Abstract
Background
Amyloid beta (Aβ) is a constituent of drusen that is a common sign of age-related macular degeneration (AMD). The purpose of this study was to investigate the effect of Aβ on human retinal pigment epithelial (RPE) cells in culture.
Methods
Cells from a human RPE cell line (ARPE-19) were exposed to 0 to 25 μM of Aβ 1–40 for 48 h, and the number of living cells was determined by WST-8 cleavage. Replicative DNA synthesis was measured by the incorporation of 5′-bromo-2′-deoxyuridine. The cell death pathway was investigated by the WST-8 cleavage assay after the addition of caspase-9 inhibitor, an anti-apoptotic factor. Real-time qRT-PCR was performed using Aβ-exposed cellular RNA to determine the level of vascular endothelial growth factor (VEGF)-A and pigment epithelium derived factor (PEDF). To determine the effect of receptor-for-advanced glycation end products (RAGE), the siRNA for RAGE was inserted into ARPE-19 treated with Aβ, and the levels of expression of VEGF-A and PEDF were determined.
Results
The number of living ARPE-19 cells was increased by exposure to 5 μM Aβ but was decreased by exposure to 25 μM of Aβ. Replicative DNA synthesis by ARPE-19 cells exposed to 25 μM of Aβ was significantly decreased indicating that 25 μM of Aβ inhibited cell proliferation. Real-time RT-PCR showed that the level of the mRNA of PEDF was increased by exposure to 5 μM Aβ, and the levels of the mRNAs of PEDF and VEGF-A were also increased by exposure to 25 μM Aβ. The addition of an inhibitor of caspase-9 blocked the decrease the number of ARPE-19 cells exposed to 25 μM Aβ. Exposure to si-RAGE attenuated the increase of VEGF-A and PEDF mRNA expression in ARPE-19 exposed to Aβ.
Conclusions
Exposure of ARPE-19 cells to low concentrations of Aβ increases the level of PEDF which then inhibits the apoptosis of ARPE-19 cells leading to RPE cell proliferation. Exposure to high concentrations of Aβ induces RPE cell death and enhances the expression of the mRNA of VEGF-A in RPE cells. The Aβ-RAGE pathway may lead to the expression VEGF-A and PEDF in RPE cells. These results suggest that Aβ is strongly related to the pathogenesis of choroidal neovascularization.
Keywords: Age-related macular degeneration, Amyloid beta, Retinal pigment epithelial cells, Vascular endothelial growth factor, Pigment epithelium-derived factor, Receptor for advanced glycation end products
Background
Age-related macular degeneration (AMD) is a progressive eye disorder that can proceed to irreversible blindness [1]. Genetic and environmental factors are known to modify the visual reduction although the relative importance of each of the factors has not been definitively determined. It has been established that one of the strongest predictors for the development of AMD is the number and size of drusen [2]. Drusen are small, yellowish-white protuberant lesions seen in the fundus ophthalmoscopically and are considered to be the initial change in eyes with AMD.
In spite of the well-known relationship between drusen and AMD, there are still some unanswered questions, e.g., how drusen form and how drusen interact with the RPE and photoreceptor cells. It was recently reported that amyloid beta (Aβ), a peptide associated with the neurodegenerative events in Alzheimer’s disease, is an important constituent of drusen [3]. Aβ is a toxic protein that affects nerve cells and is associated with many neurodegenerative disorders [4].
In the human eye, several Aβ reservoirs have been found in the retina. Elevated Aβ levels have been found in aged retinas, and Aβ has been shown to be associated with the progression of AMD [5]. Aβ 1–40 is the one of the most common and relatively less toxic form of Aβ [6]. Isas et al. reported that the concentration of Aβ1–40 is the highest among the different forms of Aβ in drusen deposits, [7] and some studies have shown how Aβ 1–40 interacts with RPE cells. For example, Liu et al. demonstrated that chronic inflammation that is an important pathogenic process in AMD is caused by the stimulation of Aβ1–40 via NF-κB activation [8], and they also demonstrated that the inflammasomes that are activated by Aβ1–40 are responsible for the upregulation of the IL-6, TNF-α, IL-1β, IL-18, caspase-1, NLRP3, and XAF1 genes in the RPE, choroid, and the neuroretina [9]. Although such findings imply that Aβ 1–40 is a potential triggering peptide for AMD, the effect of Aβ 1–40 on RPE cells remains undetermined especially on the relationship between the concentration of Aβ 1–40 and toxicity. Its effects on RPE cells need to be understood to determine its role in the development of AMD.
Thus, the purpose of this study was to determine the effects of Aβ 1–40 on human adult RPE (ARPE) cells in culture. To accomplish this, we exposed cells from an ARPE cell line to different concentrations of Aβ 1–40 and determined the number of living ARPE cells. We also examined the pathways that might be involved in the Aβ 1–40 effects on the ARPE cells.
Methods
Cell cultures
ARPE-19 cells (ATCC® CRL-2302™), a human RPE cell line [10], were obtained from American Type Culture Collection (Manassas, VA, USA). The cells were maintained in Dulbecco’s modified Eagles medium (Gibco®, Life Technologies, Carlsbad, CA) and F12 medium (Gibco®) 1:1 supplemented with 10% (v/v) fetal bovine serum (FBS), 100 units/ml penicillin G, and 100 μg/mL streptomycin (Wako Pure Chemical Industries, Ltd., Osaka, Japan). To examine the effects of Aβ on the ARPE-19 cells, several concentrations of Aβ 1–40 (Peptide Institute, Inc., Osaka, Japan) were added to the culture medium. Equivalent amount of DMSO (Wako Pure Chemical Industries), a solvent for Aβ1–40, was added to the culture medium in the control group.
