Abstract
In this paper, we examine brain lateralization patterns for a complex visual-spatial task commonly used to assess general spatial abilities. Although spatial abilities have classically been ascribed to the right hemisphere, evidence suggests that at least some tasks may be strongly bilateral. For example, while functional neuroimaging studies show right-lateralized activations for some spatial tasks (e.g., line bisection), bilateral activations are often reported for others, including classic spatial tasks such as mental rotation. Moreover, constructive apraxia has been reported following left- as well as right-hemisphere damage in adults, suggesting a role for the left hemisphere in spatial function. Here, we use functional neuroimaging to probe lateralization while healthy adults carry out a simplified visual-spatial construction task, in which they judge whether two geometric puzzle pieces can be combined to form a square. The task evokes strong bilateral activations, predominantly in parietal and lateral occipital cortex. Bilaterality was observed at the single-subject as well as at the group level, and regardless of whether specific items required mental rotation. We speculate that complex visual-spatial tasks may generally engage more bilateral activation of the brain than previously thought, and we discuss implications for understanding hemispheric specialization for spatial functions.
Keywords: visual-spatial functions, fMRI, construction task, lateralization, parietal lobe
1. Introduction
Hemispheric specialization, i.e., the notion that the brain’s two hemispheres differ with regards to the functions they subserve, the types of stimuli they prefer, and their computational makeup, has long been a topic of interest for those studying the brain, its cognitive functions, and its behavioral output. The topic has found its way into conventional wisdom (albeit often in distorted form) and continues to be a matter of lively discussion (e.g., Efron, 1990; Hugdahl & Westerhausen, 2010). Some of the earliest indicators that the two hemispheres are not created equal were the observations by Paul Broca (1861) and Carl Wernicke (1874) of “language areas” in the left hemisphere. It has also long been known that basic sensory and motor function cross over on their way from the body periphery to the cerebral cortex: The primary motor cortices of the two hemispheres control movement of the contralateral extremities and the somatosensory cortices receive tactile input from the contralateral side of the body (Penfield & Boldrey, 1937). Similarly, the visual cortices receive visual input from the contralateral side of the visual field (Holmes, 1918).
These known lateralizations enabled Sperry et al.’s (1969) crucial research on “split-brain” patients. In these patients, the fibers of the corpus callosum (and often also the anterior commissure) were cut to treat intractable epilepsy. As a result, direct inter-hemispheric communication was impossible, so that sensory information from one side of the body and the visual field was only available to the contralateral hemisphere. This provided a unique opportunity for investigating what one hemisphere can do on its own with the available information. For example, Sperry and colleagues found that the patients could not produce the names of objects presented visually to solely the right hemisphere. This is consistent with the idea that language is largely left-lateralized, at least in adults, for which there is converging evidence from countless studies using different methodologies (e.g., Broca, 1861; Lenneberg, 1967; Binder et al., 1996; Stromswold et al., 1996; Bookheimer et al., 1997).
In 1965, Bogen and Gazzaniga introduced another paper on split-brain cognition and hemispheric specialization by stating that “an increasing accumulation of clinical data suggests that complementary functions in man may be verbal v. visuospatial” (p. 394), thus attributing visual-spatial function to the right hemisphere. In further support of this notion, they reported that two split-brain patients could perform visual-spatial construction tasks with the left hand (steered by the right hemisphere), but not with the right hand (steered by the left hemisphere). This notion of a verbal left and a spatial right hemisphere is also reflected in the “Hemispheric Crowding” hypothesis (Teuber, 1974), according to which early left-hemisphere lesions result in visual-spatial impairments because verbal skills are assumed by the right hemisphere and thus “crowd out” the visual-spatial abilities it normally supports.
Much research has followed these initial findings and resulted in more detailed articulations of hemispheric lateralization. With respect to language, it is now known that while certain aspects, such as syntax and semantics, indeed rely predominantly on the left hemisphere, others, such as prosody, involve the right hemisphere (Weintraub et al., 1981; George et al., 1996). With respect to visual-spatial functions, two theoretical frameworks have embraced the idea that they may be differentially localized to the right vs. left hemisphere as a consequence of the nature of information-processing preferences in the two hemispheres. Kosslyn (1987) proposed that the left hemisphere has a processing preference for categorical spatial information (e.g., the difference between categories ‘above’ and ‘below’), while the right hemisphere tends to process coordinate spatial information (i.e., the detailed information required for reaching and navigation). Ivry and Robertson (1998) proposed that visual and auditory information undergo differential filtering by the two hemispheres, resulting in processing biases such that the left hemisphere tends to achieve representations with more ‘local’ detail while the right hemisphere achieves representations that are more ‘global’ in nature. Empirical studies have lent support to both views (e.g., Kosslyn et al., 1989, 1998; Laeng, 1994; Ivry & Robertson, 1998), and the two frameworks and their predictions are compatible with each other (Okubo & Mitchimata, 2004; Borst & Kosslyn, 2010). Notably, both frameworks emphasize that these hemispheric processing preferences are relative, not absolute. Despite these more detailed articulations of hemispheric lateralization, the general idea articulated by Bogen and Gazzaniga − that language and space are preferentially represented by the left vs. right hemispheres − has permeated the literature.
However, from our reading of the literature, the evidence is much less consistent regarding right-lateralization of visual-spatial functions than it is regarding left-lateralization of language. On one hand, there is evidence in favor of right-lateralization. Behavioral studies show that tasks tapping memory for spatial location are performed better for stimuli presented to the left hand (Witelson, 1976) or in the left visual field (Kimura, 1969; Durnford & Kimura, 1971; Tucker et al., 1999; Postma et al., 2006). Lesion studies indicate that injury to the right hemisphere, especially the parietal lobe, results in dramatic impairments in the spatial domain that are evident in drawing, construction, and orientation tasks as well as in left-right disorientation and apraxia for dressing (Brain, 1941; McFie et al., 1950; Hecaen et al., 1956; Vallar, 1998). Hemispatial neglect, in which patients have difficulty perceiving stimuli or parts of stimuli contralateral to their lesion site, is much more common after lesions to the right than lesions to the left hemisphere (Bisiach & Luzzatti, 1978; Vallar, 1998). A telltale sign is a rightward bias in the line bisection task: When asked to mark the center of a horizontal line, patients with right-parietal lesions place their mark too far to the right (Schenkenberg et al., 1980), whereas healthy adults are quite accurate and if anything tend to have a small leftward bias (Jewell & McCourt, 2000). The same rightward bias can be induced experimentally by temporarily disrupting right parietal cortex through repetitive transcranial magnetic stimulation (rTMS); left-sided rTMS has no spatially biasing effect (Fierro et al., 2000). Lastly, functional neuroimaging studies requiring line bisection judgments (Fink et al., 2001; Cicek et al, 2009) reveal activations predominantly in right parietal and premotor cortex. All this points to significant right-hemisphere lateralization for certain spatial functions.
On the other hand, there is also ample evidence for left-hemisphere involvement in some visual-spatial tasks. Lesion studies have reported impairments in visual-spatial skills, especially visual-spatial constructive functions, following left-hemisphere lesions (McFie et al., 1960; Arrigoni & De Renzi, 1964; Gainotti et al., 1977). Returning to the evidence derived from split-brain patients mentioned above, Gazzaniga’s 1995 review qualifies the initial report (Bogen & Gazzaniga, 1965) on two split-brain patients who could perform visual-spatial tasks with their left hand (right hemisphere) but not their right hand (left hemisphere) by noting that in other patients, neither hemisphere by itself could perform well on visual-spatial tasks, and in yet other patients, the left hemisphere performed better. Similarly, the occipito-parietal (dorsal) “where” pathway (Ungerleider & Mishkin, 1982) for localizing and/or interacting with objects in space is bilaterally represented (Haxby et al., 1991), although there is some evidence that the two hemispheres differ with respect to the way in which they represent object location, with the right hemisphere favoring a metric (coordinate) and the left hemisphere favoring a relative (categorical) approach (Kosslyn et al., 1989, 1998).
Most relevant to the present study is the functional neuroimaging literature on the most classic of all spatial tasks − mental rotation (Shepard & Metzler, 1971). In this task, participants are presented with pictures of two three-dimensional objects and asked to judge whether they are identical (true if one is a rotated view of the other) or not (false if one is a reflected version of the other). Various versions of this task have been widely used to gauge spatial abilities in children and adults (Vandenberg & Kuse, 1978; Kosslyn et al., 1990; Frick et al., 2013) and to evaluate gender differences in spatial abilities (Voyer et al., 1995; Peters, 2005). The related functional neuroimaging literature often reports bilateral, rather than right-lateralized, parietal activations (Cohen et al., 1996; Richter et al., 1997, 2000; Vingerhoets et al., 2002).1 Support for the possibility that mental rotation often engages bilateral areas, and certainly is not unequivocally right-lateralized, also comes from two meta-analyses of mental rotation neuroimaging studies (Zacks, 2008; Tomasino & Gremese, 2016).
Notably, the latter meta-analysis (Tomasino & Gremese, 2016) also revealed that the degree of lateralization can be modulated by stimulus type and strategy: If the task involves bodily as opposed to non-bodily stimuli (e.g., hands vs. objects) and if participants used motor-based as opposed to visual imagery-based strategies (e.g., “imagine rotating the object” vs. “imagine the object rotating in space”), activation becomes more bilateral compared to the right-lateralized activations observed for non-bodily stimuli and non-motor strategies. This is consistent with dissociations observed in patients with unilateral brain lesions, where right-sided lesions are associated with mental rotation impairments for objects (but not hands) and non-motor (but not motor-based) rotation strategies, whereas the opposite holds for left-sided lesions (Tomasino et al., 2003; Tomasino & Rumiati, 2004). It is also consistent with similar findings on line bisection, where activation becomes more bilateral (due to increasing left-sided activations) if stimuli are presented in near vs. far space (i.e., within reach) and if the bisection task is active (i.e., involving a motor component) rather than purely perceptual (Weiss et al., 2003).
In sum, the mixed pattern of lateralization results for visual-spatial tasks contrasts with the relatively unequivocal evidence for language lateralization, highlighting that we do not yet have a full understanding of whether and how spatial functions are lateralized in the brain. In part, this may be due to the fact that there is no monolithic spatial system that parallels the intricately organized system of language. Indeed, the range of spatial functions that one can enumerate includes those that engage recognition of specific types of stimuli from distinctly different domains (e.g., faces, bodies, scenes, biological motion), those that engage non-spatial domains that may be deeply entwined with spatial representation (e.g., number), and those that engage mental operations that transform objects in space (e.g., mental rotation, expansion/contraction). Based on the existing literature, it seems unlikely that these many spatial functions share just one lateralization profile; empirical studies will be required in order to understand whether the classic view of spatial functions as right-lateralized is still viable. In this paper, we contribute to this goal by examining lateralization patterns in a complex visual-spatial task that is a hallmark of spatial cognition: visual-spatial construction.
