Abstract
Background
CEA, CYFRA21-1 and NSE are tumor markers used for monitoring the response to chemotherapy in advanced adenocarcinoma, squamous cell carcinoma and small-cell lung cancer, respectively. Their role in cancer immunotherapy needs to be elucidated.
Methods
Patients with advanced non-small cell lung cancer (NSCLC) were treated with nivolumab 3 mg/kg every 2 weeks within the Italian Nivolumab Expanded Access Program. Blood samples were collected at baseline, at each cycle up to cycle 5 and then every two cycles until patient’s withdrawn from the study. All patients underwent a CT-scan after every 4 cycles of treatment and responses were classified according to RECIST 1.1. The biomarkers serum levels were measured with a chemiluminescent microparticle immunoassay for CEA and with an immuno radiometric assay for CYFRA21-1 and NSE. The markers values at baseline and after 4 cycles were used to analyze the relationship between their variation over baseline and the tumor response, evaluated as disease control rate (DCR: CR + PR + SD), and survival (PFS and OS).
Results
A total of 70 patients were evaluable for the analysis. Overall, a disease control was obtained in 24 patients (35.8%, 4 PR + 20 SD). After 4 cycles of nivolumab a CEA or CYFRA21-1 reduction ≥ 20% over the baseline was significantly associated with DCR (CEA, p = 0.021; CYFRA21-1, p < 0.001), PFS (CEA, p = 0.028; CYFRA21-1, p < 0.001) and OS (CEA, p = 0.026; CYFRA21-1, p = 0.019). Multivariate analysis confirmed the ability of CYFRA21-1 reduction ≥ 20% to predict DCR (p = 0.002) and PFS (p < 0.001).
Conclusion
The reduction in serum level of CYFRA21-1 or CEA might be a reliable biomarker to predict immunotherapy efficacy in NSCLC patients. NSE was not significant for monitoring the efficacy of nivolumab.
Keywords: NSCLC, CYFRA21-1, CEA, Immunotherapy, Tumor response, Survival
Background
Advanced lung cancer remains the leading cause of cancer related deaths worldwide being the treatment of disease still challenging [1]. Immunotherapy is a standard of treatment in advanced non-small cell lung cancer (NSCLC) patients progressing after a first-line chemotherapy or as first-line treatment in combination with chemotherapy or as single agent in patients with high expression of PD-L1. Several agents targeting immune checkpoints have been tested with remarkable results on survival and manageable toxicity [2]. Nivolumab (BMS-936558) is a fully human IgG4 programmed cell death 1 (PD-1) immune checkpoint inhibitor that enhances the immune T cell response by blocking the interaction between the PD-1, an inhibitory receptor on activated T lymphocytes, and the programmed cell death ligand 1 (PD-L1) expressed on cancer cells. Two randomized Phase III studies have been reported on squamous (CheckMate 017) and non-squamous (CheckMate 057) NSCLC [3, 4] leading to drug approval by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for advanced or metastatic NSCLC after prior chemotherapy. This improvement in the management of advanced NSCLC has required the identification of prognostic and/or predictive biomarkers to select the best candidates to immunotherapy and to monitor the tumor response [5]. PD-L1 expression has been widely explored as a potential marker but its role in the clinical setting is still controversial [6]. Serological biomarkers such as carcinoembryonic antigen (CEA), cytokeratin fragment 19 (CYFRA21-1) and neuron-specific enolase (NSE), have been mainly investigated as prognostic or predictive markers in NSCLC patients treated with chemotherapy [7, 8]. CEA is a serum glycoprotein and currently is the most widely used marker for colorectal, breast and lung cancer. Increased levels of CEA are observed in smokers and in presence of non-neoplastic disease [9, 10]. CYFRA21-1 is a fragment of cytokeratin 19 that is abundant in the pulmonary tissue. Serum concentrations are particularly elevated in the carcinoid tumors and in squamous cell carcinoma of the lung where it correlates with the tumor size, lymph node status and the stage of disease [11, 12]. As a result, CEA and CYFRA21-1 have been identified as useful prognostic factors [7–13], as predictors of efficacy for targeted therapy [14, 15] or chemotherapy [8] and as markers of postoperative recurrence and metastasis [16–18]. NSE is a cytosolic enzyme expressed at high levels in the brain and preferentially in neurons and neuroendocrine cells [19]. As a specific serum marker of neuronal injury, elevated levels of NSE have been found in cancers of neuroendocrine cellular origin, including small-cell lung cancer (SCLC) where it correlates with the extent of disease [20, 21]. For SCLC the NSE has a specificity around 85% and is useful for prognosis of survival, monitoring of treatment and prediction of relapse [16, 21, 22]. Increased levels of NSE have also been reported in NSCLC where its role as predictive and prognostic marker is still under debate. Tiseo et al. reported a significant correlation between higher baseline serum NSE levels and response to standard first-line chemotherapy in advanced NSCLC whereas did not find a prognostic role [23]. A recent meta-analysis including 2389 NSCLC patient has confirmed the lack of prognostic significance for NSE [24]. In addition, in a recent study Fiala et al. have showed a negative predictive role of high baseline NSE levels in NSCLC patients treated with epidermal growth factor tyrosine kinase inhibitors (EGFR-TKIs) [25]. The role of CEA, CYFRA21-1 and NSE in monitoring the response to immunotherapy in NSCLC patients needs to be elucidated. In the present study we tested the hypothesis that their variation compared to the baseline may act as indicators of treatment efficacy and survival in advanced NSCLC patients treated with nivolumab.
