Abstract
This article is one of ten reviews selected from the Annual Update in Intensive Care and Emergency Medicine 2019. Other selected articles can be found online at https://www.biomedcentral.com/collections/annualupdate2019. Further information about the Annual Update in Intensive Care and Emergency Medicine is available from http://www.springer.com/series/8901.
Introduction
Extracorporeal systems are increasingly used in severe hypoxemic and/or hypercapnic respiratory failure [1]. Although recent data have shown an advantage of high-flow veno-venous extracorporeal membrane oxygenation (VV-ECMO) in severe acute respiratory distress syndrome (ARDS) [2, 3], there is a paucity of evidence regarding the utility of extracorporeal CO2 removal (ECCO2R, often similarly used: low-flow ECMO) in patients with respiratory failure. Despite this fact, the number of available systems is dramatically increasing. In this chapter, we therefore provide an overview of the currently used technologies with advantages and disadvantages in the light of physiology.
Physiology of carbon dioxide (CO2)
Most of the CO2 of our body is stored as bicarbonate (HCO3−) in slow reacting compartments such as bones and therefore not directly accessible for CO2 removal. Only 1–5% of the total CO2 content is dissolved in the blood and thus can be removed with an extracorporeal system. The only way to increase the soluble CO2 content in plasma is to further dissolve it from HCO3−. This has been achieved experimentally through electrodialysis, an interesting technology with the ability to increase the efficiency of CO2 removal with low blood flow rates [4]. However, the diffusion capacity for CO2 is far higher than for oxygen, facilitating the opportunity for effective CO2 removal even with lower blood flow rates than required for oxygenation. Moreover, in regard the different compartments of CO2 storage, long-term CO2 removal may at least theoretically reduce the amount of stored of CO2 in the body, which is approximately 120 L and therefore about 10 times more than oxygen.
Among all the physiological effects of CO2, the effect on the pulmonary vasculature is of major importance. CO2 is one of the strongest vasoconstrictors of the pulmonary arterial vessels. Removing CO2 extracorporeally may therefore lead to lowering of the mean pulmonary arterial pressure by vasodilatation of the pulmonary vessels [5], but might have the disadvantage that reversing the vasoconstrictive effect may lead to unselective vasodilatation in all areas of the lung, leading to increased shunt fraction in some patients with atelectasis.
Control of respiratory drive
A further effect of CO2 is its function as the strongest stimulus of the central respiratory drive [6], which is sometimes hard to control in daily clinical practice in patients with severe lung failure. Therefore, ECCO2R may serve as a powerful tool to control this drive. This might be important in patients with ARDS [7] particularly by reducing high transpulmonary pressure and therefore ‘spontaneous breathing induced, and ventilator-associated’ lung failure (i.e., patient self-inflicted lung injury) [8]. Furthermore, ECCO2R may facilitate spontaneous breathing even in patients with severe respiratory failure being bridged to lung transplantation [9]. However, clinical experience supports the notion that regulation of the respiratory drive is independent of CO2 in some critically ill patients (e.g., patients with stiff or fibrotic lungs and strong respiratory drive stimulated by the Hering-Breuer reflex) [10, 11].
ECCO2R systems and cannulas
Increasing numbers of different ECCO2R systems are becoming available on the market, nearly all of them for veno-venous access. Historically, these systems evolved on the one hand from very low-flow renal-replacement therapy, operating at blood flow rates between 200 and 400 mL/min and driven by roller pumps [12], and on the other hand from high-flow ECMO systems with variable blood flow rates, driven by centrifugal pumps. The membrane lungs (often similarly used: oxygenator) available are usually not specifically designed for ECCO2R systems. Therefore, a large variety of membrane lungs with surface areas from 0.32 m2 to more than 1 m2 are available on the market and in use. Most systems outside the operating room are coated with heparin and, as an alternative, with phosphorylcholine/phosphatidylcholine or albumin. Furthermore, many membrane lungs designed for ECMO offer the opportunity to heat the patient. Although this is often not necessary in low-flow systems, some patients (e.g., with low body mass index [BMI]), such as some patients with chronic obstructive pulmonary disease (COPD), tend to have a decrease in body temperature even with low blood flow rates.
