Abstract
The aqueous cashew leaves extract obtained was investigated for the preparation of gold nanoparticle (AuNPs). The obtained AuNPs were characterized by UV–Visible spectroscopy, FTIR and XRD analysis. Results indicated that the green synthesized AuNPs showed good antibacterial effect against Escherichia coli and Bacillus subtilis and exhibited 74.47% viability on PBMC and 23.56% viability on MCF-7 cell lines at a maximum concentration of 100 µg/ml. Therefore, future studies on antibacterial application in food packing, wound dressing and antihelmintic applications will be studied.
Keywords: Green synthesized gold nanoparticle, Anacardium occidentale, Antibacterial, Cytotoxicity
1. Introduction
Nanotechnology is a promising arena dealing with the design, manipulation and application of nanoparticles (NP) in generating new applications (Chen and Schluesener, 2008). The unique property of NP includes its extreme microscopic size, high surface to volume ratio, an optical, thermal and catalytic property that promotes interactions between microbes and biological agents (Ahmed et al., 2016, Reuveni et al., 2011). Since 2015, nano-medicine involving gold and silver NP has become a new sensation for researchers in developing alternative solutions for biological applications (Wang and Astruc, 2014). Gold nanoparticle (AuNPs) in particular has been used extensively in biomedical and imaging procedures (Iqbal et al., 2016). The biggest choice of using AuNP for biological application is that it can be synthesized easily and have less toxicity (Rad et al., 2011). Among the several methods available for the synthesis of AuNPs, the procedures using naturally occurring plant extracts are consider to be bio compatible, cost effective and requires less labor (Quelemes et al., 2013, Kim et al., 2007, Li et al., 2016). Green synthesis involves the presence of flavonoids and polyphenols which act as reducing agents and stabilizing and capping agents (Keshavamurthy et al., 2018).
In the current study, we have selected Anacardium occidentale (leaves), a tropical evergreen tree that produces cashew seeds and cashew apple. Earlier phytochemical investigation of A. occidentale extracts revealed the presence of flavonoids, tannins, alkaloids and sugars. Cashew nut oil prepared from the seeds of A. occidentale have applied in different disease such as cancer, diabetic, arthritis, vascular diseases and liver inflammation associated illness (Omanakuttan et al., 2012) and it contains the phytochemical components such as cardol and anacardic acid reported to possesses medical importance (Mukunthan and Balaji, 2012, Hemshekhar et al., 2012). In a previous study, photosynthesis of AuNPs using leaves of A. occidentale was reported (Sheny et al., 2011). Many reports claimed that the NP synthesized using green approaches have many advantages over the chemical method of synthesizing the NP. The conversion of NP was environmental friendly and has fewer side effects to the human. Alternatively the green synthesized NP mode of action was highly active. However, there has been no previous report on antibacterial activity against Escherichia coli and Bacillus subtilis and cytotoxic effect against PBMC and MCF-7 cells. Therefore, in the current study, we have synthesized and characterized the AuNP applying A. occidentale leaves extract and assessed their antibacterial and cytotoxicity.
2. Experimental
2.1. Chemicals and reagents
Chemicals of analytical grade were procured from Sigma Aldrich USA, laboratories. A. occidentale leaves were collected from the National Institute of Traditional Medicine (NITM), Karnataka, India. The microbial strains Bacillus subtilis and Escherichia coli were revived from glycerol stocks available at Anna University (CBT), Chennai, India.
2.2. Preparation of dried biomass
Freshly collected leaves of A. occidentale were thoroughly washed with distilled water, shade dried (5 days), pulverized using ball mill and the dried biomass was stored at 40 °C.
