098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Li C., Liu R. Indirect interactions between arbuscular mycorrhizal fungi and Spodoptera exigua alter photosynthesis and plant endogenous hormones. Mycorrhiza. 2017;27(6):525–535. doi: 10.1007/s00572-017-0771-2. [DOI] [PubMed] [Google Scholar]
- Iijima M., Yamane K., Izumi Y., Daimon H., Motonaga T. Continuous application of biochar inoculated with root nodule bacteria to subsoil enhances yield of soybean by the nodulation control using crack fertilization technique. Plant Prod. Sci. 2015;18(2):197–208. [Google Scholar]
- Ippolito J.A., Laird D.A., Busscher W.J. Environmental benefits of biochar. J. Environ. Qual. 2012;41:967–972. doi: 10.2134/jeq2012.0151. [DOI] [PubMed] [Google Scholar]
- Jatav K.S., Agarwal R.M., Tomar N.S., Tyagi S.R. Nitrogen metabolism, growth and yield responses of wheat (Triticumaestivum L) to restricted water supply and varying potassium treatments. J. Indian Bot. Soc. 2014;93:177–189. [Google Scholar]
- Jemo M., Sulieman S., Bekkaoui F., Olomide O.A.K., Hashem A., Abd_Allah E.F., Alqarawi A.A., Tran L.S.P. Comparative analysis of the combined effects of different water and phosphate Levels on growth and biological nitrogen fixation of nine cowpea varieties. Front. Plant Sci. 2017;8:2111. doi: 10.3389/fpls.2017.02111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Wang Y.L. Hemoglobin in the root nodules of leguminous plants. Nature. 1945;155:227–229. doi: 10.1038/159692a0. [DOI] [PubMed] [Google Scholar]
- Kong Jing, Pei Zongping, Du Min, Sun Gan, Zhang Xin. Effects of arbuscular mycorrhizal fungi on the drought resistance of the mining area repair plant Sainfoin. Int. J. Min. Sci. Tech. 2014;24(4):485–489. [Google Scholar]
- Kumar A., Sharma S., Mishra S., Dames J.F. Arbuscular mycorrhizal inoculation improves growth and antioxidative response of Jatropha curcas (L.) under Na2SO4 salt stress. Plant Biosyst. 2013;149:260–269. [Google Scholar]
- Kumar A., Sharma S., Mishra S. Evaluating effect of arbuscular mycorrhizal fungal consortia and Azotobacter chroococcum in improving biomass yield of Jatropha curcas. Plant Biosyst. 2016;150(5):1056–1064. [Google Scholar]
- Lehmann J. A handful of carbon. Nature. 2007;447 doi: 10.1038/447143a. https://www.nature.com/articles/447143a 143 141. [DOI] [PubMed] [Google Scholar]
- Liu H., Tang C., Li C. The effects of nitrogen form on root morphological and physiological adaptations of maize, white lupin and faba bean under phosphorus deficiency. AoB Plants. 2016;8:plw058. doi: 10.1093/aobpla/plw058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau A.E., Utami S.R. Effects of biochar amendment and arbuscular mycorrhizal fungi inoculation on availability of soil phosphorus and growth of maize. J. Degrade. Min. Land Manage. 2014;2(1):69–74. [Google Scholar]
- Mehari Z.H., Elad Y., Rav-David D., Graber E.R., Harel Y.M. Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant Soil. 2015;395:31–44. [Google Scholar]
- Milla O.V., Rivera E.B., Huang W.J., Chien C.C., Wang Y.M. Agronomic properties and characterization of rice husk and wood biochars and their effect on the growth of water spinach in a field test. J. Soil Sci. Plant Nutr. 2013;13(2):251–266. [Google Scholar]
- Mirzaee M., Moieni A., Ghanati F. Effects of Drought stress on the lipid peroxidation and antioxidant enzyme activities in two canola (Brassica napusL.) cultivars. J. Agr. Sci. Tech. 2013;15:593–602. http://jast.modares.ac.ir/article-23-9016-en.pdf [Google Scholar]
- Navarro J.M., Perez-Tornero O., Morte A. Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on the rootstock salt tolerance. J. Plant Physiol. 2013;171(1):76–85. doi: 10.1016/j.jplph.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Nelson DW., Sommers LE., 1996. Total carbon, organic carbon, and organic matter. Methods of soil analysis part 3—chemical methods. (methodsofsoilan3):961–1010.
