Abstract
The distinctive anatomy of the crocodylian skull is intimately linked with dietary ecology, resulting in repeated convergence on blunt- and slender-snouted ecomorphs. These evolutionary shifts depend upon modifications of the developmental processes which direct growth and morphogenesis. Here we examine the evolution of cranial ontogenetic trajectories to shed light on the mechanisms underlying convergent snout evolution. We use geometric morphometrics to quantify skeletogenesis in an evolutionary context and reconstruct ancestral patterns of ontogenetic allometry to understand the developmental drivers of craniofacial diversity within Crocodylia. Our analyses uncovered a conserved embryonic region of morphospace (CER) shared by all non-gavialid crocodylians regardless of their eventual adult ecomorph. This observation suggests the presence of conserved developmental processes during early development (before Ferguson stage 20) across most of Crocodylia. Ancestral state reconstruction of ontogenetic trajectories revealed heterochrony, developmental constraint, and developmental systems drift have all played essential roles in the evolution of ecomorphs. Based on these observations, we conclude that two separate, but interconnected, developmental programmes controlling craniofacial morphogenesis and growth enabled the evolutionary plasticity of skull shape in crocodylians.
Keywords: Crocodylia, skull, geometric morphometrics, embryonic ontogeny, heterochrony, evolutionary developmental biology
1. Introduction
Crocodylians are instantly recognizable by their flattened skulls with tooth-filled jaws, a morphological design that allows them to capture prey in aquatic and terrestrial environments. Historically, skull shape has been used to categorize species into distinct ecomorphs (groups with ecologically associated anatomical traits) [1,2]: slender-snouted (longirostrine) forms that primarily consume fish; blunt-snouted (brevirostrine) forms that process harder prey like molluscs or turtles; and moderate-snouted (generalized) forms that take down large animals at the water's edge. Although these ecomorphs where once thought to reflect evolutionary relationships, phylogenetic analysis has revealed many of these morphological similarities are due to convergence [3,4]. Further, rigorous quantitative exploration of adult cranial anatomy has demonstrated a continuum of shape exists—from short and wide to narrow and elongate—and that shape is tightly linked to mechanical performance, overpowering phylogenetic and biogeographic signals [5,6]. These results indicate that crocodylian skull shape is evolutionarily labile but directed by ecological specializations related to feeding and foraging [7,8].
Through their post-hatching ontogeny, crocodylians undergo substantial changes in size, habitat and diet. For example, Alligator mississippiensis shows strong bite-force allometry associated with shifts in dietary composition during post-hatching growth [9], corroborating the close association between ecology and developmental patterns of skull shape change [10,11]. Comparisons of ontogenetic trajectories in blunt-snouted forms (e.g. Osteolaemus tetraspis, Paleosuchus trigonatus, Melanosuchus niger) have revealed indistinguishable growth patterns, potentially signalling developmental constraints associated with ecological specialization [12]. In contrast, slender-snouted crocodylians (e.g. Gavialis gangeticus, Tomistoma schlegelii, Mecistops cataphractus, Crocodylus acutus) show late-stage developmental convergence in which adults of different species are most similar, while younger individuals are morphologically different [12,13]. These conflicting results make it difficult to infer and generalize potential developmental mechanisms driving ecomorphological convergence.
Embryonic development may provide key insights into crocodylian skull shape evolution as changes in early ontogeny can have important implications for the resulting adult phenotype [14,15]. Comparative embryologists have long recognized the conserved ‘phylotypic’ stage of development, a point at which embryos of different species show remarkable morphological and molecular similarity [16,17]. Modern developmental genetic analyses have further demonstrated that certain minor modifications to the position, timing or amount of genetic signalling or cellular interactions during early embryonic development can cause major modifications to the eventual anatomy (e.g. [18,19]). Phylogenetic analysis of ontogenetic trajectories has revealed that change in the timing and/or rate of developmental events, phenomena collectively known as heterochrony, is an important mechanism of evolutionary change [20–23]. For instance, heterochronic shifts have been implicated in evolutionary transformations of the cranial anatomy in major vertebrate lineages, such as fish, birds and mammals, including primates and humans [24–27]. However, relatively little is known about crocodylian embryonic development or whether heterochrony has played an important role in shaping crocodylian cranial diversity.
To understand how crocodylian skull shape changes during embryonic development and how ontogenetic transformations contribute to adult diversity, we incorporate embryonic specimens into an analysis of crocodylian skull shape variation and calculate trajectories of shape change across embryonic and post-hatching ontogeny. By sampling throughout ontogeny we seek to determine if species of different ecomorphs share a conserved skull shape at any stage during skeletal development. We also investigate whether species of the same ecomorph share common ontogenetic patterns or if there are multiple pathways to achieve similar adult forms. Further, we reconstruct ancestral ontogenetic trajectories at multiple points during crocodylian evolution, and test to what extent heterochrony facilitated ecological and anatomical convergence. By integrating evolutionary, developmental, and ecological perspectives, we aim to identify potential ontogenetic mechanisms underlying cranial ecomorphological diversification and convergence and to enhance our understanding of how the cranial developmental programme itself may evolve.