2.3. Synthesis of gold nanoparticles
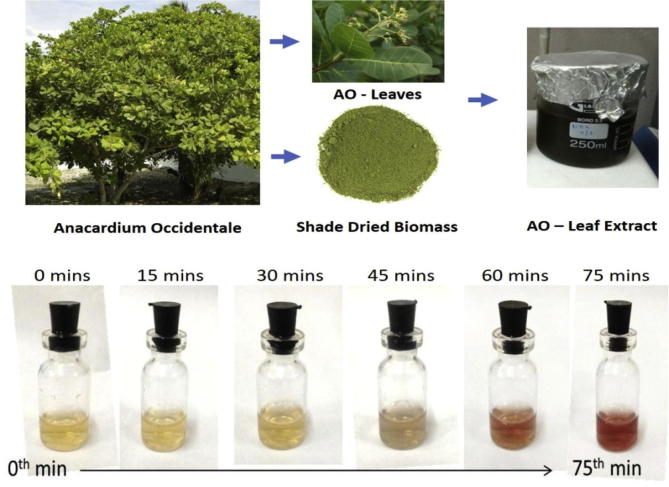
Dried biomass (10 g) of A. occidentale and 50 ml of double distilled water was blended in a beaker, stirred on a magnetic stirrer (500 rpm) overnight (RT). After incubation, the extract was filtered through a muslin cloth and lyophilized to obtain moisture free powders. The lyophilized extract powder (0.5 g) was reconstituted in double distilled water. To the 1 ml of A. occidentale extract, 20 ml of chloroauric acid (0.01 M) was added drop wise under continuous stirring (500 rpm) on a magnetic stirrer and the whole mixture was incubated overnight (Singh et al., 2014). The color change (yellow to ruby red) visible after the incubation period and was subjected for spectroscopic and bioactivity study.
2.4. Characterization of green synthesized AuNPs
UV–Visible spectroscopy (T90+ UV/VIS spectrophotometer), particle size analyzer (PSA) (Malvern Instruments zeta sizer), Transmission Electron microscope TECHNAI (FEI Company, Eindhoven, Netherlands), X-ray diffraction analysis using XRD-6000 (Shimadzu, Kyoto, Japan) were used for characterization of the green synthesized AuNPs. Further, functional groups present in the AuNPs were identified using FTIR (JASCO FT/IR-6600 machine) analysis.
2.5. Study of antibacterial activity of AuNPs
This study was performed by following (Kim et al., 2007). The bacterial cultures were grown on nutrient agar broth and were swabbed on petri plates containing sterile nutrient agar medium. Three wells were bored on the agar surface using a sterile well cutter (6.0 mm diameter) on each agar plate. Thereafter, the AuNPs suspension (20 µl; 20 µg/ml and 40 µl; 40 µg/ml) was poured into two wells of the agar plates and 5 µl (20 µg/ml) of tetracycline standard was added. The plates were incubated at 37 °C and the zone of inhibition (in mm) was evaluated after incubation.
2.6. In-vitro cyto-toxicity assays of the AuNPs on MCF 7 and PBMC cells
Cell line and Peripheral Blood Mononuclear Cells (PBMC) were procured from NCCS, India. The culturing was done in Amphotericin B (1 mg/mL), Gentamycin (100 µg/ml), Penicillin/Streptomycin (250 µg/ml) and 10% FBS. Next, cells (1 × 105/well) were plated in 24-well plates and incubated to reach confluence. Further, cell control (Con), vehicle control (VC- distilled water), and different concentrations (10 ng, 100 ng, 1 µg, 10 µg, 100 µg/ml) of green synthesized AuNPs were loaded and incubated for 24 h. After incubation, the samples were removed and rinsed with PBS buffer. The cells were incubated for a period of 4 h. The absorbance (570 nm) were measured and the cell viability was calculated in percentage:
Graphs concentration Vs cell viability was plotted on the X and Y-axis respectively. To compare the assessments the control and vehicle control samples are included in the assay (Mosmann, 1983, Evelyn et al., 2012).
2.7. Statistics
Data represents mean ± SD of three independent experiments performed in triplicates. Statistical analysis for the experiments were conducted by GraphPad Prism software version 7.04. Comparison of the means with control was done using the Dunnett’s and Tukey test.
3. Results and discussion
3.1. Green synthesis of AuNP and characterization
The color conversion from yellow to ruby red (Fig. 1) indicates the development of AuNPs. This reaction may be due to surface Plasmon resonance (SPR) displayed by the green synthesized particles (Iqbal et al., 2016, Li et al., 2016). The change of gold chloride and the change in color is due to the existence of chemical constituents in A. occidentale leaves extract (Herizchi et al., 2016).
