Abstract
In many taxa, the most common form of sex-biased migration timing is protandry—the earlier arrival of males at breeding areas. Here we test this concept across the annual cycle of long-distance migratory birds. Using more than 350 migration tracks of small-bodied trans-Saharan migrants, we quantify differences in male and female migration schedules and test for proximate determinants of sex-specific timing. In autumn, males started migration about 2 days earlier, but this difference did not carry over to arrival at the non-breeding sites. In spring, males on average departed from the African non-breeding sites about 3 days earlier and reached breeding sites ca 4 days ahead of females. A cross-species comparison revealed large variation in the level of protandry and protogyny across the annual cycle. While we found tight links between individual timing of departure and arrival within each migration season, only for males the timing of spring migration was linked to the timing of previous autumn migration. In conclusion, our results demonstrate that protandry is not exclusively a reproductive strategy but rather occurs year-round and the two main proximate determinants for the magnitude of sex-biased arrival times in autumn and spring are sex-specific differences in departure timing and migration duration.
Keywords: annual cycle, geolocator, long-distance migrant, migration phenology, protandry, protogyny
1. Introduction
Billions of migratory animals travel vast distances between their breeding and non-breeding regions to exploit seasonal changes in resource availability and secure their survival while increasing reproductive opportunities [1–3]. Natural selection favours individuals that best match their annual schedules with the phenology of their current environment [4] and gain prime access to resources [5]. In many species, factors like intrasexual competition, sex-specific breeding roles, and individual tolerance to suboptimal environmental conditions can induce differences in migration timing between males and females [5–8].
Sex-biased migration timing has most often been demonstrated for arrival to the breeding sites in spring. Protandry—the earlier arrival of males at the breeding location—is the most common form of sex-biased migration timing in many taxa [9], while protogyny—female arrival ahead of males—is less common and typically found in some species with reversed sex roles [10–12]. Protandry and protogyny have primarily been considered as reproductive strategies and seven explanations have been brought forward of how natural selection can shape spring arrival protandry [9]. Among those, the three principal adaptive hypotheses explaining protandry in birds are: (i) the mate opportunity hypothesis, (ii) the rank advantage hypothesis, and (iii) the susceptibility hypothesis [5–7,9,13]. Under these hypotheses, protandry should prevail in territorial species with a high degree of extra-pair paternity, in species with relatively larger male body size compared to females, and in populations with male-biased sex ratio and higher fecundity for early breeding females. The level of protandry also varies with migration strategy, with smaller differences between the sexes in long-distance migrants and larger differences in facultative and short-distance migrants [13–18], suggesting that processes other than reproduction play a role.
Measuring sex biases upon arrival at the breeding sites [16,19–21] provides only brief snapshots of the full annual cycles of migratory animals. Since life-history stages of migrants are inextricably linked and shaped by environmental conditions at various locations [21–25], we need a full annual perspective to better understand the driving forces that underlie sex-biased migration timing and the consequences it may have for individuals and populations [26]. Several recent studies have looked into sex-biased migration timing also at other annual stages, e.g. [21,27–33] frequently showing earlier male departure from the non-breeding sites in spring, but ambiguous patterns for autumn migration. However, sample sizes of such case studies are often small, and confirmation of sex biases in migration timing (or lack thereof) may often be masked by low statistical power. Thus, whether sex-biased migration timing is a general pattern across the entire annual cycle of migratory birds remains to be shown [34].
Furthermore, owing to difficulties in following individual migrants year-round, the proximate causes behind sex biased spring arrival times often remain obscure [35]. With advancing tracking technologies, however, we gain more data on entire migration schedules of individual birds allowing for detailed descriptions of sex-specific migration patterns, e.g. [29,36–38], and testing for proximate causes that drive differences in spring arrival times. The three main proximate determinants, that could explain sex biases in arrival timing, are differences in (i) departure timing from non-breeding areas, (ii) migration distance, and (iii) migration speed [35]. The causes are not mutually exclusive but their relative contributions remain largely unknown for most species (but see [14,39]). If proximate causes for arrival timing are similar for autumn and spring migration, we expect a similar pattern of sex-biased arrival timing (i.e. protandry) and similar strength of the ‘domino effect’ (a situation when the timing of one annual phase affects the timing of any subsequent phase [33,40]) between migratory departure and arrival in both seasons.
To gain a general insight into migration timing of males and females across the entire annual cycle, we compiled already published and unpublished tracking data on complete annual schedules of various Afro-Palaearctic long-distance migrant landbirds.
-
(i)
We test by how much and how consistently males migrate ahead of females in spring [5,9,13,35] and whether the timing of autumn migration is also sex biased. If protandry is solely a reproductive strategy [9], we expect it in spring, but not in autumn; if sex-biased timing prevails also in other parts of the annual cycle, additional processes besides breeding should be in play.
-
(ii)
We evaluate multiple proximate causes—departure timing, migration distance, duration and speed—as potential drivers for sex-biased migration timing [13,35]. If departure timing is the primary proximate driver for spring arrival protandry [14,39], we expect a clear domino effect between timing of different migration stages.
2. Methods
We studied migration phenology of male and female long-distance migratory landbirds travelling within the Afro-Palaearctic bird migration system. For our analyses, we used data from studies where individual birds had been tracked between breeding and non-breeding sites using light-level geolocators or solar-powered PTT-tags (for common cuckoo Cuculus canorus and roller Coracias garrulus from Spain; see [41,42]). We included only individuals with complete annual track recordings from which information on all four major migration transition times could be extracted—departure from breeding site, arrival at (first) non-breeding site, departure from (last) non-breeding site, and arrival at breeding site. This allowed for a year-round comparison of relative migration timing of the same individuals. Since annual migration schedules can vary considerably between years in response to varying environmental conditions at breeding and non-breeding sites as well as en route [24,43], we only included data from years where at least one male and one female had been tracked from the same breeding population. Our dataset included 14 passerine and near-passerine species from 25 European breeding populations which had been tracked between 2009 and 2017 (electronic supplementary material, table S1). The breeding sites spanned across Europe ranging from 37° N to 60° N latitude and from 8° W to 28° E longitude (electronic supplementary material, figure S1).