Methods
Patient’s enrollment
Between May 2015 and May 2016, 74 consecutive patients with advanced NSCLC previously treated with at least one line of chemotherapy were prospectively enrolled in a single-institutional translational research study at the Ospedale Policlinico San Martino in Genova, Italy, within the Italian Nivolumab Expanded Access Program. This study was approved by the Ethics Committee of Liguria Region (Italy) (P.R.191REG2015) and conducted in compliance with the principle of the Declaration of Helsinky; a written informed consent was acquired from all patients. All the patients were treated with nivolumab at the dose of 3 mg/kg every 2 weeks until disease progression, unacceptable toxicity, patient refusal, or death. Baseline assessments were done with a computed tomography scan (CT scan) of the chest and abdomen within 2 weeks before treatment and then after 4 cycles of treatment. The tumor response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST ver 1.1) [26]. Each patient’s response was classified into one of the following categories: responders, including case of complete response (CR), partial response (PR) and stable disease (SD), and non-responders including cases of disease progression (PD). Disease control rate (DCR) was defined as those patients who had obtained a CR, a PR or a SD. For patients who achieved a PD, an additional assessment was performed after 2 further cycles to confirm PD; if PD was confirmed, treatment was discontinued.
Specimen collection and tumor marker assays
The tumor markers were determined collecting a blood sample before treatment initiation (baseline visit), at each cycle up to cycle 5 and then every two cycles until patient’s withdrawn from the study. Serum levels of CEA were detected using a commercially available chemiluminescent microparticle immunoassay (Architect CEA Reagent kit, Abbott Diagnostics Division) whereas CYFRA21-1 and NSE were detected using a commercially available immuno radiometric assay (Cytokeratin 19 Fragment IRMA Kit and NSE IRMA Kit, Beckman Coulter Inc.) according to the manufacturer’s instructions. The reference range was 0 to 5 ng/ml for CEA, 0 to 3.3 ng/ml for CYFRA 21-1, 0 to 13.4 ng/ml for NSE. Hemolyzed samples were excluded from the analysis. The markers levels at baseline and after 4 cycles of nivolumab were used to analyze the relationship between their variation over the baseline and the tumor response, considered as disease control rate (DCR), progression free survival (PFS) and overall survival (OS). On the basis of the results from an our previous study in advanced NSCLC patients treated with standard first-line chemotherapy [8], a post-treatment drop in serum concentration ≥ 20% over baseline was used as cut-off level for defining a marker response. In addition, a sub-analysis of the three markers in the different histological types was further investigated.
Statistical analysis
Variables were summarized as median (range) for continuous variables and number (%) for categorical variables. Relationships between categorical variables were examined by means of the Chi square test. Patients were categorized according to median age (≤ 70 and > 70 years) and histology (adenocarcinoma vs squamous cell carcinoma). Non-parametric tests were used to check differences between the two groups and to compare the markers values at baseline and after 4 cycles of treatment. Odds Ratios (OR) and the corresponding 95% confidence intervals (95% CI) for a set of individual and clinical variables were computed to predict therapy response in a multiple logistic analysis. Univariate and multivariate analyses were performed to evaluate the prognostic impact on PFS and on OS; PFS was calculated from the start of nivolumab to the date of PD or death or last follow-up; OS was calculated from the start of nivolumab to the date of death or last follow-up. The Kaplan–Meier method was applied to estimate survival probabilities and the log-rank test was carried out to assess heterogeneity within each prognostic factor. Cox’s proportional hazards regression model was carried out as multivariate analysis to assess the prognostic role of the markers adjusted for the possible confounding effect of all other factors included in the same model. All statistical test were two-sided, and variables that had p-values of less than 0.05 were considered significant.
Results
Patients and tumor characteristics
Seventy out of 74 patients were evaluable for serum markers and response assessment after 4 cycles of nivolumab (4 patients were excluded from the analysis for hemolyzed baseline samples). Three patients stopped nivolumab for toxicity before the first CT scan evaluation. The clinicopathological characteristics are summarized in Table 1. The median age was 70 years (range 44–85) and 69% of patients were male. NSCLC included 54 adenocarcinomas (77%), 15 squamous cell carcinomas (22%) and one case of not otherwise specified (NOS) type (1%). The majority of the patients were smokers (87%), had metastatic disease (96%) and ECOG PS 0–1 (92%). The median number of prior lines of treatment was 2 (range 1–6). The median value of the serum levels of the three markers at baseline (pre-treatment) was 6.6 ng/ml for CEA (range 0.8–2615), 5 ng/ml for CYFRA21-1 (range 0.2–126.4) and 7.5 ng/ml (range 3.1–46.8) for NSE. Pre-treatment values over the upper normal limit of CEA, CYFRA 21-1 and NSE were detected in 40 (57%), 45 (64%), and 14 (20%) patients, respectively. At cycle 2 and cycle 3 data on CEA were available for 59 and 54 patients, respectively, while data on CYFRA 21-1 were available for 57 and 54 patients, respectively. At the same time points data on NSE were available for 58 and 50 patients, respectively.
Table 1.