Fig. 1.
Green synthesis of gold nanoparticles from Anacardium occidental leaves extract. The colour change from gold to ruby red indicates the formation of gold nanoparticles.
3.2. Characterization of AuNPs
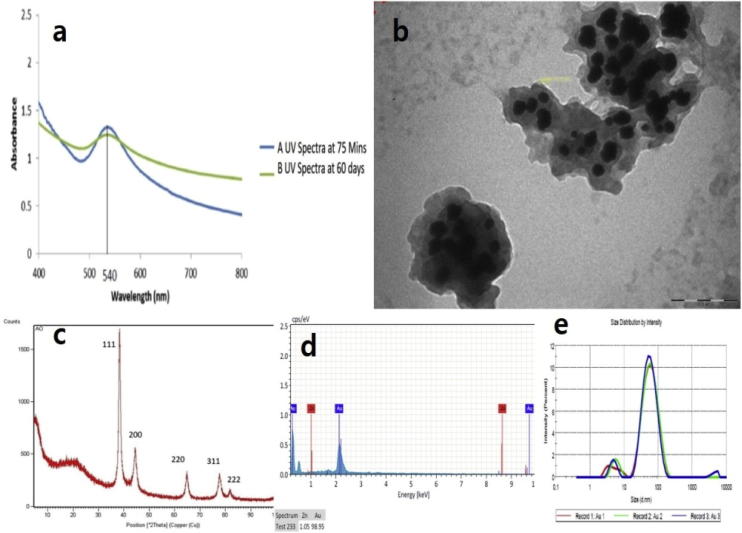
Fig. 2(a) shows the maximum peak corresponding to the SPR at 540 nm. The result confirms the formation of AuNPs, since the peak at 540 nm is specific to AuNPs (Ghosh et al., 2012). The UV analysis was repeated after 60 days to analyze the stability of the AuNPs and the biosynthesized NP were stable after 60 days. Fig. 2(e) shows the particle size analysis (PSA) of the green synthesized AuNPs. The average sizes of the NPs were 40 nm and poly dispersity index (PDI) was found to be 0.442. A PDI below 0.7 indicate that the width of the molecular weight distributions is narrow and high number of NP was <100 nm. The particle density and the bioavailability of NP will be higher at this range and this will significantly increase the activity of NP (Raza et al., 2016, Pal et al., 2007). Next, TEM analysis, the size are in between 10 and 30 nm and spherical in shapes (Fig. 2b). There are several reports mentioning spherical NP have increased antibacterial and cytotoxic effect (Raza et al., 2016, Mostafa et al., 2018). Additionally, our TEM results are in agreement with PSA result with NP size.
Fig. 2.
(a) Ultraviolet–visible spectroscopy analysis of synthesized gold nanoparticles taken at two different time (75 min and at 60 day). The maximum peak was noted at 540 nm. (b) Transmission electron microscope image of AuNPs synthesised by Anacardium occidentale leaves extract. (c) X-ray diffraction patterns of green synthesised AuNPs. (d) EDX Spectrum of AuNPs synthesized from Anacardium occidentale. (e) Particle diameter distribution measured through a particle size analyser. The average size of nanoparticles was found to be 40 nm (n = 3).
The XRD pattern of the green synthesized AuNPs showed 5 major peaks at 38.4°, 44.6°, 64.7°, 77.7°, and 81.5°, which are assigned to (1 1 1), (2 0 0), (2 2 0), (3 1 1) and (2 2 2) indicating structure (Fig. 2c). Moreover, the results from EDX analysis (Fig. 2d) display powerful gold signals (98.95%) and other impurities are noted to be phytochemicals present in the plant extracts (Herizchi et al., 2016, Philip, 2010).