(a). Compilation of individual migration data
In addition to individual migration schedules, we extracted coordinates of breeding and estimated non-breeding sites for each individual. If individuals resided at multiple non-breeding sites, we considered the first non-breeding site as the arrival site in autumn and the last non-breeding site as the departure site in spring. We calculated individual migration distances (great circle distances between individual breeding and non-breeding sites), migration duration (days) and migration speed (km day−1). Because individual duration of pre-departure fuelling cannot be quantified using current tracking technologies, migration duration was defined as the time between departure and arrival at the final destination and should not be considered as total migration duration [44]. Consequently, individual migration speed is defined as migration distance divided by migration duration, which is probably an overestimate and should not be viewed as absolute migration speed sensu stricto [44]. Furthermore, locations of non-breeding sites as inferred from light-level geolocators inherently include positional error of up to a few hundred km [45], slightly affecting the estimates of individual migration distances and speeds.
Since we found an effect of age on the timing of autumn migration with juvenile birds migrating later than adult conspecifics (β = −10.56 ± 4.65 s.e., t1,66 = −2.27, p = 0.026), we restricted our analyses to adult birds and excluded 12 juvenile hoopoes (Upupa epops) from the dataset. Thus, our final sample size consisted of 354 complete annual tracks (195 males; 159 females) of 340 individuals (repeated tracks: eight males, six females; electronic supplementary material, table S1).
We also compiled information on the species' morphological and ecological traits (data source: [46]), namely sexual size dimorphism (SSD; using wing length as a proxy for overall body size), moult strategy (region where complete post-breeding moult is undertaken—Europe or Africa), and foraging mode (aerial or terrestrial feeder). Phylogenetic relatedness between the species was assessed using the Ericson-backbone tree from Jetz et al. [47] downloaded from www.birdtree.org.
(b). Data analyses
As species and populations may differ in migration timing, distance, duration and speed, we used their relative values (Δx) as inferred from tracking data, i.e. individual migration parameters were expressed as the difference to their species-, population- and year-specific means. Values of Δx < 0 represent relatively earlier migrations, shorter distances and durations, or slower migration speeds, while Δx > 0 represent relatively later migrations, longer distances and durations, or faster migration speeds. All data analyses were done in R [48].
We first tested for differences in migration timing between males and females and then whether these differences could be explained by differences in departure time, migration duration, distance or speed. For both tests, we used mixed-effect models (LMM) and accounted for the non-independence of hierarchical data by including species, population (nested within species) and year (nested within species and population) as random factors. LMM analyses were run with the R-package ‘lme4’ [49]; p-values were obtained via R-package ‘lmerTest’ [50]. Finally, we also evaluated the relationship between individual migratory departure and arrival times (relative values Δx) across the annual cycle using simple linear regressions.
Using the R-package ‘MCMCglmm’ [51], we tested the roles of several biological species traits in explaining the average differences in male and female migration timing (in days) for each species. Foraging strategy and moulting region were included in the models as binary variables, while SSD was a continuous variable. Phylogenetic relatedness between the species was included in the model as a random effect, thus, we could account for non-independence of data owing to shared ancestry of the species. In all models, we used inverse-Gamma priors (V = 1, nu = 0.002) as non-informative priors.
As the number of male and female tracks differed between species, populations and years, our ultimate sample was male-biased, which may potentially have confounded mean and relative migration parameters. To test whether this affected our results, we repeated the analyses with a reduced dataset that contained a random sample of individuals of the more common sex to match the number of the less common sex. Consequently, this reduced dataset contained a balanced number of males and females from each population and year and thus, the same total number of individuals per sex (n = 128 males + 128 females). To avoid effects from the identity of these individuals in the selection, we repeated the random selection and analyses 99 times. Using this reduced dataset, we recalculated the relative values for migration timing, distance, duration and speed. Results from the reduced dataset analyses are presented in the electronic supplementary material.
3. Results
(a). Annual schedules
Our analyses revealed that migration schedules of males and females differed in both migration seasons, i.e. in spring and autumn (figure 1). In autumn, males departed from their respective breeding sites on average 1.7 days earlier than females (LMM with species, population and year as random effects: β = −1.73 ± 0.85 s.e., t1,352 = −2.03, p = 0.043; figure 1, electronic supplementary material, figure S2). However, we found no significant differences in relative arrival dates at the non-breeding sites between males and females originating from the same breeding sites (β = 0.17 ± 1.13 s.e., t1,352 = 0.15, p = 0.881). Note that the non-breeding sites are individual-specific, and birds of the same breeding origin did not necessarily migrate to the same destination. In spring, males departed from their non-breeding sites on average 2.9 days earlier than females (β = −2.94 ± 1.16 s.e., t1,352 = −2.52, p = 0.012). The difference in relative arrival times at the breeding site was even greater with males arriving on average 3.9 days earlier than females (β = −3.86 ± 0.98 s.e., t1,352 = −3.94, p < 0.001).
Figure 1.