Measurements of surviving cells by water-soluble tetrazolium salt WST-8
ARPE-19 cells were seeded in 96 well plates at 1.0 × 104 cells/100 μL per well. The cells were cultured with 0.5 to 25 μM of Aβ 1–40 for 48 h. After the exposure, the number of living cells was determined with the Cell Counting kit-8 (Dojindo Laboratories, Mashikimachi, Japan) according to the manufacturer’s protocol [11–13]. In brief, ARPE-19 cells were incubated with WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)- 2H-tetrazoliummonosodium salt) solution at 37 °C for 1 to 2 h. After the exposure, the optical density at 450 nm was measured with the i-Mark™ microplate reader (Bio-Rad Laboratories, Inc., California, USA).
Investigation of cell death pathway
To investigate the cell death pathway, ARPE-19 cells (1.0 × 104 cells/100 μL/well) exposed to 5 to 25 μM of Aβ- were cultured with an anti-necroptotic factor, e.g., necrostatin-1 (50 mM) or caspase-8 (1 mM) or caspase-9 inhibitors (1 mM), for 48 h [14, 15]. After that, the number of living cells was determined by WST-8 cleavage.
Measurement of replicative DNA synthesis
To determine the extent of cell proliferation, we assayed the degree of incorporation of thymidine analog BrdU (5′-bromo-2′-deoxyuridine) into newly synthesized DNA by using the DNA-BrdU Labeling and Detection kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s protocol [16, 17]. Briefly, ARPE-19 cells were cultured in 96-well plates at 1.0 × 104 cells/100 μL with different concentrations of Aβ 1–40. The cells were exposed to the BrdU solution Aβ 1–40 for 48 h. After the exposure, the optical density at 450 nm was measured with the i-MarkTM microplate reader (Bio-Rad Laboratories).
VEGF-A and PEDF messenger RNA induction
After 48 h of incubation with Aβ 1–40, the NucleoSpin RNA kit (Takara Bio, Inc., Kusatsu, Japan) was used to extract the total RNA from the ARPE-19 cells (1.0 × 104 cells/100 μL per well) according to the manufacturer’s protocol. cDNAs were synthesized from the total RNA with the Exscript RT Reagent kit (Takara Bio, Inc). The PCR primers corresponding to nucleotides 2465–2536 of the mRNA of human VEGF-A (NM_001025366) and 489–630 for PEDF mRNA (NM_002615) were synthesized by the Takara Bio, Inc. as described in detail [16–21]. Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed using SYBR® Premix Ex Taq™II (Takara Bio, Inc.,) and LightCycler® (Roche Diagnostics, Mannheim, Germany). For internal control, 1043–1228 for human ß-actin mRNA (NM_001101) (Takara Bio, Inc.,) was used.
PEDF concentration in culture medium
To quantify the degree of expression of PEDF by ARPE-19 cells, the cells were seeded in 96 well plates at 1.0 × 104 cells/100 μL per well and were cultured with 5 to 25 μM of Aβ 1–40 for 48 h. The concentration of PEDF in the culture medium was measured with the human PEDF enzyme-linked immunosorbent assay (ELISA) kit (BioProducts MD, LLC., Middletown, US) according to the manufacturer’s protocol.
RNA interference for receptor of advanced glycation end products (RAGE)
A small interfering RNA (siRNA) against RAGE was synthesized by Silencer® Select predesigned siRNA (Santa Cruz Biotechnology). The target siRNA for RAGE, sc-36,374, and a human scrambled siRNA, sc-37,007, were purchased from Santa Cruz Biotechnology as control siRNA. Transfection of ARPE-19 cells by the siRNAs was performed according to the manufacturer’s protocol.
Statistical analyses
The results are expressed as the means ± standard error of the means (SEMs). Student’s unpaired t-tests were performed to determine the statistical significance of the differences using the Graph Pad Prism software (GraphPad Software, La Jolla, CA).
Results
Exposure of ARPE-19 cells to 5 μM of Aβ 1–40 increases number of living ARPE-19 cells but exposure to 25 μM decreases number of living ARPE cells
To evaluate the effects of Aβ 1–40 on the proliferation and death of ARPE-19 cells, the cells were exposed to different concentrations of Aβ 1–40 for 48 h. The number of living cells was determined by the WST-8 assay. The results showed that the number of living cells was significantly higher after exposure to 5 μM Aβ 1–40 than in the controls. However, the number of living cells was decreased significantly by exposure to 25 μM of Aβ 1–40 (Fig. 1).
Fig. 1.
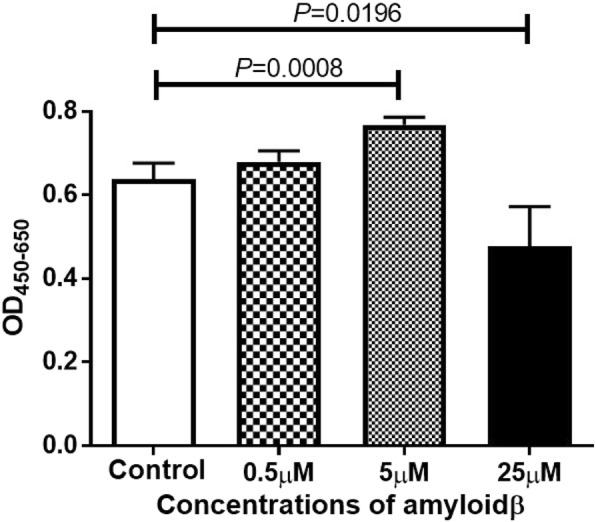
Effect of amyloid beta (Aβ) 1–40 exposure on the cells of a human adult retinal pigment epithelial cell (ARPE-19) line in culture. The ARPE-19 cells were incubated for 48 h with different concentrations of Aβ 1–40, and the number of living cells were determined by the WST-8 assay. The number of living cells was increased by exposure to 5 μM Aβ 1–40 but the number of living cells was decreased by 25 μM of Aβ 1–40 compared to the control group. Data are the means ± standard error of the means (SEMs) for each group (n = 4)
Exposure of ARPE-19 cells to 25 μM of Aβ 1–40 decreases replicative DNA synthesis and induces apoptosis of RPE cells
The extent of replication of ARPE-19 cells was determined by the incorporation of BrdU. The replicative DNA synthesis by ARPE-19 cells exposed to 25 μM of Aβ 1–40 was significantly decreased (P < 0.04; 25 μM Aβ 1–40 vs control) indicating that 25 μM of Aβ 1–40 inhibited cellular proliferation (Fig. 2).