2. Material and methods
(a). Specimen sampling, imaging and landmarking
Our sample (n = 300) included 23 species of extant crocodylians, with 18 species represented by embryonic and post-hatching ontogenies (electronic supplementary material, table S1). Embryonic specimens were subdivided into two developmental periods based on their degree of skeletal development [28]: mid-skeletal period (Ferguson stages 20–24) and late-skeletal period (Ferguson stages 25–28). Post-hatching specimens were subdivided into four periods of ontogeny based on age and skull length: hatchling, juvenile, subadult, and adult. Our analysis focused on skull shape as seen in dorsal view, as recent studies have identified this anatomical view to be the most variable aspect of cranial shape within extant crocodylians [4,5]. Specimens were positioned with their palate in the horizontal plane prior to imaging either with a Nikon D90 digital camera or in VGStudio Max 2.3 (Volume Graphics GmbH, Heidelberg, Germany) for CT scans (electronic supplementary methods).
Skull shape was quantified using a 2D geometric morphometric approach in R, primarily using the geomorph package [29–31]. A set of 14 fixed landmarks positioned on one side of the skull (electronic supplementary methods and electronic supplementary material, figure S1) were chosen to maximize our ability to sample craniofacial anatomy in the incompletely ossified skulls of embryos. Comparisons with previously published analyses found that these 14 cranial landmarks captured similar axes of adult/subadult skull shape variation and distribution of taxa/ecomorphs in morphospace, despite the reduction in the number of landmarks (electronic supplementary analyses and electronic supplementary material, figures S2) [5]. Full Procrustes superimposition (gpagen) was used to correct for differences in size and alignment of specimens, allowing for comparisons of pure shape differences [32,33].
(b). Quantifying shape and ecomorph classification
Principal components analysis (PCA; prcomp, plotTangentSpace) was employed to construct distinct axes of shape variation which describe differences among species and changes during development. First, we ran a PCA on an adults/subadults-only dataset to test distinctions between previously assigned ecomorph categories [2,5] and how they compared to our blunt, moderate, and slender morphotypes (electronic supplementary analyses and electronic supplementary material, figure S3). Based on this morphospace, we reclassified Alligator sinensis and both species of Paleosuchus as moderate; Crocodylus acutus, C. intermedius, and C. novaeguineae as slender; Melanosuchus niger as blunt; and assigned Caiman crocodilus and Caiman yacare to the blunt ecomorph (electronic supplementary material, table S2). Second, we ran a PCA on the total dataset (adults, subadults, juveniles, hatchlings, and embryos) to quantify skull shape variation throughout ontogeny and calculate ontogenetic trajectories for further analysis. Differences in skull shape between (1) ontogenetic periods, (2) ecomorph groupings and (3) ecomorph groupings within ontogenetic periods (as separate factors) were tested using Procrustes ANOVA (P-ANOVA:shape; advanced.procD.lm). Pairwise comparisons of Procrustes variance (morphol.disparity) were also made among groups to determine whether skull shape disparity changes across ontogeny or among ecomorphs.
(c). Characterization of ontogeny
Ontogenetic trajectories of skull shape were calculated using allometric regressions of Procrustes-aligned coordinates (all pure shape variation) against log transformed centroid size (a proxy for ontogenetic progression). Procrustes ANOVA was used to test the effect of size (log(centroid size), covariate) and species (factor) on multivariate skull shape (P-ANOVA:allometry; procD. allometry). The interaction term (Size:Species) was used to determine whether species differed in their trajectories. This approach uses residual randomizations (iterations = 9999) to test significance and is demonstrated to be effective with high-dimensionality data [34]. Pairwise Procrustes ANOVAs of ontogenetic trajectories were also conducted to determine which species pairs differed significantly using the same method, correcting for repeated comparisons with the Bonferroni adjustment (p-adjust). Additionally, differences in ontogenetic variability among ecomorphs were tested by comparing Procrustes variance of residuals around ontogenetic regressions. To visualize ontogenetic trajectories in morphospace and highlight the major axes of shape variation, PC1 and PC2 scores (the two largest axes of shape variance) were regressed on log transformed centroid size (lm), though all statistical tests were fully multivariate. For all plots, PC scores are from the total ontogenetic dataset.
