Abstract
This article is one of ten reviews selected from the Annual Update in Intensive Care and Emergency Medicine 2019. Other selected articles can be found online at https://www.biomedcentral.com/collections/annualupdate2019. Further information about the Annual Update in Intensive Care and Emergency Medicine is available from http://www.springer.com/series/8901.
Introduction
Despite decades of intense pre-clinical and clinical research there is still much uncertainty regarding the volume-expanding efficacy of different fluid resuscitation strategies across a range of disease states, particularly for the critically ill [1]. New concepts in vascular permeability promise to change the way we approach fluid resuscitation and ultimately lead to improvements in its efficacy. Central to these new concepts is the endothelial glycocalyx, which lines the luminal aspect of the vascular endothelium. Knowledge of the endothelial glycocalyx has permitted revision of the classic Starling principle to one that better explains the observed flux of fluid across the endothelial barrier [2].
This new model of endothelial permeability largely explains the difference in the predicted (1:3–1:5) versus the observed (approximately 1:1.3–1:1.4) ratio of colloid to crystalloid required to achieve similar hemodynamic end-points in clinical trials [1]. It also explains why the infusion of an iso-oncotic colloid fluid will not reverse existing interstitial edema [3], and may in some situations result in less volume expansion and greater tissue edema than a crystalloid in critically ill patients [4]. The volume expanding effects of infused fluids also differ depending on the rate of infusion, the degree of vasoconstriction, the integrity of the endothelial glycocalyx and the volume status. Because of this, the effectiveness of fluid resuscitation is said to be context-sensitive.
Damage to the endothelial glycocalyx, termed shedding, occurs in a number of critical illnesses, including sepsis and severe trauma, and the degree of shedding is associated with poor outcomes [5]. It is likely, but not yet proven, that protecting and restoring the endothelial glycocalyx in these conditions will improve outcomes. Several pharmacologic therapies are under investigation, but these are in the pre-clinical phase of development and there is not yet enough evidence to support their clinical use [6]. However, there is growing evidence that commonly used resuscitation fluids protect and restore the endothelial glycocalyx and modulate endothelial permeability, but differ in their ability to do so. It is therefore important that, when choosing resuscitation fluids for particular patients, clinicians consider factors additional to oncotic properties, including ability to protect and repair the endothelial glycocalyx.
The endothelial glycocalyx
The endothelial glycocalyx consists of a scaffolding network of proteoglycans, predominantly the transmembrane bound syndecan and the membrane bound glypican. Bound to these are five types of glycosoaminoglycan side chains, predominantly heparan sulfate, with chondroitin sulfate and hyaluronan less abundant [7]. Glycoproteins are also attached to the endothelium. These are diverse in function and include the cell adhesion molecules, receptors in intercellular signaling and receptors involved in fibrinolysis and coagulation. Incorporated into the scaffolding network are numerous endothelial and plasma-derived soluble molecules [7] (Fig. 1).
Fig. 1.
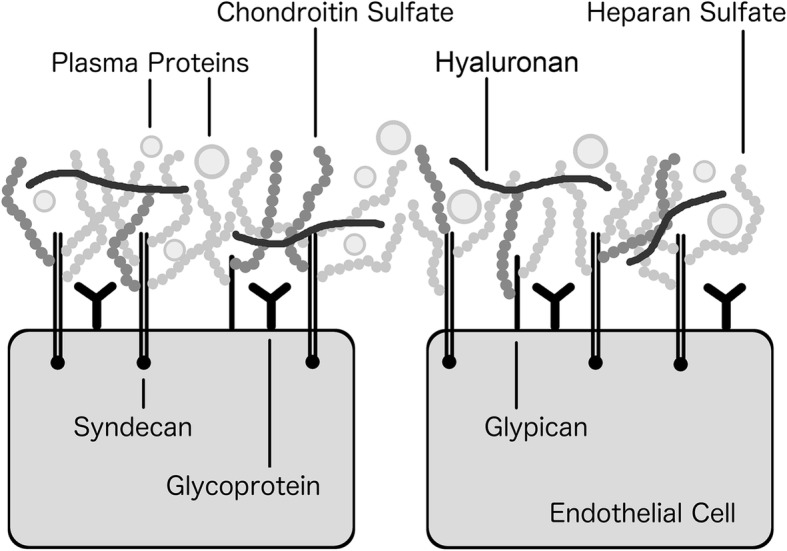
The structure of the endothelial glycocalyx
The endothelial glycocalyx is a key regulator of endothelial function. Most is known about its role in regulating vascular permeability, but it is also integral to cell-vessel wall interactions, blood rheology, mechanotransduction, inflammation, coagulation and fibrinolysis [7]. The fragile structure and small dimensions of the endothelial glycocalyx make it difficult to detect and quantify. Experimentally, the endothelial glycocalyx can be directly visualized by a number of techniques including electron microscopy, intravital microscopy, comparison of the volumes of distribution of endothelial glycocalyx permeable and non-permeable tracers, confocal microscopy, and immunohistochemical staining [8]. These techniques are all invasive and not suitable for repeated measurements, if at all, in clinical applications.
For clinical purposes, detecting endothelial glycocalyx breakdown products in plasma or serum has been widely used in a research context, but is not yet routinely available for clinical practice, and indeed the clinical significance of elevated levels has not been validated. The most commonly measured is syndecan-1 (SDC-1), the main structural backbone of the endothelial glycocalyx [7]. Heparan sulfate, chondroitin sulfate and hyaluronan have also been used to detect endothelial glycocalyx damage [8]. Alternatively, visualization with a side-stream dark field (SDF) camera or its predecessor orthogonal polarization spectral imaging (OPS) has been used to detect endothelial glycocalyx thickness in the nail fold or oral mucosa in a clinical research context. These cameras estimate endothelial glycocalyx thickness based on the speed and deformation of passing red blood cells (RBCs) and leukocytes [8].
Loss of the endothelial glycocalyx, or glycocalyx shedding, occurs commonly in a number of diseases including trauma and sepsis, and has been associated with poor patient outcomes [5]. However, it is unclear whether endothelial glycocalyx shedding is simply a marker of disease severity or if it contributes directly to poor outcomes. There are a number of biologically plausible pathways whereby endothelial glycocalyx shedding could cause harmful effects, but no clinical study has attempted to restore the endothelial glycocalyx, and in animal studies there are no data on outcomes after restoration [9].