For vascular access, different cannulas are in use. Very low-flow systems often utilize dual-lumen dialysis catheters. Although the price is very low, the recirculation rate is high with these catheters [13], limiting the efficiency of CO2 removal. For higher blood flow rates, specifically designed double lumen cannulas can be used, usually in the range from 14.5 to 20 Fr. It is important to note that adapters for these smaller cannulas are often 1/4 in. (0.6 cm) whereas larger cannulas (> 20 Fr) usually use 3/8 in. (1 cm) adapters, as is used for high flow ECMO. As an alternative to expensive and specifically designed double lumen cannulas, two small single lumen cannulas can be used offering the advantage of lower implantation risk and cost, and nearly no recirculation, whereas the main disadvantage is that two vessels have to be cannulated. Finally, smaller diameter tubing is often more flexible than typical 3/8 in. tubing, leading sometimes to unexpected kinking in daily clinical practice.
Pump technology
As mentioned earlier, ECCO2R systems with blood flow rates between 200 and 400 mL/min are typically driven by roller pumps—with the exception of the specifically designed Hemolung RAS system (ALung Technologies, Pittsburgh, PA, USA)—and those with blood flow rates above 500 mL/min are usually driven by centrifugal pumps, also called rotary blood pumps. A direct comparison of the hemolysis, coagulation, and inflammatory response potential, and thus a universal preference between roller pumps and centrifugal pumps appears difficult due to the various systems and versatile fields of application. However, centrifugal pumps play a major role in extracorporeal lung assist systems, especially due to their ability to increase the blood flow far above 400 mL/min if necessary.
From an engineering perspective, several critical aspects related to the functionality of centrifugal pumps at different operating conditions are important. As with the large, industrially used turbomachines, rotary blood pumps are developed for a specific design point. The respective components of the pump are dimensioned for this design point to allow for optimal flow guidance, as loss-free and efficient as possible. In contrast to the large turbomachinery in industry, blood pumps operate in a wide range of flow rates and pressures (e.g., 0.5–10 L/min, 0–800 mmHg) instead of a specific operating or design point. This broad application range can be achieved, in very simplified terms, by shifting the design point to high pump flows and by oversizing the hydraulic pump components. Although this reduces the flow-induced friction losses with large pump flows, it can show increased blood damage at small pump flows [14].
The hemolysis index (HI), defined as the ratio of the increase in plasma free hemoglobin (ΔHb) to the total hemoglobin concentration, is dependent on the operating point of rotary blood pumps (Fig. 1a). The HI appears to increase in a nonlinear fashion for decreasing flows. It can be expressed as a functional relationship of the main factors exposure time, t (estimated as the ratio of priming volume and pump flow), and the effective stress, τ [15]:
Fig. 1.
Operating points of rotary blood pumps with corresponding hemolysis index (HI) (panel a), shear stress (panel b) and exposure time (texp) (panel c) RPM revolutions per minute
Whereas the shear stress increases with increasing pump speed and pump flow (Fig. 1b), the exposure time is positively associated with pump flow (Fig. 1c) and is correlated with the HI at low pump flows [16].
