Abstract
The roots of Codonopis bulleynana Forest ex diels (cbFed), locally known as Tsoong, have been used as a tonic food. Tsoong has wide range of pharmacological effects, including anticancer effects. In the present study, the anticancer activity of Tsoong and its potential molecular mechanisms were investigated. Using high throughput sequencing the apoptotic pathway was ranked as one of the most important pathways and the differential expressions of apoptosis-related genes such as Casp3, Casp6 and Apaf1 were identified. The following experiments were qRT-PCR which were used to verify the genes. In vitro, cell counting kit-8 (CCK-8) assays and flow cytometry in HCT116 and SW480 colon cancer cell were used to assess the anti‐proliferation and apoptosis-promoting activities of Tsoong. In vivo, the antitumor effect of Tsoong was assessed in colon cancer-bearing nude mice as a xenograft model. H&E staining was performed with oxaliplatin set as a positive control. The results showed that Tsoong up-regulated apoptosis-related genes, inhibited tumor cell proliferation, promoted tumor cellapoptosis in a dose-dependent manner and restrained the growth of colon neoplasm. The effects of a high dose of Tsoong on colon cancer cells were similar to those of oxaliplatin. Our results may ultimately help in the development of diagnostic and therapeutic strategies to control this devastating disease. Therefore, Tsoong may be a promising Chinese herbal compound for development for use in cancer therapy.
Keywords: Tsoong, Colon cancer, Casp3, Casp6, Apaf1, Apoptosis
1. Introduction
Colon cancer is one of the most common malignant tumors with a high incident rate in the 40–50 age group and is a serious threat to human life and health. In China, due to changes in living conditions and diet structure in recently years, the incidence of colorectal cancer has been significantly increasing, and ranks the fourth place among malignant tumors at present (Xiaolei et al., 2014).
High fat diets, obesity, alcohol consumption, chronic inflammation of the gastrointestinal tract, and chronic constipation are considered to be the main causes of colorectal cancer (Jingru et al., 2012, Zackular et al., 2013, Yunpeng et al., 2016, Schulz et al., 2014, Pfalzer et al., 2016). An enormous amount of researches have been concentrated upon the exact mechanism of colon cancer, and the effective prevention and treatment of colon cancer has become a focus of medical investigations (Katz et al., 2016).
Traditional Chinese medicine is appreciated for its 5000-year-old history and still holds an important position in primary health care in China, and it has also become more popular among cancer patients in the western world (Xin et al., 2012). In addition to traditional surgery, radiotherapy and chemotherapy, the combining of traditional Chinese medicine and Western medicine treatment strategies offer potential in the treatment of the colon cancer (Sommerer and Zeier, 2016, Liu and Liang, 2017). At present, natural medicine has become a focus of clinical anticancer drug investigation, due to its multi-target, multi-link and multi-channel antitumor effects.
Codonopsis (Campanulaceae, C.) is represented in China by 39 species, some of which are commonly used as herbal remedies due to their tonic effects, such as C. pilosula and C. tangshen (Hong et al., 1983). Codonopis bulleynana Forestex diels (cbFeD) is herbaceous plant found in Yunnan, Tibet, and Sichuan Provinces. Its roots, locally known as Tsoong, have been used as a food in Yunnan Province since ancient times (Hu et al., 2012). Meanwhile, this species has become an important economic plant widely cultivated in several areas of Yunnan Province (Chen et al., 2006, Sun et al., 2016). Due to the characteristics of Tsoong being unique as a national medicinal herb, few reports exist in international journals, although there have been a wide range of investigations in China. Studies investigating the pharmacodynamics and acute toxicity of Tsoong have shown that it can enhance gastrointestinal peristalsis, improve tolerance to fatigue and hypoxia, and can promote the recovery of hemoglobin, red blood cells, IgG and the immunosuppressive effect in hemorrhagic blood deficient mice (Dong et al., 2015). Tsoong can enhance the immune function of mice with xenograft tumors and enhance the phagocytic functions of the reticuloendothelial system (Chen et al., 2012). It has been suggested that Tsoong has a positive effect on chemotherapy, reducing toxicity and enhancing immune function. The latest research literature shows that Tsoong can induce apoptosis and inhibits proliferation, migration and invasion of pancreatic ductal adenocarcinoma cells (Luan et al., 2018).
In present study, the possible effects and mechanisms of Tsoong against colon cancer was explored. Kunming mice were adopted to establish colon cancer model and Tsoong treatment model. Intestinal tissue samples from Kunming mice were used for high-throughput transcriptomic sequencing and the sequencing results were verified by qRT-PCR. The results showed that apoptosis-related genes were significantly down regulated in the disease model group of Kunming mice, and significantly increased in the Tsoong treatment group. The results suggested that Tsoong can promote the apoptosis of colon cancer cells and inhibit the intestinal cancer. Then in vitro and in vivo experiments were used to validate the inhibitory mechanism of Tsoong on intestinal cancer.
Loperamide acts on opioid receptors in the intestinal wall to inhibit the release of acetylcholine and prostaglandin, resulting in inhibition of intestinal peristalsis and prolonging of the retention time of intestinal contents, which lead to chronic constipation. Kunming mice were treated with Loperamide to establish chronic constipation model. After the successful establishment of constipation model the mice were continuously treated with Loperamide to establish colon cancer model. Anatomy showed that the mice with chronic constipation would have sarcomas in the intestines. Visible sarcomas under naked eyes were firstly found in the intestines of the colon cancer model group at the 9th week. There would be 6–12 tumors in the intestines in the 12th weeks. This meant that the colon cancer model was established successfully (Luan et al., 2017).
Oxaliplatin is a third-generation platinum anticancer drug, following cisplatin and carboplatin. Oxaliplatin induces autophagy and promotes the apoptosis of colon cancer cells (Tan et al., 2015). Oxaliplatin was used as control in the present study, in which the cytotoxic and antiproliferative effects of Tsoong on human colon cancer HCT116 and SW480 cell lines were examined. The present study also investigated apoptosis in HCT116 and SW480 cell in response to Tsoong treatment and examined the consequences of Tsoong treatment using in vivo colon cancer models to obtain therapeutic insights.