Mirroring the diverse range of conditions that are associated with endothelial glycocalyx shedding is the diverse range of mediators known to cause shedding. These include, but are not limited to, tumor necrosis factor (TNF)-α, reactive oxygen species (ROS), heparanase, hypoperfusion, hyperglycemia, bacterial toxins and growth factors [10]. The final common pathway for many initiators of shedding is the activation of proteases that cleave endothelial glycocalyx components from the cell surface [10].
Role in regulating vascular permeability: The revised Starling principle
The movement of fluid across the endothelium has, until recently, been explained by the classical Starling principle, which describes the filtration rate as being a function of two opposing forces—hydrostatic pressure and osmotic pressure—across the vessel wall [11]:
where Jv/A is the outward filtration force for a given area, Lp is the membrane hydraulic conductivity, Pc is the luminal hydrostatic pressure, Pi is the interstitial hydrostatic pressure, σ is the macromolecule reflection coefficient of the membrane, Πc is the luminal osmotic pressure and Πi is the interstitial osmotic pressure.
When Starling first described his theory in 1896 [11], the model was consistent with experimental data available at the time. However, in recent years, modern technology has enabled the observation of a number of contradictions to the classic equation. Specifically, there is no venous reabsorption of fluid, transcapillary flow rate is lower than predicted, and the interstitial protein concentration has a minimal effect on fluid flux [2]. This has led to four major modifications to the Starling model, with the endothelial glycocalyx central to these modifications.
No absorption in the steady state
Starling theorized that after being filtered out from the arterial end of a capillary (the segment under high Pc), fluid was then reabsorbed at the venous end (the segment under low Pc). However, experiments have found that while there is an initial transient response where fluid is absorbed after a sudden decrease in Pc, this rapidly changes back to outward filtration, even at the venous end of the capillary. This transient absorption phase lasts approximately 15–30 min in humans following acute hemorrhage, allowing the absorption, or ‘auto-resuscitation’, of approximately 0.5 L of interstitial fluid [2, 12]. However, in the steady state, no absorption is seen along the entire length of most capillaries, regardless of the Pc (the “no absorption rule”) [2, 12]. Instead, fluid is removed from the interstitium via the lymphatic system [12]. Only in certain unique organs, notably those of the renal, intestinal and lymphatic systems, is absorption seen in the steady state due to mechanisms that maintain a low Πi and a raised Pi [2].
The no absorption rule explains why the intravenous administration of iso- or hyper-oncotic colloid fluids will not reverse existing interstitial edema [3], and is due to the inverse relationship between capillary filtration rate and the interstitial protein concentration gradient adjacent to the vessel wall. After the initial drop in Pc, the balance of forces directs fluid inwards into the vessel lumen. This movement of fluid concentrates the interstitial proteins, increasing Πi, which opposes the absorption force inwards. Eventually, a new steady state is reached, at which point the balance of forces always results in outward filtration [2].
The sub-glycocalyx space
Starling’s original theory assumes that Πi is substantially lower than Πc. This is not correct. The interstitium is packed with proteins due to the physiological extravasation of plasma proteins, possibly through large pores located in the venular segments of capillaries, which results in Πi approaching Πc [2, 4]. But solving the original equation with the measured Πi and Πc values predicts a much higher filtration rate than that measured experimentally [13]. Furthermore, modifying Πi experimentally only has a minor impact on filtration rate [2].
The discrepancies between predicted and measured filtration rates are resolved by revising the Starling equation and replacing Πi with the osmotic pressure in a small protein-free zone between the endothelial glycocalyx and the endothelial cells [2] (Fig. 2). That is:
where Πg is the sub-glycocalyx osmotic pressure. As the osmotic pressure counteracting Πc is Πg, and not Πi, changes in Πi will have little impact on the filtration rate, as has been observed [2]. Πg is almost negligible in comparison to Πc, so the osmotic pressure gradient approaches Πc.
Fig. 2.
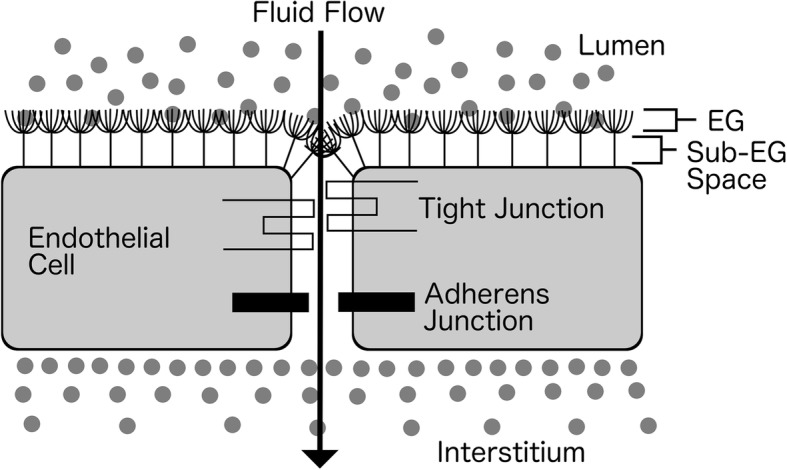
The sub-glycocalyx space. EG: endothelial glycocalyx
The sub-glycocalyx space is maintained protein-free by the constant outward filtration of fluid as explained by the no absorption rule and the plasma protein filtering effect of an intact endothelial glycocalyx. The resulting ultra filtrate flows through the sub-glycocalyx space, and then out through the intercellular clefts via breaks in the tight junction strands [2]. Due to their narrow width, the velocity in the breaks is high even at low filtration rates, which prevents movement of interstitial protein back into the sub-glycocalyx space [2].
The endothelial glycocalyx is a determinant of hydraulic conductivity
The endothelial glycocalyx is also an important determinant of hydraulic conductivity. Hydraulic conductivity (Lp) is the change in filtration rate for a given change in transendothelial pressure, and can be thought of as the ease with which water passes across the vessel wall. Far from being a static variable, Lp is dynamically influenced by the endothelial glycocalyx and the endothelium. The endothelial glycocalyx reduces Lp by mechanically resisting fluid flow [2, 4]. It also affects Lp by mechanotransducing shear force to the underlying endothelial cells, which respond to increased shear stress by releasing nitric oxide (NO) and altering junctional proteins resulting in an increase in Lp [14]. This process is physiologically relevant when meeting the increased demand for metabolic substrates to skeletal muscle during exercise, but the relevance in a critically unwell patient, where in most cases the endothelial glycocalyx is degraded and the shear stress is low, remains to be seen.