Clinicopathological characteristics
| No. of patients (70) | % | |
|---|---|---|
| Age, median (range, year) | 70 (44–85) | |
| Gender | ||
| Male | 48 | 69 |
| Female | 22 | 31 |
| Histology | ||
| Adenocarcinoma | 54 | 77 |
| Squamous | 15 | 22 |
| NOS | 1 | 1 |
| Stage | ||
| IIIB | 3 | 4 |
| IV | 67 | 96 |
| ECOG PS | ||
| 0 | 25 | 36 |
| 1 | 39 | 56 |
| 2 | 6 | 8 |
| Smoking habits | ||
| Never smoker | 9 | 13 |
| Former smoker | 35 | 50 |
| Smoker | 26 | 37 |
| Prior lines of therapy | Median 2 (range 1–6) | |
| 1 | 28 | 40 |
| 2 | 20 | 29 |
| 3 | 13 | 19 |
| ≥ 4 | 9 | 12 |
| CEA (ng/ml) baseline | ||
| Median (range) | 6.6 (0.80–2615) | |
| Normal (< 5) | 30 | 43 |
| Elevated (≥ 5) | 40 | 57 |
| CYFRA 21-1 (ng/ml) baseline | ||
| Median (range) | 5.0 (0.2–126.4) | |
| Normal (< 3.3) | 25 | 36 |
| Elevated (≥ 3.3) | 45 | 64 |
| NSE (ng/ml) baseline | ||
| Median (range) | 7.5 (3.1–46.8) | |
| Normal (< 3.3) | 56 | 80 |
| Elevated (≥ 3.3) | 14 | 20 |
NOS, not otherwise specified; ECOG, Eastern cooperative oncology group; PS, performance status; CEA, carcinoembryonic antigen; CYFRA21-1, cytokeratin fragment 19; NSE, neuron-specific enolase
Correlation between serum markers levels, clinic-pathologic features and tumor response
No significant correlation was found between baseline markers serum levels and age or gender. Abnormal baseline CEA levels were found in current smokers (p = 0.048) and in adenocarcinomas (p < 0.001). Abnormal, but not significant, baseline CYFRA21-1 levels were found in squamous tumors. No association was found between baseline NSE levels and patient and cancer characteristics (data not shown). On average, patients received 6 cycles of nivolumab (range 1–36) and a first CT scan evaluation was performed after a median time of 6.9 weeks, corresponding to 4 cycles of nivolumab,. Overall, a disease control was obtained in 24/67 patients (35.8%, 4 PR and 20 SD). Age, gender, histology, stage, ECOG PS, smoking habit and baseline serum levels did not correlate with response to nivolumab (data not shown). After 4 cycles of nivolumab the median CEA and NSE levels remained rather stable compared to baseline (5.1 ng/ml and 7.4 ng/ml, respectively) while the median CYFRA 21-1 levels dropped to 2.7 ng/ml. Interestingly, in those patients who obtained a DCR we observed a decline of all three serum markers with a significant difference between responders and no-responders (Table 2). Overall, CEA, CYFRA 21-1 and NSE reduction ≥ 20% occurred in 13/49 (26%), 17/50 (34%) and 16/44 (36%) patients, respectively, and a CEA and CYFRA 21-1 reduction were associated with favorable DCR (Table 3). With RECIST, a decrease ≥ 20% of CEA was achieved in 43.5% of responders and in 11.5% of no-responders (p = 0.021), while a decrease ≥ 20% of CYFRA21-1 occurred in 62.5% of responders and in 7.7% of no-responders (p < 0.001). Interestingly, we observed that a tumor response occurred in 87.5% of patients with a CYFRA21-1 reduction ≥ 20% already presents after the 1st cycle (p = 0.008) and in 80% of patients with a CEA reduction ≥ 20% already presents after the 2nd cycle (p = 0.033) (data not shown).
Table 2.
CEA, CYFRA 21-1 and NSE variation according to response to nivolumab
| Median (%) | Range (%) | p-value | |
|---|---|---|---|
| CEA | |||
| No responder | + 31 | − 79; + 498 | 0.005 |
| Responder | − 9 | − 92: + 88 | |
| CYFRA21-1 | |||
| No responder | + 72 | − 62; + 508 | < 0.001 |
| Responder | − 37 | − 98; + 2220 | |
| NSE | |||
| No responder | + 20 | − 64; + 182 | 0.012 |
| Responder | − 14 | − 79; + 71 | |
Table 3.
Markers reduction ≥ 20% over baseline and tumor response (R)
| No-R n (%) |
R n (%) |
p-value | |
|---|---|---|---|
| CEA reduction ≥ 20% | |||
| No | 23 (88.5) | 13 (56.5) | 0.021 |
| Yes | 3 (11.5) | 10 (43.5) | |
| CYFRA21-1 reduction ≥ 20% | |||
| No | 24 (92.3) | 9 (37.5) | < 0.001 |
| Yes | 2 (7.7) | 15 (62.5) | |
| NSE reduction ≥ 20% | |||
| No | 17 (73.9) | 11 (52.4) | 0.21 |
| Yes | 6 (26.1) | 10 (47.6) | |
Multivariate analysis, including variables for age, gender, CEA and CYFRA reduction ≥ 20%, revealed that CYFRA21-1 reduction ≥ 20% was an independent positive predictor factor for DCR (HR 4.36, 95% CI 1.7 to 11.3, p = 0.002) (Table 4). Interestingly, we observed that the reduction ≥ 20% of the tumor markers was already evident at the beginning of the therapy. In particular, the decrease of at least 20% had already evident after the 1th, 2nd and 3rd cycle in 5%, 20%, and 28% of patients for CEA, in 15%, 37% and 39% of patients for CYFRA21-1 and in 26%, 22%, and 34% of patients for NSE, respectively (data not shown). Finally, analyzing the tumor markers on the basis of histotype we observed that patients with adenocarcinoma reached a DCR when CEA and CYFRA21-1 reduction was ≥ 20%, with a significant difference in response compared to the patients with marker reduction < 20% (CEA, 77% vs 40%, p = 0.043; CYFRA21-1, 92% vs 35%, p = 0.001). Among the patients with squamous cell carcinoma, we observed a reduction ≥ 20% for CYFRA21-1 and NSE but only a CYFRA21-1 reduction resulted in DCR (p = 0.033). In both histological types NSE reduction ≥ 20% did not show to be significantly associated with DCR (data not shown).
Table 4.
