Abstract
Background
Skin flap grafting is one of the most common tissue transplantations for wound repair and organ reconstruction. Thus, improving the survival rate of the transplanted skin flap is important. Platelet-rich plasma (PRP) is an autologous platelet concentrate obtained from whole blood. It has been widely used in repairing tissue defects. Considering that the PRP gel has similar biological characteristics, this study used PRP gel for skin flap transplantation.
Material/Methods
PRP gel from Sprague-Dawley (SD) rats was prepared and the growth factor concentration was determined. A rat skin flap model was established to evaluate the survival rate of skin flap. Morphologic evaluation was also done.
Results
We found that the PRP gel increased the survival rate of the skin flap. In addition, it reduces the inflammation response in skin flap transplantation and has better effects in terms of generating new soft tissue.
Conclusions
The effectiveness PRP gel in skin flap transplantation is satisfactory. The possible mechanisms by which PRP gel promotes the survival of the skin flap includes platelets, growth factors, immune activity factor, and fibrin. PRP could be a new clinical method for promoting skin flap survival.
MeSH Keywords: Graft Survival, Platelet-Rich Plasma, Surgical Flaps
Background
Skin flap grafting is one of the most common tissue transplantations in plastic surgery for wound repair and organ reconstruction [1]. Owing to the complexity of the biological process of wound healing, decreased activity or deficiency in the number of local growth factors, or the loss of regulation of multiple factors, wound healing becomes difficult and thus restricts skin flap application in plastic surgery [2].
Among the many factors that influence the repair of tissue defects, growth factors and cytokines are reported to promote healing [3]. Exogenous cytokines have been clinically used to promote healing. However, limited by process technology, only a single kind of growth factors or cytokines is used most frequently. This can only promote a certain stage of wound healing. Theoretically, a combination of multiple cytokines conforming to the biological process of tissue healing could promote healing most effectively. A combination technique for various functional bioactive molecules in vitro is an important research focus in the field of clinical application of growth factors [4,5].
Previous studies found that platelets are often accompanied by growth factors [6,7]. After the activation of platelets, various growth factors that are similar to those in vivo with respect to types and proportions can be released continuously for a certain period. Platelet-rich plasma (PRP) is an autologous platelet concentrate obtained from fresh whole blood by centrifugation. PRP contains a high concentration and an appropriate proportion of functional bioactive molecules for wound healing, such as TGF-β1, TGF-β2, EGF, FGF, PDGF, VEGF, and IL-1 [8,9]. Recently, PRP has also been widely studied for its applications in various fields, and PRP gel has been reported to be effective in repairing and reconstruction of fractures and healing of bone defects [10,11].
Considering the similar biological characteristics in repairing tissue defects, several studies [12–14] have revealed application of PRP in skin flap transplantation. However, none of them applied PRP directly onto the flap basal surface, which should be the easiest and most convenient method for clinical use, without any direct harm, such as puncture and suppression, to the skin flap.
Due to the lack of evidence on the role of PRP, we applied PRP gel directly onto the base of skin flap graft in situ and determined its effectiveness and the possible mechanism by which PRP gel promotes skin flap survival in this study. This study was conducted to lay the foundation for clinical exploration of new methods for promoting survival of skin flaps.
Material and Methods
Preparation of PRP gel
Preparation of PRP
Eight adult Sprague-Dawley (SD) rats (n=8) were anesthetized by intraperitoneal injection of ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (5 mg/kg). A 10-ml syringe with 100 μl 8% EDTA-K2 anticoagulants was used to take 10 ml of arterial blood from the rat heart under direct vision. We used 2 ml of the blood to determine the platelet count with an automatic blood cell analyzer and another 2 ml was stored at −80°C in a refrigerator and used for growth factor determination. The remaining blood sample was shaken and placed in a centrifuge tube. The modified Curasan method was used to prepare PRP by centrifuging 2 times. First, the centrifuge tube was centrifuged for 10 min at 2400 rpm at 24°C. The blood sample was divided into 3 layers from top to bottom as follows: the supernatant, boundary layer, and red cell layer. Then, the supernatant and the boundary layer were absorbed with a tube, and the following 2-mm red blood cell layer was placed into another centrifuge tube and centrifuged 15 min at 3600 rpm. Visible red blood cells deposited on the bottom, while the platelets were deposited above this layer, which looked like a white film. The top layer contained plasma with a few non-sedimentary platelets. The final step was absorbing the uppermost level of plasma, allowing the remainder to settle for 30 min and then shaking the centrifuge tube to resuspend the red cells and platelets in the remaining plasma to form PRP. All experimental protocols were approved by the Ethics Committee of the First Affiliated Hospital of Soochow University and were carried out in strict accordance with Declaration of Helsinki (1964) and the Laboratory Animal Guidelines for Ethical Review of Animal Welfare (GB/T 35892-2018, China), approval number 378/2017.