Endothelial cells have a significant role in regulating Lp. The tight and adherens junctions contribute to the high hydraulic resistance of the intercellular space. Breakdown of these junctions occurs in response to a variety of mediators, such as vascular endothelial growth factors (VEGF) and cytokines, increasing Lp [15]. In addition, apoptosis, mitosis and transcellular pathways, such as aquaporins, may contribute to increased Lp depending on the vascular bed and the prevailing pathophysiological conditions [15]. The trans- and para-cellular pathways that mediate endothelial permeability to fluid, solutes and cells in a number of disease processes are complex and not completely understood, and are reviewed elsewhere [15].
The modified Starling model is non-linear at low filtration rates
The effect of capillary hydrostatic pressure, Pc, on filtration rate is more complex than previously thought. The original and modified Starling equations both describe the relationship between Jv/A and Pc as linear, when the other variables are constant. This relationship is described by the linear equation:
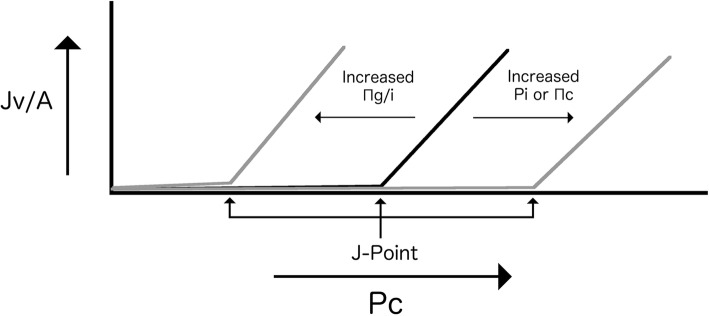
However, due to the no absorption rule, at low values of Jv/A in the steady state the flow rate only approaches zero, never actually reaching zero or becoming negative. This results in an asymptotic curve at low values of Jv/A and a linear curve at higher values. Woodcock and Woodcock [16] have described the inflection point as the J-point, and theorized that at Pc values below the J-point both crystalloids and colloids will have almost the same volume expanding effects due to the filtration rate of both being near zero. The x-intercept, that is, Pc when Jv/A is zero (or rather what it would be if the curve was linear at a Jv/A of zero), approximates the J-point, and is given by:
Increasing Pi or Πc will shift the J-point to the right, whereas increasing Πg/i will have the opposite effect (Fig. 3). Contextualizing this clinically, a right shift in the J-point is advantageous for increasing intravascular volume—more fluid can be infused (and crystalloid or colloid will have similar volume expanding effects) before the hydrostatic pressure threshold for movement of fluid interstitially is reached; whereas a shift to the left is deleterious for volume expansion—fluid will move interstitially at a lower Pc and hence lower intravascular volume. An increase in Pi is usually due to edema formation, which is not desirable, so the best way to achieve a right shift is to increase Πc and avoid increases in Πg/i.
Fig. 3.
The relationship between capillary lumen hydrostatic pressure (Pc) and the outward filtration force for a given area (Jv/A) showing the J-point, below which both crystalloids and colloids have almost the same volume expanding effect. Pi interstitial hydrostatic pressure, Πc, luminal osmotic pressure, Πi interstitial osmotic pressure, Πg sub-glycocalyx osmotic pressure
In an intact endothelial glycocalyx, Πg is negligible, and the colloid particles in an infused colloid fluid remain in the intravascular space due to the filtration effect of the endothelial glycocalyx. This results in either no change or a rightward shift of the J-point, a low filtration rate, and sustained plasma volume expansion from the infused fluid. Whether a colloid compared to a crystalloid results in greater volume expansion in this context depends on the Pc. If the Pc is below the J-point, then as the filtration rate is near zero, both crystalloids and colloids will have a similar volume expanding effect. If the Pc is above the J-point, then colloids will persist in the intra-vascular space for longer than crystalloids.
While there is currently no way to measure Pc at the bedside (there is a poor correlation between measurable macrocirculatory parameters such as blood pressure and those of the microcirculation [17]), there are indications that in healthy volunteers, the J-point does appear to approximate the Pc in what is regarded as normovolemia. When 900 mL of blood was removed from normotensive human volunteers, the volume of crystalloid required to restore normovolemia was estimated to be somewhere between one and two times the hemorrhaged volume [18], depending on the rate of replacement. By comparison, in experiments where crystalloid was infused to achieve hypervolemia, as little as 17 ± 10% was found to remain intravascularly [19].
In contrast, if the endothelial glycocalyx is damaged, the J-point is shifted to the left due to the higher Πi replacing Πg [20], and the plasma expanding efficacy of any infused fluid is reduced (that is, the outward filtration rate will be above zero at a lower plasma volume). Paradoxically, while it might initially seem that in this context (with the Pc being more likely to be above the J-point) a colloid fluid would persist in the intravascular space longer than a crystalloid, this is not necessarily the case as the colloid particles are free to move interstitially, further increasing Πi and worsening the leftward shift [20]. Endothelial glycocalyx shedding also decreases σ, making the J-point more dependent on Pi and less dependent on the osmotic pressure difference.
This concept has been demonstrated experimentally by Jacob et al. [4] who measured perfusion pressure (approximating Pc) and transudate flow (approximating Jv/A) in ex vivo guinea pig hearts. The Pc of the estimated J-point after a crystalloid infusion was approximately 10 cmH2O, and this was the same before and after enzymatically degrading the endothelial glycocalyx (although the protein-free perfusate likely resulted in endothelial glycocalyx shedding prior to the enzymatic degradation). The positive J-point was likely caused by the increased Pi from fluid movement interstitially. After a colloid infusion, this point was 0 cmH2O in an intact endothelial glycocalyx, but was reduced to − 12 cmH2O after enzymatic degradation—the interstitium was essentially ‘sucking’ fluid from the intravascular space. The negative J-point was likely due to the increased Πi from the movement of colloid particles interstitially. In a similar study there were similar levels of tissue edema after infusing a colloid compared to a crystalloid through endothelial glycocalyx denuded vessels [20]. And in another, the increase in Πi resulted in an increase in the filtration rate of subsequent fluid infusions [21].