(d). Reconstruction of heterochronic shifts
To explore how cranial ontogenetic trajectories evolved, we used species-specific slope and intercept coefficients to reconstruct ancestral trajectories on the crocodylian phylogeny using maximum-likelihood estimation (contMap and anc.ML). Ancestral trajectories were reconstructed for PC1 and PC2 ontogenies separately to allow us to interpret how modifications to trajectories impacted specific aspects of skull shape. For each species, bootstrap resampling of specimens was used to construct 95% confidence intervals for slope and elevation at each species's mean centroid size. Heterochronic shifts were identified if descendants differed significantly in slope or elevation from their ancestor. Increases or decreases in slope signified acceleration or deceleration, whereas increases or decreases in elevation signified pre- or post-displacement [23,35]. Acceleration and deceleration represent evolutionary modifications to the overall rate of shape change during ontogeny, whereas pre- or post-displacement indicate skull shape is more adult-like or further juvenilized uniformly throughout ontogeny. To understand the link between heterochrony and adult ecomorph evolution, PC scores and wireframes were calculated for ‘embryonic’ and ‘adult’ stages from the reconstructed ontogenies at ancestral nodes, based on the average centroid size for extant crocodylian embryos and adults/subadults. To account for uncertainty in the phylogenetic position of Gavialis gangeticus [36], we analysed our dataset using two time-calibrated crocodylian phylogenies that reflect the topologies preferred by molecular and morphological datasets (electronic supplementary material, figure S4) [37,38]. Results are primarily discussed with reference to the molecular tree; comparisons to the morphology tree can be found in the electronic supplementary analyses. Heterochronic shifts were reconstructed based only on the 18 species with full ontogenies (including embryos or hatchlings), because differential sampling of ontogenetic stages may affect the ontogenetic reconstruction.
3. Results
(a). Morphospace of crocodylian cranial ontogeny
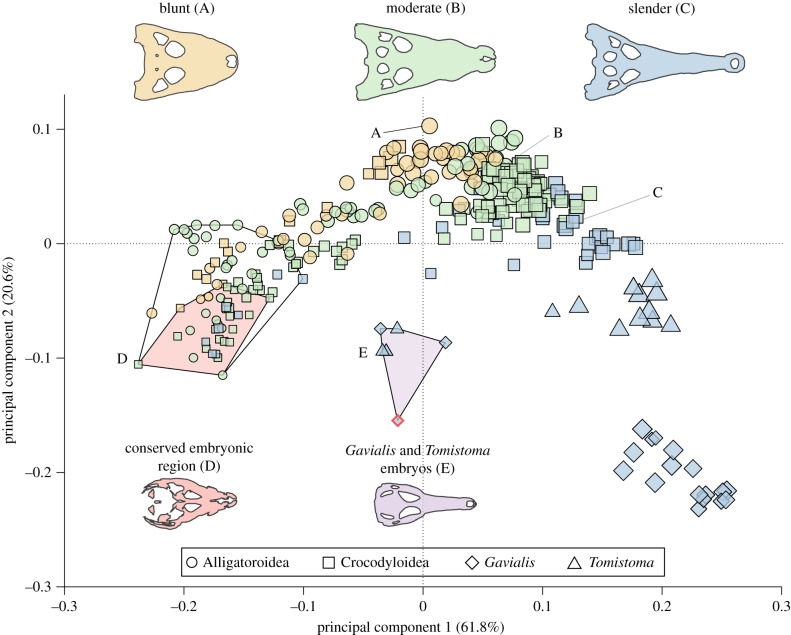
PCA revealed substantial variation in dorsal skull shape throughout ontogeny (figure 1; electronic supplementary material, figures S5 and S6). In the full ontogeny PCA, the first two PCs explained 82.4% of the variation in skull shape (PC1: 61.8%, PC2: 20.6%) and were the only PC axes significantly associated with the common allometric component of shape that captured meaningful amounts of variance (all p < 0.05; electronic supplementary material, table S3). PC1 captured changes in the relative length of the snout, size of skull table, and orientation of the orbits, while PC2 characterized changes in the width of the snout and degree of nasal retraction. Along PC1, positive values correlate with an elongated snout, smaller braincase, and more posterolaterally located orbits and negative values with a short snout, enlarged braincase, and anterior-posteriorly expanded orbits. Along PC2, negative scores correlate with narrow snouts that are straight-sided with retracted nasals and positive values more triangular skulls with wider snouts and more anteriorly extending nasals.
Figure 1.
Ontogenetic morphospace of extant crocodylian dorsal skull shape, showing differences among blunt (yellow), moderate (green) and slender (blue) ecomorphs. The conserved embryonic region (CER) is shaded in red, late-skeletal period embryos of non-gavialids are contained within the black, unshaded polygon. Gavialis gangeticus and Tomistoma schlegelii embryos occupy a unique region (shaded purple); within, the red outlined symbol represents the single mid-skeletal period embryo of Gavialis. Silhouettes are from the lettered specimens in the morphospace and the size of symbol is scaled by ontogenetic period. See electronic supplementary material, figures S5 and S6 to view morphospace labelled by species and developmental period.