Fig. 2.
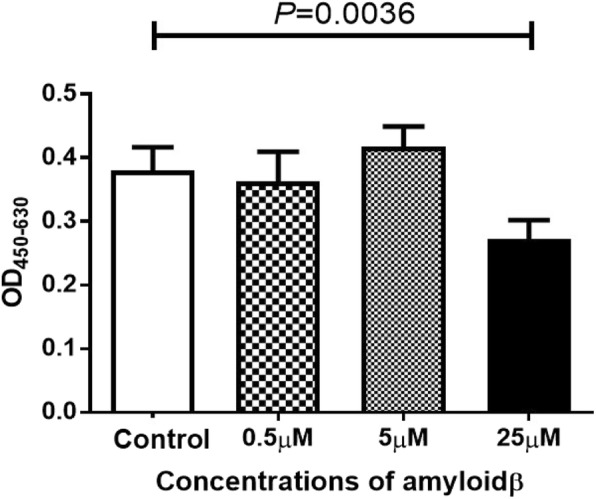
Effect of Aβ 1–40 on the DNA synthesis of ARPE-19 cells incubated for 24 h with Aβ 1–40. With 25 μM Aβ, there was a significant decrease in the DNA synthesis in the ARPE-19 cells. Data are the means ± SEMs for each group (n = 4)
Inhibitory assays for apoptosis and necroptosis were performed to determine whether Aβ increased the degree of apoptosis. The results showed that the addition of a caspase-9 inhibitor suppressed the decrease in the number of ARPE-19 cells exposed to 25 μM Aβ. This suggested that the addition of Aβ 1–40 leads to apoptosis of the RPE cells through the caspase-9 cascade. (Fig. 3).
Fig. 3.
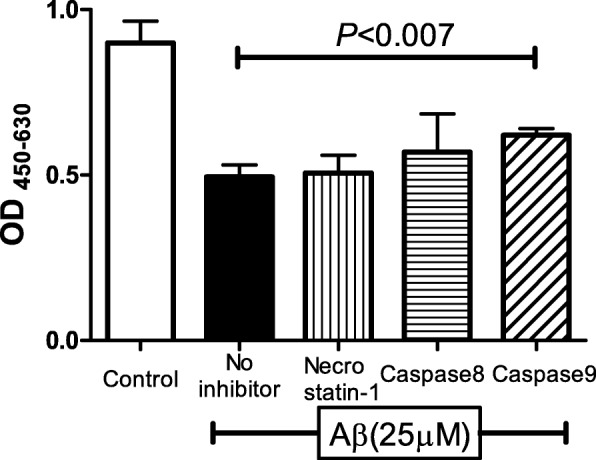
Apoptosis of ARPE-19 cells exposed to Aβ 1–40. The addition of a caspase-9 inhibitor suppresses the decrease in the number of living ARPE-19 cells exposed to 25 μM Aβ. This indicates that the apoptosis of ARPE-19 cell by 25 μM Aβ exposure was through the caspase-9 pathway. Data are the means ± SEMs for each group (n = 4)
On the other hand, exposure of the caspase-9 inhibitor to 5 μM of Aβ 1–40 increased the number of living ARPE-19 cells, but the difference in the replicative DNA synthesis between the control group and the 5 μM Aβ group was not significant. These results indicate that 5 μM of Aβ 1–40 suppressed the apoptosis of RPE cells.
Exposure to Aβ 1–40 changes level of expression of mRNA of VEGF-A and PEDF in ARPE-19 cells
It has been established that VEGF-A plays an important role in the pathology of AMD including RPE proliferation and choroidal neovascularization [22]. The level of expression of the mRNA of VEGF-A was determined by real-time RT-PCR. The results showed that the expression of VEGF-A mRNA was significantly increased only in the 25 μM Aβ 1–40 group (Fig. 4a).
Fig. 4.