Visual-spatial construction tasks, such as the classic Block Design Test (Kohs, 1920), require participants to assemble parts into a specified pattern (see Figure 1C for an illustration of patterns and constituent parts). These tasks are used to track the development of spatial abilities (Del Giudice et al., 2000; Stiles, 2012), measure individual and sex differences in neurologically healthy children and adults (Levine et al., 1999; Hegarty & Waller, 2005), and assess spatial impairments following from genetic disorders or brain damage (Cornish et al., 1998; Hoffman et al., 2003; Stiles, 2012), which makes them an important tool for understanding brain function. They are also commonly included as sub-tests of standardized IQ tests (e.g., the various derivations, for children and adults, of the Bellevue Intelligence Scales introduced by David Wechsler in 1939, and the Differential Ability Scales by Colin D. Elliot, 1990), which indicates that visual-spatial skills are viewed as an important component of overall intelligence. The widespread use of visual-spatial construction tasks is not surprising considering their obvious face validity: Constructing a complex figure out of various sub-components clearly requires several core spatial processes, including mental parsing of the target shape into constituent components, identifying those components among the available set, understanding how those parts have to be positioned relative to each other to form the target shape, and using executive control functions to interleave these processes (Hoffman et al., 2003; Ballard & Hayhoe, 1997). Given that individual parts must often undergo rotation and/or translation in order to fit correctly into the overall shape, the task also draws on the classic spatial function of mental rotation as well as other mental transformations.
Figure 1: Experimental Design.
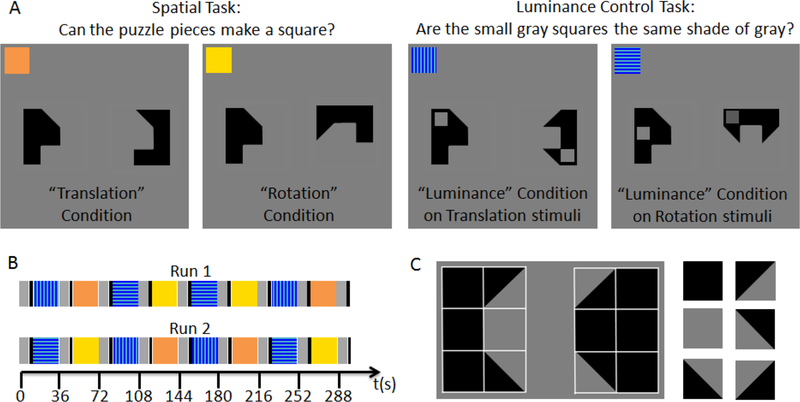
(A) Example stimuli for the different experimental and control conditions. In the Spatial Task, participants indicated whether the two puzzle pieces could be combined to form a square. The Translation condition required that participants mentally translate (slide) one shape into the other; the Rotation condition required mentally rotating one of the shapes by 90 degrees prior to the mental translation step. In the Luminance Control Task, participants indicated whether the gray squares superimposed on the puzzle pieces were the same shade of gray. (B) Schematic illustrating the time course of the two functional MRI runs. Rest periods are shown in gray, instruction periods in black, and the different task conditions in the colors shown on the inserts in A: orange for the Translation condition, yellow for the Rotation condition, and blue for the Luminance control condition. There were two types of luminance control blocks, one using the puzzle pieces from the Translation condition (vertical stripes) and one using the puzzle pieces from the Rotation condition (horizontal stripes). (C) Illustration of how the puzzle pieces used here are composed of the same elements as the figures in the Wechsler Block Design task.
To our knowledge, only one imaging study to date has investigated brain activations during a construction task. Meyer-Lindenberg and colleagues (2004) designed an fMRI task that heavily drew on visual-constructive function without requiring participants to actually assemble the design (which would not be feasible inside the scanner due to the motion artifacts it would create). On each trial, participants saw two shapes composed of complex polygons, side by side. In a Match condition, participants pushed one of two buttons to indicate whether the two shapes were identical (in which case they were also oriented in the same way). In the Construction condition, participants judged whether the two polygons could be combined to form a square, which required both mental rotation and translation of the pieces. The Match and Construction conditions were compared to each other and to a Motor control condition in which participants saw pairs of identical polygons and were instructed to push the “same” button on all trials. The focus of the Meyer-Lindenberg study was to compare activation between individuals with Williams Syndrome (who have severely impaired visual-spatial skills, exemplified by poor performance in pattern construction tasks) and typically-developing controls. Controls showed bilateral occipitoparietal activations when comparing the Construction to the Match condition, whereas people with WS did not. Group differences reached significance in the right parietal lobe when comparing Match and Motor control, and in the left parietal lobe when comparing Construction and Match, demonstrating hypoactivation in people with Williams syndrome, which the authors argue reflects the behaviorally observed deficit in the construction task. The results suggest bilateral parietal involvement in the Construction task. However, because the Construction task required mental rotation, which, as reviewed above, is often associated with bilateral parietal activations (Zacks, 2008; Tomasino & Gremese, 2016), it is not clear whether the results are attributable to mental rotation per se, or to the more general spatial demands of mentally combining two parts to form a complex pattern.
Here we adapt Meyer-Lindenberg’s design to examine lateralization patterns in neurologically healthy young adults. We make three crucial changes to the design. First, to disentangle the effects of visual-spatial construction (i.e., mental combination of parts to create a whole pattern) compared to the specific function of mental rotation, we created two Construction conditions. In the Translation condition, the polygons were positioned so that deciding whether they fit together required participants to mentally translate the parts, “sliding” one toward the other to decide. In the Rotation condition, the polygons were positioned so that participants had to mentally rotate one of the puzzle piece by 90 degrees prior to the mental translation step. While this condition requires both Rotation and Translation, we will refer to it as the “Rotation” condition, both for brevity and to emphasize what distinguishes it from the Translation condition. Second, we included Luminance Control conditions which closely matched the two Construction conditions with respect to visual stimulation and motor demands. (The conditions are described in detail below and illustrated in Figure 1.) Third, to make the task more similar to those commonly used to assess spatial skills behaviorally, we used polygons composed of the same geometric primitives as in the Block Design subtest of the Wechsler IQ tests.
Based on the literature showing bilateral activations for mental rotation but right-lateralized activations for line bisection judgments, we hypothesized that complex spatial tasks in general (and not just mental rotation) might evoke bilateral activations. If this is correct, we would expect bilateral activations not only when comparing the Rotation condition to the Luminance control condition, but also when comparing the Translation condition to the Luminance Control condition. We also directly compared activations in the Translation and the Rotation conditions to investigate whether adding mental rotation to the task demands specifically increases left-hemisphere activations.
2. Materials and Methods
2.1. Participants
Participants were 35 members of the Georgetown University community, recruited via on-campus flyers and by word of mouth. They ranged in age from 18 to 37 (mean 23, SE 0.81 years). There were 19 women and 16 men.2 All except 6 were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). While it is known that certain aspects of brain organization differ in some left-handers (for a recent review, see Willems et al., 2014), we did not exclude left-handers from this study.3 All participants provided informed consent prior to study participation and were compensated for their time.
2.2. Design, Stimuli, and Procedures
Following consenting, assessment of handedness, and MRI safety screening, participants were familiarized with the experimental task outside the scanner until they had demonstrated proficiency and felt comfortable performing it. They then completed two five-minute runs of the task inside the scanner. Each run contained two 24-second blocks of the “Translation” and the “Rotation” conditions, and their respective “Luminance Control” counterparts, as described below, for a total of eight blocks, interleaved with nine short periods of rest. Figure 1 provides an illustration of the experimental conditions (Figure 1 A) and the time course of the two functional runs (Figure 1 B). The screen background was gray throughout the experiment (RGB 128, 128, 128) because pilot work determined that participants perceived a white background as uncomfortably bright.
During Rest blocks (shown in gray in Figure 1B), participants were instructed to rest their eyes on a fixation cross displayed at screen center and did not push any buttons. Each Rest block concluded with a written instruction screen (displayed for 3 seconds) announcing the upcoming task (either “PUZZLE block. Look at the puzzle pieces. Button 1 = they fit together. Button 2 = they don’t fit together.” or “COLOR block. Look at the gray squares. Button 1 = they are equally bright. Button 2 = they look different.”). The instructions were well-known to the participants due to pre-scan training, so that recognizing them within 3 seconds did not pose any difficulty.
All experimental conditions required participants to push one of two buttons held in their right hand. For the Spatial Task (Translation and Rotation conditions, shown in orange and yellow in Figure 1), they pushed one button if the two black shapes displayed on the screen could be combined to form a square, and the other button if that was not possible. For the Luminance Control Task, they pushed one button if the two small gray squares displayed on the two black shapes were the same shade of gray, and the other button if they were different shades of gray. To ensure that participants were continuously engaged throughout the block, the next trial appeared immediately following a button push or if a response had not occurred within 3 seconds of trial onset. Participants were familiarized with this time limit prior to scanning and rarely timed out. To avoid cutting off participants right after the beginning of a trial, the last trial that began before the end of each 24 s task block was not aborted at the end of the 24 s task block, but lasted until the participant responded (or up to 3 seconds if there was no response). Thus, block duration could theoretically vary between 24 and 27 s, and participants completed as many trials as possible within that time period.4 The rest period following each task block was shortened depending on how much the block had exceeded 24 seconds so that the onset between subsequent task blocks was constant at 36 seconds.
To maximize similarity to the Wechsler Block Design task, the shapes displayed on the screen were composed of the same geometric primitives represented on the Wechsler blocks (squares and triangles). Each shape was effectively constructed from six Wechsler blocks, as illustrated in Figure 1 C. We presented all shapes that could be constructed, given the constraint that one row of blocks (the outer edge of the to-be-assembled square) always consisted of three solid black blocks, and the second row (the “seam” of the to-be-assembled square) never consisted of three solid blocks. Shapes that resembled letters (e.g., L) or objects (e.g., a house) were excluded from the stimulus set. Trials were randomly drawn from a set containing an equal number of stimulus pairs that could or could not be combined to form a square.
In the Translation condition, the shapes were always arranged such that combining them to form a square only required mentally translating them along the horizontal axis. During training, this was described to the participants as, “You can slide these together to form a solid black square,” spoken with an illustrative bimanual gesture indicating the horizontal movement of the shapes towards each other. In the Rotation condition, the left shape retained its position, but the right one was rotated 90 degrees counterclockwise. This was described to the participants as, “This time you have to first rotate this one and then see if you can slide them together,” again spoken with an illustrative bimanual gesture.
In the Luminance Control conditions, we presented the same shapes as in the Translation and Rotation conditions, but superimposed a small gray square on each shape. There were only two shades of gray (RBG 100, 100, 100 and RGB 60, 60, 60), both darker than the screen background. During training, participants were familiarized with what “same” and “different” shades of gray looked like. The gray square could appear inside any of the three solid black squares making up the outer edges of the to-be-assembled squares. We chose superimposing small squares, rather than altering the luminance of the shapes themselves, because pilot work indicated that if the two shapes themselves were of different luminance, the task could be accomplished “in one glimpse,” resulting in near-perfect accuracy and reaction times that were much faster than the Translation condition. The presence (or absence) of superimposed gray squares also served as a trial-by-trial reminder of the task, thus preventing participants from accidentally performing the Spatial Task during Luminance Control blocks (and vice versa). Participants were explicitly instructed to ignore the underlying puzzle pieces and focus exclusively on the gray squares during the Luminance Control blocks.