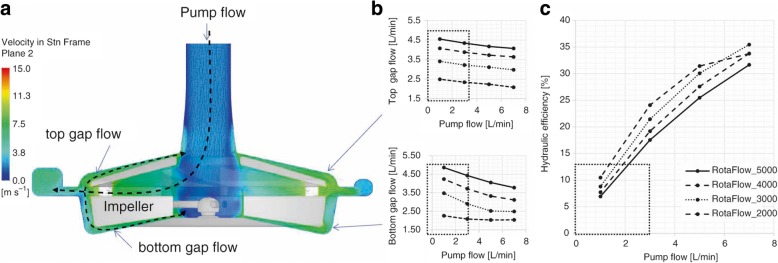
A high priming volume, as is required for the desired application in a large operating range of blood pumps, can therefore negatively affect the exposure time and hence the HI. Another reason why the HI is dependent on the operating point of rotary blood pumps is the high degree of blood recirculation in the gaps at low flows (the ratio of main pump flow to gap flow and or pump-internal return flow), which is also displayed as low hydraulic efficiency. The Rotaflow pump (Getinge AB, Gothenburg, Sweden) illustrates why rotary blood pumps do not operate equally well at every operating point from a hemocompatibility point of view. The gap flows increase for high speeds and corresponding high pressures and towards low flow rates, whereby the ratio between pump flow and the sum of the two gap flows can easily be 1–10 (Fig. 2a, b). This means that the majority of blood recirculates through the gaps multiple times before leaving the pump, significantly reducing the hydraulic efficiency of the pump to < 10% (Fig. 2c). This multiple exposure flow can lead to increased hemolysis in the blood.
Fig. 2.
Gap flow (panels a and b) and hydraulic efficiency (panel c) of rotary blood pumps
In conclusion, the authors recommend that industry consider the design of new low-flow pumps featuring smaller filling volumes (Fig. 1a) to decrease the amount of flow recirculation and the exposure time and ultimately reduce the risk of hemolysis at low flow rates.
Membrane lung technology
Nearly all ECCO2R systems use established membrane lungs, typically designed for different applications than ECCO2R. However, this may lead to increased clotting as observed in some clinical reports [17]. Therefore, specifically designed membrane lungs for ECCO2R may be of particular importance. From an engineering point of view, adequate gas exchange and high hemocompatibility are the main goals of the design process of a membrane lung. Depending on the application, a low pressure drop and low priming volume would also be favorable.
Gas exchange is mainly determined by the membrane surface area in artificial lungs [18]. The exchange area is commonly estimated based on empirical values or simulated oxygen transfer predictions [19] and then validated experimentally. However, a large membrane surface area has increased thrombotic potential due to its artificial character. Progressive or acute clot formation in the membrane lung is the reason for an acute or elective system exchange in up to a third of cases [20]. The thrombotic potential of membrane lungs correlates with low flow states [21, 22]. Therefore, sufficient washout and homogeneous flow distribution is the key to reduce the risk of thrombus formation and clots.
As a preliminary indicator, the theoretical washout, Nwo, depends on the priming volume and determines the washout capabilities in the intended flow range of different membrane lungs:
In this equation, is the flow rate and Vpr is the priming volume of the membrane lung. The theoretical washout describes the amount of volume exchange at a given flow rate. An overview of different commercially available devices and their volume exchange rate at maximum and, most importantly, minimum flow rate is given in Table 1. Of note, the minimum theoretical washout is typically around 2/min. However, for current devices, many of which were designed for ECMO applications, operating at such low flows is associated with an elevated risk of thrombus formation inside the devices with all the associated problems: elevated pressure drop, decreased gas transfer efficiency, or ultimately mechanical failure and the need for a system exchange. For example, operating a Hilite 7000 LT (see Table 1) at a flow of 0.5 L/min would yield a theoretical, potentially insufficient, washout < 2/min.
Table 1.