Ability of CEA and CYFRA 21-1 to predict DCR (CR + PR + SD) in a multivariate analysis
| Odds ratio | 95% CI | p-value | |
|---|---|---|---|
| Gender | |||
| Male | 1.0 | 0.13 | |
| Female | 1.85 | (0.8–4.1) | |
| Age | |||
| ≤ 70 | 1.0 | 0.48 | |
| > 70 | 1.31 | (0.6–2.8) | |
| CEA reduction ≥ 20% | |||
| No | 1.0 | 0.32 | |
| Yes | 1.58 | (0.6–3.9) | |
| CYFRA 21-1 reduction ≥ 20% | |||
| No | 1.0 | 0.002 | |
| Yes | 4.36 | (1.7–11.3) | |
Association between CEA, CYFRA 21-1, NSE and PFS
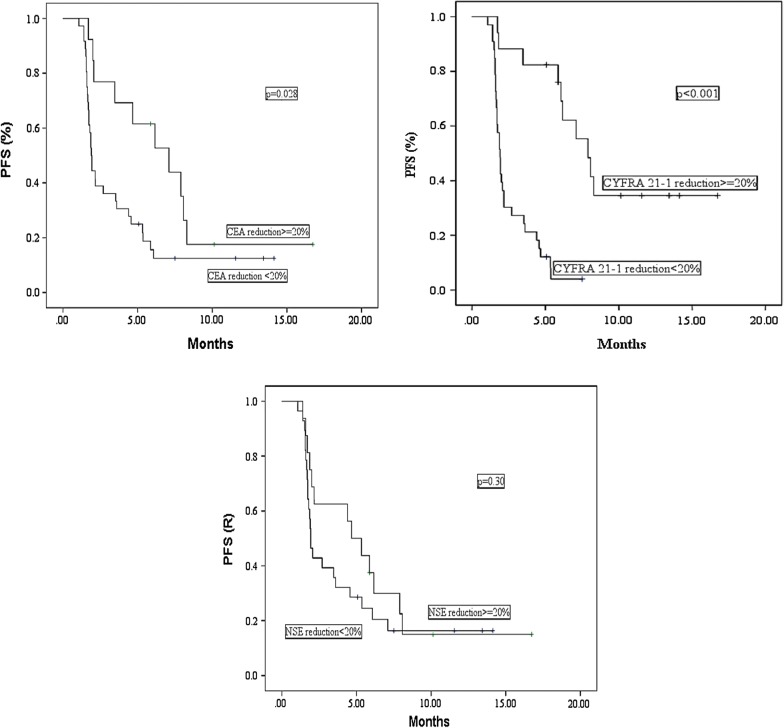
Overall, median PFS on 67 patients was 1.9 months (95% CI 1.7–2.2 months). Age, sex, histology, PS, smoking, prior treatment lines and baseline serum marker levels were not associated with PFS. In contrast, a longer PFS was observed in patients with normal baseline CEA values (2.7 months vs 1.7 months, p = 0.026) and with a CEA and CYFRA21-1 reduction ≥ 20% after 4 cycles of nivolumab (CEA: 7.1 vs 1.9 months, p = 0.028; CYFRA21-1: 7.9 vs 1.9 months, p < 0.001). No significant association was found between NSE reduction ≥ 20% and PFS (4.7 vs 1.9 months, p = 0.300) (Fig. 1). Multivariate analysis including terms for gender, age, CEA, CYFRA21-1 and NSE reduction ≥ 20% confirmed the positive prognostic role only for CYFRA21-1 reduction ≥ 20% (HR = 0.35, 95% CI 0.20–0.60, p < 0.001) (data not shown). Considering histology, a marker’s reduction improved PFS in adenocarcinoma patients (CEA,7.1 vs 1.9 months, p = 0.013; CYFRA21-1, 7.9 vs 1.9 months p < 0.001; NSE 5.9 vs 1.9 months, p = 0.067), while in patients with squamous carcinoma PFS was improved only in patients with CYFRA21-1 reduction ≥ 20% (6.1 vs 1.7 months, p = 0.032).
Fig. 1.
Progression-free survival according to CEA, CYFRA21-1 and NSE reduction ≥ 20%
Association between CEA, CYFRA 21-1, NSE and OS
For the whole study population, median follow up was 10.7 months (range 5.0–16.8) for censored patients and 3.1 months (range 0.1–13.2) for deceased patients. The association between clinicopathological characteristics and serum markers with OS is shown in Table 5. Median survival time was 9.2 months (95% CI 5.3–13.2). During the study period, 40 patients (57.1%) died. In the univariate analysis, a statistically significant prognostic effect was found for number of prior lines of treatment (n = 1, 6.1 months, 95% CI = 3.6–8.5; n ≥ 2, 12.2 months, 95% CI = 8.2–13.3, p = 0.036) and for response to therapy (13.5 months for responders vs 6.4 months for no-responders, p < 0.001). At baseline, normal markers levels were significantly associated with better OS: 12.1 months for CEA < 5 ng/ml vs 5.6 months for CEA ≥ 5 ng/ml, p = 0.035; 13.2 months for CYFRA21-1 < 3.3 ng/ml vs 5.6 months for CYFRA21-1 ≥ 3.3 ng/ml, p = 0.005 and 10.0 months for NSE < 13.4 ng/ml vs 2.2 months for NSE ≥ 13.4 ng/ml, p = 0.028.
Table 5.