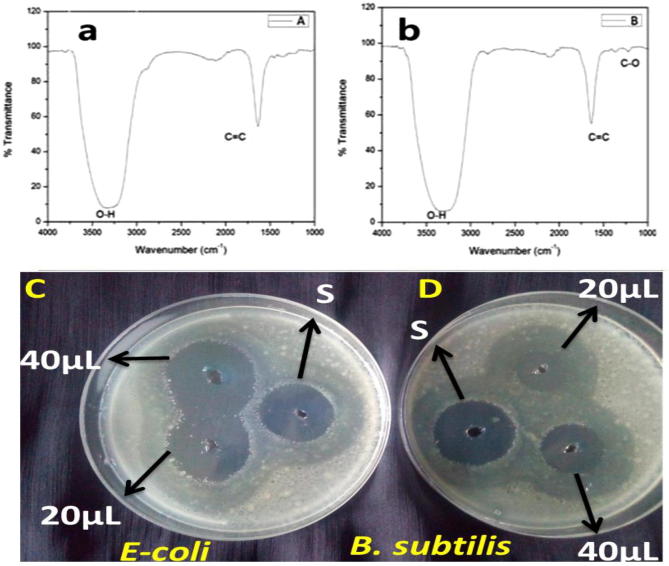
The FT-IR spectra for the green synthesized AuNPs were recorded from 1000 cm−1 to 4000 cm−1 (Fig. 3a and b). The observed strong peaks corresponding to O—H group in the spectra indicates that there may be phenolic groups present in the NP (Fig. 3b). We also observed peaks for C C in the spectra which may be due to the presence of flavonoids in the plant (Patil et al., 2018). Phenolic compounds possess antioxidant activity, act as protective agents against cancer and heart diseases, also neutralize the free radicals, and prevent them from causing cellular damage (Patil et al., 2018). In addition, the broad peak at 3250 cm−1–3500 cm−1 in the plant (Fig. 3a). The sharp peak at 1660 cm−1 indicates the fingerprint region of C C. Compared to the leaf extract, spectra of NP contains a reduced peak at 1300 cm−1, which represents C—O group. Thus, the C—O group in the plant leaves extract of A. occidentale has contributed to the formation of AuNPs and indicate the presence and binding of phenolic groups to the synthesized NP (Singh et al., 2014, Patil et al., 2016). Therefore, the investigation advice that phytochemical compounds (phenols, flavonoids) present in A. occidentale extract promotes the generation of AuNPs (Keshavamurthy et al., 2018, Herizchi et al., 2016).
Fig. 3.
(a) FT-IR spectra of Anacardium occidentale, (b) FT-IR spectra of P-AuNPs. Antibacterial assay by well diffusion method Escherichia coli (c), Bacillus subtilis (d). S- Standard. Arrows indicate the zones of inhibition of P-AuNPs and standard.
3.3. Antibacterial analysis
Table 1 displays the zone of inhibition (mm) and NP show inhibitory effect at all concentrations (Fig. 3c, d). The reason for choosing these two strains have been previously associated with food borne diseases, urinary tract infections and other wide range of ailments (Huang et al., 2001). Various antibacterial agents against them have been extensively studied; however, they exist in places where bacterial mutations are possible (Baek and An, 2011). In our study, the green synthesized AuNPs inhibited the bacterial growth and the proposed antimicrobial mechanism may be due to cell wall and membrane damage (Arokiyaraj et al., 2017), damage in ribosomes and mitochondria (Pal et al., 2007), inhibition of thiol group in bacterial cells and cause cell decease (Velusamy et al., 2016). The increased activity of AuNPs could be due to the capping of the NP that have been confirmed by FTIR analysis. In addition, the antibacterial activities of the AuNPs were higher in Gram-negative bacteria. This could be Gram-negative bacteria have a thinner cell wall in comparison with Gram-positive bacteria and AuNPs have a durable electrostatic attractiveness to the negatively charged bi-layer, thereby facilitating the diffusion of P-AuNPs and cell lysis (Zarei et al., 2014, Goodman et al., 2004).
Table 1.
Zone of inhibition of green synthesized AuNPs against Escherichia coli and Bacillus subtilis.
| Bacteria | Zone of inhibition (mm) |
||
|---|---|---|---|
| AuNPs |
Tetracycline |
||
| 20 µl | 40 µl | 5 µl | |
| Escherichia coli | 18 ± 1.3 | 24 ± 2.1 | 18 ± 1.2 |
| Bacillus subtilis | 8 ± 0.5 | 10 ± 0.8 | 20 ± 1.8 |
The values represented in the table are average of results of three independent experiments.