Platelet counting
The whole blood reserved in the preparation of PRP and the prepared PRP were absorbed by a hemoglobin pipet to make a 10-μl sample. Platelet diluents were used to dilute the sample to 2 ml. The platelet count was performed by the Sysmex F-820 automatic platelet analyzer after mixing. Then, the platelet enrichment rate of the 2 samples was compared.
Determination of growth factor concentration
The concentrations of the 2 growth factors, TGF-β1 and PDGF, in the whole blood reserved in the preparation of PRP and the prepared PRP, were determined. The TGF-β1 and PDGF concentrations were assessed by ABC-ELISA. The anti-rabbit TGF-β1 monoclonal antibody was coated on an enzyme-labeled plate. First, the TGF-β1 in the samples were combined with the monoclonal antibody, forming an immune complex to connect to the plate. Then, streptomycin antibiotic protein labeled with horseradish peroxidase combined with biotin was added to the enzyme substrate and it turned yellow. Finally, the reaction was terminated with liquid sulfuric acid. Value of Absorbance (A) was measured at 492 nm. The TGF-β1 concentration was proportional to the Value of Absorbance (A) and was measured according to the standardized curve. The PDGF concentration was determined with the same method.
Preparation of the PRP gel
The prepared PRP was mixed with an activator (a mixture of thrombin and 10% CaCl2 in the ratio of 10: 1) and then shaken at room temperature. PRP gel formed after 2 min.
In vivo experiment
Rat skin flap model
The SD rats were anesthetized by intraperitoneal injection of ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (5 mg/kg). The SD rats were placed on the operating table, and their limbs were fixed. We used 8% sodium sulfide to remove the fur from the backs of the rats. The skin flaps were designed and constructed on both sides of the rat’s back: the distance from midline to the flap was 1 cm, and the size was 2.5 cm diameter, with a 1×1 cm pedicle.
Survival experiment of the rat skin flap
A total of 32 SD rats were randomly divided into 2 groups (n=16). The SD rats were anesthetized by intraperitoneal injection of ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (5 mg/kg). In the experimental group, after hemostasis, 1 ml of prepared PRP gel was applied on the wound surface (0.1 ml/cm2). After making sure that the whole wound was covered with the PRP gel, the flap was sutured. In the control group, only the flap was sutured after hemostasis, and the rats were not given any other special treatments.
Evaluation
Morphological evaluation and survival rate
All the rats were killed (n=4 in each group each day) with an overdose of carbon dioxide on days 1, 3, 5, and 7 after the operation. The color, texture, edema, and hair growth of the 2 groups with flaps were observed and recorded, as well as the survival of the skin flap. If the color of the skin flap became black and withered, and needling did not produce bleeding, the skin flap was diagnosed as necrotic.
The full layer of skin was removed to evaluate the survival rate of the skin flap. All the flaps were photographed with a digital camera and analyzed using image processing software (Image-Pro Plus 6). The survival area, area of necrosis, and total flap area were determined for each flap to calculate the survival rate of each flap, and the survival rate was calculated as: (flap survival area/flap total area) ×100%.
Histological evaluation
Rat skin flap tissue samples were collected on days 1, 3, 5, and 7 after the operation. All the skin flap samples were fixed with 10% formalin, and 10% HCL was used for decalcification. The flaps were sliced and embedded in paraffin and then stained with hematoxylin-eosin (HE). At the same time, they were stained with Masson trichrome to show some special structures.
Histological scoring and semi-quantitative analysis
Previous studies on skin flap survival [15,16] performed general histological scoring and semi-quantitative analysis. To comprehensively assess the histology, we chose 5 parameters (fiber structure, fiber arrangement, inflammation, vascularity, and density of cells) based on HE staining. For Masson staining, 2 parameters (collagen structure and collagen arrangement) were assessed. These parameters were semi-quantitatively graded according to a 4-point scoring system (0=normal, 1=slightly abnormal, 2=moderately abnormal, and 3=markedly abnormal). The evaluation was blindly performed by 2 observers. All these data are presented as mean ± standard deviation.