OS according to clinicophathological characteristics
| Mean OS (95% CI)a (months) | p-value | |
|---|---|---|
| Overall | 9.2 (5.3–13.2) | |
| Age (years) | ||
| ≤ 70 | 6.1 (0.3–11.8) | 0.27 |
| > 70 | 10.0 (7.2–12.8) | |
| Gender | ||
| Male | 8.9 (5.1–12.8) | 0.76 |
| Female | 9.2 (2.3–16.1) | |
| Histology | ||
| Adenocarcinoma | 9.2 (4.6–13.9) | 0.56 |
| Squamous | 9.8 (2.5–17.2) | |
| PS ECOG | ||
| 0 | 9.2 (5.6–12.8) | 0.65 |
| > 1 | 2.0 (0.1–5.4) | |
| Smoke | ||
| Never smoker | 9.9 (0.1–20.5) | 0.80 |
| Smoker | 8.9 (4.7–13.2) | |
| Prior treatment lines, n | ||
| 1 | 6.1 (3.6–8.5) | 0.036 |
| ≥ 2 | 12.2 (8.2–13.3) | |
| RECIST response | ||
| No response | 6.4 (4.8–8.0) | < 0.001 |
| Response | 13.5 (11.2–15.7) | |
aMedian survival not reached
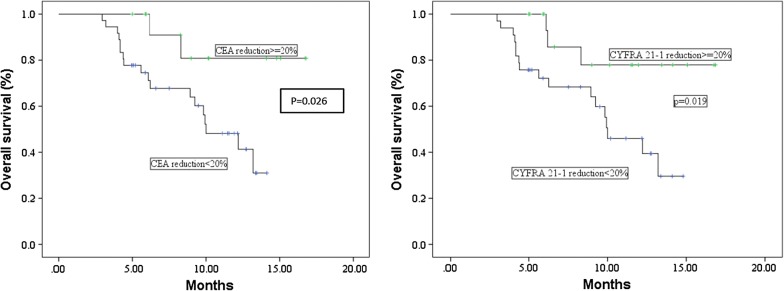
In addition, also a reduction ≥ 20% of CEA or CYFRA21-1 after 4 cycles of nivolumab represented a positive prognostic factor (Table 6). In particular, Kaplan–Meier survival curves showed that patients with CEA or CYFRA21-1 reduction ≥ 20% survived longer than patients with no marker reduction (15 months vs 9.9 months, p = 0.026 and 14.6 months vs 10 months, p = 0.019, respectively) (Fig. 2). Multivariate analysis taking into account gender, age, prior lines of therapy and baseline CEA, CYFRA21-1 and NSE levels showed a better prognosis for patients with a higher number of therapies (≥ 2 lines: HR = 0.67, 95% CI 0.48–0.94, p = 0.022) and with normal baseline CEA or CYFRA21-1 levels (CEA ≤ 5 ng/ml: HR = 0.70, 95% CI 0.49–1.01, p = 0.057; CYFRA21-1 ≤ 3.3 ng/ml: HR = 0.68, 95% CI 0.46–1.01, p = 0.055). Multivariate analysis taking into account CEA and CYFRA21-1 reduction ≥ 20% did not show statistically significant results but a tendency towards a better prognosis for patients with a CYFRA21-1 reduction ≥ 20% (HR = 0.55, 95% CI 0.28–1.07, p = 0.079) (data not shown). Finally, with regard to histologic subtypes, no significant difference in OS was observed between patients with adenocarcinoma compared to squamous carcinoma (median OS, 9.2 vs 9.8 months). Of note, OS was significantly increased only among adenocarcinoma patients with CEA reduction ≥ 20% (median OS, 14.8 vs 9.9 months, p = 0.054) (data not shown).
Table 6.
OS according to baseline serum levels and CEA, CYFRA and NSE reduction ≥ 20%
| Mean OS (95% CI)a (months) | p-value | |
|---|---|---|
| Baseline CEA | ||
| < 5 | 12.2 (8.1–16.0) | 0.035 |
| ≥ 5 | 5.6 (2.9–8.2) | |
| Baseline CYFRA21-1 | ||
| < 3.3 | 13.2 (11.0–14.3) | 0.005 |
| ≥ 3.3 | 5.6 (3.4–7.7) | |
| Baseline NSE | ||
| < 13.4 | 10.0 (6.2–13.7) | 0.028 |
| ≥ 13.4 | 2.2 (0.2–5.0) | |
| CEA reduction ≥ 20% a | ||
| No | 9.9 (8.5–11.3) | 0.026 |
| Yes | 15.0 (12.7–17.3) | |
| CYFRA21-1 reduction ≥ 20% a | ||
| No | 10.0 (8.4–11.6) | 0.019 |
| Yes | 14.6 (12.4–16.8) | |
| NSE reduction ≥ 20% a | ||
| No | 11.6 (9.9–13.4) | 0.950 |
| Yes | 12.4 (9.8–15.0) | |
aMedian survival not reached
Fig. 2.