Additional theoretical considerations
Vasoconstriction and vasodilation via exogenous or endogenous mechanisms also affect Pc and the filtration rate but in a somewhat unpredictable fashion, as these are dependent on the balance of constriction/dilation in the venules and arterioles [17]. In addition, an increased rate of intravenous fluid infusion should, theoretically, lead to increased fluid extravasation from a transient increase in Pc, but the experimental data are not conclusive. The relationship between rapid fluid infusion rate, Pc, filtration rate and poor clinical outcomes is therefore still poorly understood. Furthermore, the permeability of the intact, or partially intact, endothelial glycocalyx to macro-molecules such as albumin and semisynthetic colloids also increases with increasing Pc, adding additional complexity to the relationship between Pi and Pc [17].
Clinical implications of the revised Starling model
Methods for measuring endothelial glycocalyx status clinically are not yet routinely available outside of a research context and have unvalidated clinical significance. However, overwhelming pre-clinical and clinical evidence suggests that the endothelial glycocalyx is likely to be damaged in critically unwell patients [5]. Infusing an iso-oncotic colloid into these patients will have a similar volume expanding effect to a crystalloid fluid. How similar will depend on the degree of endothelial glycocalyx shedding, the endothelial glycocalyx permeability, the patient’s pre-infusion volume status, the infusion rate, and the degree of vasoconstriction, and is difficult to predict clinically due to the complexity of the interactions between the variables involved. This could explain why in large clinical trials of critically unwell patients the volume expanding effects of colloids compared to crystalloids are much less than predicted. For example, in the Crystalloid versus Hydroxyethyl Starch Trial (CHEST) [22] and the Saline versus Albumin Fluid Evaluation (SAFE) [23] clinical trials, the observed ratio of colloid to crystalloid to achieve the same hemodynamic resuscitation end-points was 1:1.3 and 1:1.4 respectively, which is markedly different to the ratio of 1:3–1:5 predicted by the classical Starling principle [1].
Using an iso-oncotic colloid for a potential, even if only marginally, greater volume expanding effect is not without risk. Infusing a colloid solution into a patient with a degraded endothelial glycocalyx comes at the expense of interstitial protein accumulation resulting in tissue edema to levels similar to that seen in crystalloid infusions [4]. Paradoxically, in some cases tissue edema could actually be higher and volume expansion lower after an infusion of colloids compared to a crystalloid infusion [4]. In addition, the use of semisynthetic colloids may have deleterious consequences (e.g., allergy, coagulopathy) beyond causing edema [1], and they appear to extravasate faster than albumin [20]. Furthermore, due to the no absorption rule, a colloid infusion cannot reverse existing interstitial edema regardless of the integrity of the endothelial glycocalyx.
All of these considerations could explain why, despite the slightly greater volume expansion properties, overall there have not been any significant mortality benefits from using a colloid over a crystalloid in clinical trials [1]. The volume expansion effect may be so marginal as to make no difference in outcomes, or the deleterious effects may counteract any advantage from greater volume expansion.
Fluids that preserve the glycocalyx
The preceding discussion has focused on the differential resuscitation effects of crystalloid and colloid according to the prevailing state of the endothelial glycocalyx, finding physiological rationale for the absence of evidence for superiority of one over the other. However, some colloids do appear to be markedly superior as resuscitation fluids. In hemorrhagic shock, for example, resuscitation with higher ratios of plasma seems to result in lower mortality than crystalloid [24], despite there being seemingly little advantage in terms of preservation of coagulation factors [25]. The explanation may lie not with the Starling equation but rather that certain colloids act to preserve the endothelial glycocalyx.
As there have been no clinical trials that have directly sought to assess whether restoring or protecting the endothelial glycocalyx changes clinical outcomes, the rationale for preserving the endothelial glycocalyx is based on observational and pre-clinical in vitro and in vivo data. Taken together, these data suggest that the early repair of the endothelial glycocalyx might improve the systemic inflammatory response, coagulopathy and volume responsiveness following a systemic ischemic or inflammatory stimulus such as severe sepsis or major trauma. The timeframe for the endothelial glycocalyx to repair itself clinically without any intervention is not clear, but data from a rat model and human endothelial cell culture experiments suggest that following the cessation of the shedding stimulus, it takes 5–7 days to restore the glycocalyx to baseline [26]. There is therefore a window in this relatively long timeframe for an intervention to stimulate earlier repair. There is growing evidence that commonly used resuscitation fluids have differing abilities to protect and restore the endothelial glycocalyx.
Albumin
A low-protein environment has long been recognized to cause a rapid breakdown, or shedding, of the endothelial glycocalyx [27]. This phenomena is independent of the effect on osmotic pressure, as, for the same intravascular osmotic pressure, plasma and albumin are more effective than semisynthetic colloids such as hydroxyethyl starch (HES) at preserving and restoring the endothelial glycocalyx, reducing vascular permeability, and reducing platelet and leukocyte adhesion in pre-clinical studies [4, 20, 28, 29]. The mechanism of the superior ‘sealing effect’ of albumin and plasma is still not entirely clear and has been termed the “colloid osmotic pressure paradox”.
Initially, it was thought that perfusing the endothelium with a protein-free solution collapsed the endothelial glycocalyx due to washout of its integrated proteins. However, immunohistochemical staining and electron microscopy have revealed that a low-protein environment causes a complete absence, rather than collapse, of the endothelial glycocalyx [27]. This appears to be caused by matrix metalloproteinase (MMP) cleavage of the endothelial glycocalyx components from the underlying endothelium [27]. The protective effect of protein might be mediated by a protein bound substance that inhibits MMP cleavage of the endothelial glycocalyx, such as the lipid mediator sphingosine 1-phosphate (S1P). In in vitro experiments, activation of the S1P1 receptor inhibits MMPs, preventing endothelial glycocalyx shedding [27, 28], while at the same time the endothelial glycocalyx is restored by the mobilization of intracellular pools of glycocalyx components via golgi-mediated translocation [4]. RBCs, followed by platelets, are the major source of S1P in the body [30]. Plasma proteins, predominantly high-density lipoprotein (HDL) and albumin, facilitate the release of S1P from these sources [30]. In the absence of albumin, up to 25 times less S1P is released from RBCs [28]. Whether S1P is the only mediator responsible for the colloid osmotic pressure paradox is not known, nor is it known whether agonizing the S1P1 receptor has any clinically relevant effect on the endothelial glycocalyx in vivo.