2.3. Imaging procedures
Neuroimaging was performed on a research-dedicated Siemens Trio Tim 3-Tesla magnetic resonance imaging scanner with a 12-channel birdcage headcoil. Participants lay in supine position with their heads at the center of the magnet. They wore headphones mounted in Bilsom ear defenders, which allowed them to hear instructions from the control room while being shielded from the scanner noise. Visual stimuli were projected onto a screen at the back of the scanner via an Epson PowerLite 5000 projector and viewed by the participants through a slanted mirror mounted on the head coil. Head position inside the head coil was stabilized by inserting foam pillows between the head coil and the ear defenders. Responses were recorded with two Cedrus fiber optic button response boxes, velcroed together so that participants could hold them in their right hand and operate both buttons with their thumb. Stimulus presentation and logging of behavioral responses was handled by E-Prime 2.0.
Scanning sessions began with a 1-minute “Localizer” scan to acquire a low-resolution anatomical scan to aid volume placement for the subsequent scans. Participants then performed two 5-minute functional runs to assess blood-oxygen-level-dependent (BOLD) signal changes associated with the experimental and control conditions (see Figure 1B). Lastly, we acquired a high-resolution anatomical scan (MPRAGE) on which to superimpose the functional data and to aid transformation of individual data into a standard stereotactic coordinate system. Scanning parameters were as follows: Functional runs (T2*-weighted): Gradient echo-planar imaging (EPI), 50 horizontal slices acquired in descending order, voxel size 3×3×2.8 mm3 with a distance factor of 7% between slices, repetition time (TR) of 3 seconds, echo time (TE) of 30 milliseconds, flip angle of 90 degrees, matrix 64 × 64, duration 5 minutes (100 volume acquisitions).
MPRAGE (T1-weighted): 176 sagittal slices, voxel size 1×1×1 mm3, TR of 2530 ms, TE of 3.5 ms, inversion time (TI) of 1100 ms, flip angle of 7 degrees, matrix 256 × 256, duration 8 minutes.
2.4. Imaging Analysis
2.4.1. Preprocessing
Imaging analyses were performed with BrainVoyager 20.2 for Mac. Anatomical data underwent inhomogeneity correction, brain extraction, and transformation into Talairach space using 9-parameter affine transformation. Manual corrections were performed where automated brain extraction failed, and landmarks for the Talairach transformation were identified manually. Functional imaging data from both runs underwent the following pre-processing steps: removal of the first two volume acquisitions to allow for T1 saturation, slice-scan time correction, removal of linear trends, 3D motion correction to the first volume of the run using rigid-body transformation, co-registration to the anatomical data using 9-parameter gradient-based alignment, transformation into Talairach space using the same transformation applied to the anatomical data, and spatial smoothing with an 8 mm FWHM Gaussian kernel.
2.4.2. Statistical analysis
For statistical data analysis, voxel time courses from both runs were combined and fitted with a general linear model. The model contained four stimulation-related predictors: one for the Instruction periods, one for Translation blocks, one for Rotation blocks, and one for Luminance blocks. (Note that while there were two types of Luminance blocks − those in which stimuli were oriented as in the Translation condition and those in which they were oriented as in the Rotation condition − preliminary analyses showed no differences between these conditions with respect to behavioral performance or brain activations, indicating that participants indeed followed instructions to ignore the puzzle pieces and did not perform mental rotation during the Luminance Control condition. We thus combined these conditions for the purpose of analyses to decrease the number of statistical comparisons.) Rest periods served as the model’s baseline. Each predictor’s time course was convolved with a standard hemodynamic response function (two gamma HRF, time to peak 5 seconds, time to undershoot peak 15 seconds). The model also included the z-transformed motion estimates and a constant predictor for each functional run as nuisance regressors.5 Analyses were constrained to voxels inside the brain (mask based on anatomical image), and voxel time courses were normalized (percent signal change transformation) and corrected for serial autocorrelations (second-order model). For group-level analyses, all participants’ beta maps were combined into a random effects (RFX) analysis treating each participant’s results as a random sample from a larger population. For inter-subject overlap maps, we computed, for each voxel, the percentage of participants whose individual t-maps showed significant activations for the contrast of interest.
Activation maps were thresholded using a single-voxel threshold of p < 0.001 (which was stricter than the single-voxel threshold required to keep the false discovery rate below 5%) in combination with a cluster-size threshold of k < 0.05, which is only passed by clusters whose size is unlikely to occur by chance in a dataset of similar extent and smoothness (as determined by a Monte-Carlo simulation with 1000 iterations, implemented in the “Cluster-Level Statistical Threshold Estimator” plugin for BrainVoyager).
To further probe the response properties of areas identified in the whole-brain analyses, we defined functional regions of interest (ROIs) from the group-level activation maps and extracted the average percent signal change across all voxels in the ROI, separately for each participant and condition. Using the resulting values, we computed paired Student’s t-tests to compare conditions, and Pearson product-moment correlation coefficients to assess potential links between activations and task performance.
2.4.3. Laterality Indices
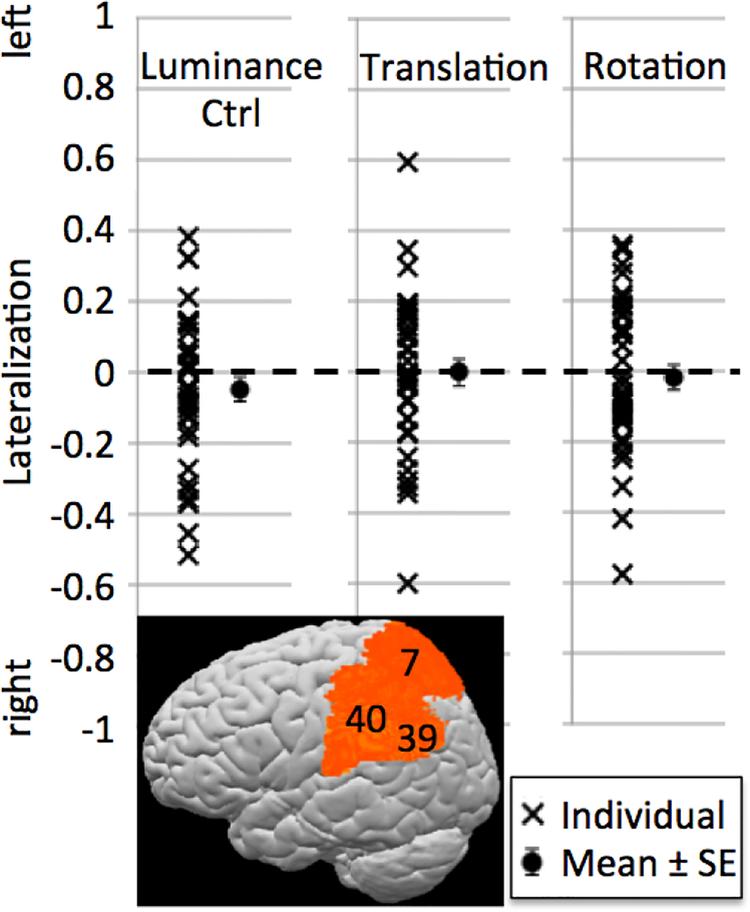
To quantify to what extent brain activation was lateralized, we employed a measure commonly used in the neuroimaging literature on language: the lateralization index (LI). While there are different ways of computing LIs, the basic idea is always the same: Quantify activation on the left and on the right, then compute the (left−right)/(left+right) ratio such that an LI of −1 indicates complete right-lateralization, an LI of 1 indicates complete left-lateralization, and an LI of 0 indicates perfect bilaterality. Because LIs are strongly dependent on the specific threshold at which activation is quantified (Wilke & Lidzba, 2007), we used a bootstrapping approach to compute a weighted mean of LIs obtained at different thresholds (Wilke & Schmithorst, 2006). Specifically, we applied 25 different t-thresholds to our activation maps, spanning the range from 0.1 to the maximum t-value in the map in equal steps. The single-voxel threshold was always combined with a cluster size threshold of 5 voxels, which, in combination with the spatial smoothing applied to the data, should prevent single-voxel activation outliers from distorting the results. LI computation was aborted for thresholds at which there were fewer than 10 active voxels on either side; otherwise, 10,000 LI estimates were computed from the sum of t-values of randomly drawn active voxels on each side (sampling ratio: 0.25), and a trimmed mean (using only the central 50% of estimates) of these 10,000 estimates was used as the robust LI estimate for this threshold. Following LI computation for each threshold, each LI was multiplied with its t-threshold value, such that LIs for stricter thresholds received higher weights. Finally, the sum of all weighted LIs was divided by the sum of all t-thresholds. Since spatial functions are usually attributed to posterior parietal cortex, LI computation was constrained to an anatomically defined bilateral posterior parietal ROI comprising BAs 7, 40, and 39. LI computation and mask generation were accomplished with an in-house script using Matlab R2015b and BVQXtools v0.8d (downloaded from the BrainVoyager support site).
3. Results
3.1. Behavioral
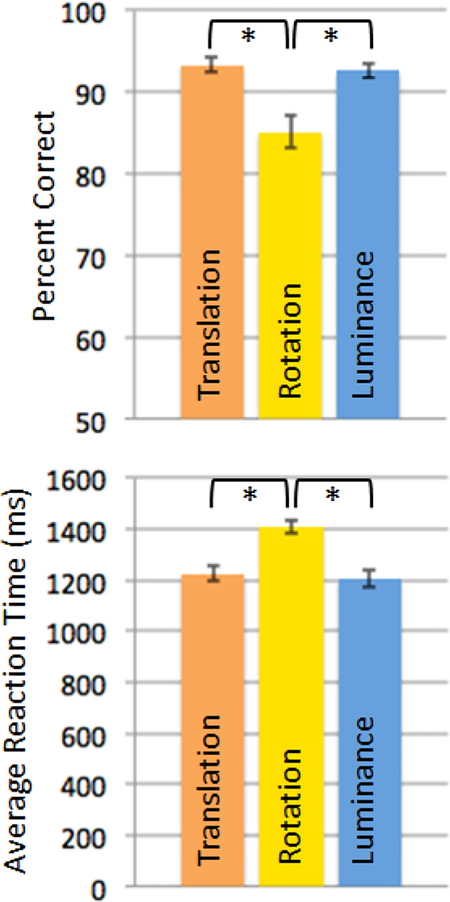
Not surprisingly, given its additional demands on mental rotation, the Rotation condition was harder than the Translation condition, showing both lower accuracy (% correct, Rotation condition: mean 85.09, SE 1.99; Translation condition: mean 93.37, SE 0.90, t(34) = 4.52, p < 0.001) and slower reaction times (RT, Rotation condition: mean 1408 ms, SE 24 ms; Translation condition: mean 1225 ms, SE 28 ms, t(34) = 10.52, p < 0.001; see Figure 2). Reaction times and accuracy data in the Luminance condition were comparable to those in the Translation condition (both t < 0.73, both p > 0.47). Like the Translation condition, the Luminance condition was easier than the Rotation condition in both accuracy (% correct, Rotation condition: mean 85.09, SE 1.99; Luminance condition: mean 92.65, SE 0.89, t(34) = 3.44, p < 0.001) and reaction times (RT, Rotation condition: mean 1408 ms, SE 24 ms; Luminance condition: mean 1205 ms, SE 32 ms, t(34) = 6.7, p < 0.001). Because the task was self-paced, the number of completed trials also differed between conditions (N trials, Translation condition: mean 57, SE 1.04; Rotation condition: mean 51, SE 0.68; Luminance condition: mean 58, SE 1.10). However, within each condition, neither the number of trials completed nor average reaction time was significantly correlated with accuracy (Rotation condition: r = 0.21 for number of trials, r = −0.17 for RT; Translation condition: r = 0.11 for number of trials, r = −0.14 for RT; Luminance condition: r = 0.16 for number of trials, r = −0.20 for RT; minimum correlation required for significance at p < 0.05 = |r| > 0.334), indicating that participants who worked faster were not necessarily better at the task. Importantly, if the number of trials completed per condition had any effect on the fMRI results, the smaller number of trials completed in the Rotation condition should lead to lower activations in that condition compared to the others. Thus, we can be confident that any excess activations observed in the Rotation condition compared to the others (as reported below) are not driven by the number of trials completed.