Real-time polymerase chain reaction
Real-time polymerase chain reaction (PCR) was performed to detect the TGF-β1, EGF, PDGF, and VEGF expression levels in the tissues on days 1, 3, 5, and 7 after the operation. Samples on days 1, 3, and 5 were performed using the punch method. Tissues were homogenized by ultrasonication, and total RNA was extracted using an RNeasy kit. RNA was reverse transcribed into cDNA by using a QuantiTect kit. Real-time PCR was performed to detect the gene expression in the Mx3000P QPCR system, and the relative TGF-β1, EGF, PDGF, and VEGF expressions were measured using the 2-AACt method.
Statistical analysis
Experimental data were analyzed with SPSS 11.0 software. Data are reported as mean ±SD. P values of <0.05 were considered significant.
Results
Blood platelet count
The number of blood platelets in the PRP was (3.74×109± 1.16×109/mL) and increased by 6.2 times as compared with that in whole blood (6.01×108±6.54×107/mL), as shown in Table 1.
Table 1.
Blood platelet count.
| Sample | Blood platelet count per milliliter | |
|---|---|---|
| Whole Blood | PRP | |
| 1 | 6.32×108 | 2.96×109 |
| 2 | 6.12×108 | 5.92×109 |
| 3 | 5.64×108 | 3.08×109 |
| 4 | 6.68×108 | 4.40×109 |
| 5 | 5.04×108 | 2.40×109 |
| 6 | 5.28×108 | 2.84×109 |
| 7 | 6.04×108 | 4.52×109 |
| 8 | 6.92×108 | 3.80×109 |
Determination of growth cytokine
ABC-ELISA was performed to measure the TGF-β1 and PDGF-BB concentrations in whole blood and PRP. As shown in Table 2, the TGF-β1 concentration in PRP (16.7±2.97 ng/mL) was 3.6 times higher than that in whole blood (4.7±1.01 ng/mL), while the PDGF-BB concentration in PRP (1428.78±322.15 pg/mL) was 1.8 times higher than that in whole blood (790.19±186.75 pg/mL).
Table 2.
Concentration of growth cytokine by ELISA.
| Sample | TGF-β1 (pg/mL) | PDGF-BB (pg/mL) | ||
|---|---|---|---|---|
| Whole blood | PRP | Whole blood | PRP | |
| 1 | 6.18×103 | 1.62×104 | 597.809 | 1634.995 |
| 2 | 3.13×103 | 2.20×104 | 858.178 | 1922.497 |
| 3 | 4.22×103 | 1.41×104 | 576.519 | 1853.538 |
| 4 | 4.81×103 | 1.53×104 | 1076.015 | 1386.845 |
| 5 | 3.69×103 | 1.23×104 | 997.261 | 1214.157 |
| 6 | 4.27×103 | 1.80×104 | 837.466 | 1204.929 |
| 7 | 5.71×103 | 1.79×104 | 758.483 | 1112.783 |
| 8 | 5.70×103 | 1.81×104 | 619.810 | 1100.515 |
Morphological evaluation and survival rate in the in vivo experiment
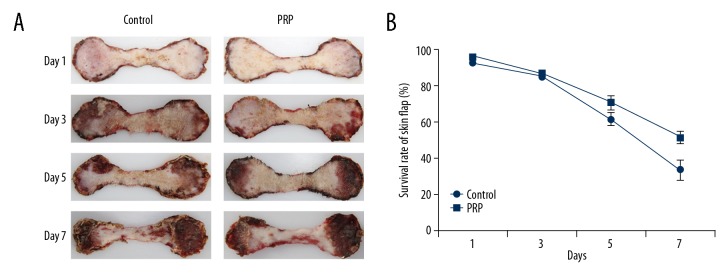
On days 1, 3, 5, and 7 after the operation, the color, texture, edema, and hair growth in the 2 flap groups were observed and the survival condition of the skin flap was recorded (Figure 1A), showing no significant difference in skin flap survival rate between the PRP and control groups on days 1 and 3. The survival rate in the PRP group was significantly higher than that in the control group on days 5 and 7 (Figure 1B, Table 3).
Figure 1.
(A) Morphology of skin flap at various time points. The texture and edema condition were better in the PRP group than in the control group on day 3, day 5, and day 7. (B) The survival rate of skin flap at various time points. There were no significant differences in the survival rate of skin flaps between the PRP and control groups on day 1 and day 3. The survival rate in the PRP group was significantly higher than in the control group on day 5 and day 7.
Table 3.