- Niu Y., Jin G., Li X., Tang C., Zhang Y., Liang Y. Phosphorus and magnesium interactively modulate the elongation and directional growth of primary roots in Arabidopsis thaliana (L.) Heynh. J. Exp. Bot. 2015;66:3841–3854. doi: 10.1093/jxb/erv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman R., Panhwar Q.A. Phosphate-solubilizing bacteria improves nutrient uptake in aerobic rice. In: Khan M.S., editor. Phosphate Solubilizing Microorganisms. © Springer International Publishing; Switzerland: 2014. [Google Scholar]
- Paetsch L., Mueller C.W., Kögel-Knabner I., von Lützow M., Girardin C., Rumpel C. Effect of in-situ aged and fresh biochar on soil hydraulic conditions and microbial C use under drought conditions. Sci. Rep. 2018;8:6852. doi: 10.1038/s41598-018-25039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J.M., Hayman D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc. 1970;55:158–161. [Google Scholar]
- Rao D.E., Chaitanya K.V. Photosynthesis and antioxidative defense mechanisms in deciphering drought stress tolerance of crop plants. Biologia. Plant. 2016;60(2):201–218. [Google Scholar]
- Razaq M., Salahuddin, Shen H.L., Sher H., Zhang P. Influence of biochar and nitrogen on fine root morphology, physiology, and chemistry of Acer mono. Sci. Rep. 2017;7:5367. doi: 10.1038/s41598-017-05721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam R.K., Deshmukh P.S., Shukla D.S. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop Sci. 1997;178:171–178. [Google Scholar]
- Sheng M., Tang M., Chen H., Yang B., Zhang F., Huang Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza. 2008;18(6–7):287–296. doi: 10.1007/s00572-008-0180-7. [DOI] [PubMed] [Google Scholar]
- Smart R.E., Bingham G.E. Rapid estimates of relative water content. Plant Physiol. 1974;53:258–260. doi: 10.1104/pp.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofo A., Scopa A., Hashem A., Abd_Allah E.F. Lipid metabolism and oxidation in plants subjected to abiotic stresses. In: Ahmad Parvaiz., editor. Water Stress and Crop Plants: A Sustainable Approach. first ed. John Wiley & Sons, Ltd.; 2016. [Google Scholar]
- Soudek P., Rodriguez Valseca I.M., Petrova S., Song J., Vanek T. Characteristics of different types of biochar and effects on the toxicity of heavy metals to germinating sorghum seeds. J. Geochem. Exp. 2017;182:157–165. [Google Scholar]
- Sparks D.L., Page A.L., Helmke P.A., Loeppert R.H., Soltanpour P.N., Tabatabai M.A., Johnston C.T., Summer M.E. Soil Science Society of America Inc; Madison, WI: 1996. Methods of soil analysis, parts 2 and 3 chemical analysis. [Google Scholar]
- Stutz Jean C., Morton Joseph B. Successive pot cultures reveal high species richness of arbuscular endomycorrhizal fungi in arid ecosystems. Can. J. Botany. 1996;74(12):1883–1889. [Google Scholar]
- Trupiano D., Cocozza C., Silvia Baronti S., CarlaAmendola C., Vaccari F.P., Lustrato G., Di Lonardo S., Fantasma F., Tognetti R., Scippa G.S. The effects of biochar and its combination with compost on lettuce (Lactuca sativa L.) growth, soil properties, and soil microbial activity and abundance. Int. J. Agr. 2017;3158207:12. [Google Scholar]
- Utobo E.B., Ogbodo E.N., Nwogbaga A.C. Techniques for extraction and quantification of arbuscular rmycorrhizal fungi, Libyan Agric. Res. Center. Int. 2011;2:68–78. [Google Scholar]
- Vurukonda S.S.K.P., Vardharajula S., Shrivastava M., SkZ A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microb. Res. 2016;184:13–24. doi: 10.1016/j.micres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Wahid F., Sharif M., Steinkellner S., Khan M.A., Marwat K.B., Khan S.A. Inoculation of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria in the presence of rock phosphate improves phosphorus uptake and growth of maize. Pak. J. Bot. 2016;48(2):739–747. file:///Users/eli/Downloads/inoculationofAMFandPSB.pdf. [Google Scholar]
- Wu Q.S., Zou Y.N., Liu W., Ye X.F., Zai H.F., Zhao L.J. Alleviation of salt stress in citrus seedlings inoculated with mycorrhiza: changes in leaf antioxidant defense systems. Plant Soil Environ. 2010;56:470–475. https://www.cabdirect.org/cabdirect/abstract/20113001150 [Google Scholar]
- Xu C.Y., Hosseini-Bai S., Hao Y., Rachaputi R.C., Wang H., Xu Z., Wallace H. Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ. Sci. Pollut. Res. Int. 2015;22(8):6112–6125. doi: 10.1007/s11356-014-3820-9. [DOI] [PubMed] [Google Scholar]
- Younis U., Athar M., Malik S.A., Shah N.H.R., Mahmood S. Biochar impact on physiological and biochemical attributes of spinach Spinacia oleracea (L.) in nickel contaminated soil. Global J. Environ. Sci. Manage. 2015;1(3):245–254. [Google Scholar]
- Yusif S.A., Dare M.O. Effect of biochar application and arbuscular mycorrhizal inoculation on root colonization and soil chemical properties. Int. Ann. Sci. 2017;1(1):33–38. [Google Scholar]
- Zarea M.J., Karimi N., Goltapeh E.M., Ghalavand A. Effect of cropping systems and arbuscular mycorrhizal fungi on soil microbial activity and root nodule nitrogenase. J. Saudi Soc. Agri. Sci. 2011;10:109–120. [Google Scholar]
- Zhao B., Xu R., Ma F., Li Y., Wang L. Effects of biochars derived from chicken manure and rape straw on speciation and phytoavailability of Cd to maize in artificially contaminated loess soil. J. Environ. Manag. 2016;184:569–574. doi: 10.1016/j.jenvman.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Zhu X.Q., Wang C.Y., Chen H., Tang M. Effects of arbuscular mycorrhizal fungi on photosynthesis, carbon content, and calorific value of black locust seedlings. Photosynthetica. 2014;52(2):247–252. [Google Scholar]