All ontogenetic periods were significantly distinct in morphospace (all p < 0.05), except adults and subadults (least-squares mean distance = 0.03, p = 0.2067). Mid-skeletal period embryonic specimens of all ecomorphs shared a similar skull shape ( electronic supplementary material, table S4) and had the smallest Procrustes variance (0.0035 excluding Gavialis gangeticus; electronic supplementary material, table S5). They clustered together in a unique region (here termed the conserved embryonic region or CER) of morphospace characterized by a short and blunt, yet narrow face with large skull table and orbital dimensions (red region in figure 1; electronic supplementary material, table S6). The mid-skeletal period embryo of Gavialis (diamond outlined in red in figure 1) was the only outlier from this region and clustered with the late-skeletal period embryos of Gavialis and Tomistoma schlegelii that have PC1 scores around zero, reflecting a more elongated snout early in ontogeny relative to the other embryos (purple region in figure 1). Through ontogeny, PC1 score and relative disparity on PC2 increased, indicating snout elongation and skull shape divergence with age (figure 1). By the late-skeletal period of embryonic development, slender forms were already significantly different from the other two ecomorphs (p < 0.05), and this trend continued through the rest of development. Adult and subadult individuals formed a spectrum of blunt (yellow symbols), through moderate (green symbols), to slender (blue symbols) skull shapes (figure 1), with significant difference between ecomorphs (p < 0.01). Gavialis was the most distinct species, with post-hatching individuals possessing extremely slender, straight-sided snouts, laterally oriented orbits and laterally expanded skull tables.
(b). Ontogenetic trajectories of cranial shape
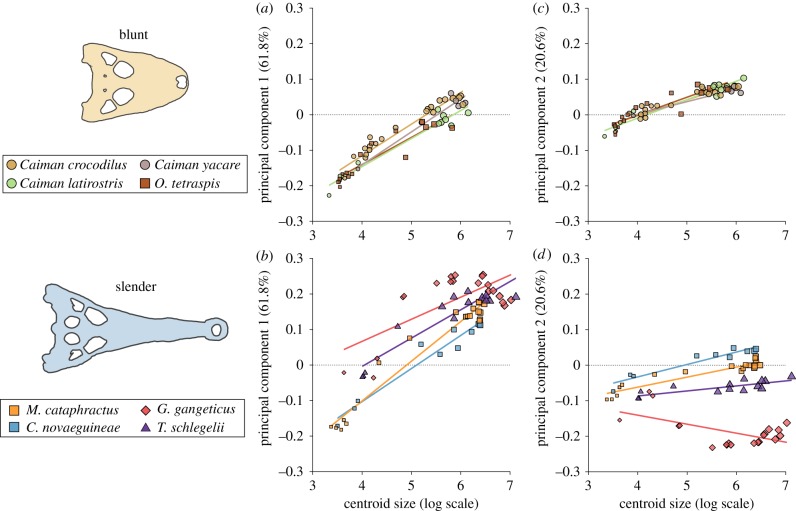
Allometric Procrustes ANOVA revealed significant differences in ontogenetic trajectories between species (p = 0.0001; electronic supplementary material, table S7 and figure S7). Pairwise comparisons further revealed 157 significant differences in ontogeny between species after correction for multiple comparisons (210 total comparisons; Bonferroni-corrected p: 0.0002381; electronic supplementary material, table S8). Although significant differences were recovered between species of different ecomorph categories (e.g. extremely slender Gavialis versus blunt Osteolaemus), the most striking differences between ecomorphs were in how variable ontogenetic trajectories were within ecomorphs (figure 2).
Figure 2.
Comparison of PC1 (a,b) and PC2 (c,d) ontogenetic trajectories for blunt and slender ecomorphs reveals more distinct ontogenies for slender forms while blunt species are nearly indistinguishable. For moderate ecomorph comparisons, see electronic supplementary material, figure S8. (Online version in colour.)
Blunt ecomorph species for which embryos were sampled showed nearly identical ontogenetic trajectories (ANOVA: allometry, all ; figure 2a,c). Only a single pairwise comparison was significantly different (Caiman crocodilus and Caiman latirostris; p = 0.021). Interestingly, both species of Paleosuchus differed significantly in ontogenetic trajectory from all but one of the blunt species (P. palpebrosus and Osteolaemus were not significantly different; p = 0.126), reinforcing our reclassification of these species as ‘moderate’ ecomorph crocodylians. A comparison of trajectories revealed that the major difference was that Paleosuchus species have steeper PC1 trajectories and achieve a slightly more elongated snout while maintaining small adult size (electronic supplementary material, table S9).
In contrast, all slender ecomorph species with embryos differed in their ontogenetic trajectories (all p = 0.021; electronic supplementary material, table S8), explaining why the slender ecomorph has significantly greater ontogenetic disparity than other ecomorphs (Procrustes variance = 0.0249, p = 0.001). Although slender forms converged on similar levels of snout elongation (PC1) at adult size, they had much more variable origins (figure 2b,d). The patterns of ontogenetic change for snout width (PC2) were strikingly different between the blunt and slender ecomorphs, as slender forms diverged as ontogeny progressed instead of following a common trajectory. Further, Gavialis followed a negative slope unlike all other crocodylian species (figure 2c,d). All moderate ecomorph alligatorids and crocodylids showed intermediate patterns, with slightly greater divergence in ontogenetic trajectories than blunt forms but less than exhibited by slender forms (electronic supplementary material, figure S8).