Survival rate of skin flap (%).
| Sample | Control | PRP | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | Day 1 | Day 3 | Day 5 | Day 7 | |
| 1 | 92.47 | 82.92 | 69.80 | 30.06 | 95.15 | 87.61 | 73.25 | 53.29 |
| 2 | 90.72 | 84.94 | 63.10 | 49.43 | 93.94 | 89.26 | 58.87 | 41.47 |
| 3 | 92.86 | 88.25 | 58.61 | 27.96 | 96.15 | 85.20 | 74.45 | 53.44 |
| 4 | 92.07 | 82.90 | 52.77 | 24.46 | 95.60 | 83.75 | 75.28 | 56.94 |
| Average | 92.03 | 84.75 | 61.07 | 32.98 | 95.21 | 86.45 | 70.46 | 51.29 |
Histological evaluation
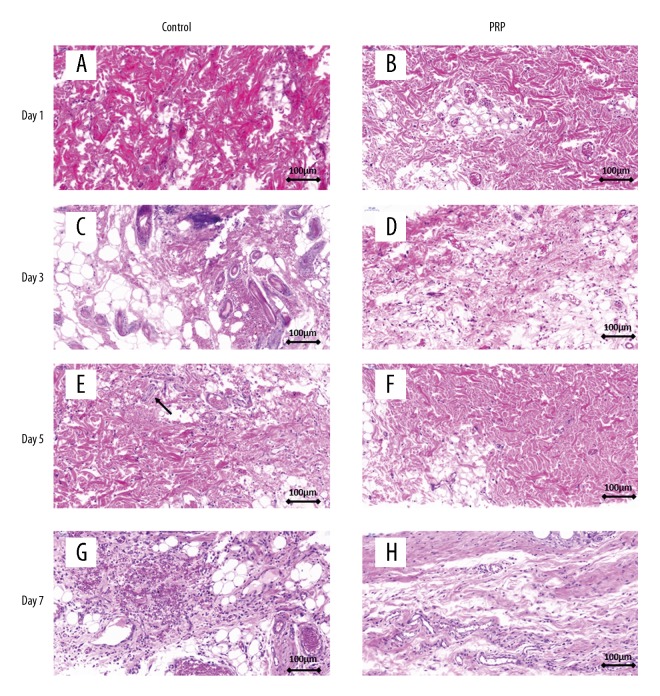
On day 1, hematoxylin-eosin (HE) staining revealed clear structures of the epiderm and derm in the PRP and control groups. Muscle fibers had an organized appearance and no obvious changes were found in the accessory organs. There were no significant differences between the 2 groups (Figure 2A, 2B). After day 3, the dermis and subcutaneous tissues in the control group presented a large area of granulation tissue repair with neutrophil infiltration (Figure 2C); the number of inflammatory cells in the PRP group was significantly decreased as compared with that in the control group, which showed lymphocyte infiltration (Figure 2D). From day 5 onward, loosely arranged muscle fibers and localized muscle fibrinolysis, necrosis, and inflammatory cell infiltration were observed in the control group (Figure 2E). On the contrary, the muscle fibers in the PRP group were evenly stained with more angiogenesis than in the control group (Figure 2F). On day 7, localized hemorrhagic foci were observed and inflammatory cells were widely present in the control group (Figure 2G), while the PRP group exhibited fewer inflammatory cells and increased neovascularization in contrast to the control group (Figure 2H).
Figure 2.
HE staining of skin flap at various time points. (A, B) The epidermis and the dermis structure were clear and muscle fiber was uniform in the PRP group and control group. (C, D) The PRP group showed less area of granulation tissue and inflammation than in the control group after day 3. (E, F) Muscle fibers in the PRP group were arranged more closely than in the control group after day 5. Locally, muscle fiber was necrosed and dissolved in the control group, accompanied by inflammatory cell infiltration (black arrow). (G, H) There were fewer inflammatory cells in the PRP group than in the control group. More new blood vessels were generated in the PRP group than in the control group.