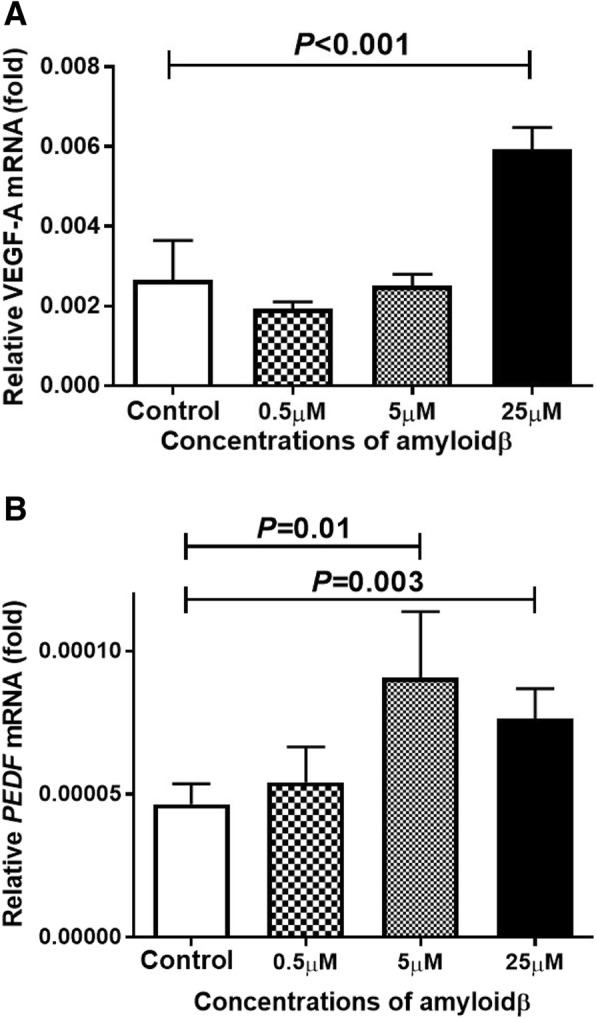
Induction of VEGF-A and PEDF expression in ARPE cells by exposure to Aβ 1–40. ARPE-19 cells were exposed to 25 μM Aβ 1–40 for 24 h, and the expressions of the mRNAs of VEGF-A and PEDF were determined by real-time RT-PCR using β-actin as an endogenous control. The level of the mRNA of VEGF-A is significantly increased only in the 25 μM Aβ group (A). On the other hand, the level of the mRNA of PEDF is increased by 5 μM Aβ 1–40 and is also increased by 25 μM Aβ 1–40 exposure (B). Data are the means ± SEMs for each group (n = 4)
PEDF was originally discovered in RPE cells, and it was found to play different roles and had anti-angiogenic, neuroprotective, and anti-apoptotic properties. We determined the expression of the mRNA of PEDF by real-time RT-PCR and found that the expression of the mRNA of PEDF in the ARPE-19 cells was increased after exposure to 5 μM Aβ 1–40 (P < 0.001 vs no addition). In addition, the levels of the mRNAs of VEGF-A and PEDF were also increased by prior exposure to 25 μM Aβ 1–40 (P < 0.001 vs no addition; P = 0.003 vs no addition respectively; Fig. 4b).
Next, ELISA was performed to confirm that PEDF was secreted into the culture medium of the ARPE-19 cells. The secretion of PEDF was significantly increased after exposure to 5 μM Aβ 1–40 (P < 0.05 vs no addition; Fig. 5).
Fig. 5.
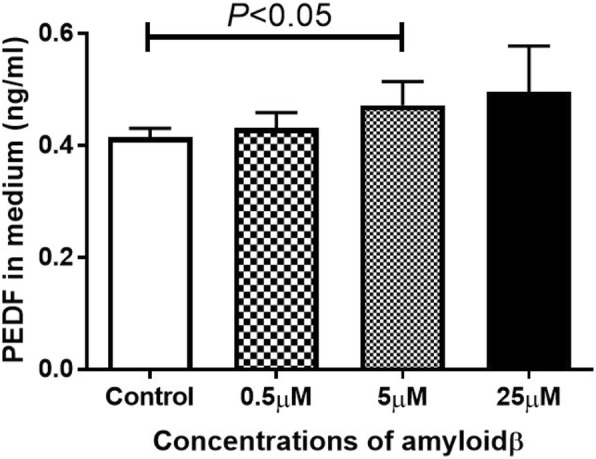
Concentrations of PEDF in the ARPE-19 cell culture medium determined by ELISA. ARPE-19 cells were exposed to Aβ 1–40 for 24 h. The concentration of PEDF is significantly increased only in the 5 μM Aβ 1–40 group. Data are the means ± SEMs for each group (n = 4)
Inhibition of PEDF signaling decreases cell proliferation
To determine whether PEDF exposure affected RPE cell proliferation, we added the PEDF receptor inhibitor, GW9662, to the Aβ 1–40-exposed ARPE-19 cells and measured the number of living cells with the WST-8 assay. The results showed that the number of living ARPE-19 cells exposed to Aβ 1–40 were significantly reduced by GW9662 (Fig. 6). This suggested that silencing the effect of PEDF decreased the number of living cells.
Fig. 6.
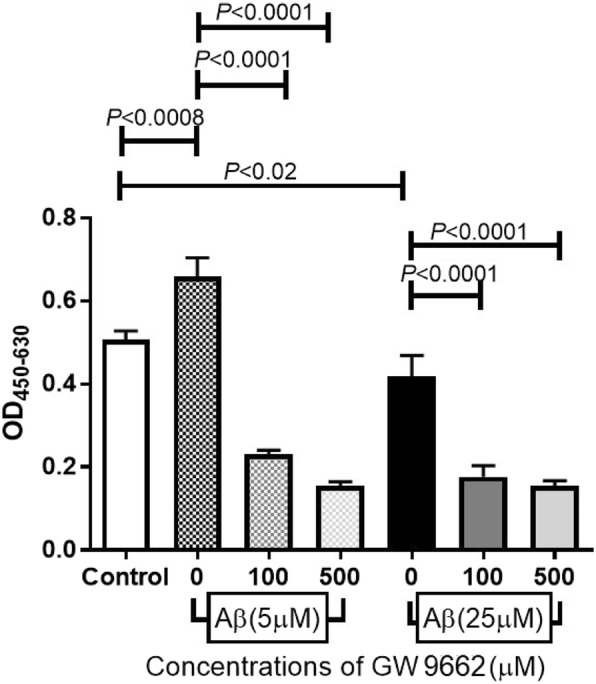
Inhibition of the PEDF signaling decreases cell proliferation. Aβ 1–40-exposed ARPE-19 cells were incubated with a PEDF intercellular inhibitor, GW9662. After 24 h, the number of living cells was measured by WST-8 cleavage. The number of Aβ 1–40-exposed ARPE-19 cells is significantly reduced by the combined addition of GW9662 and Aβ 1–40 compared to addition of Aβ 1–40 alone. Data are the means ± SEMs for each group (n = 4)
Knockdown of RAGE by siRNA attenuated changes of VEGF, PEDF expression, and living cell numbers caused by Aβ
It has been shown that Aβ can activate different signaling pathways and thereby activate a series of cell surface receptors. The results of several studies have shown that RAGE, a multi-ligand receptor for AGE, plays an important role as a receptor for Aβ. To determine whether RAGE was involved in the Aβ-stimulated RPE cell proliferation, we inserted a siRNA against RAGE into ARPE-19 cells, and then exposed them to Aβ 1–40. Our results showed that a knockdown of RAGE attenuated the increase and decrease of VEGF and PEDF expressions caused by the exposure to Aβ (Fig. 7a and b). In addition, Si-RAGE attenuated the change of viable RPE cell numbers induced by the addition of Aβ (Fig. 7c). These results indicated that Aβ caused a change in the viable cell number, and this stimulation is mediated mainly by RAGE.