3.4. Cytotoxicity analysis of the AuNPs
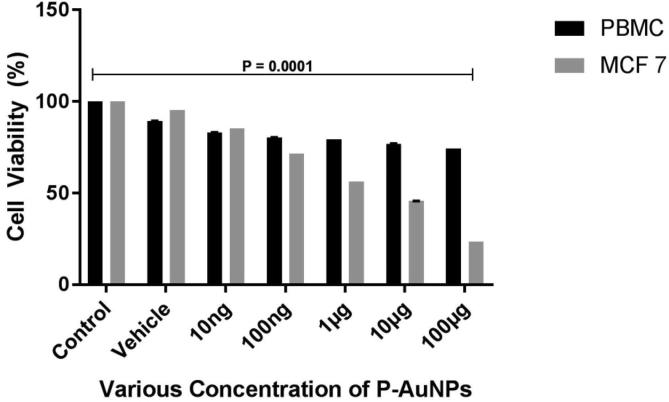
To determinate cytotoxicity of the AuNPs, MTT assays were carried out. We observed cell viability of 80.3% at a concentration of 100 ng on PBMC cells and 71.5% on MCF-7 cells. Further, at maximum concentration (100 µg/ml), the cell viability of normal cells was at 74.4% and 23.5% in cancer cell lines (Fig. 4). The IC50 values were calculated and were found to be 600 µg/ml for PBMC cells and 6 µg/ml for MCF-7 cells. The most important factor for developing an anticancer drug is its selective toxicity on cancer cells. Therefore, our results indicate that green synthesized AuNPs treatment have shown selective cytotoxicity towards cancer cells than normal cells. Here in, we used MCF-7 cell line for cancer cell line for the studies on breast cancer for decades. Since, they maintain a strong similarity to the mammalian epithelium (Lee et al., 2015). The cytotoxic mechanism can be due to the generation of free radicals (ROS) by the green synthesized AuNPs (Khalil et al., 2018, Fu et al., 2014). Earlier reports of green synthesis of AuNPs from Sasa borealis (Patil et al., 2018), Rhuschinensis (Patil et al., 2016), Illicum versus (Sathishkumar et al., 2015) etc. providing evidence on cytotoxic effects on liver, gastric, cervical and bone cancer cells (Alkilany and Murphy, 2010). However, this study has certain limitations: such as cytotoxic mechanism (apoptosis study) of action by green synthesized AuNPs is lacking. To overcome this issue, further experiments will be conducted to evaluate their mode of action.
Fig. 4.
Cytotoxicity analysis of Anacardium occidentale AuNPs on PBMC and MCF-7 Cells. The results indicate the selective cytotoxicity of AuNPs against MCF-7 CELLS. The normal PBMC were not affected drastically even at a high concentration of 100 µg/ml. The results were statistically significant with the untreated cells with a p < 0.0001.
4. Conclusion
This study reported synthesis of AuNPs by applying the leaf extracts of A. occidentale. The synthesized AuNPs were of 10–60 nm and spherical in shape. The green AuNPs elicited an increased activity against the pathogens, provided permissible levels of cytotoxicity towards normal cells and demonstrated high cytotoxicity towards MCF-7 cancer cells. The results indicate that the AuNPs synthesized by A. occidentale (Leaves) can be safely applied for antibacterial applications.
Conflicts of interest
We acknowledge no conflicts.
Acknowledgments
The work was funded by Junior Research Fellowship provided by Maulana Azad National Fellowship, University Grants Commission, Govt. of India to the research scholar Mrs. Veena S, fellowship No. MANF-2014-15-CHR-TAM-40072. We whole-heartedly thank Dr. S. L. Hoti, Officer in Charge, NITM, Indian Council of Medical Research, Belagavi for helping us in collecting the plant materials. We express our thanks to Sejong University, Republic of Korea for their support.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Devasena Thiyagarajan, Email: tdevasenabio@gmail.com.
Arokiyaraj Selvaraj, Email: arokiyaraj16@gmail.com.