It is unclear whether the infusion of albumin in vivo has the same endothelial glycocalyx restoration effect as that seen in vitro. Animal studies have yielded conflicting results—in a mouse model of hemorrhage where fresh frozen plasma (FFP) attenuated the increase in vascular permeability, human albumin had almost no effect [31], whereas in a rat model of hemorrhage, albumin restored the glycocalyx thickness to 81 ± 31% of the baseline, compared to the full restoration achieved by FFP, but better than the 42 ± 21% of that from 0.9% saline [32]. Permeability in this study was restored to baseline after both FFP and albumin infusion. Furthermore, in clinical trials, there is only a narrow, if any, survival benefit of using albumin as a resuscitation fluid, although there is weak evidence that there may be an advantage of albumin in septic patients [1]. There are also concerns about the safety of albumin in traumatic brain injury (TBI), although a recent study has suggested that the harm in this patient population might actually be caused by the hypotonicity of the carrier fluid, not by the albumin itself [1].
There are a number of possible explanations for these contradictory data. It could be that albumin restores the endothelial glycocalyx, but this restoration does not change clinical outcomes. It could also be that circulating albumin levels are required to drop below a critical level before supplementation has any clinically significant effect. Or, it could be that it is not albumin itself that is a mediator of endothelial glycocalyx repair, but rather another mediator contained in the albumin solution, such as S1P. Supporting this hypothesis is a study in which albumin exposed to RBCs for 20 min or a solution without albumin but containing S1P maintained normal vessel permeability in rat microvessels, but albumin not conditioned by RBCs did not, nor did Ringer’s solution that was conditioned to RBCs [28]. Commercially available sources of albumin for pre-clinical research use, such as fetal calf serum and bovine serum albumin, contain physiologically active levels of S1P [28], which may account for their efficacy in protecting and restoring the endothelial glycocalyx in in vitro experiments. The levels of S1P in human albumin manufactured for clinical purposes have not been reported, and the albumin used in the aforementioned studies was not analyzed for any other potential mediators. Differing levels of these mediators could account for the difference in observed effects. Artificially S1P-enriched human albumin would be an attractive solution for trials in human resuscitation.
Fresh frozen plasma
The evidence for the endothelial glycocalyx-restoring properties of FFP is more compelling. In cell culture and animal models of endothelial glycocalyx injury, FFP consistently attenuates glycocalyx shedding and the associated increase in vascular permeability and leukocyte adhesion, and in animal models it also attenuates acute lung injury and gut inflammation following hemorrhagic shock [29]. In a clinical study of 33 non-bleeding critically-ill patients given 12 mL/kg FFP as pre-procedure prophylaxis, SDC-1 blood levels were significantly lower following the administration off FFP, indicating that FFP had reduced the degree of endothelial glycocalyx shedding [9]. FFP starts repairing the endothelial glycocalyx within 1 h, and this appears to be mediated via not only the cessation of ongoing shedding, but also by up-regulation of endothelial glycocalyx component production. Hemorrhagic shock reduces the expression of SDC-1 mRNA, and resuscitation with crystalloid reduces this expression even further, while FFP returns SDC-1 mRNA expression back to baseline [33].
The increase in endothelial glycocalyx production by FFP could confound the clinical detection of glycocalyx shedding with blood levels of glycocalyx components. In a rat model of hemorrhage and TBI, SDC-1 blood levels at 23 h were higher in the FFP resuscitated group compared to the 0.9% saline resuscitated group, suggesting higher levels of endothelial glycocalyx shedding in the FFP group [34]. The authors suggested the low levels of SDC-1 at 23 h actually most likely reflected a reduction in endothelial glycocalyx production in the saline group, not a reduction in endothelial glycocalyx shedding.
The mechanism by which FFP restores the endothelial glycocalyx, reduces endothelial permeability, and attenuates early inflammation is not clear. It is not known if the same mediator is responsible for the endothelial glycocalyx repairing properties of both FFP and albumin. Similar to albumin, the pre- and post-transfusion levels of S1P in FFP approved for clinical use have not been measured. In addition, FFP’s effects are pleiotropic: for instance it also repairs the endothelial adherens junction, which would account for some of the improvement in permeability [35]. This is not surprising given that plasma contains over 1000 proteins and numerous soluble mediators [36]. S1P in FFP may play an important role in preserving and restoring the endothelial glycocalyx, but so may other mediators of protease activity such as TIMP3 (tissue inhibitor of metalloproteinase 3) [37] or ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) [9]. However, there is as yet less evidence for these mediators in the pathogenesis of endothelial glycocalyx shedding than S1P.
The components of FFP that repair the endothelial glycocalyx may also be present in plasma-derived products such as prothrombin complex concentrate (PCC). Pati et al. demonstrated that in a mouse model of hemorrhagic shock, PCC attenuated the increase in vascular permeability with equal efficacy as FFP [31]. Interestingly, the PCC was not as effective as FFP in an endothelial cell culture model [31]. It may be that multiple components of plasma are required to act synergistically to mediate their restorative effects. This study did not measure the endothelial glycocalyx, so it can only be speculated that the permeability reducing effect was mediated via glycocalyx restoration. Lyophilized and spray-dried plasma also appear to have the same endothelial protective properties as FFP [9].
Other variations in the processing and storage of FFP may not preserve its endothelial glycocalyx repairing properties, and as it is not known what mediates these properties it is difficult to predict how these may differ with different processes. For example, the protective effects of FFP are substantially diminished by post-thaw storage at 4 °C for 5 days [9]. Furthermore, the timing of resuscitation could also be important. In a cell culture model, resuscitation with plasma immediately following injury restored the endothelial glycocalyx and vessel permeability, whereas resuscitation with plasma at 3 h following injury had no protective effect [38].