Priming volume and theoretical washout (data for commercially available devices were obtained from manufacturers’ manuals)
| Device | Priming volume (mL) | Flow range (L/min) | Theoretical washout (/min) |
|---|---|---|---|
| Novalung iLA | 175 | 0.5–4.5 | 2.86–25.71 |
| Quadrox-i Pediatric | 99 | 0.2–2.8 | 2.02–28.28 |
| Quadrox-i Small Adult | 175 | 0.5–5 | 2.86–28.57 |
| Quadrox-i Adult | 215 | 0.5–7 | 2.33–32.56 |
| Capiox RX05 | 43 | 0.1–1.5 | 2.36–34.88 |
| Hilite 800 LT | 55 | 0.1–0.8 | 1.82–14.55 |
| Hilite 2400 LT | 95 | 0.35–2.4 | 3.68–25.26 |
| Hilite 7000 LT | 320 | 1–7 | 3.13–21.88 |
There are two principle configurations of membrane lungs that determine the flow path (see Table 2). The fiber matrices are either wound around an inner cylinder or stacked and glued into a rectangular housing. Examples of a wound membrane lung are the Hilite product line (Xenios AG, Heilbronn, Germany) and CAPIOX Oxygenator (Terumo, Tokyo, Japan). One of the advantages is the possibility of countercurrent gas flow, which allows a constant driving force for the diffusion of the gas. On the other hand, the fiber length is usually longer. The stacked design has a wide cross-sectional area and a rather short flow path through the fiber bundle. The bigger cross-sectional area makes this design comparably more resistant to clogging through washed in debris and it offers lower pressure drop. However, it is obviously more challenging to distribute a tubular flow profile at the inlet connector equally over the full cross-sectional area of the fiber bundle. Consequently, the corners experience lower blood flow velocities and thus are usually prone to clotting. The Quadrox product line (Getinge AB, Gothenburg, Sweden) and iLA (Xenios AG, Heilbronn, Germany) are prominent examples of stacked membrane lung design.
Table 2.
Advantages and disadvantages of most common configurations of membrane lungs for CO2 retention applications
| Stacked | Wound | |
|---|---|---|
| Advantages | −Low pressure drop through higher permeability in flow direction −Unidirectional flow −Increased convectional gas transfer through cross-layered fiber arrangement |
−Countercurrent flow possible |
| Disadvantages | −Inhomogeneous wash out of the corners and higher risk of thrombus formation −Allows only crosscurrent flow |
−Prone to clogging through washed in debris due to its smaller cross-sectional area −Mostly long fibers and long flow path |
| Examples | −iLA (Novalung, Germany) −Quadrox series (Maquet, Germany) |
−Hilite product line (Medos Medizintechnik AG, Germany) −CAPIOX oxygenator (Terumo, Japan) |
For CO2 retention applications, further design parameters require attention. The driving force for the gas exchange according to Fick’s law of diffusion is the partial pressure difference between blood and sweep gas. Although the pressure gradient for oxygen is approximately 650 mmHg [23], the gradient for CO2 is usually only 45–90 mmHg. Along the fiber, the CO2 content will adjust to the blood content and the diffusive driving force declines. To obtain an efficient membrane lung with the lowest necessary amount of membrane surface, a design incorporating short fibers and allowing high sweep gas ratios keeps the gradient over the full length of the fiber at the highest possible level.
In addition, various attempts to increase CO2 elimination on membrane lungs have been made. Eash et al. recognized that the major resistance to gas transfer in membrane lungs lay in the blood-sided laminar boundary layer on the membrane surface and was proportional to the thickness of this layer [24]. Active and passive approaches have been investigated to disrupt the boundary layer and overcome the diffusive resistance. The most prominent example for active mixing is the Hemolung RAS device (ALung Technologies Inc., Pittsburgh, PA, USA). Svitek et al. documented an increase in CO2 removal by 133% by rotation of the fiber bundle with 1500 RPM [25]. A passive approach was evaluated by developing a membrane lung with circular blood flow paths that promote gas transfer through secondary flow [26].
In conclusion, the authors recommend that membrane lungs should be specifically designed for ECCO2R with target blood flow rates < 500 mL/min and 500–1500 mL/min with optimized blood flow paths aiming for faster washout and lowest possible clotting rate.