2. Materials and methods
2.1. Establishment of colon cancer model and Tsoong treatment model
2.1.1. Materials
72 Kunming mice were provided by Kunming Medical University. Main equipment for experiments included Leica stereomicroscope (Leica Company, Germany), optical microscope (Nikon Company, Japan), and microscopic imaging system (Nikon Company, Japan). Loperamide hydrochloride (Xian-Janssen Pharmaceutical Ltd., performance standard: YBH04562010, specifications: 2 mg/tablet, batch number: 141111266), and all other needed regents were analytical reagents (Xilong Chemical Co., Ltd.).
2.1.2. Preparation of Loperamide
For constipation induction experiments, commercial Loperamide hydrochloride (2 mg/tablet) was dissolved in physiological saline to a concentration of 0.25 mg/mL, adjusted pH to 7.0, mixed well and stored at −20 °C for further use.
2.1.3. Experimental procedures
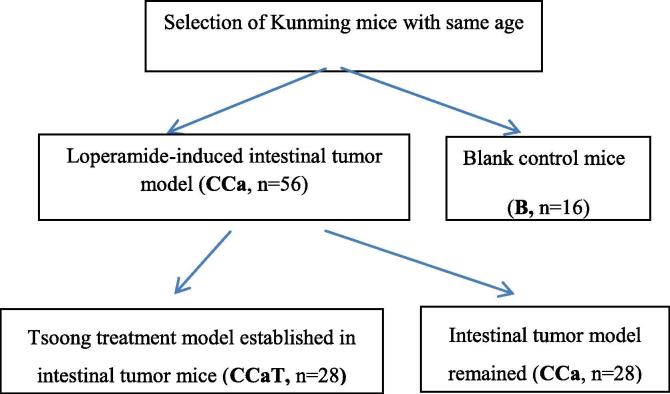
A total of 72 healthy Kunming mice with same age were screened and randomly divided into experimental group (CCa, n = 56) and blank control group (B, n = 16) (Fig. 1). For induction of constipation model, the CCa group was treated by intragastric administration of Loperamide according to the intragastric volume per unit of body weight of 2.5 mg/(Kg * d) for 2 consecutive weeks; the B group received intragastric administration with equal volume of sterile saline solution. Two weeks later, the constipation model was established successfully (Luan et al., 2017).
Fig. 1.
Experimental flow chart. CCastands for the experimental group gavaged with1/2 original dosage of Loperamide to induce intestinal tumor after the successful establishment of constipation model (n = 56); CCaT stands for Tsoong treatment mice group in CCa (n = 28); The rest mice without Tsoong treatment remained CCa (n = 28).
2.1.4. Establishment of colon cancer model based on constipation model
After the successful establishment of constipation model, the CCa group with constipation were continuously treated with 1/2 original dosage Loperamide, which lead to colon cancer (Fig. 1). Stereo-microscope was used to observe lesion morphology, and lesions were fixed by 10% formalin solution and sliced into 5 μm sections according to the paraffin section method. Anatomy showed that the mice with chronic constipation would have sarcomas in the intestines. Visible sarcomas under naked eyes was firstly found in the intestines of the CCa group at the 9th week. There would be 6–12 tumors in the intestines in the 12th weeks. This meant that the colon cancer model was established successfully.
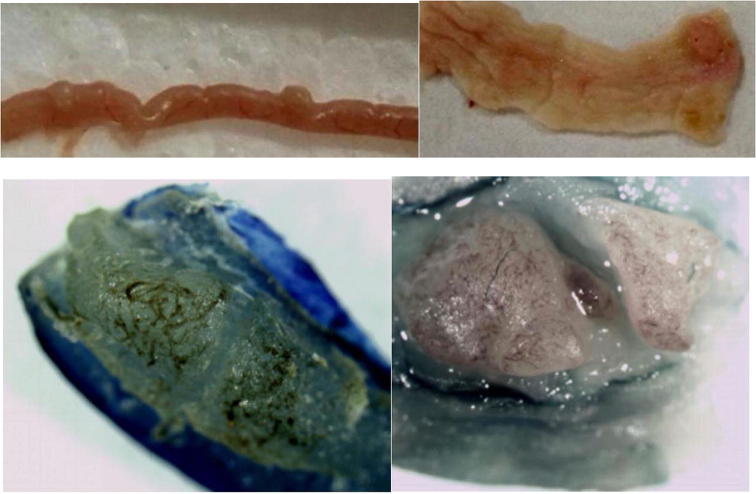
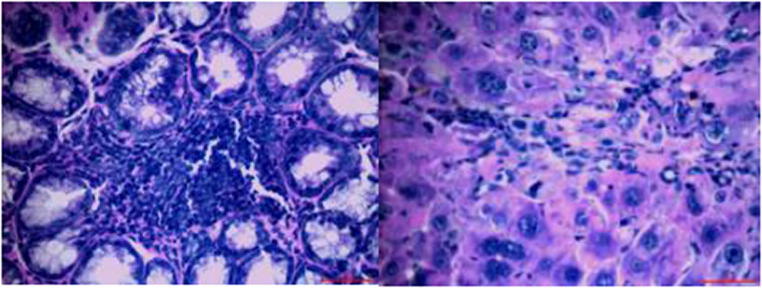
After the lesions in the intestines were fixed by 10% neutral formalin solution, sarcomas under the stereomicroscope presented uneven surface and abundant blood vessels in staggered and irregular arrangement (Fig. 2). Observation of pathological sections under high-power microscope revealed that cells showed irregular shape, inconsistent size, irregular nuclear shape, nuclear hyperchromatism, prominent nucleolus and nuclear division at lesion sites, which were confirmed as tumor cells when compared with relevant data (Fig. 3).
Fig. 2.
Sarcoma morphology in the external and internal wall of the intestine (below images present visible sarcomas under naked eyes in the internal wall of the intestine under stereomicroscope after fixation by 10% neutral formalin solution).
Fig. 3.
Intestinal tumors under 40X microscope (H&E staining).
2.1.5. Establishment of Tsoong treatment model
After the successful establishment of colon cancer model, the CCa group was randomly divided into 2 groups. One group (CCaT, n = 28) was treated with Tsoong and the other group (CCa, n = 28) remained colon cancer model. After 12 weeks of Tsoong treatment, the tumors were markedly reduced. This meant that Tsoong treatment model was established successfully.