(c). Evolutionary trends in cranial ontogeny and ecomorph
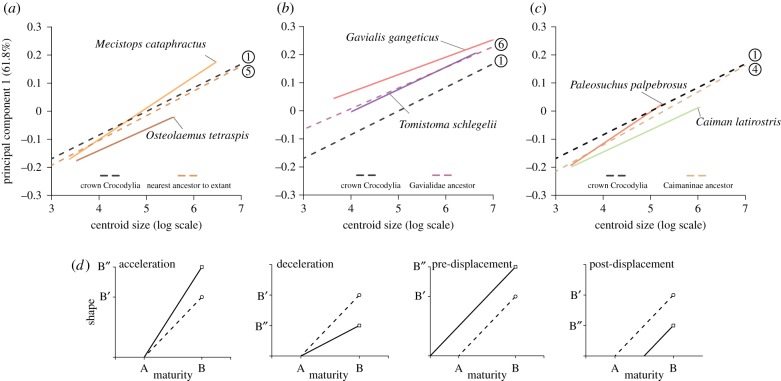
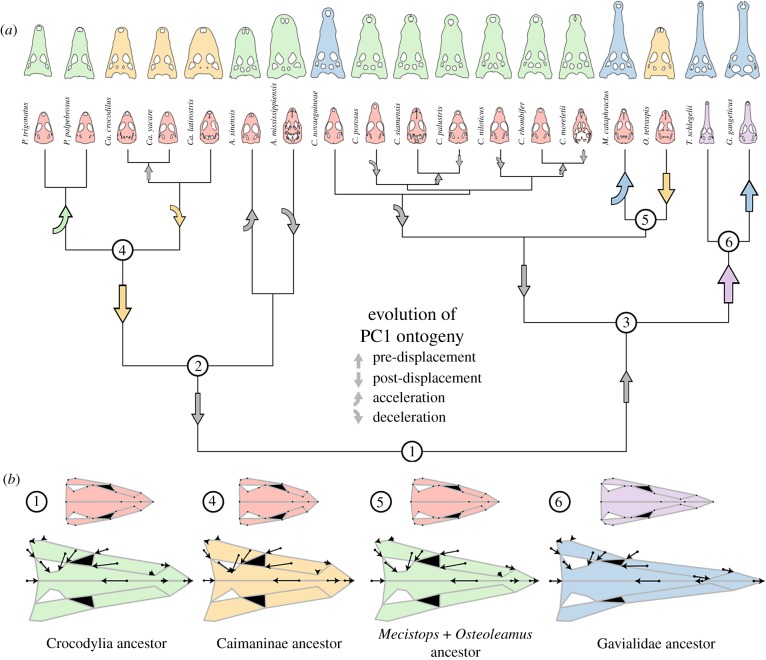
The ancestral crocodylian was reconstructed with an ontogenetic trajectory within the range observed for the moderate ecomorph based on the molecular topology (figure 3; electronic supplementary material, figures S9–S11). The reconstruction based on the morphological topology differed slightly, suggesting instead that the ancestral crocodylian would possess a more-slender ontogeny (less negative PC1 and PC2 intercepts, and flatter PC2 slope). However, both reconstructions were concordant that ecomorph convergence during crocodylian evolution occurred via modifications to slope, elevation, or both aspects of ontogeny (electronic supplementary material, tables S9–S14, and figures S10 and S11), although changes to the elevation were less common for PC2 ontogenies. Nevertheless, ontogenetic trajectories also changed when adult skull shape was relatively static (e.g. within Crocodylus) as a result of minor fluctuations in slope or elevation that offset each other. Although less common, multiple branches showed no change in ontogenetic trajectory (e.g. prior to Osteolaemus and Mecistops split or the Tomistoma terminal branch). Importantly, both reconstructions agreed that the most significant changes to ontogeny were associated with the convergent evolution of slender and blunt ecomorphs (figure 4; electronic supplementary material, figure S12).
Figure 3.
Reconstructed evolution of PC1 ontogenies of exemplar slender (a,b), blunt (a,c), and moderate (c) species. Theoretical heterochronic shifts (d) are displayed for comparison. In all plots, dashed lines are reconstructed ancestral ontogenies (numbers correspond to nodes in molecular phylogeny as shown in figure 4) while solid lines are extant species' ontogenies. (Online version in colour.)
Figure 4.
Reconstructed heterochronic modifications to PC1 ontogenetic trajectories across the crocodylian phylogeny (a). Shifts that change adult ecomorph are shown in colour of novel ecomorph. Reconstructed embryonic and adult shapes for key ancestral ontogenies are shown (b) with arrows on the adult shape depicting changes from the embryonic shape. Reconstructed heterochronic modification to PC2 ontogenies can be found in electronic supplementary material, figure S12. A list of heterochrony statements can be found in electronic supplementary material, table S13. (Online version in colour.)