References
- Ahmed S., Ahmad M., Swami B.L., Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. of Adv. Res. 2016;7:17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkilany A.M., Murphy C.J. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J. Nanopart Res. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arokiyaraj S., Vincent S., Saravanan M., Lee Y., Oh Y.K., Kim K.H. Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. Artif Cells Nanomed. Biotechnol. 2017;45:372–379. doi: 10.3109/21691401.2016.1160403. [DOI] [PubMed] [Google Scholar]
- Baek Y.W., An Y.J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 2011;409:1603–1608. doi: 10.1016/j.scitotenv.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Chen X., Schluesener H.J. Nanosilver: a nanoproduct in medical application. Toxicol. Lett. 2008;176:1–12. doi: 10.1016/j.toxlet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Evelyn M.L.P., Fernando W.C., Araujo I.A., Souza I.V.G.A., Bastos T.G., Silva S.C., Nascimento G.C.G., Militaao L.A.L., Soares H.S., Xavier S.J.M. Pharmacological screening and acute toxicity of bark roots of Guettardaplatypoda. Braz. J. Pharmacogn. 2012;22:1315–1322. [Google Scholar]
- Fu P.P., Xia Q., Hwang H.M., Ray P.C., Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J. Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Patil S., Ahire M., Kitture R., Gurav D.D., Jabgunde A.M., Kale S., Pardesi K., Shinde V., Bellare J., Dhavale D.D., Chopade B.A. Gnidiaglauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J. Nanobiotechnol. 2012;10:1–9. doi: 10.1186/1477-3155-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C.M., McCusker C.D., Yilmaz T., Rotello V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- Hemshekhar M., Sebastin Santhosh M., Kemparaju K., Girish K.S. Emerging roles of anacardic acid and its derivatives: a pharmacological overview. Basic Clin. Pharmacol. Toxicol. 2012;110:122–132. doi: 10.1111/j.1742-7843.2011.00833.x. [DOI] [PubMed] [Google Scholar]
- Herizchi R., Abbasi E., Milani M., Akbarzadeh A. Current methods for synthesis of gold nanoparticles. Artif. Cells NanomedBiotechnol. 2016;44(2016):596–602. doi: 10.3109/21691401.2014.971807. [DOI] [PubMed] [Google Scholar]
- Huang S.L., Weng Y.M., Chiou R.Y. Survival of Staphylococcus aureus and Escherichia coli as affected by ethanol and NaCl. J. Food Prot. 2001;64:546–550. doi: 10.4315/0362-028x-64.4.546. [DOI] [PubMed] [Google Scholar]
- Iqbal M., Usanase G., Oulmi K., Aberkane F., Bendaikha T., Fessi H., Zine N., Agusti G., Errachid E.S., Elaissari A. Preparation of gold nanoparticles and determination of their particles size via different methods. Mat. Res. Bull. 2016;79:97–104. [Google Scholar]
- Keshavamurthy M., Srinath B.S., Ravishankar Rai V. Phytochemicals mediated green synthesis of gold nanoparticles using PterocarpusSantalinus L. (Red Sanders) bark extract and their antimicrobial properties. Particul. Sci. and Tech. 2018;36:785–790. [Google Scholar]
- Khalil A.T., Ovais M., Ullah I., Ali M., Shinwari Z.K., Hassan D., Maaza M. Sageretiathea (Osbeck.) modulated biosynthesis of NiO nanoparticles and their in vitro pharmacognostic, antioxidant and cytotoxic potential. Artif. Cells Nanomed. Biotechnol. 2018;46:838–852. doi: 10.1080/21691401.2017.1345928. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Kuk E., Yu K.N., Kim J.H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwang C.Y., Kim Y.K., Lee Y.S., Jeong D.H., Cho M.H. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Lee A.V., Oesterreich S., Davidson N.E., Natl J. MCF-7 cells—changing the course of breast cancer research and care for 45 years. J. Natl. Cancer Inst. 2015;107:1–4. doi: 10.1093/jnci/djv073. [DOI] [PubMed] [Google Scholar]