Blood flow rates and treatment goals
Considering all the possible modifications of the circuit, the blood flow rate is the main determinant and easiest way to increase the CO2 removal rate. Human and animal data suggest that a blood flow rate of 250 mL/min removes 40–60 mL CO2/min [27–32], accounting for 20–25% of total CO2 production in patients at rest, whereas an increase in the blood flow rate up to 1000 mL/min removes approximately 150 mL CO2/min. In most patients, this blood flow rate is enough to remove approximately 50–60% of total CO2, which may be associated with an important clinical impact. Furthermore, the CO2 removal capacity is independent of the oxygen content of the sweep gas flow, with even 21% oxygen (ambient air) sweep gas having no impact on CO2 removal capacity compared to 100% oxygen (own unpublished data). Therefore, under defined circumstances, ambient air can be used for ECCO2R systems, only loosing 10–30 mL oxygen transfer/min compared to use of 100% oxygen as sweep gas. By contrast, in high-flow ECMO, CO2 removal capacity is linearly correlated with the sweep gas flow rate; sweep gas flow rates > 5–6 L/min have only a minor impact on CO2 removal in low flow systems, and this is more pronounced in systems with large membrane lung surfaces [13, 18].
In general, in choosing the ‘right’ system for the ‘right’ patient, one has to consider that the discrepancy between a low blood flow rate and large surface area may lead to more clotting, since the passing time through the membrane lung is slow. To avoid the vicious circle of clotting, associated loss of coagulation factors (particularly fibrinogen) and secondary bleeding, anticoagulation with a target activated partial thromboplastin time (aPTT) of 1.8–2 times the reference is often necessary. Nevertheless, optimizing the membrane lung surface area and new developments focusing on specific designs for low blood flow rates with faster transit times through the membrane lung represent promising opportunities to avoid the potential adverse effects of anticoagulation. Importantly, use of lower blood flow rates and systems is not necessarily less invasive or less risky!
Which CO2 removal capacity is appropriate?
In our opinion, there is no right or wrong blood flow rate. The amount of CO2 removal is dependent on the treatment goals for the patient. A blood flow rate of 1000 mL/min removes half of the CO2 and may correct even severe respiratory acidosis [13], whereas very low blood flow rates have a less pronounced effect. Thus, low flow rates may be more appropriate in patients with less severe respiratory acidosis or to reduce the invasiveness of mechanical ventilation in ARDS. However, there are currently no high-quality data for an evidence-based recommendation in COPD or in ARDS. The authors therefore recommend attempting to include all patients with potential indications for ECCO2R into large observational registries or clinical trials when possible.
Conclusion
Despite the lack of rigorous data, the use of extracorporeal support devices for respiratory failure is increasing at an exponential rate around the world. ECCO2R represents a promising technology that may be useful in patients with hypercapnic respiratory failure, or to reduce the intensity of mechanical ventilation delivered to patients with ARDS. Current systems available on the market are typically hybrid constructs, made up of components that were not necessarily designed or optimized for the delivery of low flow rates. The development of purpose-driven ECCO2R devices—with custom pumps, membrane lungs, cannula, and tubing—may improve the risk/benefit profile for these devices. Clinical trials are urgently needed to confirm the potential efficacy of ECCO2R in patients with respiratory failure.
Acknowledgements
We are grateful to Sascha Groß-Hardt, Institute of Applied Medical Engineering, Helmholtz Institute Aachen, RWTH Aachen University, Germany for his contribution to the current work. The current work was supported by the German Federal Ministry of Education and Research (13GW0219B; Verbundprojekt tragbare Langzeitunterstützung der Lunge zur Behandlung der schweren COPD [p-ECCO2R]).