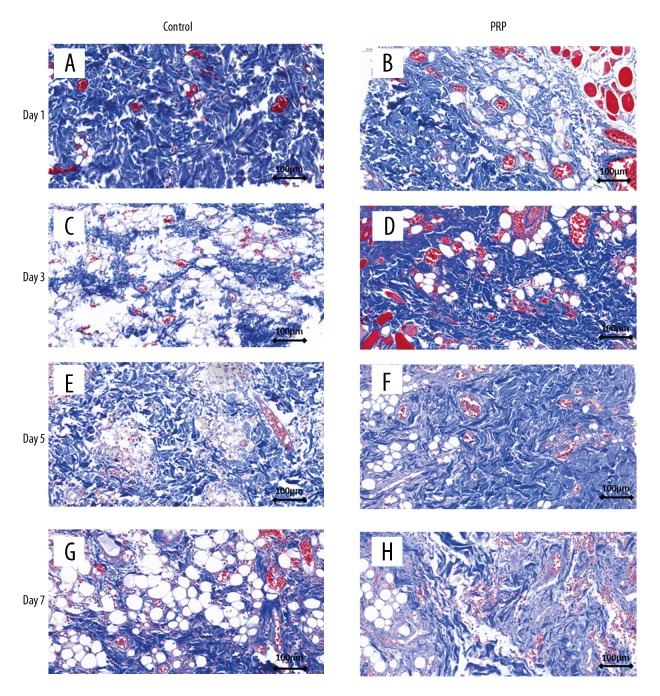
Masson staining revealed no obvious structural changes in the control and PRP groups on day 1 (Figure 3A, 3B). On the following day, collagen fibers in the dermal layer were loosely arranged in the control group (Figure 3C, 3E, 3F) but were tightly organized in the PRP group (Figure 3D, 3F, 3H); no interstitial abnormalities were observed in the 2 groups.
Figure 3.
Masson staining of skin flap at various time points. (A, B) No difference was found between the control group and PRP group on day 1. (C–F) Dermal collagen fibers in the control group were arranged more closely in the PRP group than in the control group. (G, H) The structure and arrangement of collagen fibers in the PRP group were better than in the control group.
Histological scoring and semi-quantitative analysis
Table 4 shows the scoring for HE staining. As vividly shown in the table, PRP enhanced the angiogenesis on day 5 and day 7, and it also revealed a significant difference in suppressing the inflammation on day 5. On day 7, PRP played its role and made a significant improvement in histological scoring, but there was no significant difference in fibrous change or cell density.
Table 4.
Histological scoring analysis for HE staining.
| Fiber structure | Fiber arrangement | Inflammation | Vascularity | Density of cells | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | PRP | Control | PRP | Control | PRP | Control | PRP | Control | PRP | Control | PRP | |
| Day 1 | 0.00± 0.00 | 0.25± 0.50 | 0.50± 0.58 | 0.50± 0.58 | 0.00± 0.00 | 0.25± 0.50 | 1.75± 0.50 | 1.50± 0.58 | 0.50± 0.58 | 0.50± 0.58 | 2.75± 0.96 | 3.00± 0.81 |
| Day 3 | 0.75± 0.50 | 0.75± 0.50 | 0.75± 0.50 | 1.00± 0.00 | 1.00± 0.82 | 0.50± 0.58 | 1.00± 0.82 | 0.75± 0.50 | 0.75± 0.96 | 0.25± 0.50 | 4.25± 1.71 | 3.25± 1.71 |
| Day 5 | 0.50± 0.58 | 0.50± 0.58 | 1.25± 0.50 | 1.25± 0.50 | 1.50± 0.58 | 0.50± 0.58* | 1.75± 0.50 | 0.50± 0.58* | 1.00± 0.00 | 0.75± 0.96 | 6.00± 0.82 | 3.50± 2.08 |
| Day 7 | 1.25± 0.50 | 1.75± 0.50 | 1.25± 0.96 | 0.75± 0.50 | 2.00± 0.82 | 1.25± 0.50 | 2.00± 0.82 | 0.50± 0.58* | 1.75± 0.50 | 1.25± 0.50 | 8.25± 1.50 | 5.50± 1.00* |
Represents significant difference (p<0.05).
Similar to HE staining, Masson staining also showed no significant difference in collagen change (Table 5). However, the mean of the PRP group score was lower than that in the control group, indicating only a slight effect of PRP. The semi-quantitative analysis results were in accordance with the histological evaluation.
Table 5.
Histological scoring analysis for HE staining.
| Collagen structure | Collagen arrangement | Total | ||||
|---|---|---|---|---|---|---|
| Control | PRP | Control | PRP | Control | PRP | |
| Day 1 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.25±0.50 | 0.00±0.00 | 0.25±0.50 |
| Day 3 | 0.75±0.95 | 0.25±0.50 | 1.00±0.81 | 0.50±1.00 | 1.75±1.50 | 0.75±0.96 |
| Day 5 | 0.50±1.00 | 0.25±0.50 | 1.00±0.00 | 0.50±0.58 | 1.50±1.00 | 0.75±0.96 |
| Day 7 | 1.75±0.96 | 0.50±0.58 | 2.00±1.15 | 1.25±0.95 | 3.75±2.06 | 1.75±0.96 |
No significant difference found (p>0.05).