Overall survival according to CEA and CYFRA21-1 reduction ≥ 20%
Discussion
Immune checkpoint inhibitors such as anti-PD1 and anti PD-L1, are a recent option of treatment widely used for advanced cancers, including NSCLC. However, a substantial proportion of patients do not respond to these agents and display severe toxicities that lead to discontinuation of treatment [27]. On the other hand, in a small proportion of patients who do response, immunotherapy appears capable of producing long-term responses with substantial survival benefits [28]. For these reasons the discovery of biomarkers able to predict efficacy would be useful to select patients who might benefit from this therapy. Recently, particularly in melanoma cancer, several studies have investigated the association between routinely available peripheral blood biomarkers and response to immunotherapy [29–35]. Baseline or post-treatment changes in absolute leucocytes count (ALC), leucocytes sub-type counts, serum lactate dehydrogenase (LDH) and CRP levels, are among the most promising aim able to predict tumor response and survival in advanced melanoma patients treated with anti-PD-1 [30, 31] or anti-CTLA4 therapy [32–35]. Conversely, in advanced NSCLC, a few blood markers have been proposed as prognostic biomarkers for nivolumab therapy. In particular, higher baseline neutrophil to lymphocytes ratio (NLR) and platelet to lymphocyte ratio (PLR) have shown significant association with worse survival outcomes [36]. In addition, a recent study has examined a panel of six blood biomarkers showing as a combination of high ALC, high absolute eosinophil count (AEC) and low absolute neutrophil count (ANC) was associated with better survival outcome in NSCLC patients treated with nivolumab [37]. The role of CEA and CYFRA21-1 in monitoring tumor response during a first-line chemotherapy has been previously demonstrated in a publication from our Institution [8] and in a recent meta-analysis [38], but their role as predictive or treatment monitoring markers with immunotherapy has not yet been elucidated. To the best of our knowledge, this is the first study focusing on the role of CEA, CYFRA21-1 and NSE as potential markers for tumor response in advanced NSCLC patients treated with nivolumab. Interestingly, after 4 cycles of nivolumab, we observed that a CEA or CYFRA21-1 reduction ≥ 20% over the baseline was significantly associated with a better response (at least a disease control) whereas high baseline markers serum levels did not correlate with response to nivolumab. Multivariate analysis confirmed the positive association between CYFRA21-1 reduction and DCR. In addition previous studies in advanced NSCLC patients had showed that changes in CEA or CYFRA21-1 levels during chemotherapy [8, 38], radiochemotherapy [39] or targeted therapy [15, 16], had a higher predictive value than baseline level alone, indicating the usefulness of both markers for treatment monitoring. In agreement with these studies, we observed a reduction of the tumor markers > 20% already at the beginning of the therapy, in particular after the first two cycles, suggesting a possible role as markers able of monitoring the tumor response in an initial phase of the treatment also with immunotherapy. We also observed a good concordance between histological types and tumor markers. In adenocarcinoma and squamous cell carcinoma a CEA and a CYFRA21-1 reduction ≥ 20%, respectively, were significantly associated with a tumor response to nivolumab. In our study high baseline values of CEA and CYFRA21-1 were associated with worse OS, and, only for CEA, also with worse PFS. In this regard, data in literature are rather controversial. A recent study [40] reported as a pretreatment serum CYFRA21-1 level ≥ 2.2 ng/ml was an independent predictor of a favorable PFS (median PFS 155 vs 51.5 days, p = 0.05), while according to other authors [41] a baseline serum CEA level ≥ 5 ng/ml was associated with worse PFS. In our study multivariate analysis showed that normal baseline CEA or CYFRA21-1 levels and a more than 2 prior lines of therapies were independent prognostic factors in patients treated with nivolumab. These results suggest that NSCLC patients with normal pretreatment CEA or CYFRA21-1 test show a better OS. In addition, we observed a significant correlation between markers reduction after 4 cycles of nivolumab and survival outcome. In particular, a CEA or CYFRA21-1 reduction ≥ 20% was significantly associated with better PFS and OS. Specifically, in the multivariate analysis the CYFRA21-1 reduction ≥ 20% contributed significantly to the prediction of PFS and had a significant trend towards a positive prognostic factor. Interestingly, in patients with adenocarcinoma we observed a positive association between CEA or CYFRA21-1 reduction ≥ 20% and longer PFS whereas in patients with lung squamous carcinoma a CYFRA21-1 reduction ≥ 20% was statistically associated with better PFS. In both the histotypes similar median OS was observed whereas longer median OS was observed only for adenocarcinoma patients with a CEA reduction ≥ 20%. Therefore, CEA and CYFRA21-1 seem to have a better performance when monitoring adenocarcinoma patients, whereas the low number of squamous carcinoma patients did not allow to draw a conclusion in this sense. These results confirm the association of CEA with adenocarcinoma and of CYFRA21-1 with squamous carcinoma reported in previous studies [42, 43]. We are aware of the limitation of our study. This was a mono-centric study in which all consecutive patients were treated with nivolumab in an expanded access program. However, since to include the patients in this program the physicians were obligated to follow some inclusion and exclusion criteria, that have not allowed to treat all the patients with nivolumab, the risk of a patient selection bias cannot be excluded. Indeed, our study included a relatively homogeneous population with the majority of the patients stage IV, male and smokers. A strength of our study, is the mono-institutional approach that ensure that all the clinical and instrumental assessments and survival data (DCR, PFS and OS) as well as the laboratory analysis were performed consistently among all the patients before and during the treatment and data were not missed. The reduced number of patients and events in our study did not allow to draw definitive conclusions and for this reason further investigations are warranted. However, the correlation of the CEA and CYFRA21-1 reduction ≥ 20% with DCR and longer PFS was highly significant. If validated, these findings may be useful to physicians to make clinical decision; for example, nivolumab treatment may be stopped in patient without an evidence of a radiologic response and without CEA or CYFRA21-1 reduction ≥ 20% at this time-point, given their poor survival outcome and their extremely low probability of achieving a controlled disease. In conclusion, CEA and CYFRA21-1 may serve as realible markers of efficacy in NSCLC patients treated with nivolumab, either when considering the determination of the markers at baseline, or a markers reduction ≥ 20% after 4 cycles of nivolumab. On the contrary, the reduction of NSE was not significant for monitoring the efficacy of nivolumab. Further studies in a large population need to be conducted to confirm these results that may predict response and survival to immunotherapy.
Conclusion
In summary, in advanced NSCLC patients we investigated the utility of analyzed three available serum tumor markers in predict tumor response and survival during the treatment with nivolumab. This is the first study that has analyzed the correlation between CEA, CYFRA21-1 and NSE reduction over the baseline and the tumor response. We found that a CEA or CYFRA21-1 reduction ≥ 20% after 4 cycles of nivolumab may serve as a reliable early marker of efficacy significantly associated with better DCR and PFS. Monitoring the changes in CEA or CYFRA21-1 during the treatment with nivolumab may be of great interest for the prediction of tumor response and survival.
Authors’ contributions
FG designed the study; AMO and MM performed the tests on the serum samples; FR performed the statistical analysis; DBMG and FR summarized the data and drafted the manuscript. All of the authors contributed to the revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
SC is a Ph.D. supported by the Italian Ministry of Health (GR2011-12; 02350922). This study was supported by the Italian Ministry of Health (CO-2016-3; 02361470).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The dataset used and analyzed within the current study is available from the corresponding author upon reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All of the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Written informed consent was obtained from each patient included in the study. The study protocol has been approved by the Ethics Committee of Liguria Region (Italy) (P.R.191REG2015).