Clinically, there is evidence that the infusion of FFP, particularly in traumatic hemorrhage, decreases early deaths, particularly when the plasma is administered early [39]. In the PAMPer trial, a 564 patient multicenter randomized controlled trial (RCT) of pre-hospital administration of FFP compared to standard care (no FFP pre-hospital), 30-day mortality was lower in the group that received pre-hospital FFP (23.2% vs. 33.0%) [39]. The improvement in mortality occurred early; there was separation in the survival curve after only 3 h following randomization [39]. FFP’s beneficial effects were independent of any attenuation of coagulopathy [40], and it could be speculated that it may instead, at least in part, have been mediated by endothelial protection.
There has historically been a reluctance to use FFP due to concern about the risk of adverse events, such as transfusion-associated acute lung injury and allergic transfusion reactions [41]. However, a variety of strategies to reduce these risks, such as using male only donor plasma and leukoreduction, have resulted in a safer product [41]. Recent RCTs have found no increased incidence of major complications including multiorgan failure, acute lung injury or sepsis with the use of FFP, and only a low incidence of minor transfusion-related reactions in patients who receive FFP [24, 39]. Interestingly, in a pilot study of 44 bleeding patients undergoing surgery for thoracic aorta dissection, patients randomized to OctaplasLG had significantly lower SDC-1 and sVE-cadherin levels (a marker of endothelial cell intercellular junction integrity) compared to patients given standard FFP [42]. OctaplasLG is a pathogen-reduced product derived from approximately 1000 plasma donations with standardized concentrations of clotting factors, and is free from damage-associated molecular patterns, cytokines, cell debris and microparticles due to several stages of microfiltration. The removal of these particles could result in a product that has fewer adverse effects, but is also more effective at restoring the endothelial glycocalyx than standard FFP. Potentially, isolating the endothelial glycocalyx protective mediator among FFP’s thousand or so proteins and soluble mediators could prove to be the most effective and safest therapy for protecting the endothelial glycocalyx.
Red blood cells
Packed RBCs (PRBCs) could, theoretically, have an endothelial glycocalyx protective effect due to their role as a source of S1P. RBCs, followed by platelets, are the major source of S1P in the body; S1P is rapidly removed from the circulation, so circulating RBCs and platelets may be integral in maintaining sufficient plasma levels [30]. A study of individually perfused rat microvessels supports this hypothesis. Albumin exposed to PRBCs for 20 min or a solution without albumin but containing S1P maintained normal vessel permeability, but albumin not conditioned by PRBCs did not [28].
However, PRBCs transfused systemically do not appear to be protective of the endothelial glycocalyx. In a rat hemorrhagic shock model, resuscitation with fresh whole blood or unwashed PRBCs, but not with washed PRBCs or lactated Ringers, increased endothelial glycocalyx thickness and reduced vascular permeability, suggesting that the residual plasma in the unwashed PRBCs is responsible for the endothelial protective effect, and not the PRBCs themselves [43]. The equivalent of approximately 4 units of blood product was transfused to each animal in this study. It could be that there were enough circulating endogenous RBCs in these animals to keep S1P levels above a critical level, so supplementation did not achieve any noticeable effect. If this were the case, then this has consequences for patients who receive massive blood transfusions where their entire circulating blood volume is replaced with exogenous blood products. For these patients, the S1P content of transfused blood products could be of great clinical significance.
Notably, aged PRBC units contain less S1P than fresh units [44]. There is high quality evidence that transfusion of fresher PRBCs does not improve patient outcomes [45]. However, the large trials that have addressed this question did not consider PRBCs towards the end of their 42-day shelf life, but were instead pragmatic responses to the emerging clinical tendency to transfuse the freshest available PRBCs in preference to units with a median age of around 20 days [45]. In addition, these trials did not specifically look at massive transfusion, and it is possible that the age of PRBCs matters in this population, but not in those who receive a small number of PRBC units.
Platelets
There is growing evidence that the transfusion of platelets early following hemorrhagic shock improves patient outcomes. Most recently, a sub-study of the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial analyzed the 261 patients in the trial who only received the first cooler of blood products, and therefore either did or did not receive platelets along with their PRBC resuscitation. Patients who received platelets had significantly lower 24-h (5.8% vs. 16.9%) and 30-day mortality (9.5% vs. 20.2%) [46]. While there are inherent limitations with such a sub-study, this finding is consistent with previous observational studies that have suggested that increased plasma and platelet to PRBC ratios improve outcomes in bleeding trauma patients [47].
Almost certainly some of the mortality benefit from platelet transfusion would be attributed to an improvement in hemostasis. However, it is also possible that the endothelial protective effects of platelets also play a role in improving outcomes. Platelets release cytokines and growth factors that preserve the integrity of the endothelial intercellular junction and thereby maintain a low vascular permeability [48]. Platelets are also a source of S1P [30], so it is possible that S1P plays a role in maintaining a low vascular permeability by protecting the endothelial glycocalyx; however the effect of transfused platelets on the endothelial glycocalyx specifically has not yet been studied.
Similar to plasma and PRBCs, the processing and storage conditions of platelets affect their ability to preserve vascular permeability. Washed platelets stored for 5 days, compared to one, have approximately 50% lower levels of S1P [49] and increase vascular permeability both in vitro and in vivo [48]. There is also significant inter-donor variability in the ability of transfused platelets to preserve endothelial permeability. In addition, washed platelets stored at 4 °C (cold stored), compared to standard storage at room temperature (22 °C), were more effective at preserving endothelial permeability in both in vitro and in vivo models [50].
Crystalloids and artificial colloids
Crystalloids have no ability to restore the endothelial glycocalyx [4, 9], although they may differ in their effects on Lp (hydraulic conductivity) primarily through the effects of calcium on endothelial cells [4]. Artificial colloids do have some protective and restorative properties through an unknown mechanism, but they are inferior to albumin and FFP in this regard. This has been demonstrated in in vivo and ex vivo animal studies of endothelial glycocalyx injury, in which HES was marginally more effective than crystalloid at restoring the endothelial glycocalyx and reducing the corresponding increase in vascular permeability, but significantly inferior to albumin and FFP [9, 20].