Quantitative PCR assay
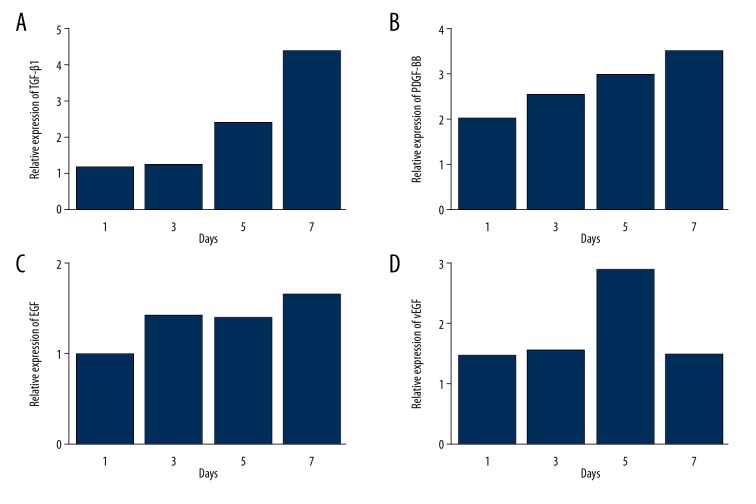
As is shown in Figure 4, we found that the TGF-β1, PDGF-BB, EGF, and VEGF expressions were higher in the PRP group than in the control group.
Figure 4.
Relative expression of (A) TGF-β1, (B) PDGF-BB, (C) EGF, and (D) vEGF in PRP group compared to control group at various time points. All types of growth cytokine were overexpressed in the PRP group, resulting in a better condition for skin flap survival.
Discussion
Wound healing is a complex pathophysiological process involving tissue regeneration and repair. Clean, small, and superficial wounds can self-heal, whereas dirty, large, and deep wounds require comprehensive systemic intervention [17,18]. Wounds cannot heal normally to obtain proper anatomical and functional integrity when there is massive infiltration of various factors; therefore, in a constant inflammatory state, long-term chronic wounds heal poorly and are known to be refractory [19]. Currently, complex refractory wounds are treated with skin flap transplantation after debridement and disinfection. Surgical treatment is preferred for wound healing because of its high-quality outcome and low recurrence rate. However, surgical treatment has certain indications: deep-tissue exposed wounds with poor blood supply; clean wounds with good blood supply that have been conservatively treated over a long period because of a cutaneous defect having a large area; and chronic wounds directly or indirectly induced by diabetic and angiolymphatic ulcers [20,21]. Surgical treatment is not suitable for patients with severe injuries, and autograft wound repair can induce great trauma and functional and visual damage [22]. Moreover, long-term dressing changes and repeated wound repair after surgery can cause a heavy burden on patients. Many kinds of cells, extracellular matrix, and cytokines are involved in the complex biological process of wound healing, which is affected by systemic and local factors. Our results show that PRP gel can efficiently increase the survival rate of the skin flap. PRP gel can also reduce the inflammation response in skin flap transplantation and produce better effects in generating new soft tissue.
In the early stage of flap transplantation, we hypothesized that platelets in PRP play an important role in hemostasis and coagulation. Through the processes of adherence, release, aggregation, contraction, and adsorption, they obtain complete hemostasis, prevent formation of subcutaneous hematoma, and protect flap survival. Then, according to our study, especially in histology analysis and PCR test, we found that PRP has further benefits via angiogenesis enhancement and inflammation inhibition.
Chronic wounds have a decreased number and activity of local related growth factors (GF); however, PRP can release many growth factors such as PDGF, TGF-β, EGF, and VEGF when activated. These growth factors play an important role in cell proliferation and vascular tissue regeneration to promote wound healing [23,24]. The growth factors combine with their corresponding receptors on cell membranes, then activate downstream signaling pathways to transduce the signal into the nucleus and thus promote cell proliferation, matrix synthesis, and angiogenesis. PDGF has been shown to promote mitosis and biological chemotaxis, and increase the formation of capillaries to help in flap healing. TGF-β can induce apoptosis of inflammatory cells, promote cell proliferation and collagen production, and induce cell epithelialization. The biological effect of VEGF is extensive and is known to promote the formation of granulation tissue. VEGF is known to increase the permeability of blood vessels, improve the function of vascular endothelial cells, prolong the life of endothelial cells, and promote angiogenesis.