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AEC
absolute neutrophil count
- ALC
absolute leucocytes count
- ANC
absolute eosinophil count
- anti-PD1
anti-programmed cell death 1
- anti-PD-L1
anti-programmed cell death ligand 1
- CEA
carcinoembryonic antigen
- 95%CI
95% confidence intervals
- CR
complete response
- CRP
C-reactive protein
- CT scan
computed tomography scan
- CTLA4
cytotoxic T-lymphocyte antigen 4
- CYFRA21-1
cytokeratin fragment 19
- DCR
disease control rate
- EGFR-TKIs
epidermal growth factor tyrosine kinase inhibitors
- ECOG
Eastern Cooperative Oncology Group
- EMA
european medicines agency
- FDA
food and drug administration
- HR
hazard ratio
- LDH
serum lactate dehydrogenase
- NLR
neutrophil to lymphocytes ratio
- NOS
not otherwise specified
- NSCLC
non-small cell lung cancer
- NSE
neuron-specific enolase
- OR
odds ratios
- OS
overall survival
- PD
disease progression
- PD-1
programmed cell death 1
- PD-L1
programmed cell death ligand 1
- PFS
progression free survival
- PLR
platelet to lymphocyte ratio
- PR
partial response
- PS
performance Status
- RECIST
response evaluation criteria in solid tumors
- SCLC
small-cell lung cancer
- SD
stable disease
Contributor Information
M. G. Dal Bello, Email: mariagiovanna.dalbello@hsanmartino.it
R. A. Filiberti, Email: rosa.filiberti@hsanmartino.it
A. Alama, Email: angela.alama@hsanmartino.it
A. M. Orengo, Email: annamaria.orengo@hsanmartino.it
M. Mussap, Email: michele.mussap@hsanmartino.it
S. Coco, Email: simona.coco@hsanmartino.it
I. Vanni, Email: irenevanni85@yahoo.it
S. Boccardo, Email: simona.boccardo@hsanmartino.it
E. Rijavec, Email: ery80x@yahoo.it
C. Genova, Email: carlo.genova1985@gmail.com
F. Biello, Email: febiello@gmail.com
G. Barletta, Email: giulia.barletta@yahoo.it
G. Rossi, Email: giovanni.rossi.1689@gmail.com
M. Tagliamento, Email: tagliamento.marco@gmail.com
C. Maggioni, Email: claudia_87m@yahoo.it
F. Grossi, Email: fg1965@yahoo.it
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.1, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11.
- 2.Rijavec E, Genova C, Alama A, et al. Role of immunotherapy in the treatment of advanced non-small-cell lung cancer. Future Oncol. 2014;10:79–90. doi: 10.2217/fon.13.145. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thakur MK, Gadgeel SM. Predictive and prognostic biomarkers in non-small cell lung cancer. Semin Respir Crit Care Med. 2016;37:760–770. doi: 10.1055/s-0036-1592337. [DOI] [PubMed] [Google Scholar]
- 6.Dal Bello MG, Alama A, Coco S, et al. Understanding the checkpoint blockade in lung cancer immunotherapy. Drug Discov Today. 2017;22:1266–1273. doi: 10.1016/j.drudis.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZH, Han YW, Liang H, et al. Prognostic value of serum CYFRA21-1 and CEA for non-small-cell lung cancer. Cancer Med. 2015;4:1633–1638. doi: 10.1002/cam4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ardizzoni A, Cafferata MA, Tiseo M, et al. Decline in serum carcinoembryonic antigen and cytokeratin 19 fragment during chemotherapy predicts objective response and survival in patients with advanced nonsmall cell lung cancer. Cancer. 2006;107:2842–2849. doi: 10.1002/cncr.22330. [DOI] [PubMed] [Google Scholar]
- 9.Stockley RA, Shaw J, Whitfield AG, et al. Effect of cigarette smoking, pulmonary inflammation, and lung disease on concentrations of carcinoembryonic antigen in serum and secretions. Thorax. 1986;41:17–24. doi: 10.1136/thx.41.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth SN, King JP, Leonard JC, Dykes PW. Serum carcinoembryonic antigen in clinical disorders. Gut. 1973;14:794–799. doi: 10.1136/gut.14.10.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molina R, Agusti C, Filella X, et al. Study of a new tumor marker, CYFRA 21-1, in malignant and nonmalignant diseases. Tumour Biol. 1994;15:318–325. doi: 10.1159/000217908. [DOI] [PubMed] [Google Scholar]
- 12.Sertić Milić H, Franjević A, Bubanović G, et al. Size, edge, and stage of NSCLC determine the release of CYFRA 21-1 in bloodstream. Wien Klin Wochenschr. 2015;127:465–471. doi: 10.1007/s00508-014-0678-2. [DOI] [PubMed] [Google Scholar]
- 13.Holdenrieder S, Nagel D, Stieber P. Estimation of prognosis by circulating biomarkers in patients with non-small cell lung cancer. Cancer Biomark. 2010;6:179–190. doi: 10.3233/CBM-2009-0128. [DOI] [PubMed] [Google Scholar]
- 14.Cai Z. Relationship between serum carcinoembryonic antigen level and epidermal growth factor receptor mutations with the influence on the prognosis of non-small-cell lung cancer patients. Onco Targets Ther. 2016;9:3873–3878. doi: 10.2147/OTT.S102199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Zheng H, Hu F, et al. Serum CYFRA21-1 is correlated with the efficacy of epidermal growth factor receptor-tyrosine kinase inhibitor in non-small cell lung cancer patients harboring EGFR mutations. Zhongguo Fei Ai Za Zhi. 2016;19:550–558. doi: 10.3779/j.issn.1009-3419.2016.08.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holdenrieder S. Biomarkers along the continuum of care in lung cancer. Scand J Clin Lab Invest Suppl. 2016;245:S40–S45. doi: 10.1080/00365513.2016.1208446. [DOI] [PubMed] [Google Scholar]
- 17.Cabrera-Alarcon JL, Carrillo-Vico A, Santotoribio JD, et al. CYFRA 21-1 as a tool for distant metastasis detection in lung cancer. Clin Lab. 2011;57:1011–1014. [PubMed] [Google Scholar]