Phylogenetic reconstruction of ontogenetic trajectories suggested that slender snouts evolved via different heterochronic mechanisms (figure 3; for PC2 ontogenies see electronic supplementary material, figure S13). Compared to the ancestral crocodylian (black dashed line in figure 3), Mecistops had an increased rate of snout elongation (steeper PC1 slope) and decreased rate of snout widening (shallower PC2 slope), resulting in a longer, more narrow snout as an adult while retaining a conserved embryonic starting point. The PC1 acceleration event was quite recent, however, having only occurred since Mecistops diverged from its shared ancestor with Osteolaemus (approx. 17 Ma; orange-brown dashed line in figure 3a). This pattern of acceleration was also recovered using the morphological topology, when Mecistops is not sister to Osteolaemus (electronic supplementary material, table S14 and figure S10). Gavialis and Tomistoma, in contrast, possessed substantially higher PC1 elevations relative to both Mecistops and the reconstructed ancestral crocodylian ontogeny. The elevation difference evolved via a pre-displacement shift which occurred in the gavialid ancestor (approx. 23 Ma; red-purple dashed line in figure 3b), resulting in longer snouts early in ontogeny. Tomistoma has essentially retained the ancestral gavialid PC1 ontogeny, while the highly apomorphic Gavialis underwent a further increase in elevation which accounts for its extremely slender snout. In addition to changes in PC1 trajectories, a decrease in PC2 slope was recovered prior to the gavialid split, resulting in more narrow snouts in later ontogeny, with Tomistoma and Gavialis subsequently undergoing distinct shifts. Taken as a whole, these data indicate that two different heterochronic mechanisms, acceleration (an increase in the rate of shape change) and pre-displacement (a more mature starting embryonic shape) have been employed independently in the evolution of the slender ecomorph (figure 4; electronic supplementary material, table S13 and figure S12).
The convergent evolution of the blunt ecomorph resulted in nearly indistinguishable ontogenetic trajectories (figure 2) which evolved via the same heterochronic shifts. Compared with the ancestral crocodylian, both genera (Caiman and Osteolaemus) had substantially lower elevations to their PC1 trajectories of snout elongation (figure 3a,c) and steeper PC2 slopes, resulting in shorter, wider snouts (for PC2 ontogenies see electronic supplementary material, figure S14). However, the Caiman lineage supports an additional decrease in the rate of snout elongation (PC1 slope) not recovered for Osteolaemus (figure 4; electronic supplementary material, table S13). The last common ancestor of Osteolaemus and Mecistops had an ontogenetic trajectory quite similar to the ancestral crocodylian, demonstrating that modifications to the ontogenetic trajectory of Osteolaemus only occurred in the last approximately 17 Myr (figure 3a; electronic supplementary material, figure S14). In contrast, the common ancestor of Caiman and its sister group Paleosuchus (green-orange dashed line in figure 3c) had already undergone two decreases in PC1 elevation (approx. 65 Ma and approx. 25 Ma), and an increase in PC2 slope resulting in a more blunt ontogeny than the ancestral crocodylian. Subsequently, the genus Caiman underwent a further decrease in PC1 slope. In contrast, the ancestor of Paleosuchus underwent an increase in both PC1 and PC2 slope, resulting in a moderate ecomorph skull shape while retaining small body size as an adult (figure 3c). Our data suggest that post-displacement (less mature starting embryonic shape) and deceleration (a decrease in the rate of shape change), either in tandem or not, facilitated the convergent evolution of the highly similar blunt ontogenetic trajectories (figure 4; electronic supplementary material, table S13 and figure S12).
4. Discussion
Any morphological variation that arises during evolution was necessarily preceded by specific alterations in developmental programmes, and identification of the specific nature of developmental change can provide insight into the deeper cellular and molecular mechanisms underlying evolution [19,39]. Although heterochrony is recognized to be only one of these processes and not the lone mechanism for deriving evolutionary novelty and diversity [40], the well-established framework for understanding changes in developmental timing, when applied to geometric morphometric analyses, is still very powerful [39]. This evo-devo approach has revealed ontogenetic trajectories often constrain cranial anatomy, because of conserved aspects of developmental programmes [24]. However, these developmental systems also facilitate the evolution of diversity because of the modularity of distinct components of ontogenetic trajectories and the semi-independence of interacting developmental programmes [24,27]. Previous studies of crocodylian cranial ontogeny have primarily assessed how well post-hatching ontogenetic trajectories reflect either phylogenetic or ecological patterns [10–14] or how embryonic ontogeny of pre-skeletal facial structures (e.g. frontonasal prominence) compare with other amniote clades [41]. Our study connects the earliest embryonic origins of cranial skeletal structures with the ontogeny of their later growth and gives new insight into the previous gaps in our understanding of the ontogeny of the crocodylian skull.