The immunological constituents of PRP can help organisms eradicate pathogens and necrotic and senile tissues, and increase their local anti-infection ability. The white blood cells, immune cells, and immunomodulatory factors in the reticular structure, such as interleukins (IL-6 and IL4) and TNF, provide PRP immune defense and local anti-inflammatory effects, which can reduce inflammatory reaction and promote tissue repair after surgery. Studies [25,26] have reported that PRP can inhibit the growth of Staphylococcus aureus, Escherichia coli, and other bacteria in vitro, indicating that PRP has antibacterial activity. In this study, according to the histological evaluation, the PRP group also showed less inflammatory response.
In addition, fibronectin and osteonectin in PRP can protect platelets from loss during injection and help tissue repair. The fibrin in PRP provides a reticular scaffold for chemotaxis, and it provides a place for collecting stem cells, supporting cell migration, and promoting cell adhesion, which induce the migration and proliferation of red blood cells, leukocytes, platelets, and antibodies into skin flaps.
Conclusions
In this study, we found that PRP gel can increase the skin flap survival rate. In addition, PRP can enhance the angiogenesis and reduce the inflammation response in skin flap transplantation. PRP has better effects in generating new soft tissue. The effectiveness of PRP gel in skin flap transplantation is satisfactory. The mechanism by which the PRP gel promotes skin flap survival includes various factors, such as platelets, growth factors, immune activity factor, and fibrin. The growth factors in PRP may enhance the growth of the skin flap. The immune activity factors may affect the antibacterial activity and reduce immune response. Platelets and fibrin accelerate coagulation and provide a scaffold for the skin flap. Therefore, PRP could be a new clinical method for improving skin flap survival rates.
Footnotes
Conflict of interests
None.
Source of support: This work was supported by the Suzhou Science and Technology Project (SYS201654)
References
- 1.Zhou X, Wang J, Qiang L, et al. Outcomes of using a modified anteromedial thigh perforator flap for repai ring the anterolateral thigh free flap donor site: A retrospective clinical review. Medicine. 2018;97(16):e0491. doi: 10.1097/MD.0000000000010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cam B, Bagdas D, Ozyigit MO, et al. Effects of adrenomedullin and glucagon-like peptide on distal flap necrosis and vascularity: The role of receptor systems and nitric oxide. Wounds. 2017;29(6):163–67. [PubMed] [Google Scholar]
- 3.Janis J, Harrison B. Wound healing: Part II. Clinical applications. Plast Reconstr Surg. 2014;133(3):383e–92e. doi: 10.1097/PRS.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 4.Philippeos C, Telerman SB, Oules B, et al. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol. 2018;138(4):811–25. doi: 10.1016/j.jid.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon YR, Kang EH, Yang CE, et al. The effect of platelet-rich plasma on composite graft survival. Plast Reconstr Surg. 2014;134(2):239–46. doi: 10.1097/PRS.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 6.Bulam H, Ayhan S, Yilmaz G, et al. The effect of subcutaneous platelet-rich plasma injection on viability of auricular cartilage grafts. J Craniofac Surg. 2015;26(5):1495–99. doi: 10.1097/SCS.0000000000001819. [DOI] [PubMed] [Google Scholar]
- 7.Güler İ, Billur D, Aydin S, Kocatürk S. Efficacy of platelet-rich fibrin matrix on viability of diced cartilage grafts in a rabbit model. Laryngoscope. 2015;125(3):E104–11. doi: 10.1002/lary.25097. [DOI] [PubMed] [Google Scholar]
- 8.Andia I, Rubioazpeitia E, Maffulli N. Platelet-rich plasma modulates the secretion of inflammatory/angiogenic proteins by inflamed tenocytes. Clin Orthop Relat Res. 2015;473(5):1–11. doi: 10.1007/s11999-015-4179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jo CH, Lee SY, Yoon KS, Shin S. Effects of platelet-rich plasma with concomitant use of a corticosteroid on tenocytes from degenerative rotator cuff tears in interleukin 1β-induced tendinopathic conditions. Am J Sports Med. 2017;45(5):1141–50. doi: 10.1177/0363546516681294. [DOI] [PubMed] [Google Scholar]