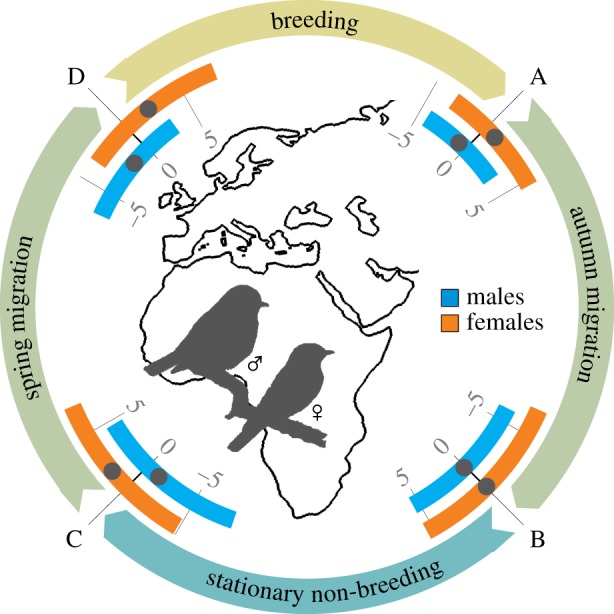
Differences in male (blue) and female (orange) migration timing of Afro-Palaearctic long-distance migratory birds (values below 0 correspond to earlier migration; measured in days). (A) Departure from the breeding site, (B) arrival at the non-breeding site, (C) departure from the non-breeding site, (D) arrival at the breeding site. Average values of relative migration times are indicated by black dots within interquartile ranges given as coloured bars.
The overall patterns were similar when using the reduced dataset; yet, the differences in male and female annual migration schedules were larger (average difference ± s.d.; breeding departure: 2.0 ± 0.5 days; arrival non-breeding: 0.4 ± 0.6 days (females earlier); departure non-breeding: 3.2 ± 0.7 days; arrival breeding: 4.1 ± 0.4 days; electronic supplementary material, figure S2 boxplots).
(b). Proximate causes of arrival timing
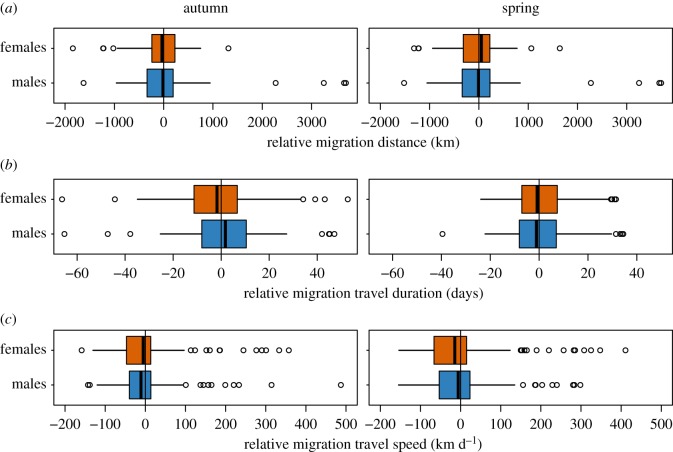
Our models identified sex-specific differences in departure timing and migration duration as the most important predictors for sex biases in arrival times (protandry or protogyny) at non-breeding and breeding sites (table 1). Migration distance and speed were similar for males and females during both migration seasons and did not account for sex-biased arrival times (table 1; figure 2).
Table 1.
Summary statistics of linear-mixed effects models examining proximate determinants of the magnitude of protandry (measured in days) at (a) autumn arrival at the non-breeding sites and (b) spring arrival at the breeding sites. (Species, population (nested in species) and tracking year (nested in species and population) were included in the models as random effects. All explanatory variables were scaled.)
| fixed effects | estimate | s.e. | t-value | p-value |
|---|---|---|---|---|
| (a) sex-specific differences in autumn arrival time | ||||
| intercept | 0.709 | 0.002 | 337.7 | <0.001 |
| departure time | 7.254 | 0.003 | 2830.0 | <0.001 |
| migration duration | 13.696 | 0.003 | 5433.2 | <0.001 |
| migration speed | 0.001 | 0.002 | 0.4 | 0.665 |
| migration distance | –0.001 | 0.002 | –0.2 | 0.876 |
| (b) sex-specific differences in spring arrival time | ||||
| intercept | –4.938 | 0.008 | –605.4 | <0.001 |
| departure time | 7.962 | 0.009 | 827.4 | <0.001 |
| migration duration | 9.641 | 0.012 | 785.8 | <0.001 |
| migration speed | –0.006 | 0.011 | –0.6 | 0.586 |
| migration distance | 0.001 | 0.009 | 0.1 | 0.898 |
Figure 2.
Comparison of relative migration (a) distance, (b) duration, and (c) speed between males and females in autumn and spring. Boxplots show median values with interquartile ranges (IQR; boxes), whiskers extend to 1.5 times the IQR, outliers are given as dots.
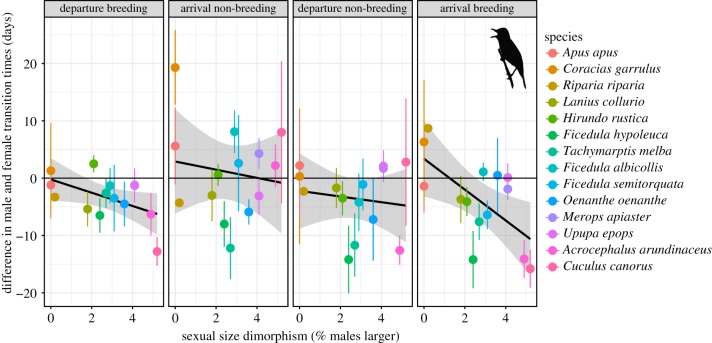
The biological trait model showed that differences between male and female migration timing were greater in species with larger SSD (figure 3), particularly upon spring arrival at the breeding sites. Foraging mode was not a significant predictor of differences in male and female migration timing throughout the entire annual cycle (electronic supplementary material, figure S3). Moult strategy was only a significant predictor for departure from non-breeding sites with species undergoing complete moult in Africa showing smaller differences between male and female spring departure timing (electronic supplementary material, figure S3).
Figure 3.
Differences in male and female migratory transition times among species (mean difference ± s.d.) and their relationship (±95% confidence interval—shaded area) with sexual size dimorphism as inferred from wing length. Differences below 0 denote cases of males being earlier, while values above 0 indicate females being earlier. The order of species in the figure legend corresponds to the order from left to right in the four individual plots. (Online version in colour.)