Fig. 7.
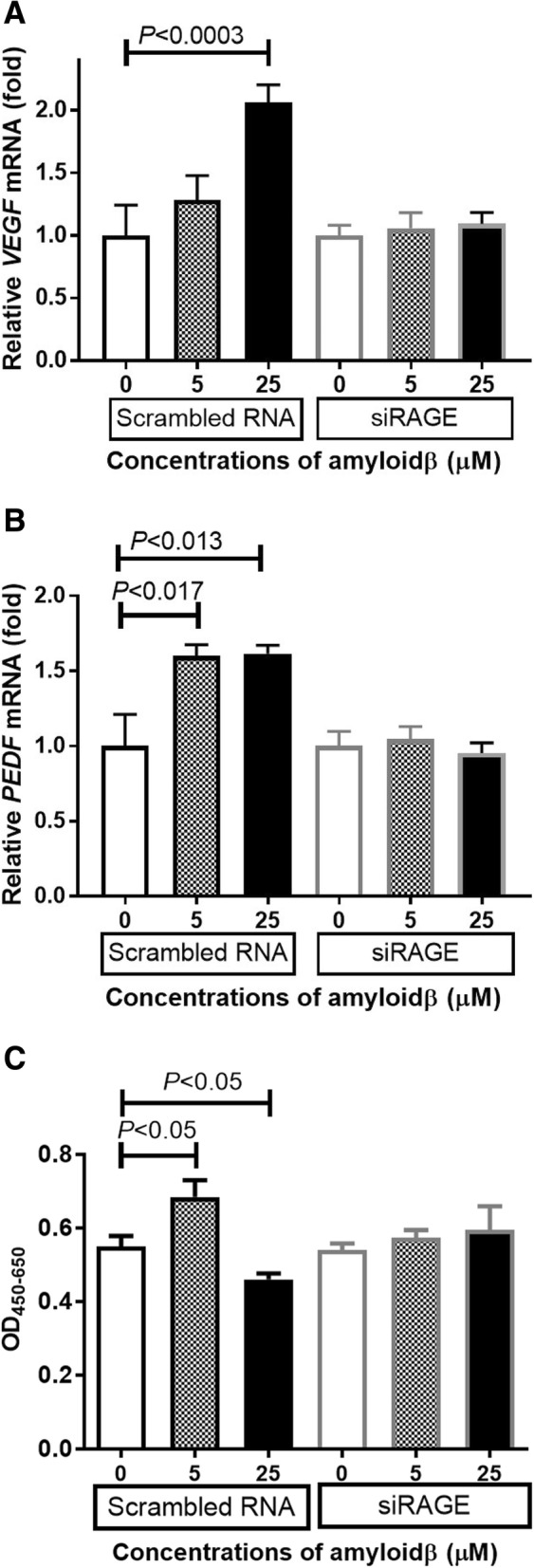
Relationship between RAGE and Aβ in the expression of VEGF and PEDF. Si RAGE-inserted ARPE-19 cells were incubated with Aβ 1–40 for 24 h, and the expression of the mRNA of VEGF-A and PEDF were measured by real-time RT-PCR using β-actin as an endogenous control. The control in each group was defined as 1 and show the number of relative comparisons in the experimental group. After 48 h of incubated with Aβ 1–40, the living cell number was measured by WST-8 assay. Knockdown of RAGE attenuated the increase and decrease of VEGF (a) and PEDF (b) expression caused by Aβ. In addition, Si-RAGE attenuated the increase and decrease of viable RPE cell number induced by Aβ addition (c). Data are the means ± SEMs for each group (n = 4)
Discussion
The Aβs are 36–43 amino acid peptides that are associated with the pathogenesis of Alzheimer’s disease. It is well-known that amyloid plaques are specific findings in Alzheimer’s disease, and the plaques consist of several types of Aβ peptides. Aβ is produced from amyloid β precursor protein cleaved by β selectase or γ selectase [23]. The normal function of Aβ has not been determined although some experimental studies have demonstrated that the lack of Aβ does not cause any loss of physiological function [24]. However, there is also some evidence that Aβ has molecular protective activities including that against oxidative stress [25, 26]. On the other hand, Aβ is associated with the progression of other diseases, and the results of several studies showed that Aβ is involved in the pathogenesis of AMD including studies that used a molecular approach [27–29]. However, the functions of Aβ in human RPE cells have not been conclusively determined.
In this study, we had predicted that high concentrations of Aβ would be necessary to study the pathogenesis of AMD, and we used Aβ 1–40 that is relatively less toxic but more commonly distributed in drusen compared to other forms of Aβ. Our results demonstrated that the concentration of Aβ 1–40 regulated the expression of PEDF and VEGF in RPE cells in culture.