(a). Crocodylian craniofacial development
The inclusion of embryos in the analysis of crocodylian skull shape variation has revealed that most mid-skeletal period embryonic crocodylians occupy a conserved embryonic region (CER) of morphospace, irrespective of their adult ecomorph category (red region in figure 1; electronic supplementary material, tables S5 and S6). However, species- and ecomorph-specific shape differences begin to appear by the latest stages of embryonic development (electronic supplementary material, table S4) and species continue to diverge during ontogeny to achieve the previously recognized continuum of adult crocodylian cranial diversity (figure 1) [5,11]. The CER recovered here is reminiscent of the mid-embryonic ‘phylotypic’ stage of development, a period of developmental constraint which causes remarkable morphological similarities across lineages [17,42,43]. Recent studies have demonstrated that disparity in gene expression is minimized [44] and evolutionarily older genes are active during this period [45]. Previous explorations of developmental trajectories of earlier stages of craniofacial ontogeny among amniotes, including crocodylians, demonstrated that shape disparity is minimized during stages when fusion of facial prominences occurs and then increases during later growth [41]. Studies within vertebrate clades as divergent as fishes [24], lizards [46] and crocodylians (this analysis) further demonstrate that, within particular clades, anatomical conservation may extend from early morphogenesis at the ‘phylotypic’ stage to later developmental stages, including the onset of skeletogenesis. The existence of CERs during later periods of development for clades at different levels of evolutionary divergence, suggests that conserved developmental processes (molecular, genetic or cellular) are operating until species-specific traits begin to appear. This observation implies that the ‘neck’ of the developmental hourglass may be significantly longer when ontogenies of closely related species are compared.
The only exceptions to the CER were the mid- and late-skeletal period embryos of Gavialis gangeticus and Tomistoma schlegelii, which occupy a distinct cluster in morphospace (purple region in figure 1). By including phylogenetically informed reconstructions, our results show that it is the gavialids which have diverged from the CER, as the ancestral crocodylian ontogeny had an origin either within or very near to the CER (electronic supplementary analyses and electronic supplementary material, figure S9). Divergence from the CER in the two extreme slender-snouted taxa may suggest that release from a developmental constraint was necessary to evolve such extreme slender-snouted morphologies. A release from constraint would be expected to coincide with an increase in disparity, matching the increased ontogenetic variability within the slender ecomorph recovered both in our analysis and prior studies [12,13]. Further, our data demonstrate slender ecomorph species converge on similar levels of snout elongation (PC1) only at the latest stages of post-hatching ontogeny, whereas snout width (PC2) diverges from a more similar embryonic starting point (figure 2). In contrast, the taxa which have convergently evolved the blunt ecomorph (e.g. Caiman and Osteolaemus) originate within the CER and are remarkably similar across both axes of shape variation, indicating convergent evolution of the same ontogenetic trajectory. Thus, our data suggest that the CER may reflect a potential general constraint on early to mid-embryonic development (see below) that has potentially biased the evolution of blunt skulls toward convergence on similar ontogenies, rather than the adult-stage convergence from divergent embryonic starting points seen in the slender ecomorph.
(b). Heterochrony drives evolution of crocodylian craniofacial ontogeny
Reconstruction of ancestral crocodylian ontogenies revealed that blunt and slender skull shapes have convergently evolved via different patterns of heterochrony from the ancestral moderate ecomorph (figure 4; electronic supplementary material, table S13 and figure S12). Slender forms establish similar adult skull shapes through diverse ontogenetic trajectories, reflecting different developmental mechanisms (figures 3 and 4). Pre-displacement of ontogeny resulted in a distinct embryonic skull shape and allowed gavialids to explore a unique portion of ontogenetic morphospace, while acceleration of the rate of snout elongation allowed Mecistops cataphractus to remain in the CER and still achieve a slender adult skull. Blunt forms show that the same heterochronic mechanisms (post-displacement and deceleration) can be employed to independently achieve indistinguishable ontogenetic trajectories (figures 3 and 4; electronic supplementary material, figure S12). These recovered heterochronic patterns, in conjunction with gene expression and growth dynamics studies (see further below), suggest two separate, but interconnected, developmental programmes facilitated crocodylian craniofacial evolution—one that operates during early to mid-stage morphogenesis (i.e. controls the origin) and another that is involved in directing later ontogenetic patterns (i.e. controls the slope). These linked developmental programmes may explain the lability and ease of convergence found in the evolution of crocodylians and their extinct relatives [4,7,47]. Analogous developmental programmes have also been shown to underlie repeated convergent evolution of craniofacial shape in lizards and birds [46,48–50]. These examples also demonstrate that individual lineages are capable of applying unique mechanisms to achieve convergence [51], as may be the case with Gavialis and Tomistoma. We hypothesize developmental pathways that connect early facial patterning and long-term facial growth have allowed crocodylians to readily modify the ontogeny of skull shape, facilitating convergence.