Funding
This study was in part funded by the German Federal Ministry of Education and Research No. 13GW0219B. Publication costs were funded by the University Witten/ Herdecke.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors’ contributions
All authors contributed to the final drafting of the manuscript, read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
C.K. received travel grants and lecture fees from Maquet, Rastatt, Germany. C.K. received an open research grant for the hospital from Maquet Cardiopulmonary, Rastatt, Germany. E.F. received fees as a consultant for MC3 Cardiopulmonary and ALung Technologies. F.H: reported no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Karagiannidis C, Brodie D, Strassmann S, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. 2016;42:889–896. doi: 10.1007/s00134-016-4273-z. [DOI] [PubMed] [Google Scholar]
- 2.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 3.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 4.Zanella A, Castagna L, Salerno D, et al. Respiratory electrodialysis. A novel, highly efficient extracorporeal CO2 removal technique. Am J Respir Crit Care Med. 2015;192:719–726. doi: 10.1164/rccm.201502-0289OC. [DOI] [PubMed] [Google Scholar]
- 5.Karagiannidis C, Strassmann S, Philipp A, Muller T, Windisch W. Veno-venous extracorporeal CO2 removal improves pulmonary hypertension in acute exacerbation of severe COPD. Intensive Care Med. 2015;41:1509–1510. doi: 10.1007/s00134-015-3917-8. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer KE. Respiratory pattern and respiratory response to CO2. J Appl Physiol. 1958;13:1–14. doi: 10.1152/jappl.1958.13.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Karagiannidis C, Lubnow M, Philipp A, et al. Autoregulation of ventilation with neurally adjusted ventilatory assist on extracorporeal lung support. Intensive Care Med. 2010;36:2038–2044. doi: 10.1007/s00134-010-1982-6. [DOI] [PubMed] [Google Scholar]
- 8.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 9.Biscotti M, Gannon WD, Agerstrand C, et al. Awake extracorporeal membrane oxygenation as bridge to lung transplantation: a 9-year experience. Ann Thorac Surg. 2017;104:412–419. doi: 10.1016/j.athoracsur.2016.11.056. [DOI] [PubMed] [Google Scholar]
- 10.Crotti S, Bottino N, Ruggeri GM, et al. Spontaneous breathing during extracorporeal membrane oxygenation in acute respiratory failure. Anesthesiology. 2017;126:678–687. doi: 10.1097/ALN.0000000000001546. [DOI] [PubMed] [Google Scholar]
- 11.Crotti S, Bottino N, Spinelli E. Spontaneous breathing during veno-venous extracorporeal membrane oxygenation. J Thorac Dis. 2018;10:S661–S669. doi: 10.21037/jtd.2017.10.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeffries RG, Lund L, Frankowski B, Federspiel WJ. An extracorporeal carbon dioxide removal (ECCO2R) device operating at hemodialysis blood flow rates. Intensive Care Med Exp. 2017;5:41. doi: 10.1186/s40635-017-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karagiannidis C, Kampe KA, Sipmann FS, et al. Veno-venous extracorporeal CO2 removal for the treatment of severe respiratory acidosis: pathophysiological and technical considerations. Crit Care. 2014;18:R124. doi: 10.1186/cc13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu GM, Jin DH, Jiang XH, et al. Numerical and in vitro experimental investigation of the hemolytic performance at the off-design point of an axial ventricular assist pump. ASAIO J. 2016;62:657–665. doi: 10.1097/MAT.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 15.Giersiepen M, Wurzinger LJ, Opitz R, Reul H. Estimation of shear stress-related blood damage in heart valve prostheses—in vitro comparison of 25 aortic valves. Int J Artif Organs. 1990;13:300–306. doi: 10.1177/039139889001300507. [DOI] [PubMed] [Google Scholar]