2.2. Transcriptome sequencing
2.2.1. RNA extraction, library preparation, and next-generation sequencing
Next-generation sequencing is rapidly becoming the method of choice for transcriptional profiling experiments. Total RNA was isolated from snap-frozen colonic tissue from the control group and tumor samples from the experimental groups. using the AllPrep DNA/RNA Mini Kit (Qiagen, Valencia, CA, USA). The quality and quantity of the extracted RNA samples were determined with bioanalyzer and ultramicro spectrophotometer, respectively. The library was constructed using the Illumina TruSeq RNA preparation kit (Illumina, San Diego, CA, USA) according to the manufacturer’s manual. The next generation sequencing platforms most frequently used for RNA-seq are the Illumina HiSeq. Samples were sequenced on the Illumina instrument with 50–75 bp paired-end reads.
2.2.2. Analysis of RNA-seq
Raw Illumina fastq files were adapter-removed by software cutadapt (https://pypi.python.org/pypi/cutadapt/), Pollution-assessed by blast+ (http://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastDocs& DOC_TYPE=Download) and quality-controled by Prinseq (Schmieder and Edwards, 2011). The reads were aligned to the mouse genome with TopHat v2.0.10 (Kim et al., 2013) and The Cufflinks (Trapnell, 2010) program cuffdiff was used to calculate expression levels. Gene Ontology classification and enrichment analysis was performed base on the genes. KEGG PATHWAY is a collection of manually drawn pathway maps representing our knowledge on the molecular interaction and reaction networks for metabolism, genetic Information Processing, human Diseases and etc (http://www.kegg.jp/). KEGG was used to annotate the genes.
2.2.3. Statistical analysis
Results were obtained by a variety of statistics and graphic software. The statistical analysis was performed with R packages, mothur (Schloss et al., 2009) and Samtools (http://samtools.sourceforge.net/). Welch’s t-tests were conducted to compare the phenotypes of the different group mice and statistical significance was set at P < 0.05. To determine the role of the chronic constipation and chemical carcinogen in the development of colon tumorigenesis, the well-established model of constipation recapitulates the progression from chronic constipation to intestinal tumor in humans.
2.3. Validation of in vitro and in vivo experiments
2.3.1. qRT-PCR experiment verification
Through bioinformatics analysis the genes that may be related to tumor therapy had been selected. The apoptosis-related genes were screened out. They were Casp3, Casp6 andApaf1. The selected gene expressions in mice intestinal tissue was measured using qRT-PCR (Quantitative real time polymerase chain reaction). The following PCR primers were used as follow: Apaf1 forward 5′-AGATTTGGGATTCTGCGACTG-3′ and reverse 5′-GAAGGTGGTTACTCTTGTTGGTG-3′; Casp3 forward 5′-TGACTGGAAAGCCGAAACTC-3′ and reverse 5′-CAAGCCATCTCCTCATCAGTC-3′; Casp6 forward 5′-CGATTGCTTCATCTGTGTCTTC-3′ and reverse 5′-GTCTCCTTTGAACAAGCCAGTC-3′.
Fluorescence PCR amplifications were performed with ABI step one plus. The qRT-PCR reactions were performed in triplicate for the three biological replicates. Relative quantification was performed using the 2−△△Ct method, including an efficiency correction for the primers using the Relative Expression Software Tool.
2.3.2. Cell culture
The HCT116 and SW480 cell lines were obtained from American Type culture collection (Manassas, VA, USA). The HCT116 and SW480 colon cancer cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS (cat. no. 10099158; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 μg/ml of streptomycin and 100 U/ml of penicillin in a humidified atmosphere of 5% CO2 at 37 °C.
2.3.3. Preparation of drug‐containing serum
The dry Tsoong was purchased from Yunnan International Pty, Ltd. (Yunnan, china). Firstly, 1 kg of dry Tsoong was soaked in cold water for 30 min, decocting twice with 1:6 w/v distilled water for1 h. Filtration was then performed to the appropriate concentrations, the first decoction comprised in 1:10 w/v distilled water for 90 min and second comprised 1:8 w/v distilled water for 60 min. A final quantity of 450 g dried powder was obtained by spray drying at room temperature, which was then sealed and stored in the dark at 4 °C. The Tsoong powder was dissolved in normal saline for the gavage experiments. The drug-containing serum solutions were collected from mice following exposure to the following treatments (once per day for 1 week): Treatment with normal saline by gastrogavage (n = 8; normal control group); 5 g/kg of Tsoong by gastrogavage (n = 8; low Tsoong group); 10 g/kg of Tsoong bygastrogavage (n = 8; mid Tsoong group), 20 g/kg of Tsoong bygastrogavage (n = 8; high Tsoong group); 5 mg/kg oxaliplatin by gastrogavage (n = 8; oxaliplatin group). The blood samples were obtained from the abdominal aorta following treatment, following which the serum was isolated by centrifuging at 1800 × g for 10 min at 4 °C and stored at −80 °C for the follow-up experiments.
2.3.4. Experimental groups
The drug-containing serum solutions from the mice were used to treat the HCT116 and SW480cells. The five treatment groups comprised the normal control group, the low Tsoong group, the mid Tsoong group, the high TSoong group, and the oxaliplatin group.
2.3.5. Cell counting kit‐8 (CCK‐8) assay
Celll proliferation was determined using the colorimetric water-soluble tetrazolium salt assay using a CCK-8 kit (Beyotime Institute of Biotechnology, co., Ltd., Haimen, china). In brief, the cells ata density of 2 × 103 cells per well was seeded in 96 well plates and incubated with the low, mid, or high dose of Tsoong, with or without oxaliplatin, for 24, 48 and 72 h. Following treatment, the culture medium was removed and replaced with 100 μl of fresh medium containing 10 μl of CCK-8 solution in each well and the cells were incubated at 37 °C for 2 h. The number of viable cells was determined by reading the absorbance at 450 nm using a Thermo Platemicroplate reader (Rayto Life and Analytical Science co., Ltd., Shenzhen, 103 china).
2.3.6. Cell cycle assay
Following treatment, the cells were harvested and resuspended at a density of 1 × 106 cells/ml. The cells were fixed with ice-cold 70% ethanol for at least 30 min. cell cycle was analyzed using a flow cytometer with propidiumiodide (PI) as a specific fluorescent dye probe. The PI fluorescence intensity of 10,000 cells was measured for each sample using a Becton-dickinson FAcScalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
2.3.7. Cell apoptosis assay
Following treatment, cell apoptosis was assessed using an Annexin V-FITC apoptosis detection kit (BD Pharmingen, San Diego, CA, USA). In brief, Annexin V‐FITC (5 μl) and PI (5 μl) were added to 100 μl cells at a concentration of 1x106 cells/ml and incubated in the dark for 15 min at room temperature. Subsequently, binding buffer was added, and apoptosis was analyzed using ow cytometry (BD Biosciences).