RAGE was originally found to be a receptor recognizing advanced-glycation end products (AGEs). RAGE consists of three superficial domains, and one of the domains recognizes AGEs [30]. After the activation of RAGE by binding to the AGEs, the AGE-RAGE pathway can lead to acute and chronic inflammatory disorders such as AMD [31]. In addition to the AGEs, there are other ligands that bind to RAGE. For example, Aβ and S100/calgranulins are also ligands of RAGE [32]. Because RAGE is also expressed on the surface of RPE cells and is increased under AMD conditions, we examined whether the Aβ-RAGE pathway is involved in the development of neovascular macular diseases with the expression of some cytokines.
Our results showed that the number of living RPE cell was significantly increased by exposure to 5 μM Aβ but markedly decreased by an exposure to 25 μM Aβ. These results indicated that the influence of Aβ on retinal pigment epithelial cells depends on its concentration. Yoshida et al. demonstrated that the concentration of VEGF in the conditioning medium of RPE cells exposed to Aβ increased in a dose dependent manner [33]. We confirmed that the expression of VEGF-A is increased in ARPE-19 cells after exposure to Aβ, and the mRNA of VEGF was elevated after exposure to higher concentrations of Aβ. Because these results cannot be explained by the change in the number of living cells, we also measured the concentration of PEDF in the culture medium. PEDF is a glycoprotein that belongs to the superfamily of serine protease inhibitors [34], and it has been shown to have a protective effect in retinal diseases especially age-related macular degeneration [35, 36]. Wang et al. reported that PEDF suppressed the apoptosis of RPE cells and the expression of inflammatory cytokines in a mouse AMD model [37]. In our study, the level of VEGF did not change but that of PEDF increased after exposure to 5 μM Aβ. Green et al. performed histopathologic studies of human CNVs, and they reported that CNVs were enveloped by proliferating RPE cells [38]. The proliferation of RPE cells in the 5 μM Aβ group may have been caused by the expression of PEDF, and it represents the proliferation of the surrounding pigment epithelial cells in eyes with age-related macular degeneration. PEDF has cellular protective effects even in the 25 μM Aβ group but the strong apoptosis signal in the 25 μ M group decreased the number of living cells. These findings are supported by the significant decrease in the number of living cells after exposure to GW 9662, an antagonist of PPAR-γ and a known nuclear inhibitor of PEDF [39].
These results predicted that apoptosis of RPE cells is high in the regions where Aβ deposits are found and VEGF enhances the development of CNVs in human eyes. On the other hand, when the number of Aβ deposits is low and RPE damage is slight, PEDF predominantly functions. Under these conditions, there is a protection and proliferation of the RPE cells.
Our results showed that the number of living cells was decreased by exposure to 25 μM of Aβ, and caspase-9 inhibition partially reduced the loss of living cells in the 25 μM Aβ group. Because caspase-9 is activated when apoptosis occurs through the caspase pathway, these results suggest that the apoptosis is mediated by caspase 9, and it is related to the death of retinal pigment epithelial cells caused by high concentration of Aβ. In addition, the siRNA knockdown study showed that Aβ probably acts by binding to RAGE. The Aβ-RAGE pathway can then lead RPE cells to caspase-9-related apoptosis.
This study was solely based on an in vitro approach in which RPE culture was the main component, and we mainly focused on molecular changes. While RPE dysfunction caused by Aβ is essential to the pathogenesis of AMD, choroidal endothelial cells may also play an important role [40]. But cross talk between the RPE cells and choroidal cells was not investigated in our study. In addition, we did not examine the functional assessments of the RPE cells including the RPE barrier or their phagocytic function. Thus, it is important to show the changes of the functional assessments of RPE. Further experiments are needed for understanding the pathogenesis of AMD.
According to a recent report [41], aducanumab, an anti-Aβ antibody, can reduce Aβ plaques in the brain of patients with Alzheimer’s disease. It has still not been tested whether this antibody is also effective on the Aβ deposits in the human retina. However, if the role played by amyloid β in age-related macular degeneration can be further determined, it may be possible that this drug could be used to treat eyes with AMD.
Conclusions
The results showed that a low concentration of Aβ increases the level of PEDF and thus inhibits the apoptosis of RPE cells and increase the number of RPE cells. A high concentration Aβ induces RPE cell death through the caspase-9 cascade and enhances VEGF-A transcription in RPE cells. We suggest that this may lead to the development of choroidal neovascularization. The Aβ-RAGE pathway may lead to the expression VEGF-A and PEDF in ARPE-19 cells.
Acknowledgements
None.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Grant-in- Aid for Encouragement of Scientists; KAKENHI Grant Number #26931058). However, the funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The all data used to support the findings of this study are available from the corresponding author upon request.
Authors’ contributions
NM, HT and NO contributed to the design of the study; NM, HT, HH, MY, conducted the study; HH, MY and TU acquired the data; NM, MY and NO prepared the manuscript; HT drafted the manuscript; and TU, and NO crucially revised the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
This research was performed on the ARPE-19 cell line in vitro and did not involve live human or animal subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Naonori Masuda, Email: masuda828@naramed-u.ac.jp.
Hiroki Tsujinaka, Email: kinstar@naramed-u.ac.jp.
Hiromasa Hirai, Email: hirai-masa@naramed-u.ac.jp.
Mariko Yamashita, Email: mariko.yamashita@naramed-u.ac.jp.
Tetsuo Ueda, Email: tueda@naramed-u.ac.jp.
Nahoko Ogata, Email: ogata@naramed-u.ac.jp.