Figure 2: Behavioral results.
Both accuracy and reaction times indicate that the Translation and Luminance condition were equally hard, and significantly easier than the Rotation condition
3.2. Imaging
3.2.1. Translation and Rotation vs. Luminance Control
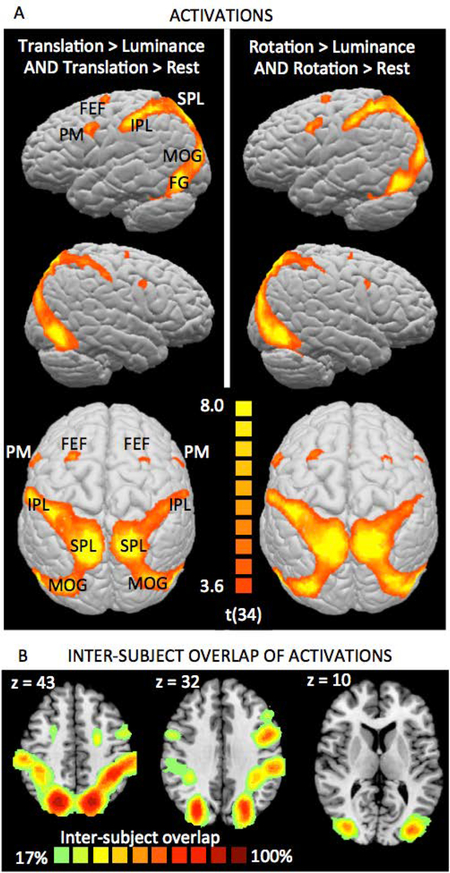
As predicted, both the Translation and the Rotation condition showed stronger activation than the Luminance Control condition in an extensive network of brain areas (Table 1, Fig. 3A), with activation peaks in superior and inferior parietal cortex (BAs 7 and 40), lateral occipital (BA 19) and inferior temporal cortex (BA 37), and premotor cortex (BA 6). All of these areas have previously been reported as showing activation in mental rotation tasks (see, for example, the meta-analyses by Zacks et al., 2008, and Tomasino & Gremese, 2016).
Table 1.
Activation clusters for Translation > Luminance and Rotation > Luminance
| Peak Tal coords, T>L | Peak Tal coords, R>L | Peak t, T>L | Peak t, R>L | side | Location description | BA |
|---|---|---|---|---|---|---|
| −21, −70, 43 | −21, −70, 43 | 13.2 | 13.2 | left | superior parietal lobe (SPL) | 7 |
| 24, −67, 40 | 12, −67, 49 | 13.5 | 11.7 | right | superior parietal lobe (SPL) | 7 |
| −39, −43, 40 | −36, −43, 43 | 12.5 | 12.1 | left | postcentral sulcus (IPL) | 7, 40 |
| 36, −40, 40 | 36, −40, 40 | 9.0 | 8.9 | right | postcentral sulcus (IPL) | 7, 40 |
| −33, −82, 7 | −33, −82, 4 | 9.5 | 9.9 | left | middle occipital gyrus (MOG) | 19 |
| 30, −82, 13 | 30, −82, 13 | 11.9 | 9.1 | right | middle occipital gyrus (MOG) | 19 |
| −45, −64, −11 | −45, −67, −8 | 12.8 | 12.1 | left | fusiform gyrus (FG) | 37 |
| 45, −58, −11 | 42, −61, −8 | 11.8 | 12.1 | right | fusiform gyrus (FG) | 37 |
| −21, −10, 49 | −21, −10, 49 | 9.9 | 9.5 | left | precentral sulcus (FEF) | 6 |
| 24, −10, 49 | 24, −10, 52 | 10.0 | 8.3 | right | precentral sulcus (FEF) | 6 |
| −45, −2, 31 | −45, −1, 31 | 9.9 | 10.1 | left | precentral sulcus (PM) | 6 |
| 48, 5, 31 | 45, 2, 28 | 5.8 | 6.0 | right | precentral sulcus (PM) | 6 |
| −3, 8, 49 | −2, 8, 52 | 6.1 | 3.4 (n.s.) | midline | medial frontal gyrus(pre-SMA) | 6 |
T>L, R>L, R>T - fMRI contrasts for the conjunctions of Translation > Luminance and Translation > Rest, and Rotation > Luminance and Rotation > Rest, respectively. BA - Brodmann area; IPL - inferior parietal lobule; FG - fusiform gyrus; FEF - frontal eye field; PM - premotor area; pre-SMA - pre-supplementary motor area.
Figure 3: Spatial Task activations.
(A) Areas displaying significantly stronger activations during the Spatial Task conditions (left: Translation condition, right: Rotation condition) than during the Luminance Control condition while also showing significant activation compared to Rest in the respective Spatial Task condition. Strong bilateral activations were observed in the superior parietal lobe (SPL) and along the postcentral sulcus (BAs 7 and 40, IPL), on the middle occipital gyrus (MOG, BA 19) and the fusiform gyrus (FG, BA 37), and in premotor cortex (PM, BA 6). For activation peaks, see Table 1. (B) Inter-subject overlap maps, illustrating that activation locations were highly consistent across participants. Activation maps are overlayed on the Colin27 brain template transformed into Talairach space, and thresholded at a p<0.001 single-voxel threshold combined with a k<0.05 cluster-size threshold.
Activations (Fig. 3 A) were strongly bilateral and in virtually identical locations for the Rotation and Translation conditions, indicating that this bilateral pattern is a signature of the mental visual-spatial transformations required by the construction tasks, regardless of whether they involve mental rotation. Similar activation patterns were observed in all participants, and there was high inter-subject agreement regarding activation locations (Fig. 3 C, supplementary Figure S1).
The only obvious difference was the presence of significant activation in the pre-supplementary motor area (pre-SMA, not visible in Figure 3A due to its location on the medial surface between the hemispheres) for Rotation > Luminance Control, but not for Translation > Luminance Control. Compared to Rest, all conditions showed activation in this area, although it was of smaller magnitude than activations in parietal and occipital cortex. Because activation was comparable in the Translation and Luminance Control conditions, it cannot reflect anything specific to visual-spatial construction. Instead, it is likely linked to task difficulty and/or effort, since it was strongest in the (most difficult) Rotation condition. This interpretation is in line with the literature, which counts the pre-SMA among a set of brain areas referred to as “Task-Positive Network” (TPN, Fox et al., 2005), “Multiple Demand Network” (Fedorenko et al., 2013), or “Effort (or Extrinsic) Mode Network” (Hugdahl et al., 2015), whose activation increases with the demands posed by an externally focused task, regardless of that task’s specific nature.
3.2.2. Rotation vs. Translation
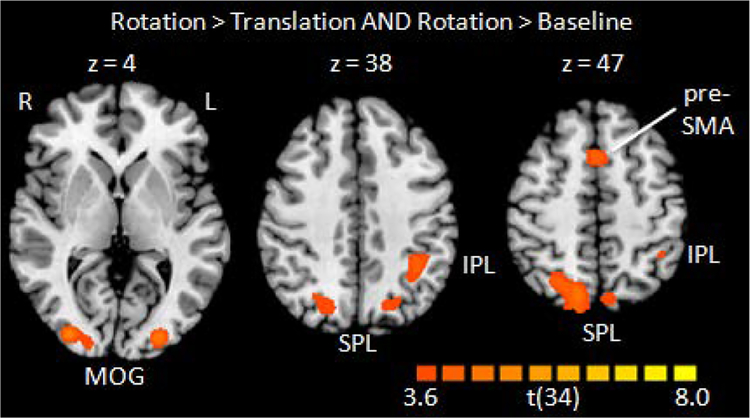
Comparing the Rotation and the Translation conditions revealed stronger activations for the Rotation condition in superior parietal cortex bilaterally (BA 7, with higher peak value and larger cluster extent on the right), left inferior parietal cortex (BAs 7 and 40), bilateral lateral occipital cortex (BA19), and the pre-SMA (Fig. 4, Table 2), thus displaying the bilateral occipitoparietal activation pattern suggested by the literature on mental rotation. The excellent correspondence between these areas and those found activated by the Rotation and Translation compared to Luminance Control condition highlights the similarity between the Rotation and Translation conditions and indicates that mental rotation is not special in the sense that it activates unique brain areas. Instead, it is one of many operations of visual-spatial processing and draws upon the same general brain areas as other aspects of visual-spatial construction. Of course it is likely that the computations underlying mental rotation rely wholly or partly on separate ensembles of neurons, but at the spatially coarse level of fMRI investigations, mental rotation appears to be “more of the same” with respect to visual-spatial construction.
Figure 4: Activations when contrasting the Rotation condition with the Translation condition.
Note that the views were chosen to best show the activations on as few slices as possible. Peak locations are listed in Table 2. Thresholds as in Fig. 3.
Table 2.
Activation peaks for Rotation > Translation
| Peak Talairach coordinates | Peak t | Cluster extent (mm3) | side | Location description | BA |
|---|---|---|---|---|---|
| −6, −73, 46 | 4.2 | 379 | left | superior parietal lobe (SPL) | 7 |
| 27, −64, 43 | 5.4 | 4238 | right | superior parietal lobe (SPL) | 7 |
| −39, −46, 43 | 4.8 | 1626 | left | postcentral sulcus (IPL) | 40 |
| −21, −67, 31 | 5.1 | 1632 | left | postcentral sulcus (IPL) | 7 |
| −18,−88,−5 | 6.0 | 5400 | left | middle occipital gyrus (MOG) | 19 |
| 30, −85, 7 | 5.7 | 4583 | right | middle occipital gyrus (MOG) | 19 |
| 0, 11, 46 | 4.6 | 862 | midline | medial frontal gyrus(pre-SMA) | 6 |
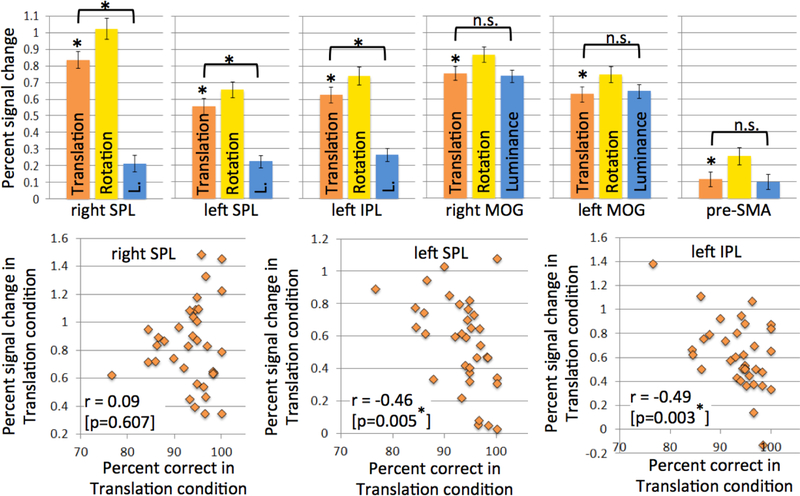
To explore whether these activation differences might be associated with sheer difficulty or effort, rather than specific to visual-spatial construction, we performed post-hoc analyses on functional ROIs created from the group-level activations. Note that because they were defined by their stronger activation in the Rotation than in the Translation condition, these ROIs are bound to show significant activation in the Rotation condition. If ROI activation is associated with visual-spatial processing, it should also be significant in the Translation condition, which also makes spatial demands. As can be seen in Figure 5 (orange bars), this was the case for all ROIs. More importantly, if ROI activation is to some extent space-specific, it should be significantly larger in the Translation condition than in the Luminance Control condition, which is equal in difficulty (judging from accuracy and reaction times) but does not make spatial demands. In contrast, equal activation in the Translation and Luminance Control conditions would be indicative of a general difficulty effect.