- 10.El Backly RM, Zaky SH, Muraglia A, et al. A platelet-rich plasma-based membrane as a periosteal substitute with enhanced osteogenic and angiogenic properties: A new concept for bone repair. Tissue Eng Part A. 2013;19(1–2):152–65. doi: 10.1089/ten.TEA.2012.0357. [DOI] [PubMed] [Google Scholar]
- 11.Rabillard M, Grand JG, Dalibert E, et al. Effects of autologous platelet rich plasma gel and calcium phosphate biomaterials on bone healing in an ulnar ostectomy model in dogs. Vet Comp Orthopaed. 2009;22(6):460–66. doi: 10.3415/VCOT-09-04-0048. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Enomoto M, Ukegawa M, et al. Subcutaneous injections of platelet-rich plasma into skin flaps modulate proangiogenic gene expression and improve survival rates. Plast Reconstr Surg. 2012;129(4):858–66. doi: 10.1097/PRS.0b013e3182450ac9. [DOI] [PubMed] [Google Scholar]
- 13.Findikcioglu F, Findikcioglu K, Yavuzer R, et al. Effect of preoperative subcutaneous platelet-rich plasma and fibrin glue application on skin flap survival. Aesthet Plast Surg. 2012;36(5):1246–53. doi: 10.1007/s00266-012-9954-6. [DOI] [PubMed] [Google Scholar]
- 14.Karayannopoulou M, Papazoglou LG, Loukopoulos P, et al. Locally injected autologous platelet-rich plasma enhanced tissue perfusion and improved survival of long subdermal plexus skin flaps in dogs. Vet Comp Orthopaed. 2014;27(5):379–86. doi: 10.3415/VCOT-14-02-0030. [DOI] [PubMed] [Google Scholar]
- 15.Zhou K, Zhang Y, Lin D, et al. Effects of calcitriol on random skin flap survival in rats. Sci Rep. 2016;6(1):18945–45. doi: 10.1038/srep18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagdas D, Etoz BC, Gul Z, et al. Chlorogenic acid enhances abdominal skin flap survival based on epigastric artery in nondiabetic and diabetic rats. Ann Plas Surg. 2016;77(2):e21–25. doi: 10.1097/SAP.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 17.Martinou E, Drakopoulou S, Aravidou E, et al. Parecoxib’s effects on anastomotic and abdominal wound healing: A randomized Controlled trial. J Surg Res. 2018;223:165–73. doi: 10.1016/j.jss.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Lokhande G, Carrow JK, Thakur T, et al. Nanoengineered injectable hydrogels for wound healing application. Acta Biomater. 2018;70:35–47. doi: 10.1016/j.actbio.2018.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnaswamy VR, Manikandan M, Munirajan AK, et al. Expression and integrity of dermatopontin in chronic cutaneous wounds: A crucial factor in impaired wound healing. Cell Tissue Res. 2014;358(3):833–41. doi: 10.1007/s00441-014-2000-z. [DOI] [PubMed] [Google Scholar]
- 20.Galat DD, Mcgovern SC, Larson DR, et al. Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am. 2009;91(1):48–54. doi: 10.2106/JBJS.G.01371. [DOI] [PubMed] [Google Scholar]
- 21.Myers WT, Leong M, Phillips LG. Optimizing the patient for surgical treatment of the wound. Clin Plast Surg. 2007;34(4):607–20. doi: 10.1016/j.cps.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi M, Muramatsu H, Nakano M, et al. Experience of using cultured epithelial autografts for the extensive burn wounds in eight patients. Ann Plast Surg. 2014;73(1):25–29. doi: 10.1097/SAP.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 23.Evans DC, Evans BG. The effects of platelet-rich plasma and activated collagen on wound healing in primary total joint arthroplasty. Orthopedics. 2018;41(2):e262–67. doi: 10.3928/01477447-20180213-05. [DOI] [PubMed] [Google Scholar]
- 24.Cieślikbielecka A, Pierchała M, Królikowska A, Reichert P. Effect of L-PRP treatment on wound healing after surgical skin incision in an experimental animal model. Connect Tissue Res. 2018;59:550–60. doi: 10.1080/03008207.2018.1424148. [DOI] [PubMed] [Google Scholar]
- 25.Mariani E, Filardo G, Canella V, et al. Platelet-rich plasma affects bacterial growth in vitro. Cytotherapy. 2014;16:1294–304. doi: 10.1016/j.jcyt.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Maghsoudi O, Ranjbar R, Mirjalili SH, Fasihiramandi M. Inhibitory activities of platelet-rich and platelet-poor plasma on the growth of pathogenic bacteria. Iran J Pathol. 2017;12:79–87. [PMC free article] [PubMed] [Google Scholar]