However, the protective effect of HES seen in pre-clinical studies does not appear to translate clinically. In clinical trials of sepsis and off-pump coronary bypass graft surgery, which both caused a significant elevation in blood concentration of SDC-1, indicating glycocalyx shedding, there was no difference in blood concentrations of SDC-1 in patients resuscitated with HES compared to those resuscitated with a crystalloid [51, 52]. In addition, large randomized clinical trials of HES in critically ill patients have found no benefit to using HES over a crystalloid, instead finding that HES is associated with the increased use of blood products and the development of acute kidney injury [1]. There is little evidence about the effects of other types of artificial colloids on the endothelial glycocalyx.
Conclusion
Until fairly recently, the theoretical advantages of one type of resuscitation fluid over another have been based on a now outdated understanding of vascular permeability. Colloid fluids were considered superior to crystalloids due to their theorized greater retention within the intravascular space, but clinical trial data have neither supported this nor convincingly demonstrated a mortality or efficacy benefit from any one fluid type over another [1]. These observations are clarified by the revised Starling equation, which explains the similar volume expanding and interstitial edema forming properties of crystalloid and colloid fluids when the endothelial glycocalyx is shed and the hydrostatic pressure is low in critically ill patients, as well as other considerations, such as the effects of colloid accumulation in the interstitial space. Future research into fluid resuscitation will benefit from an updated understanding of the determinants of vascular permeability, and perhaps most promising is the identification of the endothelial glycocalyx as a possible therapeutic target. Resuscitation fluids differ in their ability to protect and restore the endothelial glycocalyx. While FFP has been identified as the most effective, further work is needed to establish the mechanisms, and to determine whether glycocalyx repair improves clinical outcomes. A fluid resuscitation strategy that protects and repairs the endothelial glycocalyx may prove to be the most effective.
Acknowledgements
Not applicable.
Funding
Publication costs were funded by the National Health and Medical Research Council Centre of Research Excellence for Patient Blood Management in Critical Illness and Trauma.
Availability of data and materials
Not applicable.
Authors’ contributions
All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Finfer S, Myburgh J, Bellomo R. Intravenous fluid therapy in critically ill adults. Nat Rev Nephrol. 2018;14:541–557. doi: 10.1038/s41581-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 2.Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87:198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 3.van der Heijden M, Verheij J, van Nieuw Amerongen GP, Groeneveld AB. Crystalloid or colloid fluid loading and pulmonary permeability, edema, and injury in septic and nonseptic critically ill patients with hypovolemia. Crit Care Med. 2009;37:1275–1281. doi: 10.1097/CCM.0b013e31819cedfd. [DOI] [PubMed] [Google Scholar]
- 4.Jacob M, Bruegger D, Rehm M, et al. The endothelial glycocalyx affords compatibility of Starling’s principle and high cardiac interstitial albumin levels. Cardiovasc Res. 2007;73:575–586. doi: 10.1016/j.cardiores.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Johansson P, Stensballe J, Ostrowski S. Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Crit Care. 2017;21:25. doi: 10.1186/s13054-017-1605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schott U, Solomon C, Fries D, Bentzer P. The endothelial glycocalyx and its disruption, protection and regeneration: a narrative review. Scand J Trauma Resusc Emerg Med. 2016;24:48. doi: 10.1186/s13049-016-0239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflug Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lekakis J, Abraham P, Balbarini A, et al. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on peripheral circulation. Eur J Cardiovasc Prev Rehabil. 2011;18:775–789. doi: 10.1177/1741826711398179. [DOI] [PubMed] [Google Scholar]
- 9.Straat M, Muller MC, Meijers JC, et al. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: a prospective substudy of a randomized trial. Crit Care. 2015;19:163. doi: 10.1186/s13054-015-0828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam EJ, Park PW. Shedding of cell membrane-bound proteoglycans. Methods Mol Biol. 2012;836:291–305. doi: 10.1007/978-1-61779-498-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19:312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levick JR. Revision of the Starling principle: new views of tissue fluid balance. J Physiol. 2004;557(Pt 3):704. doi: 10.1113/jphysiol.2004.066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levick JR. Capillary filtration-absorption balance reconsidered in light of dynamic extravascular factors. Exp Physiol. 1991;76:825–857. doi: 10.1113/expphysiol.1991.sp003549. [DOI] [PubMed] [Google Scholar]
- 14.Yen WY, Cai B, Yang JL, et al. Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. PLoS One. 2015;10:e0117133. doi: 10.1371/journal.pone.0117133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trani M, Dejana E. New insights in the control of vascular permeability: vascular endothelial-cadherin and other players. Curr Opin Hematol. 2015;22:267–272. doi: 10.1097/MOH.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 16.Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108:384–394. doi: 10.1093/bja/aer515. [DOI] [PubMed] [Google Scholar]
- 17.Tatara T. Context-sensitive fluid therapy in critical illness. J Intensive Care. 2016;4:20. doi: 10.1186/s40560-016-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn RG. Fluid therapy in uncontrolled hemorrhage--what experimental models have taught us. Acta Anaesthesiol Scand. 2013;57:16–28. doi: 10.1111/j.1399-6576.2012.02763.x. [DOI] [PubMed] [Google Scholar]
- 19.Jacob M, Chappell D, Hofmann-Kiefer K, et al. The intravascular volume effect of Ringer’s lactate is below 20%: a prospective study in humans. Crit Care. 2012;16:R86. doi: 10.1186/cc11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob M, Bruegger D, Rehm M, Welsch U, Conzen P, Becker BF. Contrasting effects of colloid and crystalloid resuscitation fluids on cardiac vascular permeability. Anesthesiology. 2006;104:1223–1231. doi: 10.1097/00000542-200606000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Borup T, Hahn RG, Holte K, Ravn L, Kehlet H. Intra-operative colloid administration increases the clearance of a post-operative fluid load. Acta Anaesthesiol Scand. 2009;53:311–317. doi: 10.1111/j.1399-6576.2008.01857.x. [DOI] [PubMed] [Google Scholar]