2.3.8. Xenograft tumors
A total of 18 female Balb/c athymic nude mice (5–6 weeks old, body weight 19–22 g) (Vital River Laboratories, Beijing, China) were housed at 25 °C in 40–70% humidity, in a 12 h light/dark cycle with free access to food and water and were subcutaneously injected in the right flank with2.0 × 106 SW480 cells in 0.1 ml PBS. When tumors had formed, the tumor volume (V) was measured using calipers daily and calculated using the following formula: V = (L × W2)/2, where L was the length and W was the width of the tumor. The mice were randomly divided into three groups (n = 5): Normal control group mice treated with normal saline via gavage; high dose of Tsoong (20 g/kg) mice treated with a high dose of Tsoong viagavage; oxaliplatin group mice with colon cancer treated with oxaliplatin (5 mg/kg via gavage). Growth curves were plotted using the average tumor volume within each experimental group every week. After six weeks, the mice were sacrificed, and the dissected tumors were collected and prepared for subsequent analyses. All animal experiments were approved by the Animal center of Southwest Forestry University (Kunming, china). All experimental procedures involving animals were performed according to the institutional ethical guidelines for animal experiments and in accordance with the Guide for the care and Use of Laboratory Animals.
2.3.9. Statistical analysis
The results are presented as the mean ± standard deviation. The statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). For comparisons Dunnett t-test or two-way analysis of variance was used. P < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. Analysis of transcriptome sequencing
3.1.1. Effects of Tsoongon the apoptosis-related genes.
The correlation between the gene expression of samples is an important indicator to reflect the reliability and the rationality of sample selection. In order to facilitate the comparison of different experimental groups, the B was used to indicate blank group, the CCa was used to indicate colon cancer group, and the CCaT was used to indicate Tsoong treatment group. The differential gene test method uses cuffdif2 in Cufflink. The software is widely used in the RNA-seq analysis with the reference genome. Filter condition was set to p-Value < 0.05. In organisms, different genes coordinate their biological functions. The most important biochemical and signal transduction pathways of differentially expressed genes are identified by pathway enrichment. KEGG (Kyoto Encyclopedia of Genes and Genomes) is the main public database of pathway.
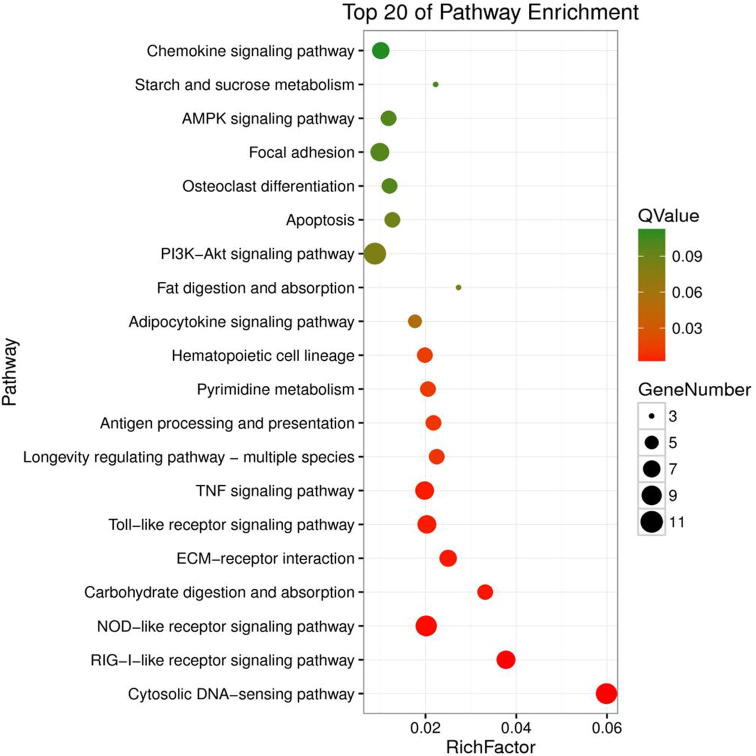
Through the comparison of B vs. CCaand CCa vs. CCaT, 272 DEGs and178 DEGswere identified, respectively. To distinguish the most affected pathways, a KEGG enrichment analysis (corrected p-Value < 0.001 and FDR < 0.001) was performed. The significantly enriched KEGG pathways in B vs. CCa are shown in Fig. 4.
Fig. 4.
The significantly enriched KEGG pathways in B vs. CCa. Apoptosis pathway was one of the most significantly enriched KEGG pathways.
It can be seen in Fig. 4 Apoptosis pathway was one of the most significantly enriched KEGG pathways about colon cancer. Some important selected genes in Apoptosis pathway are shown in Tables 1 and 2. They were Casp3 (Caspase3), Casp6 (Caspase6) and Apaf1.
Table 1.
The selected genes in Apoptosis pathway in B vs. CCa.
| Gene | Fold change | pvalue | B_fpkm | CCa_fpkm |
|---|---|---|---|---|
| Casp3 | 0.500 | 0.015 | 27.07 | 13.51 |
| Casp6 | 0.858 | 0.602 | 118.65 | 101.76 |
| Apaf1 | 0.255 | 0.020 | 12.64 | 3.21 |
Table 2.
The selected genes in Apoptosis pathway in CCavs. CCaT.