Figure 5: ROI analyses probing the response profile of areas displaying significantly stronger activation during the Rotation than the Translation condition (cf Figure 4).
If the ROI’s activation reflects visual-spatial construction (as opposed to general task) demands, it should show significant activation during the Translation condition (which requires visual-spatial construction) and significantly less activation during the Luminance Control condition. This is the case for the three parietal ROIs (top left), but not for the occipital and the frontal ROIs (top right), all of which activate similarly for the Translation and the Luminance Control condition. Of the parietal ROIs, the left ones additionally showed significant negative correlations between activation and performance in the Translation condition (bottom row), but not in the Luminance Control condition, which further confirms their role in visual-spatial construction. All correlation values can be found in supplementary Table S1.
As can be seen in Figure 5 (top), all parietal ROIs showed significant Translation>Luminance activation differences, which demonstrates their specific role in visual-spatial construction. In addition, the left parietal ROIs also showed strong negative correlations between activation and performance in the Translation condition (Figure 5, bottom; supplementary Table S1); participants whose error rates suggest that they found the task more difficult also showed the strongest parietal activations. No significant performance correlations were observed for the Luminance condition, and correlations in the Translation condition significantly exceeded those in the Luminance condition (see supplementary Table S1, last row, for results of a Fisher r-to-z transformation and the associated p-values), suggesting a space-specific effect. Note that while no space-specific difficulty-related activation increases were observed for the Translation condition in right SPL, this should not be taken as evidence that right SPL activation is not space-specific. Right SPL passed the crucial test for space-specific activation, i.e., that activation in the Translation condition be significantly larger than in the equally difficult Luminance condition. The absence of a significant negative correlation between performance and activation in the Translation condition and the stronger activation of right SPL compared to left SPL and IPL could suggest that while right SPL is always involved in spatial tasks (and more so if they require mental rotation), the involvement of left SPL is modulated at least partly by the difficulty of the spatial task (even if it does not require mental rotation). However, this interpretation requires independent confirmation in an experiment that explicitly manipulates difficulty in a within-subject design.
In contrast, the occipital ROIs and the pre-SMA were equally activated in the Translation and the Luminance Control condition, thus violating the crucial requirement for space-specific activation, and showed similar correlations between performance and activation in the Translation and the Luminance Control condition that did not differ significantly (see supplementary Table S1). Taken together, these findings suggest that occipital and pre-SMA activations were not specific to visual-spatial construction demands, but more generally to engagement in a visual task and modulated by task difficulty.
3.2.3. Laterality Indices
While the activations shown in Figure 2A provide strong qualitative evidence that visual-spatial construction tasks are associated with bilateral, not right-lateralized activations, we also addressed this question quantitatively by computing laterality indices (LIs) for individual participants. As described in the methods section, LIs range from −1 (maximally right-lateralized) to 1 (maximally left-lateralized), with values around 0 indicating bilaterally symmetric activation.
Figure 6 illustrates the LIs for the different conditions. While there was considerable variation across subjects, LIs generally clustered around 0 and did not differ significantly from 0 at the group level, indicating bilateral rather than lateralized activation for all conditions. There were no LI differences between the conditions (paired t-tests, all p > 0.1). We also computed Pearson correlations between performance and LI and subjected them to non-directional significance tests because we had no a-priori expectations about how lateralization might change as a function of performance. There were no significant correlations between accuracy or reaction times and LIs in the Luminance Control condition (r = 0.07 [p = 0.689] for % correct and r = 0.09 [p = 0.607] for RT). For the two Spatial Task conditions, correlations for accuracy and RTs trended in opposite directions, as would be expected since increasing difficulty should be reflected in lower accuracy and longer RTs (Translation condition: r = −0.33 [p = 0.053] for accuracy, r = 0.20 [p = 0.249] for RT; Rotation condition: r = −0.18 [p = 0.301] for accuracy, r = 0.25 [p = 0.147] for RT). While most of these correlations did not reach significance, the tendency for high-performing participants to show more right-lateralized LIs is consistent with the observation of negative correlations between left-parietal activations and accuracy during the ROI analyses (Fig. 5). They could cautiously be interpreted as suggesting that the left parietal lobe becomes increasingly involved if the task is difficult for the particular participant. Such increases in bilateral activation with increased task difficulty have been observed across task domains (e.g., Just et al., 1996, for language; Klingenberg et al., 1997, for working memory; Helton et al., 2010, for vigilance).
Figure 6: Lateralization indices.
Lateralization was computed for a bilateral posterior parietal ROI comprising BAs 7, 40, and 39. While there was considerable variation across individuals, group means (shown with standard error bars) did not differ from zero, indicating bilaterality. There were no significant LI differences between conditions.
4. Conclusions and Discussion
This study was designed to investigate the neural basis of a complex visual-spatial task: in particular, a visual-spatial construction task similar to those commonly used to assess spatial abilities on the behavioral level. Based predominantly on the observation that impairments in the visual-spatial domain, such as hemispatial neglect, occur more frequently after lesions to the right than the left hemisphere (Bisiach & Luzzatti, 1978; Vallar, 1998), visual-spatial skills have often been ascribed to the right hemisphere, and the parietal lobe in particular (Gazzaniga & Ladavas, 1987). However, a close look at the literature reveals several challenges to this view. For example, spatial impairments are also observed in adults with left-hemisphere lesions (McFie et al., 1960; Arrigoni & De Renzi, 1964; Gainotti et al., 1977), and functional neuroimaging studies of mental rotation, a core visual-spatial function, often reveal bilateral activations (Zacks, 2008; Tomasino & Gremese, 2016). Moreover, studies that use spatial “localizer” tasks such as distance judgments to identify spatial processing regions of the dorsal stream have identified bilateral parietal ROIs (e.g., Haxby et al., 1991; Zachariou et al., 2015; 2016). Based on this, we asked whether complex visual-spatial construction tasks would evoke bilateral activations or clear lateralization favoring right parietal cortex. We further asked whether the presence or absence of mental rotation demands would alter lateralization.
To answer these questions, we conducted an fMRI study in which neurologically healthy young adults were exposed to two Construction conditions (one requiring mental Translation only, and one requiring mental Rotation and Translation) and a Luminance Control condition that was visually similar and of similar difficulty to the Translation condition. By contrasting brain activations in these different conditions, we showed that both Construction conditions were associated with bilateral activation increases in the same parietal and lateral occipital brain areas. This pattern was evident not only at the group level, but also in individual subjects, with lateralization indices clustering around zero. Compared to the Translation condition, the Rotation condition evoked stronger activations in superior parietal cortex bilaterally and in left inferior parietal cortex, within the same areas activated when contrasting either condition to the Luminance Control condition. Left parietal activations tended to increase with increasing task difficulty (as reflected in lower accuracy and slower reaction times) specifically in the Spatial Task conditions, but not the Luminance Control condition, which further confirmed the functional role of left parietal cortex in this complex visual-spatial construction task.
Taken together, our findings suggest bilateral parietal involvement in complex visual-spatial tasks requiring the mental manipulation of objects in space, regardless of whether these manipulations include rotation. Below, we put these findings in perspective, discussing how they intersect with previous views of spatial lateralization of function as well as the empirical findings that have been offered as support. We argue that our findings of bilateral involvement in the spatial construction task are consistent with many previous findings but, importantly, support some re-interpretation of previous strongly-held views about the lateralization of function for space (compared to language) more generally.
4.1. Lesion studies: More evidence for bilaterality
If complex visual-spatial skills require two intact hemispheres, one would expect to see impairments of visual-spatial skills after lesions of either hemisphere in adults. At first glance this appears to be at odds with clinical findings that right parietal lesions more frequently produce marked and lasting spatial impairments (Brain, 1941; McFie et al., 1950; Hecaen et al., 1956; Bisiach & Luzzatti, 1978; Ratcliff, 1979; Vallar, 1998). However, a careful examination of the lesion literature presents a more complex picture, consistent with our present findings. While lasting disorders of spatial attention, such as hemineglect, are indeed more common with right-sided lesions (Vallar, 1993), left-sided lesions can also impair aspects of spatial cognition, especially visual-spatial construction abilities (McFie et al., 1960; Arrigoni & De Renzi, 1964; Gainotti et al., 1977). According to McFie et al. (1950), it was once “the generally accepted view that spatial disorientation and kindred symptoms are to be ascribed to a lesion of the dominant [i.e., left] hemisphere” (p. 169, explanation in brackets added) and “Impaired performance on visual-constructive tasks is extremely common in cases with occipital, occipito-parietal, or parieto-temporal lesions of the left cerebral hemisphere; indeed the most striking instances of constructional apraxia have been reported in cases of left-sided cerebral involvement” (p. 188). Moreover, dissociations have been shown for the same visual-spatial task depending on stimulus type and strategy: Whereas patients with right-sided lesions could mentally rotate hands but not non-bodily stimuli, the opposite was true for patients with left-sided lesions (Tomasino et al., 2003). Similarly, patients with left-sided lesions showed impairments when instructed to imagine rotating the stimulus, but not when instructed to imagine the stimulus rotating by itself (Tomasino & Rumiati, 2004).
We suspect that the emphasis on the right hemisphere’s role in spatial cognition in the more recent literature is due to the fact that right-hemisphere lesions produce very obvious and striking impairments such as hemispatial neglect, which are related to the spatial domain but are also heavily loaded on attention, whereas the impairments from left-hemisphere lesions, such as constructional apraxia, are less evident in everyday life and only revealed by tasks that make very specific spatial demands, such as mental rotation or construction. Moreover, because left-hemisphere lesions often result in language impairments, patients may be unable to follow the instructions for tasks that would reveal more subtle spatial deficits.