- Li J., Li Q., Ma X., Tian B., Li T., Yu J., Dai S., Weng Y., Hua Y. Biosynthesis of gold nanoparticles by the extreme bacterium Deinococcus radiodurans and an evaluation of their antibacterial properties. Int. J. Nanomed. 2016;11:5931–5944. doi: 10.2147/IJN.S119618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mostafa A.A., Al-Askar A.A., Almaary K.S., Dawoud T.M., Sholkamy E.N., Bakri M.M. Antimicrobial, anticoagulant, and cytotoxic evaluation of multidrug resistance of new 1,4-dihydropyridine derivatives. Saudi J. Biol. Sci. 2018;25:361–366. doi: 10.1016/j.sjbs.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukunthan K.S., Balaji S. Cashew apple juice (Anacardium occidentale L.) speeds up the synthesis of silver nanoparticles. Int. J. Green Nano Tech. 2012;4:71–79. [Google Scholar]
- Omanakuttan A., Nambiar J., Harris R.M., Bose C., Pandurangan N., Varghese R.K., Kumar G.B., Tainer J.A., Banerji A., Perry J.J., Nair B.G. Anacardic acid inhibits the catalytic activity of matrix metalloproteinase-2 and matrix metalloproteinase-9. Mol. Pharmacol. 2012;82:614–622. doi: 10.1124/mol.112.079020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S., Tak Y.K., Song J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. environ. microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M.P., Jin X., Simeon N.C., Palma J. Anticancer activity of Sasa borealis leaf extract-mediated gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018;46:82–88. doi: 10.1080/21691401.2017.1293675. [DOI] [PubMed] [Google Scholar]
- Patil M.P., Ngabire D., Pham Thi H.H., Kim M.D., Kim G.D. Eco-friendly synthesis of gold nanoparticles and evaluation of their cytotoxic activity on cancer cells. J. clust Sci. 2016;16:1051–1056. [Google Scholar]
- Philip D. Rapid green synthesis of spherical gold nanoparticles using Mangiferaindica leaf. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010;77:807–810. doi: 10.1016/j.saa.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Quelemes P.V., Araruna F.B., de Faria B.E., Kuckelhaus S.A., da Silva D.A., Mendonca R.Z., Eiras C., Dos S., Soares M.J., Leite J.R. Development and antibacterial activity of cashew gum-based silver nanoparticles. Int. J. Mol. Sci. 2013;14:4969–4981. doi: 10.3390/ijms14034969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad A.G., Abbasi H., Afzali H.M. Gold nanoparticles: synthesising, characterizing and reviewing novel application in recent years. Phys. Procedia. 2011;22:203–208. [Google Scholar]
- Raza M.A., Kanwal Z., Rauf A., Sabri A.N., Riaz S., Naseem S. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials (Basel) 2016;6:1–15. doi: 10.3390/nano6040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni T., Motiei M., Romman Z., Popovtzer A., Popovtzer R. Targeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo study. Int. J. Nanomed. 2011;6:2859–2864. doi: 10.2147/IJN.S25446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar M., Pavagadhi S., Mahadevan A., Balasubramanian R. Biosynthesis of gold nanoparticles and related cytotoxicity evaluation using A549 cells. Ecotoxicol. Environ. Saf. 2015;114:232–240. doi: 10.1016/j.ecoenv.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Sheny D.S., Mathew J., Philip D. Phytosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011;79:254–262. doi: 10.1016/j.saa.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Singh S., Vidyarthi A.S., Nigam V.K., Dev A. Extracellular facile biosynthesis, characterization and stability of gold nanoparticles by Bacillus licheniformis. Artif. Cells Nanomed. Biotechnol. 2014;42:6–12. doi: 10.3109/21691401.2012.759122. [DOI] [PubMed] [Google Scholar]
- Velusamy P., Venkat Kumar, G.V., Jeyanthi J., Das R., Pachaiappan R. Bio-inspired green nanoparticles: synthesis, mechanism, and antibacterial application. Toxicol. Res. 2016;32:95–102. doi: 10.5487/TR.2016.32.2.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Astruc D. Magnetically recoverable ruthenium catalysts in organic synthesis. Molecules. 2014;19:4635–4653. doi: 10.3390/molecules19044635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M., Jamnejad A., Khajehali E. Antibacterial effect of silver nanoparticles against four foodborne pathogens. Jundishapur J. Microbiol. 2014;7:e8720. doi: 10.5812/jjm.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]