(c). Relationship between individual timing of consecutive migration episodes
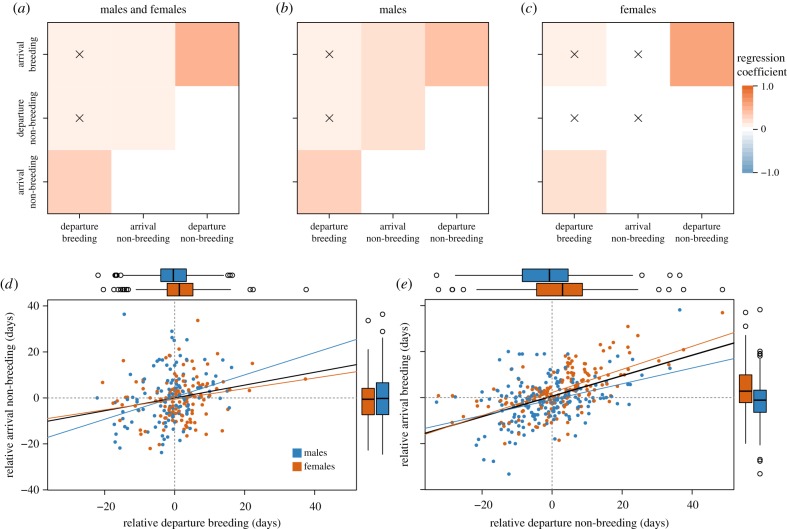
We found the strongest positive relationships between breeding site departure and non-breeding site arrival time as well as between non-breeding site departure and breeding site arrival time (autumn: β = 0.20 ± 0.04 s.e., F1,352 = 25.7, R2 = 0.07, p < 0.001; spring: β = 0.58 ± 0.05, F1,352 = 115.6, R2 = 0.25, p < 0.001; figure 4). Thus, the strongest domino effect between timing of migration events was found within, rather than across, autumn and spring migrations. There were also positive relationships between arrival and departure time at non-breeding sites, and non-breeding site arrival and breeding site arrival time—yet, to a lesser extent (figure 4a). In males, domino effects of migration timing were similar to the general pattern described above with the strongest relationship between non-breeding site departure and breeding site arrival time in spring (β = 0.47 ± 0.08, F1,193 = 37.3, r2 = 0.16, p < 0.001; figure 4b). In females, migration timing in autumn and spring was not related, yet departure from non-breeding and arrival at breeding sites were strongly related (β = 0.71 ± 0.08, F1,157 = 80.3, r2 = 0.33, p < 0.001; figure 4c). Analyses of the reduced dataset yielded similar results (electronic supplementary material, figure S4).
Figure 4.
Relationships between individual timing of migration events. (a) A matrix showing simple linear regressions between individual migratory departure and arrival times in autumn and spring for males and females combined, (b) for males only, and (c) for females only. Non-significant regressions are marked with ‘X’. A detailed example of the relationship between individual timing of migration departure and arrival is presented for autumn (d) and spring (e). Lines correspond to simple linear regressions: black for both sexes combined, blue—males, orange—females. Boxplots show median ± interquartile range (IQR—boxes; whiskers extend to values within 1.5 times the IQR and dots depict outliers) of x- and y-axis values for each sex.
4. Discussion
(a). Sex biases in annual schedules
Taking a full annual perspective on sex-biased timing of migration, we observed earlier male migration for three out of four main migration stages. Protandry in breeding site arrival was largely explained by an earlier departure of males from the non-breeding sites [14,35] and sex-specific differences in migration duration, whereas migration speed and distance contributed insignificantly. In autumn, males departed earlier from the breeding sites than females, but since the sexes also differed in migration duration, timing of arrival at the non-breeding sites was similar for both sexes. The species composition in our study comprise various taxonomic orders and families with variable moulting strategies, degree of territoriality, foraging modes, and SSD, and therefore, we feel confident to generalize our results to most long-distance migrants.
Our findings suggest that in Afro-Palaearctic migratory landbirds, males arrive at the breeding sites on average only a few days ahead of females. Earlier male arrival in spring has been shown in many migratory species with differences ranging between two weeks in some short-distance migrants and 2–8 days in long-distance migrants [18,20]. Furthermore, protandry in spring arrival is largely caused by males departing earlier from the non-breeding sites. This finding confirms the suggestion of several earlier case studies [14,21,27,39,52,53]. Earlier departure in males seems to be endogenously driven, as under constant day length conditions males show earlier onset of migratory restlessness than females [54]. Additionally, our findings also shed new light on sex-specific differences in migration duration as a primary contributor to sex-biased arrival timing. Migration duration is clearly an interaction between migration speed and distance, thus, these three parameters are partially masked within one another. However, the differences between average migration speed and distance of males and females were negligible, contributing only insignificantly towards sex-biased migration arrival times.
We also found that in autumn, males generally depart from the breeding sites earlier than females, but these differences ceased upon arrival at the non-breeding sites. Hitherto, our understanding of sex-biased timing of bird autumn migration has largely been based on data from ringing stations. Several of such studies reported no sex-differences or even protogyny (earlier female migration) in long-distance migrants during autumn [12,55], which would be in contrast to our results. However, an inherent pitfall of data from ringing stations is that they capture birds on passage and typically their origin and destination are unknown. Thus, any differences between the sexes that such ringing-station data might suggest, could be cofounded by variable migration timing of individuals that come from, or head to, different locations. Naturally, this is resolved in our dataset (and individual tracking data in general) and we can directly compare breeding site departure and non-breeding site arrival of individuals from the same breeding populations.