4.2. Functional neuroimaging
To investigate lateralization of spatial function, the neuroimaging literature to a large extent relies on variations of the so-called “landmark task” (Fink, 2000), which is modeled after the line bisection task that clinicians use to diagnose hemispatial neglect (see Introduction). Given that hemispatial neglect is both more common and more persistent after lesions to the right than to the left hemisphere, it is not surprising that this task yields right-lateralized (although not exclusively right) activations at group level as well as in most individual participants (Fink, 2000, 2001; Çiçek et al., 2009; Cavézian et al., 2012). However, it is important to note that hemispatial neglect can also be viewed as a disorder of attention rather than one of spatial representation (Bartolomeo, 2014). According to this view, patients’ biases on the line bisection task result from an inability to direct attention to the contralesional side of the line, which causes them not to perceive the leftmost part of the line. Support for this view comes from a phenomenon described as “extinction,” where neglect of stimuli on the contralesional side is only evident in the presence of a competing stimulus on the ipsilesional side. This has been interpreted as demonstrating a problem with disengaging attention from the ipsilesional side and directing it to contralesional space, rather than with accurate spatial representation of contralesional space per se. Additional evidence for the attentional nature of hemispatial neglect comes from the observation that it is often accompanied by non-spatial attention deficits, such as disruptions of the temporal dynamics of visual processing (Husain et al., 1997) and a general difficulty with sustaining attention over longer periods of time, even in the auditory domain (Robertson et al., 1997). Since parts of the fronto-parietal attention system are right-lateralized (Corbetta et al., 1993; Corbetta & Shulman, 2002), right-lateralization of activation in the landmark task may thus be attributable to right-lateralization of spatial attention in particular, rather than right-lateralization of visual-spatial functions in general.
Another commonly imaged spatial task is mental rotation. As already touched upon in the Introduction, parietal brain activations reported for this task are bilateral in the majority of papers (Cohen et al., 1996; Tagaris et al., 1997; Richter et al., 1997, 2000; Jordan et al., 2001; Vingerhoets et al., 2002; Vanrie et al., 2002; Lamm et al., 2007), bilateral but stronger on the right in some (Harris et al., 2000; Podzebenko et al., 2002), and exclusively right (Halari et al., 2006) or exclusively left (Alivisatos & Petrides, 1997; Vingerhoets et al., 2001) in very few.6 A 2008 meta-analysis concluded that “Activity was observed bilaterally in most areas; however, in the parietal cortex, activity was somewhat more consistently observed in the right hemisphere” (Zacks, 2008). Similarly, a 2010 meta-analysis concluded that the mental rotation network included activations in the inferior and superior parietal lobule bilaterally, although laterality was modulated by stimulus type and strategy such that activation was more right-lateralized for non-bodily-related stimuli and non-motor strategies (Tomasino & Gremese, 2016). The fact that when strong lateralization is observed, it is usually in studies with small numbers of participants and those that split participants into sub-groups based on sex, in combination with the inconsistent results, may indicate that the observed lateralization effects are spurious or not very robust, with the more robust finding being that of bilaterality and potentially a slight right-bias.
All in all, our impression is that while our results are at odds with a generally held notion of the right hemisphere as the spatial one, they are perfectly consistent with the existing literature reviewed above. This conclusion suggests that many complex spatial tasks may engage both hemispheres to a similar degree. This may follow from the many requirements of such tasks. For example, in our puzzle task, people must represent the individual parts accurately, carry out a mental operation on these parts, and make a judgment of whether the two parts can compose a square after these operations. Other complex spatial cognitive tasks might similarly draw on multiple spatial functions (e.g., parsing, object representation, mental operation). If any or all of these functions engage bilateral parietal areas, then the result will be bilateral activation. This proposal is not inconsistent with theoretical formulations that propose specialization of the two hemispheres for different types of spatial processing (e.g., Kosslyn, 1987; Ivry & Robertson, 1998). For one thing, those proposals focus on earlier aspects of visual processing and the consequences of these for later spatial representation. More importantly, however, even the puzzle task requires that people represent the final model in terms of both local and global features − the local individual parts and the global sum of the parts. Even given spatial processing specializations within each hemisphere, the full puzzle task would require the cooperation of both kinds of processing and thus both hemispheres.
4.3. Caveats, Implications, and Future Directions
An important caveat when interpreting the present findings is that activation increases in particular brain regions during an fMRI task are not by themselves indicative that the brain regions are required for performing the task. One might argue that the left parietal activations observed here were functionally redundant and merely collateral to those observed in the right hemisphere. Another possibility is that the left parietal activations reflected participants’ attempts to use linguistic encoding for more difficult parts of the task. Although we doubt that even complex linguistic encodings would have beneficial effects (and the absence of activations in classic language areas, such as left inferior frontal cortex, speaks against complex inner monologue), it is possible that simple encodings such as “rotate”, “slide”, or “three peaks” could have been deployed while carrying out the construction tasks, especially if participants were having difficulty. The observed correlations between left parietal activations and performance during the construction task speak against the idea that these activations were merely collateral; however, definitive conclusions require studies that systematically manipulate the engagement of the left parietal lobe. For example, one could introduce specific variations in stimuli and instructions to see whether this results in changed activation and/or performance (see Tomasino & Gremese, 2016). Another approach is to experimentally disrupt the left parietal lobe, for example by means of transcranial magnetic stimulation (TMS), in order to see whether this leads to declines in performance, suggesting a critical role of this region. While we know of no such study focused on visual-spatial construction, there are studies investigating the effects of parietal rTMS on mental rotation performance. One showed a slowing of reaction times only with disruptive stimulation of the right parietal lobe (Harris & Miniussi, 2003), whereas another (Bestmann et al., 2002) demonstrated slowing of reaction times for visuomotor mental rotation for rTMS of the left and right parietal lobe equally. More studies are urgently needed, but the existing evidence seems to support the idea of bilateral involvement.
Importantly, we do not mean to suggest that all visual-spatial functions are bilateral. For example, as mentioned above, both clinical assessments and neuroimaging studies implicate the right but not the left hemisphere in line bisection tasks, and other specialized functions such as the perception of faces and biological motion are known to be right-lateralized (faces: Kanwisher et al., 1997; Pitcher et al., 2007; biological motion: Pelphrey et al., 2003; Grosbras et al., 2012). In line with the notion that some, but not all spatial skills require bilateral involvement, a meta-analysis of studies on lateralization of visual-spatial functions (Vogel et al., 2003) concluded that, while across all types of studies and populations the right hemisphere was dominant for spatial processing, findings depended strongly on the specific task, the population studied, and the method of study. In addition, stimulus type and strategy appear to play a significant role even within the same spatial task, with increasing left – and thus more bilateral – activations for bodily stimuli and for strategies that draw upon motor imagery (Tomasino & Gremese, 2016).
Over the past decades, impairments in the spatial domain have been called by different names, classified into different sub-categories, ascribed to perceptual or motor-related difficulties, and attributed to different hemispheres and locations within hemispheres. Clarifying this picture will require additional decades of research. This should include an effort to create theoretically motivated taxonomies of spatial functions that help us understand the complexity embodied in the creation, manipulation, and use of spatial representations. These should be used to categorize the effects of naturally occurring lesions and integrate the results with findings from behavioral and functional neuroimaging studies. Lastly, hypothesized distinctions should receive independent confirmation with experimentally induced lesions (e.g., with transcranial magnetic stimulation). Until then, we would argue that, for understanding the brain basis for spatial functions, it would be helpful to use tasks that are complex enough to engage a number of different spatial functions, of which some may activate only narrow regions of the brain, but others might activate broad networks of brain areas related to spatial processing. We believe that the task introduced here can help to serve that purpose.
Supplementary Material
Highlights.
visual-spatial functions are predominantly attributed to the right parietal lobe
behaviorally, these functions are often assessed using construction tasks
we performed functional MRI during a visual-spatial construction task
fMRI revealed bilateral, not right-lateralized parietal activations
visual-spatial functions should not generally be thought of as right-lateralized
Acknowledgements
We thank Ms. Serena F. Pu for help with data collection and analysis.
This research was supported in part by an NIH CTSA Scholars Award through the Georgetown and Howard Universities Center for Clinical and Translational Science (NCATS KL2 TR001432) to ASG, Georgetown’s “Music for the Mind” Young Investigator Award to ASG, T32 Postdoctoral Research Fellowship through NIH (5T32 HD 046388) to KF, NIH grant K18 DC014558 to ELN, by funds to BL as a George Bergeron Visiting Scholar, by the NIH-funded DC Intellectual and Developmental Disabilities Research Center (U54 HD090257), and by funds from the Center for Brain Plasticity and Recovery at Georgetown University and MedStar National Rehabilitation Hospital.
Footnotes
While some studies indicate that lateralization may differ between sexes and at different points in the menstrual cycle (Gur et al., 2000; Hugdahl et al., 2006; Schöning et al., 2007; Zhu et al., 2015), the results are inconsistent across studies and may be due to small sample sizes. For example, some studies (Thomsen et al., 2000; Levin et al., 2005) found stronger right parietal activations for male than female participants, whereas other studies (Jordan et al., 2002; Clements et al., 2006) reported stronger right parietal activations in women.
Given the inconsistent reports in the literature about sex differences in mental rotation activation, we ensured sample sizes large enough that groups of male and female participants could be analyzed separately. However, initial analyses did not reveal any significant sex differences. We thus report results across all subjects.
We did, however, complete the group analyses with and without the left-handed participants, and despite small quantitative differences (as one would expect by chance), the results remained qualitatively the same. We also compared individual activation maps between left and right-handers and found no obvious differences (supplementary Figure S1).
In practice, variations in block duration were small (mean block duration 24.89 s, SD 0.29 s, min 24.15 s, max 25.74 s), and block durations did not differ significantly between conditions (Rotation: mean 24.95 s, SE 0.57 s; Translation: mean 24.85 s, SE 0.48 s; Luminance: mean 24.88 s, SE 0.31 s; all pairwise t(34) < 1.59, p > 0.12), nor were they correlated with performance in any of the conditions (all |r| < 0.25).
As mentioned above, we allowed participants to work at their own pace to ensure continuous task-related activations in all conditions. As a consequence, reaction times (and the number of trials completed) differed between conditions. To investigate whether this had any bearing on the results, we also ran a GLM including reaction times as a nuisance regressor. The results of this analysis were not significantly different from the ones presented here.
Note that “exclusive” activation on one side simply means that no significant activation increases were observed on the other side at the particular threshold chosen. If effect sizes are overall small, activation might reach significance on only one side purely by chance, so that these papers are better counted among the “bilateral, but stronger on the right/left” categories.