- 18.Lee DS, Kim SJ, Kang JH, et al. Serum carcinoembryonic antigen levels and the risk of whole-body metastatic potential in advanced non-small cell lung cancer. J Cancer. 2014;5:663–669. doi: 10.7150/jca.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson BJ, Reilly JP, Shashaty MG, et al. Admission plasma levels of the neuronal injury marker neuron-specific enolase are associated with mortality and delirium in sepsis. J Crit Care. 2016;36:18–23. doi: 10.1016/j.jcrc.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasprzak A, Zabel M, Biczysko W. Selected markers (chromogranin A, neuron-specific enolase, synaptophysin, protein gene product 9.5) in diagnosis and prognosis of neuroendocrine pulmonary tumours. Pol J Pathol. 2007;58:23–33. [PubMed] [Google Scholar]
- 21.Holdenrieder R, Marrades RM, Augé JM, et al. Assessment of a combined panel of six serum tumor markers for lung cancer. Am J Respir Crit Care Med. 2016;193:427–437. doi: 10.1164/rccm.201404-0603OC. [DOI] [PubMed] [Google Scholar]
- 22.Barak V, Holdenrieder S, Nisman B, Stieber P. Relevance of circulating biomarkers for the therapy monitoring and follow-up investigations in patients with non-small cell lung cancer. Cancer Biomark. 2010;6:191–196. doi: 10.3233/CBM-2009-0129. [DOI] [PubMed] [Google Scholar]
- 23.Tiseo M, Ardizzoni A, Cafferata MA, et al. Predictive and prognostic significance of neuron-specific enolase (NSE) in non-small cell lung cancer. Anticancer Res. 2008;28:507–513. [PubMed] [Google Scholar]
- 24.Yan HJ, Tan Y, Gu W. Neuron specific enolase and prognosis of non-small cell lung cancer: a systematic review and meta-analysis. J BUON. 2014;19:153–156. [PubMed] [Google Scholar]
- 25.Fiala O, Pesek M, Finek J, et al. The role of neuron-specific enolase (NSE) and thymidine kinase (TK) levels in prediction of efficacy of EGFR-TKIs in patients with advanced-stage NSCLC. Anticancer Res. 2014;34:5193–5198. [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Borghaei H, Brahmer J, Horn L, et al. Nivolumab vs Docetaxel in Advanced NSCLC: checkMate 017/0572-Y Update and exploratory cytokine profile analysis: track: immunotherapy. J Thorac Oncol. 2016;11:S237–S238. doi: 10.1016/j.jtho.2016.08.106. [DOI] [Google Scholar]
- 28.Barbee MS, Ogunniyi A, Horvat TZ, Dang TO. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 2015;49:907–937. doi: 10.1177/1060028015586218. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins AM, Rowland A, Kichenadasse G, et al. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer. 2017;117:913–920. doi: 10.1038/bjc.2017.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura Y, Kitano S, Takahashi A, et al. Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget. 2016;7:77404–77415. doi: 10.18632/oncotarget.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diem S, Kasenda B, Spain L, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114:256–261. doi: 10.1038/bjc.2015.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2018;29:524. doi: 10.1093/annonc/mdx059. [DOI] [PubMed] [Google Scholar]
- 34.Kelderman S, Heemskerk B, van Tinteren H, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother. 2014;63:449–458. doi: 10.1007/s00262-014-1528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simeone E, Gentilcore G, Giannarelli D, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother. 2014;63:675–683. doi: 10.1007/s00262-014-1545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Tanizaki J, Haratani K, Hayashi H, et al. Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol. 2018;13:97–105. doi: 10.1016/j.jtho.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 38.Holdenrieder S, Wehnl B, Hettwer K, et al. Carcinoembryonic antigen and cytokeratin-19 fragments for assessment of therapy response in non-small cell lung cancer: a systematic review and meta-analysis. Br J Cancer. 2017;116:1037–1045. doi: 10.1038/bjc.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Zhang N, Li B, Wang Z, Sun H, Yi Y, Huang W. Decline of serum CYFRA21-1 during chemoradiotherapy of NSCLC: a probable predictive factor for tumor response. Tumour Biol. 2011;32(4):689–695. doi: 10.1007/s13277-011-0169-2. [DOI] [PubMed] [Google Scholar]
- 40.Shirasu H, Ono A, Omae K, et al. CYFRA 21-1 predicts the efficacy of nivolumab in patients with advanced lung adenocarcinoma. Tumour Biol. 2018;40:2. doi: 10.1177/1010428318760420. [DOI] [PubMed] [Google Scholar]
- 41.Kataoka Y, Hirano K, Narabayashi T, et al. Carcinoembryonic antigen as a predictive biomarker of response to nivolumab in non-small cell lung cancer. Anticancer Res. 2018;38:559–563. doi: 10.21873/anticanres.12259. [DOI] [PubMed] [Google Scholar]
- 42.Lee S, Lee CY, Kim DJ, et al. Pathologic correlation of serum carcinoembryonic antigen and cytokeratin 19 fragment in resected nonsmall cell lung cancer. Korean J Thorac Cardiovasc Surg. 2013;46(3):192–196. doi: 10.5090/kjtcs.2013.46.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molina R, Filella X, Augé JM, et al. Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Biol. 2003;24(4):209–218. doi: 10.1159/000074432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used and analyzed within the current study is available from the corresponding author upon reasonable request.