| Gene | Fold change | pvalue | CCa_fpkm | CCaT_fpkm |
|---|---|---|---|---|
| Casp3 | 1.100 | 0.699 | 13.51 | 14.99 |
| Casp6 | 1.040 | 0.602 | 101.766 | 105.4 |
| Apaf1 | 1.542 | 0.02 | 3.21 | 4.94 |
3.1.2. Verification to effects of Tsoong treatment using qRT-PCR experiments
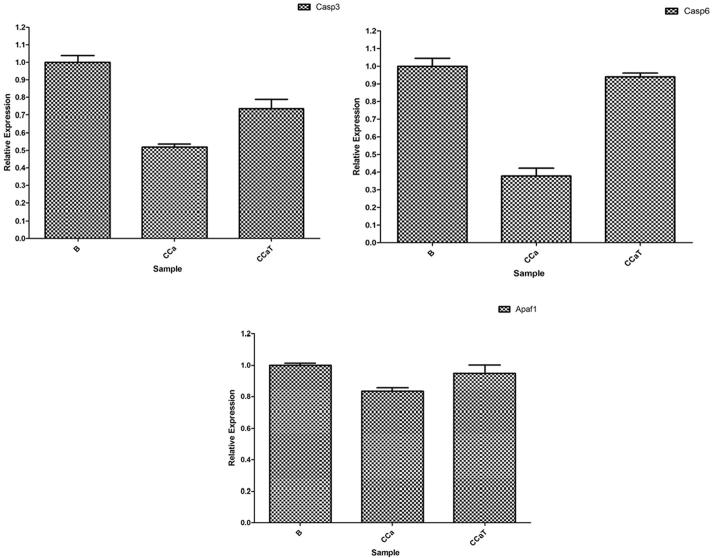
Because some data did not meet the requirements (p-value < 0.05) the qRT-PCR were conducted to verify the high throughput sequencing results. Differentially expression of the above 3 genes in B group, CCa group and CCaT group were studied with qRT-PCR (quantitative real-time Reverse Transcription Polymerase Chain Reaction) experiments to verify the above results and efficacy of Tsoong. The qRT-PCR results are shown in Fig. 5. Through high throughput sequencing and qRT-PCR experiment verification these apoptosis-related genes (Casp3, Casp6 and Apaf1) were downregulated in the colon cancer model group (CCa) and they were obviously recovered in the Tsoong treatment group (CCaT).
Fig. 5.
Differentially expression of Casp3, Casp6 and Apaf1 in B group, CCa group and CCaT group were verified with qRT-PCR experiments. They are the most important apoptosis-related genes. The qRT-PCR experiments verified that the Tsoong restored the expression of apoptosis genes.
3.2. In vitro experiments
3.2.1. Effects of Tsoong on the proliferation and cell cycle of HCT116 117 and SW480 cells
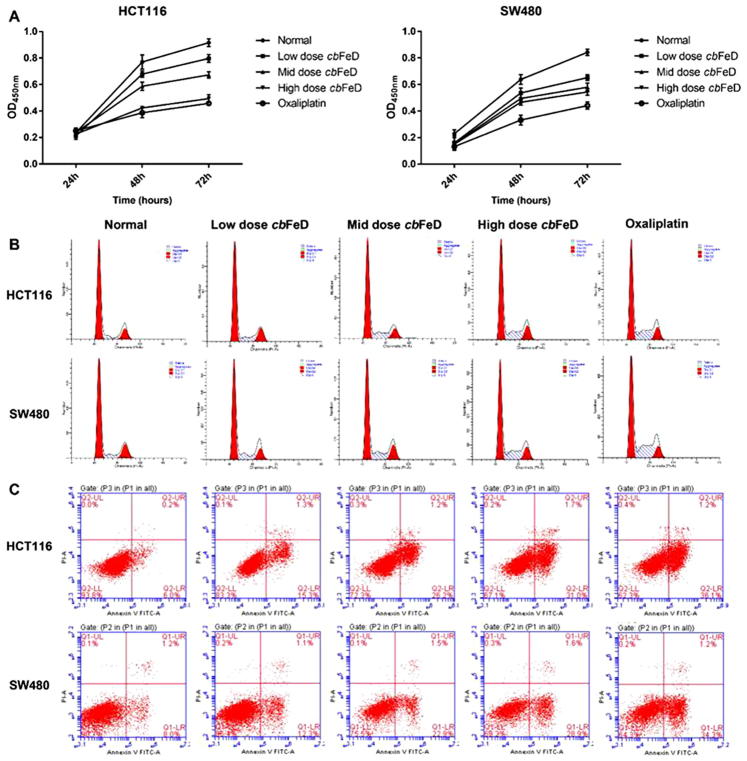
Codonopsis can induce cell cycle arrest and apoptosis in human colon tumor (Wang et al., 2011). cbFed is a species of the Codonopsis and Tsoong is the root of cbFed. The Tsoong-containing serum solutions were prepared from mice by gastrogavage with saline (control), or5 (low), 10 (mid) and 20 (high) g/kg of Tsoong. To determine the role of Tsoong on cell proliferation, cell cycle and cell apoptosis, the HCT116 and SW480 cells were treated with these Tsoong-containing serum solutions. The results showed that Tsoong inhibited the proliferation of HCT116 and SW480cells at 48 and 72 h. The cell proliferation rate was decreased with increasing concentrations of Tsoong-containing serum solutions, suggesting that Tsoong inhibited the cell proliferation in a dose-dependent manner (Fig. 6A). The cell proliferation in the high Tsoong group was similar to that in the oxaliplatin group. Similarly, Tsoong decreased the proportion of cells in the G1 phase cells but increased the proportion of cells in the S phase, suggesting that Tsoong induced S phase arrest (Fig. 6B), which was similar to the effect of oxaliplatin. Therefore, Tsoong inhibited cell proliferation in adose-dependent manner and induced cell cycle arrest at the Sphase.
Fig. 6.
Effect of Tsoong on the proliferation and cell cycle of HCT116 and SW480 cancer cells. The cells were treated with Tsoong-containing serum solutions prepared from mice treated by gastrogavage with saline (control), or 5 (low), 10 (mid) or 20 (high) g/kg of Tsoong for indicated durations. (A) cell proliferation was detected using a cell counting kit‐8 assay, and (B) cell cycle was detected using flow cytometry. (C) Cell apoptosis was analyzed using flow cytometry. Oxaliplatin was used as a control. P < 0.05 vs. control; P < 0.01 vs. control. Tsoong or cbFeD, Codonopis bulleynana Forest ex diels.
3.2.2. Effects of Tsoong on the apoptosis of HCT116 and SW480 cells
The apoptotic rates of HCT116 and SW480 cells following Tsoong treatment were also analyzed (Fig. 6C). The apoptotic rates of the HCT116 cells treated with low, mid, and high doses of Tsoong were 16.6, 27.4 and 32.7%, respectively, whereas the apoptotic rate of the HCT116 cells in the control group was only 6.2%. Tsoong treatment produced the same effects on the SW480 cells. The results suggested that the apoptotic rate induced by Tsoong was increased with dose. Treatment of the cells with oxaliplatin confirmed the apoptosis of the HCT116 and SW480 cells.