Two issues could be raised about our results and their interpretation, namely that (i) tracking devices might affect females more than males and thus delay their migration; and (ii) earlier arriving males might be easier to recapture than late arriving ones. Although it has been shown that tracking devices can have more negative effects on female rather than male apparent survival in aerial foragers [56], no sex-specific effects on the timing of migration have been found [57]. As to the recapture probability of early- and late-arriving individuals, most of our study species breed in nest-boxes or natural cavities, which are regularly inspected during the entire breeding season. Thus, late-arriving breeders are as likely to be recaptured as early-arriving breeders. However, recapture probabilities might differ if late-arriving males are unable to breed, e.g. if all territories are already occupied [21]. We recognize that a general constraint inherent to individual-based archival bio-logging devices is that the dataset contains only successfully migrating and surviving individuals and cannot infer or analyse the migration timing of unsuccessful birds.
(b). Full annual perspective on adaptive hypotheses for protandry
Protandry has primarily been considered a reproductive strategy [9] and therefore, most research has focused on sex biases in arrival times at the breeding site, largely neglecting the timing of other annual stages. We further discuss the three leading adaptive hypotheses for protandry in migratory birds [13] and put them in the context of full annual cycles.
The susceptibility hypothesis predicts that males arrive earlier in spring because they are better able to withstand adverse weather conditions (e.g. owing to their larger body size) en route or at the breeding sites early in the season [7]. In long-distance migrants, however, this applies only to the breeding site arrival in spring as Afro-Palaearctic migratory birds typically do not experience cold conditions at other parts of the annual cycle. Thus, the susceptibility hypothesis alone cannot explain the observed differences in male and female migration timing at other annual stages.
In the mate opportunity hypothesis, earlier arrival of males provides direct fitness benefits via polygyny, and theoretical models have convincingly demonstrated the mate opportunity hypothesis to be the most plausible explanation for spring protandry in migratory animals [6]. If males and females migrate at similar speeds and over similar distances (as shown in figure 2), this hypothesis also justifies why males should depart from the non-breeding sites ahead of females. However, applying this hypothesis to explain the protandry pattern during autumn migration is not that straightforward. Because no mating takes place after autumn migration, the mate opportunity hypothesis predicts no sex-biased arrival times at the non-breeding site which is in line with our findings. The mate opportunity hypothesis, however, fails to explain why males should leave the breeding sites earlier than females.
The rank advantage hypothesis argues that male–male competition for access to prime breeding sites is the main driver of spring arrival protandry [5]. While this hypothesis could also explain why males start spring migration earlier than females, an extension of the rank-advantage model by also including female–female competition sometimes resulted in protogyny, rather than protandry—contrasting our findings [6]. This is because early in spring, female–female competition can be stronger than male–male competition, as females compete for a resource that is relatively scarcer—territories occupied by males—than the resource contested for by males—vacant territories. Autumn migration is additionally characterized by the presence of male–female competition for access to high quality non-breeding sites, as spending the non-breeding residency period in good conditions can be of utmost importance for survival, preparing for spring migration, and future reproductive success [58]. Introducing intersexual competition in the rank-advantage model eliminates sex-biased arrival at the non-breeding sites—a pattern found in our study—as both sexes are expected to advance their arrival up to a point where increased costs of premature or excessively fast migration counteract the benefits of an even earlier arrival [5]. Competition for resources at the non-breeding sites would also lead to early departure from the breeding sites in autumn, as early-departing individuals (or populations) would gain a head-start over those who depart later [30]. Thus, both sexes should advance their departure date from the breeding sites to arrive early at the non-breeding sites. Earlier departure of males found in our study may be attributed to females investing more energy and/or time in reproduction, which delays their post-nuptial moult and preparation for migration [22]. Indeed, for species that moult before post-breeding migration, males have been shown to start post-nuptial moult earlier than females [59,60]—an important prerequisite for timely departure from the breeding sites in autumn. Thus, timing of moult might set an important constraint for timing of migration across the annual cycle generating sex-biased migration schedules (see the electronic supplementary material, figure S3).
(c). Links between consecutive annual stages
In both migratory seasons, timing of departure and arrival at the destination were positively correlated, indicating that late departure from one site cannot be fully compensated for but rather leads to late arrival at the next site with potential downstream consequences [40,61,62]. Such cascading effects have been shown in barn swallows, where females that departed early from the non-breeding areas also bred earlier and had higher fecundity; yet, no such relationships were found in males [63]. Thus, the start of spring migration bears stronger consequences for reproductive success in one sex than the other, which is in line with our finding of a tighter relationship between spring departure and arrival dates in females compared to males.
In females, spring migration schedules were not dependent on the timing of their previous autumn migration, while in males, arrival time at the non-breeding site and timing of spring migration were still positively related. Studies on short-lived migrant species suggest that effects from the previous migration season do not carry over to influence the timing of the subsequent spring migration [21,22,33,64,65]. The non-breeding period potentially serves as a buffer dissolving the rank order of individuals from the autumn migration. The sample size of these case studies, however, may sometimes be insufficient for comparing different demographic groups within the populations. Our results suggest that males and females experience different levels of domino effects between timing of consecutive migration seasons [63].
5. Conclusion
Our study has advanced the knowledge of a long-debated subject—differences in year-round migration schedules of males and females in long-distance migratory birds. We show that sex-biased timing is not restricted to spring arrival at breeding sites, but males and females differ in migration schedules across the annual cycle. The magnitude of spring arrival protandry is primarily driven by earlier male departure from the non-breeding sites and sex-specific differences in migration duration. Earlier male departure in autumn, however, does not translate into earlier arrival at the non-breeding sites. Although, our understanding of the selective advantages of spring protandry and their trade-offs has advanced during the last decades, e.g. [39,62,63,66] the ultimate causes of sex-biased autumn migration timing remain to be empirically tested. A potential prime candidate might be rank advantage in acquiring non-breeding territories or home ranges for optimal moult and maintenance of good body condition.