- 22.Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 23.Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 24.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan S, Brohi K, Chana M, et al. Hemostatic resuscitation is neither hemostatic nor resuscitative in trauma hemorrhage. J Trauma Acute Care Surg. 2014;76:561–567. doi: 10.1097/TA.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 26.Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res. 2009;104:1318–1325. doi: 10.1161/CIRCRESAHA.108.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng Y, Adamson RH, Curry FRE, Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol. 2014;306:H363–H372. doi: 10.1152/ajpheart.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamson RH, Clark JF, Radeva M, Kheirolomoom A, Ferrara KW, Curry FE. Albumin modulates S1P delivery from red blood cells in perfused microvessels: mechanism of the protein effect. Am J Physiol Heart Circ Physiol. 2014;306:H1011–H1017. doi: 10.1152/ajpheart.00829.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barelli S, Alberio L. The role of plasma transfusion in massive bleeding: protecting the endothelial glycocalyx? Front Med. 2018;5:91. doi: 10.3389/fmed.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ksiazek M, Chacinska M, Chabowski A, Baranowski M. Sources, metabolism, and regulation of circulating sphingosine-1-phosphate. J Lipid Res. 2015;56:1271–1281. doi: 10.1194/jlr.R059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pati S, Potter DR, Baimukanova G, Farrel DH, Holcomb JB, Schreiber MA. Modulating the endotheliopathy of trauma: factor concentrate versus fresh frozen plasma. J Trauma Acute Care Surg. 2016;80:576–585. doi: 10.1097/TA.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 32.Torres LN, Chung KK, Salgado CL, Dubick MA, Torres Filho IP. Low-volume resuscitation with normal saline is associated with microvascular endothelial dysfunction after hemorrhage in rats, compared to colloids and balanced crystalloids. Crit Care. 2017;21:160. doi: 10.1186/s13054-017-1745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozar RA, Peng ZL, Zhang RZ, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112:1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genet GF, Bentzer P, Ostrowski SR, Johansson PI. Resuscitation with pooled and pathogen-reduced plasma attenuates the increase in brain water content following traumatic brain injury and hemorrhagic shock in rats. J Neurotrauma. 2017;34:1054–1062. doi: 10.1089/neu.2016.4574. [DOI] [PubMed] [Google Scholar]
- 35.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One. 2011;6:e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schenk S, Schoenhals GJ, de Souza G, Mann M. A high confidence, manually validated human blood plasma protein reference set. BMC Med Genet. 2008;1:41. doi: 10.1186/1755-8794-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozar RA, Pati S. Syndecan-1 restitution by plasma after hemorrhagic shock. J Trauma Acute Care Surg. 2015;78(6 Suppl 1):S83–S86. doi: 10.1097/TA.0000000000000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diebel LN, Martin JV, Liberati DM. Microfluidics: a high-throughput system for the assessment of the endotheliopathy of trauma and the effect of timing of plasma administration on ameliorating shock-associated endothelial dysfunction. J Trauma Acute Care Surg. 2018;84:575–582. doi: 10.1097/TA.0000000000001791. [DOI] [PubMed] [Google Scholar]
- 39.Sperry JL, Guyette FX, Brown JB, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315–326. doi: 10.1056/NEJMoa1802345. [DOI] [PubMed] [Google Scholar]
- 40.Brown LM, Aro SO, Cohen MJ, et al. A high fresh frozen plasma: packed red blood cell transfusion ratio decreases mortality in all massively transfused trauma patients regardless of admission international normalized ratio. J Trauma. 2011;71(2 Suppl 3):S358–S363. doi: 10.1097/TA.0b013e318227f152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52(Suppl 1):65S–79S. doi: 10.1111/j.1537-2995.2012.03663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stensballe J, Ulrich AG, Nilsson JC, et al. Resuscitation of endotheliopathy and bleeding in thoracic aortic dissections: the VIPER-OCTA randomized clinical pilot trial. Anesth Analg. 2018;127:920–927. doi: 10.1213/ANE.0000000000003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres LN, Sondeen JL, Dubick MA, Filho IT. Systemic and microvascular effects of resuscitation with blood products after severe hemorrhage in rats. J Trauma Acute Care Surg. 2014;77:716–723. doi: 10.1097/TA.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 44.Selim S, Sunkara M, Salous AK, et al. Plasma levels of sphingosine 1-phosphate are strongly correlated with haematocrit, but variably restored by red blood cell transfusions. Clin Sci. 2011;121:565–572. doi: 10.1042/CS20110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McQuilten ZK, French CJ, Nichol A, Higgins A, Cooper DJ. Effect of age of red cells for transfusion on patient outcomes: a systematic review and meta-analysis. Transfus Med Rev. 2018;32:77–88. doi: 10.1016/j.tmrv.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Cardenas JC, Zhang X, Fox EE, et al. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. Blood Adv. 2018;2:1696–1704. doi: 10.1182/bloodadvances.2018017699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holcomb JB, Zarzabal LA, Michalek JE, et al. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 Suppl 3):S318–S328. doi: 10.1097/TA.0b013e318227edbb. [DOI] [PubMed] [Google Scholar]
- 48.Baimukanova G, Miyazawa B, Potter DR, et al. Platelets regulate vascular endothelial stability: assessing the storage lesion and donor variability of apheresis platelets. Transfusion. 2016;56(Suppl 1):S65–S75. doi: 10.1111/trf.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pienimaeki-Roemer A, Ruebsaamen K, Boettcher A, et al. Stored platelets alter glycerophospholipid and sphingolipid species, which are differentially transferred to newly released extracellular vesicles. Transfusion. 2013;53:612–626. doi: 10.1111/j.1537-2995.2012.03775.x. [DOI] [PubMed] [Google Scholar]
- 50.Baimukanova G, Miyazawa B, Potter DR, et al. The effects of 22 degrees C and 4 degrees C storage of platelets on vascular endothelial integrity and function. Transfusion. 2016;56(Suppl 1):S52–S64. doi: 10.1111/trf.13455. [DOI] [PubMed] [Google Scholar]
- 51.Muller RB, Ostrowski SR, Haase N, Wetterslev J, Perner A, Johansson PI. Markers of endothelial damage and coagulation impairment in patients with severe sepsis resuscitated with hydroxyethyl starch 130/0.42 vs ringer acetate. J Crit Care. 2016;32:16–20. doi: 10.1016/j.jcrc.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 52.Kim TK, Nam K, Cho YJ, et al. Microvascular reactivity and endothelial glycocalyx degradation when administering hydroxyethyl starch or crystalloid during off-pump coronary artery bypass graft surgery: a randomised trial. Anaesthesia. 2017;72:204–213. doi: 10.1111/anae.13642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.