3.3. In vivo experiments
3.3.1. Tsoong suppresses tumorigenicity in vivo
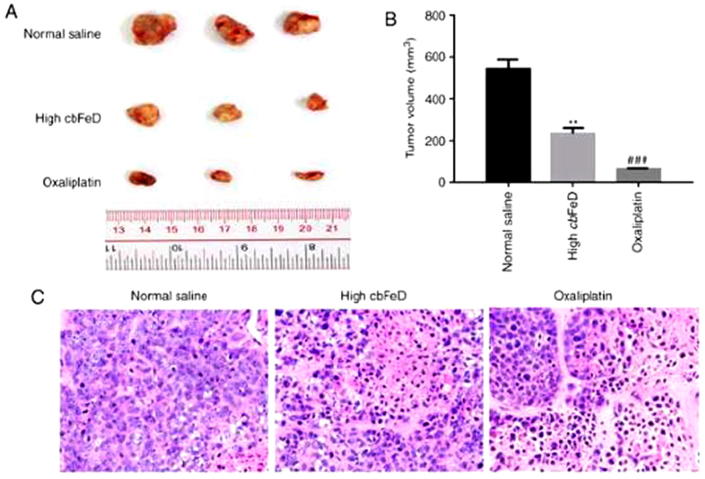
To confirm the above findings, particularly the results of the CCK‐8 assay (Fig. 6A), and due to the fact that SW480 cells have been used in the establishment of xenograft tumors in previous studies (Chen et al., 2016, Jiang et al., 2017), SW480 cell were used to establish a nude-mouse transplanted tumor model in the present study. A high dose of Tsoong or oxaliplatin were administered to nude mice by gastrogavage or injection, 6 weeks following intragastric administration, these two groups exhibited significantly smaller tumors, compared with those in the normal saline group (Fig. 7A and B). The H&E staining showed that the Tsoong induced a higher level of inflammatory cell in filtration (Fig. 7C).
Fig. 7.
Effects of Tsoong and oxaliplatin on growth of xenograft tumors. Nude mice were subcutaneously injected in the right flank with 2.0 × 106 SW480 cells in 0.1 ml PBS. Once tumors had formed, tumor volume was measured. The mice were then randomly divided into three groups (n = 5): Normal control group, mice treated with normal saline via gavage; high Tsoong group, mice treated with a high dose (20 g/kg) of Tsoong via gavage; oxaliplatin group, mice with colon cancer treated with oxaliplatin (5 mg/kg) via gavage or injection. After 6 weeks, the dissected tumors were collected, and H&E were performed. (A) Tumor size. (B) tumor volume. (C) H&E staining of xenograft tumors. Magnification, x400. Oxaliplatin was used as a control. P < 0.01 vs. control; P < 0.001 vs. control. Tsoong or cbFeD, Codonopis bulleynana Forestex diels.
4. Discussion
Caspases are a family of protease enzymes playing essential roles in apoptosis. Caspase deficiency has been identified as a cause of tumor development. Casp3 (Caspase3) and Casp6 (Caspase6) are the members of the Caspase family. They play central roles in the execution-phase of cell apoptosis. Casp3 and Casp6 can promote apoptosis in tumor cells and inhibit tumor (Han et al., 2017, Li et al., 2017, Suita et al., 2017, Suboj et al., 2012).
There are many kinds of proteases in caspase family, which can be stepwise hydrolyzed and activated and promote the progress of apoptosis jointly. The pro-apoptosis factor Casp3 is one of the most important members of the cysteine protease family, and it exists in cytoplasm in the form of an inactive zymogen. The original structural domain of Casp3 will be cleaved off by proteolysis under the stimulation of apoptotic signals and be activated through the formation of a tetramer (Abbas et al., 2018, Ayaz et al., 2018). The activated Casp3 participates in a series of cell change processes related to apoptosis, including the degradation of intracellular protein substrates, inactivation of apoptosis inhibitors, aggregation of chromatin and promotion of apoptotic body formation (Braton et al., 2000, Bumidin et al., 2018). Activated Casp3 is able to cleave the peptide bonds after aspartate residues, leading to degradation and inactivation of important proteins present in cytoplasm, nucleus, and cytoskeleton. Therefore, it is considered the most critical enzyme in apoptosis of mammalian cells as well as the core protease in the protease cascade (Bossy-WetzeI and Green, 1999, Jamal et al., 2018).
Casp6 gene is an effector molecule of Caspase and is the most important apoptotic executor besides Casp3. It turns into an active form only after the activation of the upstream Caspase. The large and small subunits aggregate to form a dimer, while two dimers can be polymerized into a more active tetramer then, which acts on substrates related to the downstream apoptosis and causes apoptosis. Apaf1 (Apoptotic protease activating factor 1) encodes a cytoplasmic protein that forms one of the central hubs in the apoptosis regulatory network. Apaf1 is one of the key regulators in the mitochondrial apoptotic pathway, and the loss of Apaf1 leads to cellular resistance against the apoptotic signals.
Apaf1 plays a pivotal role in cellular apoptosis and is involved in the mitochondria-mediated apoptosis pathway. Different forms of cellular stress induce the release of cytochrome c from the gap of mitochondrial inner membrane into the cytoplasm directly. With the presence of dATP/ATP, cytochrome c binds to Apaf1 in the cytoplasm and recruits procasp9 (procaspase9) after the oligomerization of Apaf1, thus an apoptotic body will be formed, in which procasp9 is activated into Casp9 (Caspase9) before the activation of Casp3 (Caspase3) and other proteins in caspase family by the apoptotic body, leading to irreversible apoptosis. It is obvious that Apaf1 is an important pro-apoptotic factor in the mitochondrial apoptosis pathway as well as the core of the apoptotic body (Paik et al., 2007, Zlobec et al., 2007, Strater et al., 2010, Chen et al., 2018a.
Natural products have been used in traditional and folk medicine for therapeutic purposes. They provide important sources of promising leads for the development of novel therapeutic drugs (Wang et al., 2011, D’Souza, 2018). The fresh roots of Codonopis bulleynana Forest ex Diels, locally known as Tsoong, have been used as a food or vegetable and herbal remedies due to their tonic effects in Yunnan Province since ancient times (Chen et al., 2006, Alvi et al., 2018). Recently, phytochemical research also revealed that the crude extract of Tsoong increased the chemotherapeutic sensitivities and attenuated and enhance immune function of tumor-bearing mice (Chen et al., 2012, Chen et al., 2018b).