Supplementary Material
Acknowledgements
We are thankful to all field assistants and volunteers that supported the individual studies underlying this manuscript and other researchers for collecting and publishing their data. Two anonymous reviewers provided helpful comments on an earlier version of the manuscript. T. Finch provided additional details on European roller data.
Data accessibility
Data are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.t78400r [67].
Authors' contributions
M.B., S.B. and S.H. conceived the idea and study design. M.B., P.A., J.A.A., J.S.C., T.E., L.G., J.K., F.L., C.M.M., P.P. and S.H. carried out individual tracking projects, analysed and provided geolocator data. M.B. analysed the data and wrote the manuscript. All authors discussed, revised and approved the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
Financial support was provided by the Swiss Ornithological Institute. The Swiss Federal Office for the Environment supported geolocator development (UTF-Nr. 254, 332, 363, 400); individual tracking projects were funded by the Czech Science Foundation (13-06451S to P.A. and P.P.), Palacký University grant scheme (IGA_PrF_2018_016 to P.A.), Fundação para a Ciência e a Tecnologia (SFRH/BPD/91527/2012 and SFRH/BD/113580/2015 to J.A.A. and J.S.C.), the Swedish Research Council (to L.G.), Institute of Vertebrate biology (RVO: 68081766 to J.K. and P.P.), and the Swiss National Science Foundation (31003A_160265 to S.H. and S.B.).
References
- 1.Bauer S, Hoye BJ. 2014. Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344, 1242552 ( 10.1126/science.1242552) [DOI] [PubMed] [Google Scholar]
- 2.Alerstam T, Hedenström A, Åkesson S. 2003. Long-distance migration: evolution and determinants. Oikos 2103, 247–260. ( 10.1034/j.1600-0706.2003.12559.x) [DOI] [Google Scholar]
- 3.Dingle H. 2014. Migration: the biology of life on the move, 2nd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Møller AP, Rubolini D, Lehikoinen E. 2008. Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl Acad. Sci. USA 105, 16 195–16 200. ( 10.1073/pnas.0803825105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokko H. 1999. Competition for early arrival birds in migratory birds. J. Anim. Ecol. 68, 940–950. ( 10.1046/j.1365-2656.1999.00343.x) [DOI] [Google Scholar]
- 6.Kokko H, Gunnarsson TG, Morrell LJ, Gill JA. 2006. Why do female migratory birds arrive later than males? J. Anim. Ecol. 75, 1293–1303. ( 10.1111/j.1365-2656.2006.01151.x) [DOI] [PubMed] [Google Scholar]
- 7.Møller AP. 2004. Protandry, sexual selection and climate change. Glob. Chang. Biol. 10, 2028–2035. ( 10.1111/j.1365-2486.2004.00874.x) [DOI] [Google Scholar]
- 8.Cristol DA, Baker MB, Carbone C. 1999. Differential migration revisited: latitudinal segregation by age and sex class. Curr. Ornithol. 15, 33–88. ( 10.1007/978-1-4757-4901-4_2) [DOI] [Google Scholar]
- 9.Morbey YE, Ydenberg RC. 2001. Protandrous arrival timing to breeding areas: a review. Ecol. Lett. 4, 663–673. ( 10.1046/j.1461-0248.2001.00265.x) [DOI] [Google Scholar]
- 10.Oring LW, Lank DB. 1982. Sexual selection, arrival times, philopatry and site fidelity in the polyandrous spotted sandpiper. Behav. Ecol. Sociobiol. 10, 185–191. ( 10.1007/BF00299684) [DOI] [Google Scholar]
- 11.Ydenberg RC, Niehaus AC, Lank DB. 2005. Interannual differences in the relative timing of southward migration of male and female western sandpipers (Calidris mauri). Naturwissenschaften 92, 332–335. ( 10.1007/s00114-005-0637-x) [DOI] [PubMed] [Google Scholar]
- 12.Mills AM. 2005. Protogyny in autumn migration: do male birds ‘play chicken’? Auk 122, 71 ( 10.1642/0004-8038(2005)122[0071:PIAMDM]2.0.CO;2) [DOI] [Google Scholar]
- 13.Morbey YE, Coppack T, Pulido F. 2012. Adaptive hypotheses for protandry in arrival to breeding areas: a review of models and empirical tests. J. Ornithol. 153, 207–215. ( 10.1007/s10336-012-0854-y) [DOI] [Google Scholar]
- 14.Schmaljohann H, et al. 2016. Proximate causes of avian protandry differ between subspecies with contrasting migration challenges. Behav. Ecol. 27, 321–331. ( 10.1093/beheco/arv160) [DOI] [Google Scholar]
- 15.Both C, Bijlsma RG, Ouwehand J. 2016. Repeatability in spring arrival dates in pied flycatchers varies among years and sexes. Ardea 104, 3–21. ( 10.5253/arde.v104i1.a1) [DOI] [Google Scholar]
- 16.Tarka M, Hansson B, Hasselquist D. 2015. Selection and evolutionary potential of spring arrival phenology in males and females of a migratory songbird. J. Evol. Biol. 28, 1024–1038. ( 10.1111/jeb.12638) [DOI] [PubMed] [Google Scholar]
- 17.Klvaňa P, Cepák J, Munclinger P, Michálková R, Tomášek O, Albrecht T. 2017. Around the Mediterranean: an extreme example of loop migration in a long-distance migratory passerine. J. Avian Biol. 39, 133–138. ( 10.1111/jav.01595) [DOI] [Google Scholar]
- 18.Tøttrup AP, Thorup K. 2008. Sex-differentiated migration patterns, protandry and phenology in north European songbird populations. J. Ornithol. 149, 161–167. ( 10.1007/s10336-007-0254-x) [DOI] [Google Scholar]
- 19.Becker PH, Schmaljohann H, Riechert J, Wagenknecht G, Zajková Z, González-Solís J. 2016. Common terns on the East Atlantic Flyway: temporal–spatial distribution during the non-breeding period. J. Ornithol. 157, 927–940. ( 10.1007/s10336-016-1346-2) [DOI] [Google Scholar]
- 20.Cadahía L, Labra A, Knudsen E, Nilsson A, Lampe HM, Slagsvold T, Stenseth NC. 2017. Advancement of spring arrival in a long-term study of a passerine bird: sex, age and environmental effects. Oecologia 184, 917–929. ( 10.1007/s00442-017-3922-4) [DOI] [PubMed] [Google Scholar]
- 21.Ouwehand J, Both C. 2017. African departure rather than migration speed determines variation in spring arrival in pied flycatchers. J. Anim. Ecol. 86, 88–97. ( 10.1111/1365-2656.12599) [DOI] [PubMed] [Google Scholar]