References
- 1.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 2.Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol. 1999;44(1):1–29. doi: 10.1016/S0039-6257(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 3.Hamley IW. The amyloid beta peptide: a chemist's perspective. Role in Alzheimer's and fibrillization. 2012 ;112(10):5147–92. doi: 10.1021/cr3000994. [DOI] [PubMed]
- 4.Ratnayaka JA, Serpell LC, Lotery AJ. Dementia of the eye: the role of amyloid beta in retinal degeneration. Eye (Lond) 2015;29(8):1013–1026. doi: 10.1038/eye.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohno-Matsui K. Parallel findings in age-related macular degeneration and Alzheimer's disease. Prog Retin Eye Res. 2011;30(4):217–238. doi: 10.1016/j.preteyeres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem 2002;30;277(35):32046–32053. [DOI] [PubMed]
- 7.Isas JM, Luibl V, Johnson LV, Kayed R, Wetzel R, Glabe CG, Langen R, Chen J. Soluble and mature amyloid fibrils in drusen deposits. Invest Ophthalmol Vis Sci. 2010;51(3):1304–1310. doi: 10.1167/iovs.09-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu RT, Wang A, To E. Gao J, Cao S, Cui JZ, Matsubara JA. Vinpocetine inhibits amyloid-beta induced activation of NF-κB, NLRP3 inflammasome and cytokine production in retinal pigment epithelial cells. Exp Eye Res. 2014;127:49–58. doi: 10.1016/j.exer.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu RT, Gao J, Cao S, Sandhu N, Cui JZ, Chou CL, Fang E, Matsubara JA. Inflammatory mediators induced by amyloid-beta in the retina and RPE in vivo: implications for inflammasome activation in age-related macular degeneration. Invest Ophthalmol Vis Sci 2013 1;54(3):2225–2237. doi: 10.1167/iovs.12-10849. [DOI] [PMC free article] [PubMed]
- 10.Yoshikawa T, Ogata N, Izuta H, Shimazawa M, Hara H, Takahashi K. Increased expression of tight junctions in ARPE-19 cells under endoplasmic reticulum stress. Curr Eye Res. 2011;36(12):1153–1163. doi: 10.3109/02713683.2011.606592. [DOI] [PubMed] [Google Scholar]
- 11.Masui T, Ota I, Itaya-Hironaka A, Takeda M, Kasai T, Yamauchi A, Sakuramoto-Tsuchida S, Mikami S, Yane K, Takasawa S, Hosoi H. Expression of REG III and prognosis in head and neck cancer. Oncol Rep. 2013;30(2):573–578. doi: 10.3892/or.2013.2521. [DOI] [PubMed] [Google Scholar]
- 12.Kyotani Y, Ota H, Itaya-Hironaka A, Yamauchi A, Sakuramoto-Tsuchida S, Zhao J, Ozawa K, Nagayama K, Ito S, Takasawa S, Kimura H, Uno M, Yoshizumi M. Intermittent hypoxia induces the proliferation of rat vascular smooth muscle cell with the increases in epidermal growth factor family and erbB2 receptor. Exp Cell Res 2013 15;319(19):3042–3050. doi: 10.1016/j.yexcr.2013.08.014. [DOI] [PubMed]
- 13.Ota H, Itaya-Hironaka A, Yamauchi A, Sakuramoto-Tsuchida S, Miyaoka T, Fujimura T, Tsujinaka H, Yoshimoto K, Nakagawara K, Tamaki S, Takasawa S, Kimura H. Pancreatic β cell proliferation by intermittent hypoxia via up-regulation of Reg family genes and HGF gene. Life Sci 2013 4;93(18–19):664–672. doi: 10.1016/j.lfs.2013.09.001. [DOI] [PubMed]
- 14.Saveljeva S, Mc Laughlin SL, Vandenabeele P, Samali A, Bertrand MJ. Endoplasmic reticulum stress induces ligand-independent TNFR1-mediated necroptosis in L929 cells. Cell Death Dis. 2015;8(6):e1587. doi: 10.1038/cddis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra D, Choy G, Deng X, Bhatia B, Daniel P, Tang DG. Association of active caspase 8 with the mitochondrial membrane during apoptosis: potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Mol Cell Biol. 2004;24(15):6592–6607. doi: 10.1128/MCB.24.15.6592-6607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi S, Akiyama T, Nata K, Abe M, Tajima M, Shervani NJ, Unno M, Matsuno S, Sasaki H, Takasawa S, Okamoto H. Identification of a receptor for reg (regenerating gene) protein, a pancreatic beta-cell regeneration factor. J Biol Chem 2000 14;275(15):10723–10726. [DOI] [PubMed]
- 17.Shervani NJ, Takasawa S, Uchigata Y, Akiyama T, Nakagawa K, Noguchi N, Takada H, Takahashi I, Yamauchi A, Ikeda T, Iwamoto Y, Nata K, Okamoto H. Autoantibodies to REG, a beta-cell regeneration factor, in diabetic patients. Eur J Clin Investig. 2004;34(11):752–758. doi: 10.1111/j.1365-2362.2004.01419.x. [DOI] [PubMed] [Google Scholar]
- 18.Ota H, Tamaki S, Itaya-Hironaka A, Yamauchi A, Sakuramoto-Tsuchida S, Morioka T, Takasawa S, Kimura H. Attenuation of glucose-induced insulin secretion by intermittent hypoxia via down-regulation of CD38. Life Sci 2012 30;90(5–6):206–211. doi: 10.1016/j.lfs.2011.11.011. [DOI] [PubMed]
- 19.Nakagawa K, Takasawa S, Nata K, Yamauchi A, Itaya-Hironaka A, Ota H, Yoshimoto K, Sakuramoto-Tsuchida S, Miyaoka T, Takeda M, Unno M, Okamoto H. Prevention of Reg I-induced β-cell apoptosis by IL-6/dexamethasone through activation of HGF gene regulation. Biochim Biophys Acta. 2013;1833(12):2988–2995. doi: 10.1016/j.bbamcr.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimoto K, Fujimoto T, Itaya-Hironaka A, Miyaoka T, Sakuramoto-Tsuchida S, Yamauchi A, Takeda M, Kasai T, Nakagawara K, Nonomura A, Takasawa S. Involvement of autoimmunity to REG, a regeneration factor, in patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2013;174(1):1–9. doi: 10.1111/cei.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami-Kawaguchi S, Takasawa S, Onogawa T, Nata K, Itaya-Hironaka A, Sakuramoto-Tsuchida S, Yamauchi A, Ota H, Takeda M, Kato M, Okamoto H. Expression of Ins1 and Ins2 genes in mouse fetal liver. Cell Tissue Res. 2014;355(2):303–314. doi: 10.1007/s00441-013-1741-4. [DOI] [PubMed] [Google Scholar]