Tumorigenesis and development is closely related to aberrant cell apoptosis process. Therefore, apoptosis is believed to play a significant role in tumorigenesis. In particular, induction of apoptosis is the main mechanism of action for anti-cancer drugs. The ability and potency for a particular drug compound to induce apoptosis also becomes an important dimension for its evaluation. Apoptosis-related genes (e.g., Casp3, Casp6 and Apaf1) can largely determine whether a cell would enter apoptosis process.
Therefore, Apaf1, Casp3, Casp6 and so on are the most important genes involved in apoptosis. In the mouse disease model group, they were significantly down-regulated, followed by a significant rebounded after Tsoong treatment. It not only was a result of high-throughput transcriptome sequencing, but also was verified by qRT-PCR experiment. Then, in vitro and in vivo experiments had further demonstrated that Tsoong could promote tumor cell apoptosis and inhibit intestinal cancer.
Tsoong has wide range of pharmacological effects, including anticancer effects. The anti cancerrole of Tsoong was confirmed in the present study. Tsoong can inhibit cell proliferation, increase the proportion of cells in the S phase, and promote the apoptosis of HCT116 and SW480 cells. The inhibition of cell proliferation, and promotion of cell cycle arrest and cell apoptosis in the HCT116 and SW480 cells by Tsoong were found to occur in a dose-dependent manner. Similarly, nude mice xenograft experiments confirmed that Tsoong could inhibit the growth of colon cancer and induce the apoptosis of xenograft tumor cells.
5. Conclusion
In conclusion, the present study demonstrated that Tsoong: (i) significantly promoted expression of apoptosis-related genes; (ii) inhibited proliferation and promoted apoptosis of colon cancer cells; (iii) restrained the growth of colon neoplasm in nude mouse. Our results may ultimately help in the development of diagnostic and therapeutic strategies to control this devastating disease. Therefore, Tsoong may be a promising Chinese herbal compound for development for use in cancer therapy.
Funding
The study was supported by the National Natural Science Foundation of China (grant no. 61462095 and 61702442).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The sampling of human peritoneal tissue was approved by the Local Ethics Committee (Yunnan Medical University, KMMU: 2017004), in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Yunpeng Luan, Email: luanyunpeng2015@126.com.
Youjie Zhao, Email: cn_caoyong@126.com.
References
- Abbas P., Hashim Y.Z.H.Y., Salleh H.M. Cytotoxic effects and response surface optimization of solvent extraction of crude extracts from aquilaria subintegra uninfected branch. Sci. Heri. J. 2018;2(2):10–15. [Google Scholar]
- Alvi M.A., Ahmad S., Aqib A.I., Tayyab M.H., Murtaza A., Ashfaq K., Rashid I., Ghafoor M., Ahmed H.A. Diagnosing post parturient hemoglobinuria in goat on the basis of hematology, serum biochemistry and treatment response. Mat. Sci. Med. 2018;2(2):34–36. [Google Scholar]
- Ayaz T., Khattak A.M., Ahmad N. Supra soft R-separation axioms. Acta Scient. Malaysia. 2018;2(2):27–31. [Google Scholar]
- Bossy-WetzeI E., Green D.R. Caspases induce cytcehrome c release from mitixhondria by activating cytosolie factors. J. Biol. Chem. 1999;274(25):17484–17490. doi: 10.1074/jbc.274.25.17484. [DOI] [PubMed] [Google Scholar]
- Braton S.B., Nlacfarlane M., Cain K. Protein complexes activate distinct caspase cascades in death receptor and stress induced apoptosis. Exp. Cell Res. 2000;256(1):27. doi: 10.1006/excr.2000.4835. [DOI] [PubMed] [Google Scholar]
- Bumidin M.S., Johari F.A., Risan N.F., Nasir M.H.M. The effect of aqueous extracts of nigella sativa on breast cancer cell line Mcf-7: an in vitro study. Sci. Herit. J. 2018;2(1):13–17. [Google Scholar]
- Chen S., Meng X., Wang Y., Sun X. Antioxidant activity and optimisation of ultra sonic-assisted extraction by response surface methodology of aronia melanocarpa anthocyanins. Mat. Sci. Pharma. 2018;2(1):06–09. [Google Scholar]
- Chen S.L., Cai S.R., Zhang X.H., Li W.F., Zhai E.T. Targeting CRMP-4 by lentivirus-mediated RNA interference inhibits SW480 cellproliferation and colorectal cancer growth. Exp. Therap. Med. 2016;12(4):2003–2008. doi: 10.3892/etm.2016.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Su Y., Huang M. Comparison and analysis of application effect of traditional paper operation method and digital information system in hemodialysis. Mat. Sci. Pharma. 2018;2(2):01–03. [Google Scholar]
- Chen Z.J., Li Y.S., Wei Q.H., Chen S.L., Chen D.X. Effects of codonopis bulleynana forest ex diels on enhancing sensitivity, reducing toxicity of chemotherapy and regulating immune function in sarcoma 180 tumor-bearing mice. Chin. Trad. Patent Med. 2012;34:1848–1851. [Google Scholar]
- Chen Z.J., Wei Q.H., Zhou J.Y. The effects of codonopis bulleynana on the growth and proliferation of HL60 cells. Yunnan J. Trad. Chin. Med. Mater. Med. 2006;27:49. [Google Scholar]
- D’Souza U.J.A. Problem based learning, student-centered active learning. Mat. Sci. Pharma. 2018;2(1):01–02. [Google Scholar]
- Dong L.D., Qian Z.G., Yang Z., Cheng Y.X. Study on the anti-hyperlipidemia, anti-fatigue and anti-anoxia effects of Codonopsis foetens Hook. Yunnan J. Trad. Chin. Med. Mater. Med. 2015;36:66–68. [Google Scholar]
- Han N.N., Zhou Q., Huang Q., Liu K.J. Carnosic acid cooperates with tamoxifen to induce apoptosis associated with caspase-3 activation in breast cancer cells in vitro and in vivo. Biomed. Pharmacother. 2017;89:827–837. doi: 10.1016/j.biopha.2017.01.084. [DOI] [PubMed] [Google Scholar]
- D.Y. Hong, Y.S. Lian, L.D. Shen, Flora of China. Science Press: Beijing, 73; 1983, p. 32.