- 22.Briedis M, Krist M, Král M, Voigt CC, Adamík P. 2018. Linking events throughout the annual cycle in a migratory bird—wintering period buffers accumulation of carry-over effects. Behav. Ecol. Sociobiol. 72, 93 ( 10.1007/s00265-018-2509-3) [DOI] [Google Scholar]
- 23.Marra PP, Hobson KA, Holmes RT. 1998. Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282, 1884–1886. ( 10.1126/science.282.5395.1884) [DOI] [PubMed] [Google Scholar]
- 24.Tøttrup AP, Klaassen RHG, Kristensen MW, Strandberg R, Vardanis Y, Lindström Å, Rahbek C, Alerstam T, Thorup K. 2012. Drought in Africa caused delayed arrival of European songbirds. Science 338, 1307 ( 10.1126/science.1227548) [DOI] [PubMed] [Google Scholar]
- 25.Both C, et al. 2006. Pied flycatchers Ficedula hypoleuca travelling from Africa to breed in Europe: differential effects of winter and migration conditions on breeding date. Ardea 94, 511–525. [Google Scholar]
- 26.Briedis M, Bauer S. 2018. Migratory connectivity in the context of differential migration. Biol. Lett. 14, 20180679 ( 10.1098/rsbl.2018.0679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arlt D, Olsson P, Fox JW, Low M, Pärt T. 2015. Prolonged stopover duration characterises migration strategy and constraints of a long-distance migrant songbird. Anim. Migr. 2, 47–62. ( 10.1515/ami-2015-0002) [DOI] [Google Scholar]
- 28.Arizaga J, Willemoes M, Unamuno E, Unamuno JM, Thorup K. 2015. Following year-round movements in barn swallows using geolocators: could breeding pairs remain together during the winter? Bird Study 62, 141–145. ( 10.1080/00063657.2014.998623) [DOI] [Google Scholar]
- 29.Briedis M, Träff J, Hahn S, Ilieva M, Král M, Peev S, Adamík P. 2016. Year-round spatiotemporal distribution of the enigmatic semi-collared flycatcher Ficedula semitorquata. J. Ornithol. 157, 895–900. ( 10.1007/s10336-016-1334-6) [DOI] [Google Scholar]
- 30.Briedis M, Hahn S, Gustafsson L, Henshaw I, Träff J, Král M, Adamík P. 2016. Breeding latitude leads to different temporal but not spatial organization of the annual cycle in a long-distance migrant. J. Avian Biol. 47, 743–748. ( 10.1111/jav.01002) [DOI] [Google Scholar]
- 31.Liechti F, et al. 2015. Timing of migration and residence areas during the non-breeding period of barn swallows Hirundo rustica in relation to sex and population. J. Avian Biol. 46, 254–265. ( 10.1111/jav.00485) [DOI] [Google Scholar]
- 32.Koleček J, et al. 2016. Cross-continental migratory connectivity and spatiotemporal migratory patterns in the great reed warbler. J. Avian Biol. 47, 756–767. ( 10.1111/jav.00929) [DOI] [Google Scholar]
- 33.Gow EA, et al. 2019. A range-wide domino effect and resetting of the annual cycle in a migratory songbird. Proc. R. Soc. B 286, 20181916 ( 10.1098/rspb.2018.1916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marra PP, Cohen EB, Loss SR, Rutter JE, Tonra CM. 2015. A call for full annual cycle research in animal ecology. Biol. Lett. 11, 20150552 ( 10.1098/rsbl.2015.0552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppack T, Pulido F. 2009. Proximate control and adaptive potential of protandrous migration in birds. Integr. Comp. Biol. 49, 493–506. ( 10.1093/icb/icp029) [DOI] [PubMed] [Google Scholar]
- 36.Bäckman J, Andersson A, Pedersen L, Sjöberg S, Tøttrup AP, Alerstam T. 2017. Actogram analysis of free-flying migratory birds: new perspectives based on acceleration logging. J. Comp. Physiol. A 203, 543–564. ( 10.1007/s00359-017-1165-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pakanen VM, Jaakkonen T, Saarinen J, Rönkä N, Thomson RL, Koivula K. 2018. Migration strategies of the Baltic dunlin: rapid jump migration in the autumn but slower skipping type spring migration. J. Avian Biol. 49, e01513 ( 10.1111/jav.01513) [DOI] [Google Scholar]
- 38.Buechley ER, McGrady MJ, Çoban E, Şekercioğlu ÇH. 2018. Satellite tracking a wide-ranging endangered vulture species to target conservation actions in the Middle East and East Africa. Biodivers. Conserv. 27, 2293–2310. ( 10.1007/s10531-018-1538-6) [DOI] [Google Scholar]
- 39.Rotics S, et al. 2018. Early arrival at breeding grounds: causes, costs and a trade-off with overwintering latitude. J. Anim. Ecol. 87, 1627–1638. ( 10.1111/1365-2656.12898) [DOI] [PubMed] [Google Scholar]
- 40.Piersma T. 1987. Hop, skip, or jump? Constraints on migration of Arctic waders by feeding, fattening, and flight speed. Limosa 60, 185–194. [Google Scholar]
- 41.Thorup K, et al. 2017. Resource tracking within and across continents in long-distance bird migrants. Sci. Adv. 3, e1601360 ( 10.1126/sciadv.1601360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodríguez-Ruiz J, De La Puente J, Parejo D, Valera F, Calero-Torralbo MA, Reyes-González JM, Zajková Z, Bermejo A, Avilés JM. 2014. Disentangling migratory routes and wintering grounds of Iberian near-threatened European rollers Coracias garrulus. PLoS ONE 9, 1–19. ( 10.1371/journal.pone.0115615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Briedis M, Hahn S, Adamík P. 2017. Cold spell en route delays spring arrival and decreases apparent survival in a long-distance migratory songbird. BMC Ecol. 17, 11 ( 10.1186/s12898-017-0121-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alerstam T. 2003. Bird migration speed. In Avian migration (eds Berthold P, Gwinner E, Sonnenschein E), pp. 253–267. Berlin, Germany: Springer. [Google Scholar]
- 45.Lisovski S, et al. 2018. Inherent limits of light-level geolocation may lead to over-interpretation. Curr. Biol. 28, R99–R100. ( 10.1016/j.cub.2017.11.072) [DOI] [PubMed] [Google Scholar]
- 46.Cramp S, Simmons K.. 2006. Birds of the western Palearctic interactive (ver. 2.0). Totnes, UK: Gostours.