- 16.Fraser KH, Zhang T, Taskin ME, Griffith BP, Wu ZJ. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. J Biomech Eng. 2012;134:081002. doi: 10.1115/1.4007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Sorbo L, Pisani L, Filippini C, et al. Extracorporeal CO2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med. 2015;43:120–127. doi: 10.1097/CCM.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 18.Karagiannidis C, Strassmann S, Brodie D, et al. Impact of membrane lung surface area and blood flow on extracorporeal CO2 removal during severe respiratory acidosis. Intensive Care Med Exp. 2017;5:34. doi: 10.1186/s40635-017-0147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Chen X, Ding J, et al. Computational study of the blood flow in three types of 3D hollow fiber membrane bundles. J Biomech Eng. 2013;135:121009. doi: 10.1115/1.4025717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubnow M, Philipp A, Foltan M, et al. Technical complications during veno-venous extracorporeal membrane oxygenation and their relevance predicting a system-exchange—retrospective analysis of 265 cases. PLoS One. 2014;9:e112316. doi: 10.1371/journal.pone.0112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gartner MJ, Wilhelm CR, Gage KL, Fabrizio MC, Wagner WR. Modeling flow effects on thrombotic deposition in a membrane oxygenator. Artif Organs. 2000;24:29–36. doi: 10.1046/j.1525-1594.2000.06384.x. [DOI] [PubMed] [Google Scholar]
- 22.Funakubo A, Taga I, McGillicuddy JW, Fukui Y, Hirschl RB, Bartlett RH. Flow vectorial analysis in an artificial implantable lung. ASAIO J. 2003;49:383–387. [PubMed] [Google Scholar]
- 23.Kaesler A, Rosen M, Schmitz-Rode T, Steinseifer U, Arens J. Computational modeling of oxygen transfer in artificial lungs. Artif Organs. 2018;42:786–799. doi: 10.1111/aor.13146. [DOI] [PubMed] [Google Scholar]
- 24.Eash HJ, Jones HM, Hattler BG, Federspiel WJ. Evaluation of plasma resistant hollow fiber membranes for artificial lungs. ASAIO J. 2004;50:491–497. doi: 10.1097/01.MAT.0000138078.04558.FE. [DOI] [PubMed] [Google Scholar]
- 25.Svitek RG, Frankowski BJ, Federspiel WJ. Evaluation of a pumping assist lung that uses a rotating fiber bundle. ASAIO J. 2005;51:773–780. doi: 10.1097/01.mat.0000178970.00971.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernando UP, Thompson AJ, Potkay J, et al. A membrane lung design based on circular blood flow paths. ASAIO J. 2017;63:637–643. doi: 10.1097/MAT.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt M, Jaber S, Zogheib E, Godet T, Capellier G, Combes A. Feasibility and safety of low-flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit Care. 2018;22:122. doi: 10.1186/s13054-018-2038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Villiers Hugo J, Sharma AS, Ahmed U, Weerwind PW. Quantification of carbon dioxide removal at low sweep gas and blood flows. J Extra Corpor Technol. 2017;49:257–261. [PMC free article] [PubMed] [Google Scholar]
- 29.Peperstraete H, Eloot S, Depuydt P, De Somer F, Roosens C, Hoste E. Low flow extracorporeal CO2 removal in ARDS patients: a prospective short-term crossover pilot study. BMC Anesthesiol. 2017;17:155. doi: 10.1186/s12871-017-0445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batchinsky AI, Jordan BS, Regn D, et al. Respiratory dialysis: reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit Care Med. 2011;39:1382–1387. doi: 10.1097/CCM.0b013e31820eda45. [DOI] [PubMed] [Google Scholar]
- 31.Allardet-Servent J, Castanier M, Signouret T, Soundaravelou R, Lepidi A, Seghboyan JM. Safety and efficacy of combined extracorporeal CO2 removal and renal replacement therapy in patients with acute respiratory distress syndrome and acute kidney injury: the pulmonary and renal support in acute respiratory distress syndrome study. Crit Care Med. 2015;43:2570–2581. doi: 10.1097/CCM.0000000000001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burki NK, Mani RK, Herth FJF, et al. A novel extracorporeal CO(2) removal system: results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest. 2013;143:678–686. doi: 10.1378/chest.12-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.