References
- Alivisatos B, Petrides M (1997) Functional activation of the human brain during mental rotation. Neuropsychologia 35:111–118. [DOI] [PubMed] [Google Scholar]
- Arrigoni G, De Renzi E (1964) Constructional apraxia and hemispheric locus of lesion. Cortex 1 (2): 170–197. [Google Scholar]
- Badzakova-Trajkov G, Corballis MC, Häberling IS (2016) Complementarity or independence of hemispheric specializations? A brief review. Neuropsychologia 93:386–393. [DOI] [PubMed] [Google Scholar]
- Ballard DH, Hayhoe MM, Pook PK, Rao RPN (1997) Deictic codes for the embodiment of cognition. Behav Brain Sci 20:723–767. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P Attention disorders after right brain damage - living in halved worlds. London, Springer, 2014. [Google Scholar]
- Basser LS (1962) Hemiplegia of early onset and the faculty of speech with special reference to the effects of hemispherectomy. Brain 85: 427–460. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Thilo KV, Sauner D, Siebner HR, Rothwell JC (2002) Parietal magnetic stimulation delays visuomotor mental rotation at increased processing demands. NeuroImage 17:1512–1520. [DOI] [PubMed] [Google Scholar]
- Binder JA, et al. (1996) Determination of language dominance using functional MRI. Neurology 46:978–984. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Luzzatti C (1978) Unilateral neglect of representational space. Cortex 14:129–133. [DOI] [PubMed] [Google Scholar]
- Bogen JE, Gazzaniga MS (1965) Cerebral commissurotomy in man: Minor hemisphere dominance for certain visuospatial functions. J Neurosurg 23:394–399. [Google Scholar]
- Bookheimer SY, et al. (1997). A direct comparison of PET activation and electrocortical stimulation mapping for language localization. Neurology 48:1056–65. [DOI] [PubMed] [Google Scholar]
- Borst G, Kosslyn SM (2010) Varying the scope of attention alters the encoding of categorical and coordinate spatial relations. Neuropsychologia 48:2769–2772. [DOI] [PubMed] [Google Scholar]
- Brain WR (1941) Visual disorientation with special reference to lesions of the right cerebral hemisphere. Brain 64: 244–272. [PMC free article] [PubMed] [Google Scholar]
- Broca P (1861) Remarks on the seat of the faculty of articulated language, following an observation of aphemia (loss of speech). Bulletin de la Société Anatomique 6:330–357. [Google Scholar]
- Cai Q, Van der Haegen L, Brysbaert M (2013) Complementary hemispheric specialization for language production and visuospatial attention. Proc Natl Acad Sci USA 110:E322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavézian C, Valadao D, Hurwitz M, Saoud M, Danckert J (2012) Finding centre: ocular and fMRI investigations of bisection and landmark task performance. Brain Res 1437:89–103. [DOI] [PubMed] [Google Scholar]
- Çiçek M, Deouell LY, Knight RT (2009) Brain activity during landmark and line bisection tasks. Front Hum Neurosci 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, et al. (1996) Changes in cortical activity during mental rotation: A mapping study using functional magnetic resonance imaging. Brain 119:89–100. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE (1993) PET study of visuospatial attention. J Neurosci 13:1202–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3(3):201–215. [DOI] [PubMed] [Google Scholar]
- Cornish KL, Munir F, Cross G (1998) The nature of the spatial deficit in young females with Fragile-X syndrome: a neuropsychological and molecular persepctive. Neuropsychologia 36:1239–1249. [DOI] [PubMed] [Google Scholar]
- Del Guidice E, Grossi D, Angelini R, Crisanti AF, Latte F, Fragassi NA, Trojano L (2000) Spatial cognition in children. I. Development of drawing-related visuospatial and sontructional abilities in preschool and early school years. Brain Dev 22:362–367. [DOI] [PubMed] [Google Scholar]
- Durnford M, Kimura D (1971) Right hemisphere specialization for depth perception reflected in visual field differences. Nature 231:394–395. [DOI] [PubMed] [Google Scholar]
- Efron R (1990) The decline and fall of hemispheric specialization. Lawrence Erlbaus Associates, Inc, Hillsdale, NJ. [Google Scholar]
- Elliot CD (1990) The nature and structure of children’s abilities: Evidence from the Differential Ability Scales. J Psychoed Assess 8:376–390. [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N (2013) Broad domain generailty in focal regions of frontal and parietal cortex. Proc Natl Acad Sci USA 110:16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro B, Brighina F, Oliveri M, Piazza A, La Bua V, Buffa D, Bisiach E (2000) Contralateral neglect induced by right posterior parietal rTMS in healthy subjects. Neuroreport 11:1519–1521. [PubMed] [Google Scholar]
- Fink GR, et al. (2000) Line bisection judgments implicate right parietal cortex and cerebllum as assessed by fMRI. Neurology 54:1324–1331. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Zilles K (2001) The neural basis of vertical and horizontal line bisection judgments. An fMRI study of normal volunteers. NeuroImage 14:S59–S67. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional netowrks. Proc Natl Acad Sci USA 102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Ferrara K, Newcombe NS (2013) Using a touch screen paradigm to assess the development of mental rotation between 3.5 and 5.5 years of age. Cogn Process 14:117. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, et al. (2007) Atypical language in lesional and nonlesional complex partial epilepsy. Neurology 69:1761–1771. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Miceli G, Caltagirone C (1977) Constructional apraxia in left brain-damaged patients: A planning disorder? Cortex 13 (2):109–118. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS (1995) Principles of human brain organization derived from split-brain studies. Neuron 14:217–228. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Ladavas E (1987) Disturbances in spatial attention following lesion or disconnection of the right parietal lobe. Advances Psychol 45:203–213. [Google Scholar]
- George MS, et al. (1996): Understanding emotional prosody activates right hemisphere regions. Arch Neurol 53:665–670. [DOI] [PubMed] [Google Scholar]
- Groen MA, Whitehouse AJ, Badcock NA, Bishop DV (2012) Does cerebral lateralization develop? A study using functional transcranial Doppler ultrasound assessing lateralization for language production and visuospatial memory. Brain Behav 2:256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras MH, Beaton S, Eickhoff SB (2012) Brain regions involved in human motion perception: A quantitative voxel-based meta-analysis. Hum Brain Mapp 33:431–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC et al. (2000) An fMRI study of sex differences in regional activation to a verbal and a spatial task. Brain Lang 74:157–170. [DOI] [PubMed] [Google Scholar]
- Halari R, Sharma T, Hines M, Andrew C, Simmons A, Kumari V (2006) Comparable fMRI activity with differential behavioural performance on mental rotation and overt verbal fluency tasks in healthy men and women. Exp Brain Res 169:1–14. [DOI] [PubMed] [Google Scholar]
- Hämäläinen H, Takio F (2010) Integrating auditory and visual asymmetry In: Hugdahl K & Westerhausen R (eds) The two halves of the brain. MIT Press, Cambridge, MA. [Google Scholar]
- Harris IM, Egan GF, Sonkkila C, Tochon-Danguy HJ, Paxinos G, Watson JD (2000) Selective right parietal lobe activation during mental rotation: a parametric PET study. Brain 123:65–73. [DOI] [PubMed] [Google Scholar]
- Harris IM, Miniussi C (2003) Parietal lobe contribution to mental rotation demonstrated with rTMS. J Cogn Neurosci 15:315–323. [DOI] [PubMed] [Google Scholar]
- Haxby JV et al. (1991) Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci USA 88:1621–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecaen H, Penfield W, Bertrand C, Malmo R (1956) The syndrome of apractognosia due to lesions of the minor cerebral hemisphere. [PubMed] [Google Scholar]
- Hegarty M, Waller D (2005) Individual differences in spatial abilities In: Shah P & Miyake A (eds): The Cambridge handbook of visuospatial thinking. Cmbridge University Press, NY. [Google Scholar]
- Helton WE, Warm JS, Tripp LD, Matthews G, Parasuraman R, Hancock PA (2010) Cerebral lateralization of vigilance: A function of task difficulty. Neuropsychologia 48:1683–1688. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Landau B, Pagani B (2003) Spatial breakdown in spatial construction: Evidence from eye fixations in children with Williams syndrome. Cogn Psychol 46:260–301. [DOI] [PubMed] [Google Scholar]
- Holmes G (1918) Disturbances of vision by cerebral lesions. Brit J Ophthal 2:353–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K, Thomsen T, Ersland L (2006) Sex differences in visuo-spatial processing: An fMRI study of mental rotation. Neuropsychologia 44:1575–1583. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Raichle ME, Mitra A, Specht K (2015) On the existence of a generalized non-specific task-dependent network. Front Hum Neurosci 9:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K, Westerhausen R (2010) The two halves of the brain. MIT Press, Cambridge, MA. [Google Scholar]
- Husain M, Shapiro K, Martin J, Kennard C (1997) Abnormal temporal dynamics of visual attention in spatial neglect patients. Nature 385:154–156. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Robertson LC (1998) The two sides of perception. MIT Press, Cambridge, MA. [Google Scholar]
- Jewell G, McCourt ME (2000) Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 38(1):93–110. [DOI] [PubMed] [Google Scholar]
- Jordan K, Heinze HJ, Lutz K, Kanowski M, Jäncke L (2001) Cortical activations during the mental rotation of different visual objects. NeuroImage 13:143–152. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR (1996) Brain activation modulated by sentence comprehension. Science 274:114–116. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM (1997) The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17:4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D (1969) Spatial localization in the left and right visual fields. Canad J Psych 23(6):445–458. [DOI] [PubMed] [Google Scholar]
- Klingenberg T, O’Sullivan BT, Roland PE (1997) Bilateral activation of fronto-parietal networks by incrementing demand in a working memory task. Cereb Cortex 7:465–471. [DOI] [PubMed] [Google Scholar]
- Kohs SC (1920) The Block-Design Test. J Exp Psychol 3:357–376. [Google Scholar]
- Kosslyn SM (1987) Seeing and imagining in the cerebral hemispheres: A computational approach. Psych Rev 94:148–175. [PubMed] [Google Scholar]
- Kosslyn SM, et al. (1989) Evidence for two types of spatial representations: Hemispheric specialization for categorical and coordinate relations. J Exp Psychol 15:723–735. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, et al. (1998) Neural systems that encode categorical versus coordinate spatial relations: PET investigations. Psychobiology 26:333–347. [Google Scholar]
- Kosslyn SM, Margolis JA, Barrett AM, Goldknopf AJ, Daly PF (1990) Age differences in imagery ability. Child Dev 61:995–110. [PubMed] [Google Scholar]
- Laeng B (1994) Lateralization of categorical and coordinate spatial functions: A study of unilateral stroke patients. J Cogn Neurosci 6:189–203. [DOI] [PubMed] [Google Scholar]
- Lamm C, Windischberger C, Moser E, Bauer H (2007) The functional role of dorso-lateral premotor cortex during mental rotation: An event-related fMRI study separating cognitive processing steps using a novel task paradigm. NeuroImage 36:1374–1386. [DOI] [PubMed] [Google Scholar]
- Lenneberg E (1967). Biological foundations of language. New York: Wiley. [Google Scholar]