- Hu Q.F., Xuesen L.X.S., Huang H.T., Mu H.X., Tu P.F., Li G.P. Phenylpropanoids from the roots of codonopsis cordifolioidea and their biological activities. Bullet.- Kor. Chem. Soc. 2012;33:278–280. [Google Scholar]
- Jamal F., Khattak A.M., Khan G.A., Abdullah S. Five separation axioms in quad soft non-linear structure. Acta Scient. Malaysia. 2018;2(2):14–20. [Google Scholar]
- Jiang H., Ju H., Zhang L., Lu H., Jie K. MicroRNA-577 suppresses tumor growth and enhances chemosensitivity in colorectal cancer. J. Biochem. Mol. Toxicol. 2017;31(6) doi: 10.1002/jbt.21888. [DOI] [PubMed] [Google Scholar]
- Jingru L., Yubin J., Mingcang C. Advances of pharmacological researches in constipation animal model. China J. Exp. Trad. Med. Form. 2012;22:353–356. [Google Scholar]
- Katz M.L., Young G.S., Zimmermann B.J., Tatum C.M., Paskett E.D. Assessing colorectal cancer screening barriers by two methods. J. Cancer Edu. 2016;4:1–8. doi: 10.1007/s13187-016-1148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Pertea G., Trapnell C. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Yang Y., Ding Y., Tang X., Sun Z. Impacts of surviving and caspase-3 on apoptosis and angiogenesis in oral cancer. Oncol. Lett. 2017;14(3):3774–3779. doi: 10.3892/ol.2017.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Liang X.C. New developments in the pharmacodynamics and pharma-cokinetics of combination of Chinese medicine and Western medicine. Chin. J. Integrat. Med. 2017;23(4):312–319. doi: 10.1007/s11655-016-2271-1. [DOI] [PubMed] [Google Scholar]
- Luan Y.P., Cao Y., Mao D.C., Li Y.M., Yue X.G., Zhao Y.J., Xiong F., Rong J., He C.Z. RNA sequencing reveals the differences between chemical and chronic constipation induction of intestinal tumor. Biomed. Res. 2017;28(22):10053–10061. [Google Scholar]
- Luan Y.P., Li Q.F., Wu S.G., Mao D.C., Deng Y.Y. Tsoong induces apoptosis and inhibits proliferation, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol. Med. Rep. 2018;17(3):3527–3536. doi: 10.3892/mmr.2017.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik S.S., Jang K.S., Song Y.S., Jang S.H. Reduced expression of Apaf-1 in colorectal adenocarcinoma correlates with tumor progression and aggressive phenotype. Ann. Surg. Oncol. 2007;12(14):3453–3459. doi: 10.1245/s10434-007-9541-2. [DOI] [PubMed] [Google Scholar]
- Pfalzer A.C., Kamanu F.K., Parnell L.D. Interactions between the colonic transcriptome, metabolome, and microbiome in mouse models of obesity-induced intestinal cancer. Physiol. Geno. 2016;48(8) doi: 10.1152/physiolgenomics.00034.2016. [DOI] [PubMed] [Google Scholar]
- Schloss P.D., Westcott S.L., Ryabin T. Introducing mother: open source, platform independent, community supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75(23):7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder R., Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27(6):863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M.D., Atay C., Heringer J. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514(7523):508–512. doi: 10.1038/nature13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerer C., Zeier M. Clinical manifestation and management of AdPKd in Western countries. Kidney Dis. 2016;2(3):120–127. doi: 10.1159/000449394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strater J., Herter I., Merkel G., Hinz U., Weitz J. Expression and prognostic significance of APAF-1, caspase-8 and caspase-9 in stage II/III colon carcinoma: caspase-8 and caspase-9 is associated with poor prognosis. Int. J. Cancer. 2010;127(4):873–880. doi: 10.1002/ijc.25111. [DOI] [PubMed] [Google Scholar]
- Suboj P., Babykutty S., Srinivas P., Gopala S. Aloe emodin induces G2/M cell cycle arrest and apoptosis via activation of caspase-6 in human colon cancer cells. Pharmacology. 2012;89(1–2):91. doi: 10.1159/000335659. [DOI] [PubMed] [Google Scholar]
- Suita H., ShinOmiya T., Nagahara Y. Caspase-6 induces 7A6 antigen localization to mitochondria during FAS-induced apoptosis of jurkat cells. Anticancer Res. 2017;37(4):1697. doi: 10.21873/anticanres.11501. [DOI] [PubMed] [Google Scholar]
- Sun J., Wang L., Wang M., Wang Z., Li F. Two new polyacetylene glycosides from the roots of codonopsis tangshen oliv. Nat. Prod. Res. 2016;30(20):1–6. doi: 10.1080/14786419.2016.1174230. [DOI] [PubMed] [Google Scholar]
- Tan S., Peng X., Peng W., Zhao Y., Wei Y. Enhancement of oxaliplatin-induced cell apoptosis and tumor suppression by 3-methyladenine in colon cancer. Oncol. Lett. 2015;9:2056–2062. doi: 10.3892/ol.2015.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28(5):511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Xu M.L., Hu J.H., Rasmussen S.K., Wang M.H. Codonopsis lanceolata extract induces G0/G1 arrest and apoptosis in human colon tumor HT-29 cells-involvement of ROS generation and polyamine depletion. Food Chem. Toxicol. 2011;49:149–154. doi: 10.1016/j.fct.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Xiaolei W., Xiaojuan L., Weihong W. The association between constipation and the risk of colorectal cancer: a meta-analysis. Chin. J. Clin. 2014;20:3634–3639. [Google Scholar]
- Xin T., Zhang F., Jiang Q., Chen C., Huang D., Li Y., Shen W., Jin Y., Sui G. The inhibitory effect of a polysaccharide from Codonopsis pilosula on tumor growth and metastasis in vitro. Int. J. Biol. Macromol. 2012;51:788–793. doi: 10.1016/j.ijbiomac.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Yunpeng L., Dechao M., Yanmei L. Effect of chronic constipation on dimethylhydrazine-induced intestinal cancer in kunming mice. J. Balkan Tribol. Assoc. 2016;12(4) [Google Scholar]
- Zackular J.P., Baxter N.T., Iverson K.D. The gut microbiome modulates colon tumorigenesis. mBio. 2013;6(4):e00692. doi: 10.1128/mBio.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlobec I., Minoo P., Baker K., Haegert D., Khetani K. Loss of APAF-1 expression is associated with tumour progression and adverse prognosis in colorectal cancer. Eur. J. Cancer. 2007;43(6):1101–1107. doi: 10.1016/j.ejca.2007.01.029. [DOI] [PubMed] [Google Scholar]