- 22.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22(1):1–29. doi: 10.1016/S1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 23.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 24.Luo Y, Bolon B, Damore MA, Fitzpatrick D, Liu H, Zhang J, Yan Q, Vassar R, Citron M. BACE1 (beta-secretase) knockout mice do not acquire compensatory gene expression changes or develop neural lesions over time. Neurobiol Dis. 2003;14(1):81–88. doi: 10.1016/S0969-9961(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 25.Zou K, Gong JS, Yanagisawa K, Michikawa M. A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage. J Neurosci. 2002;22(12):4833–4841. doi: 10.1523/JNEUROSCI.22-12-04833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baruch-Suchodolsky R, Fischer B. Abeta40, either soluble or aggregated, is a remarkably potent antioxidant in cell-free oxidative systems. Biochemistry. 2009;26;48(20):4354–4370. doi: 10.1021/bi802361k. [DOI] [PubMed]
- 27.Prasad T, Zhu P, Verma A, Chakrabarty P, Rosario AM, Golde TE, Li Q. Amyloid β peptides overexpression in retinal pigment epithelial cells via AAV-mediated gene transfer mimics AMD-like pathology in mice. Sci Rep 2017 12;7(1):3222. doi: 10.1038/s41598-017-03397-2. [DOI] [PMC free article] [PubMed]
- 28.Sun J, Huang P, Liang J, Li J, Shen M, She X, Feng Y, Luo X, Liu T, Sun X. Cooperation of Rel family members in regulating Aβ1-40-mediated pro-inflammatory cytokine secretion by retinal pigment epithelial cells. Cell Death Dis. 2017;8(10):e3115. doi: 10.1038/cddis.2017.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynn SA, Keeling E, Munday R, Gabha G, Griffiths H, Lotery AJ, Ratnayaka JA. The complexities underlying age-related macular degeneration: could amyloid beta play an important role? Neural Regen Res. 2017;12(4):538–548. doi: 10.4103/1673-5374.205083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto Y, Kato I, Doi T, Yonekura H, Ohashi S, Takeuchi M, Watanabe T, Yamagishi S, Sakurai S, Takasawa S, Okamoto H, Yamamoto H. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest. 2001;108(2):261–268. doi: 10.1172/JCI11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsujinaka H, Itaya-Hironaka A, Yamauchi A, Sakuramoto-Tsuchida S, Ota H, Takeda M, Fujimura T, Takasawa S, Ogata N. Human retinal pigment epithelial cell proliferation by the combined stimulation of hydroquinone and advanced glycation end-products via up-regulation of VEGF gene. Biochem Biophys Rep. 2015;29(2):123–131. doi: 10.1016/j.bbrep.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma W, Lee SE, Guo J, Qu W, Hudson BI, Schmidt AM, Barile GR. RAGE ligand upregulation of VEGF secretion in ARPE-19 cells. Invest Ophthalmol Vis Sci. 2007;48(3):1355–1361. doi: 10.1167/iovs.06-0738. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida T, Ohno-Matsui K, Ichinose S, Sato T, Iwata N, Saido TC, Hisatomi T, Mochizuki M, Morita I. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J Clin Invest. 2005;115(10):2793–2800. doi: 10.1172/JCI24635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53(3):411–414. doi: 10.1016/0014-4835(91)90248-D. [DOI] [PubMed] [Google Scholar]
- 35.Ogata N, Wada M, Otsuji T, Jo N, Tombran-Tink J, Matsumura M. Expression of pigment epithelium-derived factor in normal adult rat eye and experimental choroidal. Invest Ophthalmol Vis Sci. 2002;43(4):1168–1175. [PubMed] [Google Scholar]
- 36.Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K, Matsumura M. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004;88(6):809–815. doi: 10.1136/bjo.2003.032466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Subramanian P, Shen D, Tuo J, Becerra SP, Chan CC. Pigment epithelium-derived factor reduces apoptosis and pro-inflammatory cytokine gene expression in a murine model of focal retinal degeneration. ASN Neuro. 2013;5(5):e00126. doi: 10.1042/AN20130028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green WR, Key SN., 3rd Senile macular degeneration: a histopathologic study. Trans Am Ophthalmol Soc. 1977;75:180–254. [PMC free article] [PubMed] [Google Scholar]
- 39.Ishibashi Y, Matsui T, Ohta K, Tanoue R, Takeuchi M, Asanuma K, Fukami K, Okuda S, Nakamura K, Yamagishi S. PEDF inhibits AGE-induced podocyte apoptosis via PPAR-gamma activation. Microvasc Res. 2013;85:54–58. doi: 10.1016/j.mvr.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Ohno-Matsui K, Nakahama K, Okamoto A, Yoshida T, Shimada N, Mochizuki M, Morita I. Amyloid beta enhances migration of endothelial progenitor cells by upregulating CX3CR1 in response to fractalkine, which may be associated with development of choroidal neovascularization. Arterioscler Thromb Vasc Biol. 2011;31(7):e11–e18. doi: 10.1161/ATVBAHA.110.215517. [DOI] [PubMed] [Google Scholar]
- 41.Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O'Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;1;537(7618):50–56. doi: 10.1038/nature19323. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The all data used to support the findings of this study are available from the corresponding author upon request.