- Levin SL, Mohamed FB, Platek SM (2005) Common ground for spatial cognition? A behavioral and fMRI study of sex differences in mental rotation and spatial working memory. Evolut Psychol 3:227–254. [Google Scholar]
- Levine SC, Huttenlocher J, Taylor A, Langrock A (1999) Early sex differences in spatial skill. Dev Psychol 35:940–949. [DOI] [PubMed] [Google Scholar]
- Lidzba K, Staudt M, Wilke M, Krägeloh-Mann I (2006) Visuospatial deficits in patients with early left-hemispheric lesions and functional reorganization of language: Consequence of lesion or reorganization? Neuropsychologia 44: 1088–1094. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Cross JH, Boyd SG, Gadian DG, Vargha-Khadem F, Baldeweg T (2004) Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study. Brain 127: 1229–1236. [DOI] [PubMed] [Google Scholar]
- Loring DW, et al. (1999) Effects of anomalous language representation on neuropsychological performance in temporal lobe epilepsy. Neurology 53:260–264. [DOI] [PubMed] [Google Scholar]
- Mazard A, Mazoyer B, Etard O, Tzourio-Mazoyer N, Kosslyn SM, Mellet E (2002) Impact of fMRI acoustic noise on the functional anatomy of visual mental imagery. J Cog Neurosci 14 (2): 172–186. [DOI] [PubMed] [Google Scholar]
- Mazard A, Tzourio-Mazoyer N, Crivello F, Mazoyer B, Mellet E (2004) A PET meta-analysis of object and spatial mental imagery. Eur J Cog Psych 16 (5): 673–695. [Google Scholar]
- McFie J, Piercy MF, Zangwill OL (1950) Visual-spatial agnosia associated with lesions of the right cerebral hemisphere. Brain 73:167–190. [DOI] [PubMed] [Google Scholar]
- McFie J, Zangwill O (1960) Visual-constructive disabilities associated with lesions of the left cerebral hemisphere. Brain 83:243–260. [Google Scholar]
- Meyer-Lindenberg A, (2004) Neural basis of genetically determined visuospatial construction deficit in Williams syndrom. Neuron 43:623–631. [DOI] [PubMed] [Google Scholar]
- Okubo M, Michimata C (2004) The role of high spatial frequencies in hemispheric processing of categorical and coordinate spatial relations. J Cogn Neurosci 16:1576–1582. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9(1):97–113. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Mitchell TV, McKeown MJ, Goldstein J, Allison T, McCarthy G (2003) Brain activity evoked by the perception of human walking: Controlling for meaningful coherent motion. J Neurosci 23:6819–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Boldrey E (1937) Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain: A journal of neurology. [Google Scholar]
- Peters M (2005) Sex differences and the factor of time in solving Vandenberg and Kuse mental rotation problems. Brain Cogn 57:176–184. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Yovel G, Duchaine B (2007) TMS evidence for the involvement of the right occipital face area in early face processing. Current Biology 17:1568–1573. [DOI] [PubMed] [Google Scholar]
- Podzebenko K, Egan GF, Watson JDG (2002) Widespread dorsal stream activation during a parametric mental rotation task, revealed with functional magnetic resonance imaging. NeuroImage 15:547–558. [DOI] [PubMed] [Google Scholar]
- Postma A, Huntjens RJC, Meuwissen M, Laeng B (2006) The time course of spatial memory precessing in the two hemispheres. Neuropsychologia 44:1914–1918. [DOI] [PubMed] [Google Scholar]
- Powell JL, Kemp GJ, García-Finaña M (2012) Association between language and spatial laterality and cognitive ability: an fMRI study. NeuroImage 59:1818–1829. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) Inaugural Article: A default mode of brain function. Proc Natl Acad Sci USA 98(2):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Milner B (1977) The role of eary left-brain injury in determining lateralization of cerebral speech functions. Annals of the New Qork Academy of Sciences 299:355–369. [DOI] [PubMed] [Google Scholar]
- Ratcliff G (1979) Spatial thought, mental rotation and the right cerebral hemisphere. Neuropsychologia 17:49–54. [DOI] [PubMed] [Google Scholar]
- Rey M, Dellatolas G, Bancaud J, Talairach J (1988) Hemispheric lateralization of motor and speech functions after early brain lesion: Study of 73 epileptic patients with intracarotid amytal test. Neuropsychologia 26:167–172. [DOI] [PubMed] [Google Scholar]
- Richter W, Ugurbil K, Georgopoulos A, Kim SG (1997). Time-resolved fMRI of mental rotation. [DOI] [PubMed] [Google Scholar]
- Richter W, et al. (2000) Motor area activity during mental rotation studied by time-resolved single-trial fMRI. J Cogn Neurosci 12 (2):310–320 [DOI] [PubMed] [Google Scholar]
- Robertson IH, et al. (1997) Auditory sustained attention is a marker of unilateral spatial neglect. Neuropsychologia 35:1527–1532. [DOI] [PubMed] [Google Scholar]
- Schenkenberg T, Bradford DC, Ajax ET (1980) Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology 30(5):509–517. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Metzler J (1971) Mental rotation of three-dimensional objects. Science 171(972):701–703. [DOI] [PubMed] [Google Scholar]
- Sperry RW, Gazzaniga MS, Bogen JE (1969) Interhemispheric relationships: the neocortical commissures; syndromes of hemisphere disconnection In: Vinken PJ and Bruyn GW, eds: Handbook of Clinical Neurology 4:273–290. John Wiley and Sons, NY. [Google Scholar]
- Staudt M, Lidzba K, Grodd W, Wildgruber D, Erb M, Krägeloh-Mann I (2002) Right-hemispheric organization of language following early left-sided brain lesions: functional MRI topography. NeuroImage 16:954–967. [DOI] [PubMed] [Google Scholar]
- Stiles J, Reilly JS, Levine SC, Trauner DA, Nass R (2012) Neural plasticity and cognitive development: Insights from children with perinatal brain injury. New York: Oxford University Press. [Google Scholar]
- Strauss E, Satz P, Wada J (1990) An examination of the crowding hypothesis in epileptic patients who have undergone the carotid amytal test. Neuropsychologia 28:1221–1227. [DOI] [PubMed] [Google Scholar]
- Stromswold K, Caplan D, Alpert N, Rauch S (1996) Localization of syntactic comprehension by Positron Emission Tomography. Brain Lang 52:452–473. [DOI] [PubMed] [Google Scholar]
- Tagaris GA, Kim SG, Strupp JP, Andersen P, Ugurbil K, Georgopoulos AP (1997) Mental rotation studied by functional magnetic resonance imaging at high field (4 Tesla): performance and cortical activation. J Cogn Neurosci 9:419–432. [DOI] [PubMed] [Google Scholar]
- Teuber HL (1974) Why two brains? In Schmitt FO & Worden FG (Eds) The Neurosciences: Third Study Program. Cambridge, Mass, MIT Press. [Google Scholar]
- Tomasino B, Toraldo A, Rumiati RI (2003) Dissociation between the mental rotation of visual images and motor images in unilateral brain-damaged patients. Brain Cogn 51:368–371. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Rumiati RI (2004) Effects of strategies on mental rotation and hemispheric lateralization: neuropsychological evidence. J Cogn Neurosci 16:878–888 [DOI] [PubMed] [Google Scholar]
- Tomasino B & Gremese M (2016) Effects of stimulus type and strategy on mental rotation network: an activation likelihood estimation meta-analysis. Front Hum Neurosci, Vol 9, Article 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DM, Hartry-Speiser A, McDougal L, Luu P, deGrandpre D (1999) Mood and spatial memory: emotion and right hemisphere contribution to spatial cognition. Biol Psych 50:103–125. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M (1982) Two cortical visual systems. In “analysis of Visual Behavior (Ingle DJ, Goodale MA, Mansfield RJW, eds), pp. 549–586. MIT Press: Cambridge, MA. [Google Scholar]
- Vallar G (1998). Spatial hemineglect in humans. Trends in Cognitive Sciences 2(3): 87–97. [DOI] [PubMed] [Google Scholar]
- Vallar G (1993) The anatomical basis of spatial hemineglect in humans In Robertson IA and Marshall JC (Eds) Unilateral Neglect: Clinical and Experimental Studies. Hillsdale, NJ, Lawrence Erlbaum Associates. [Google Scholar]
- Vandenberg SG, Kuse AR (1978) Mental rotations, a group test of three-dimensional spatial visualization. Percept Motor Skills 47:599–604. [DOI] [PubMed] [Google Scholar]
- Vanrie J, Beatse J, Sunaert S, Van Hecke P (2002) Mental rotation versus invariant features in object perception from different viewpoints: an fMRI study. Neuropsychologia 40:917–930. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, Santens P, Van Laere K, Lahorte P, Dierckx R, De Reuck J (2001) Regional brain activity during different paradigms of mental rotation in healthy volunteers: a positron emission tomography study. NeuroImage 13:381–391. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, de Lange FP, Vandemaele P, Deblaere K, Achten E (2002) Motor imagery in mental rotation: An fMRI study. NeuroImage 17:1623–1633. [DOI] [PubMed] [Google Scholar]
- Vogel JJ, Bowers CA, Vogel DS (2003). Cerebral lateralization of spatial abilities: A meta-analysis. Brain and Cognition 52 (2): 197–204. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP (1995) Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull 117:250–270. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1939) Wechsler-Bellevue intelligence scale. New York: The Psychological Corporation. [Google Scholar]
- Weintraub S, Mesulam MM, Kramer L (1981) Disturbances in prosody. A right-hemisphere contribution to language. Arch Neurol 38:742–744. [DOI] [PubMed] [Google Scholar]
- Weiss PH, Marshall JC, Zilles K, Fink GR (2003) Are action and perception in near and far space additive or interactive factors? NeuroImage 18:837–846. [DOI] [PubMed] [Google Scholar]
- Wernicke C (1874) Der aphasische Symptomenkomplex Eine psychologische Studie auf anatomischer Basis. Breslau: M: Cohn & Weigert. [Google Scholar]
- Wilke M & Lidzba K (2007) LI-toolbox: A new toolbox to assess lateralization in functional MR-data. J Neurosci Methods 163: 128–136. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ (2006) A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. NeuroImage 33: 522–530. [DOI] [PubMed] [Google Scholar]
- Willems RM, Van der Haegen L, Fisher SE, Francks C (2014) On the other hand: including left-handers in cognitive neuroscience and neurogenetics. Nat Rev Neurosci 15:193–201. [DOI] [PubMed] [Google Scholar]
- Witelson SF (1976) Sex and the single hemisphere: specialization of the right hemisphere for spatial processing. Science 193 (4251):425–427. [DOI] [PubMed] [Google Scholar]
- Woods BT (1980) The restricted effects of right-hemisphere lesions after age one; Wechsler Test data. Neuropsychologia 18:65–70. [DOI] [PubMed] [Google Scholar]
- Woods RP, Dodrill CB, Ojemann GA (1988) Brain injury, handedness, and speech lateralization in a series of amobarbital studies. Ann Neurol 23:510–518. [DOI] [PubMed] [Google Scholar]
- Woods BT, Teuber HL (1973) Early onset of complementary specialization of cerebral hemispheres in man. Transactions of the American Neurological Association 98:113–117. [PubMed] [Google Scholar]
- Zachariou V, Nikas CV, Safiullah ZN, Behrmann M, Klatzky R, Ungerleider LG (2015) Common dorsal stream substrates for the mapping of surface texture to object parts and visual spatial processing. J Cogn Neurosci 27:2442–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Nikas CV, Safiullah ZN, Gotts SJ, Ungerleider LG (2016) Spatial mechanisms within the dorsal visual pathway contribute to the configural processing of faces. Cerebral Cortex 1–15. doi: 10.1093/cercor/bhw224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM (2008) Neuroimaging studies of mental rotation: a meta-analysis and review. J Cogn Neurosci 20:1–19. [DOI] [PubMed] [Google Scholar]
- Zhu X, Kelly TH, Curry TE Jr, Lal C, Joseph JE (2015) Altered functional brain asymmetry for mental rotation: effect of estradiol changes across the menstrual cycle. NeuroReport 26:814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.