- 47.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 48.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 49.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 50.Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. ( 10.18637/jss.v082.i13) [DOI] [Google Scholar]
- 51.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.1002/ana.22635)20808728 [DOI] [Google Scholar]
- 52.Tøttrup AP, et al. 2012. The annual cycle of a trans-equatorial Eurasian-African passerine migrant: different spatio-temporal strategies for autumn and spring migration. Proc. R. Soc. B 279, 1008–1016. ( 10.1098/rspb.2011.1323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Briedis M, Hahn S, Krist M, Adamík P. 2018. Finish with a sprint: evidence for time-selected last leg of migration in a long-distance migratory songbird. Ecol. Evol. 8, 6899–6908. ( 10.1002/ece3.4206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maggini I, Bairlein F. 2012. Innate sex differences in the timing of spring migration in a songbird. PLoS ONE 7, e31271 ( 10.1371/journal.pone.0031271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lehikoinen A, Santaharju J, Pape Møller A. 2017. Sex-specific timing of autumn migration in birds: The role of sexual size dimorphism, migration distance and differences in breeding investment. Ornis Fenn. 94, 53–65. [Google Scholar]
- 56.Scandolara C, et al. 2014. Impact of miniaturized geolocators on barn swallow Hirundo rustica fitness traits. J. Avian Biol. 45, 417–423. ( 10.1111/jav.00412) [DOI] [Google Scholar]
- 57.Brlík V. et al. Accepted Weak effects of geolocators on small birds: a meta-analysis controlled for phylogeny and publication bias. J. Anim. Ecol. ( 10.1111/1365-2656.12962) [DOI] [PubMed]
- 58.Rushing CS, Marra PP, Dudash MR. 2016. Winter habitat quality but not long-distance dispersal influences apparent reproductive success in a migratory bird. Ecology 97, 1218–1227. ( 10.1890/15-1259.1/suppinfo) [DOI] [PubMed] [Google Scholar]
- 59.Borowske A, Gjerdrum C, Elphick C. 2017. Timing of migration and prebasic molt in tidal marsh sparrows with different breeding strategies: comparisons among sexes and species. Auk 134, 51–64. ( 10.1642/AUK-16-116.1) [DOI] [Google Scholar]
- 60.Flinks H, Helm B, Rothery P. 2008. Plasticity of moult and breeding schedules in migratory European stonechats Saxicola rubicola. Ibis 150, 687–697. ( 10.1111/j.1474-919X.2008.00833.x) [DOI] [Google Scholar]
- 61.Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S. 2011. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80, 4–18. ( 10.1111/j.1365-2656.2010.01740.x) [DOI] [PubMed] [Google Scholar]
- 62.Wiggins DA, Pärt T, Gustafsson L, Part T. 1994. Seasonal decline in collared flycatcher Ficedula albicollis reproductive success: an experimental approach. Oikos 70, 359 ( 10.2307/3545773) [DOI] [Google Scholar]
- 63.Saino N, Ambrosini R, Caprioli M, Romano A, Romano M, Rubolini D, Scandolara C, Liechti F. 2017. Sex-dependent carry-over effects on timing of reproduction and fecundity of a migratory bird. J. Anim. Ecol. 86, 239–249. ( 10.1111/1365-2656.12625) [DOI] [PubMed] [Google Scholar]
- 64.van Wijk RE, Schaub M, Bauer S. 2017. Dependencies in the timing of activities weaken over the annual cycle in a long-distance migratory bird. Behav. Ecol. Sociobiol. 71, 73 ( 10.1007/s00265-017-2305-5) [DOI] [Google Scholar]
- 65.Senner NR, Hochachka WM, Fox JW, Afanasyev V. 2014. An exception to the rule: carry-over effects do not accumulate in a long-distance migratory bird. PLoS ONE 9, e0086588 ( 10.1371/journal.pone.0086588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lerche-Jørgensen M, Korner-Nievergelt F, Tøttrup AP, Willemoes M, Thorup K. 2018. Early returning long-distance migrant males do pay a survival cost. Ecol. Evol. 8, 11 434–11 449. ( 10.1002/ece3.4569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Briedis M, et al. 2019. Data from: A full annual perspective on sex-biased migration timing in long-distance migratory birds Dryad Digital Repository. ( 10.5061/dryad.t78400r) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Briedis M, et al. 2019. Data from: A full annual perspective on sex-biased migration timing in long-distance migratory birds Dryad Digital Repository. ( 10.5061/dryad.t78400r) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.t78400r [67].