Our analysis also recovered significant, if minor, heterochronic modifications to crocodylian cranial ontogeny that did not result in deviations from the ancestral moderate ecomorph. Within the genus Crocodylus, for example, we recovered 10 significant heterochronic shifts without any associated shift in adult ecomorph (figure 4; electronic supplementary material, table S13 and figure S12). It is possible that some of these shifts in ontogenetic trajectory are a result of the ancestral state reconstruction method; however, such shifts are also consistent with a phenomenon known as ‘developmental systems drift’ [52]. The divergence of developmental programmes is a logical consequence of lineage divergence, and just as genes and phenotypes are capable of evolving via drift mechanisms so too can ontogeny. Developmental studies have revealed developmental systems drift to be a common phenomenon that explains how characters known to be homologous have diverged in their gene regulatory underpinnings [52]. For example, drift in signalling pathways have been implicated in the reverse development of salamander digits [53], enhancer evolution in Drosophila segmentation [54] and formation of similar sexual structures in roundworms [55,56]. The connection between our proposed early and long-term developmental programmes offers a clue as to how drift in a developmental programme may result in changes (minor or major) to ontogeny, as either system is capable of independent changes in different crocodylian lineages. However, the fact that in some clades (e.g. Crocodylus) significant heterochronic shifts did not modify the resulting adult ecomorph suggests that common selection pressures to maintain skull shape (e.g. diet, skull strength) may play an overriding role in offsetting the effects of developmental systems drift.
(c). Potential developmental mechanisms underlying crocodylian evolution
The morphometric patterns recovered here are fundamentally established by underlying developmental processes at the genetic, molecular, cellular, and tissue levels. Although little is known yet about the developmental genetics of craniofacial morphogenesis in crocodylians beyond broad comparisons among amniotes (e.g. [14,15]), insight into which pathways might be involved can be gleaned from studies on similar evolutionary processes in fish, mammals, and birds. For example, Bone morphogenic protein 4 (Bmp4) is one of several key genes known to be involved with early facial patterning in the frontonasal prominence across vertebrates [57,58] with earlier and/or increased levels of expression in craniofacial primordia reported to be responsible for wider/deeper beaks in Darwin's finches [48], broader/larger bills in ducks [59] and more robust jaws in cichlid fishes [58]. Sonic hedgehog (Shh) signalling has also been shown to mediate BMP activity in the frontonasal prominence, directing both proximodistal and mediolateral growth of the middle and upper face [60]. Two different gene pathways appear to be critical in controlling craniofacial length in amniotes, with Calmodulin (CaM) directing early embryonic cartilage differentiation and proliferation, and Indian hedgehog (Ihh) driving growth zones between cranial bones later in ontogeny [18,48,58]. Relevantly, increases in the expression of a CaM homologue in fishes have been associated with a heterochronic shift during the early growth of elongated jaws in halfbeak and needlefish [61].
Within crocodylians, evolution of the most extreme slender and blunt skull shapes (figures 3 and 4) was achieved by heterochronic modifications to embryonic starting shape (i.e. pre- and post-displacement). This suggests that shifts in the early facial programmes directing initial snout elongation and lateral expansion (e.g. changes in CaM or SHH and BMP signalling) have the potential to play key roles in ecomorphological convergence. However, changes to the slope of cranial shape ontogeny (i.e. acceleration and deceleration) appear more commonly across the two primary axes of shape (figure 4; electronic supplementary material, table S13 and figure S12) and indicate that modifications in the later growth programme (e.g. regulation of bone growth by IHH signalling) may be the more likely evolutionary scenario. For example, the shift in embryonic cranial shape in the common ancestor of Gavialis and Tomistoma (figures 3b and 4) implies a potential modification to the early facial patterning programme, such as a delayed or reduced level of BMP activity, resulting in a more narrow embryonic snout. The elongated snouts of gavialid embryos also suggest that an earlier onset or increased amount of CaM activity may also have been involved. Alternatively, the acceleration of snout elongation from the CER observed in Mecistops could be explained by an expanded region or increased level of IHH activity causing increased proliferation in growth zones of the snout (figures 3a and 4). Conversely, morphometric patterns in blunt lineages suggest that modification to pathways upstream of both modules could result in coordinated shifts in both developmental programmes. A greater understanding of the molecular patterning of various aspects of cranial development in crocodylians, particularly during mid-to-late embryonic stages, will be required to test these hypothesized developmental mechanisms and to understand how they have influenced crocodylian craniofacial diversity and convergence.
5. Conclusion
Our study demonstrates that crocodylians have a highly conserved cranial shape during embryonic development (mid-skeletal period), analogous to the earlier ‘phylotypic’ stage, which is probably maintained by a set of conserved developmental interactions. However, by late embryonic development we detect differences in ontogenetic trajectories indicative of various types of heterochronic changes relative to the ancestral ontogeny, which are associated with the emergence of clade- and species-specific morphological characteristics. Interestingly, the relationship between particular modes of heterochrony (e.g. pre-displacement or acceleration) and particular types of morphological transformations (e.g. elongation or widening of the skull) appears to be very flexible. The evolution of ontogenetic trajectories suggests that minor heterochronic shifts are quite commonplace and that even lineages with moderate skull shapes may not remain developmentally ‘static’ but rather experience small magnitude shifts in opposing directions, thus offering evidence of developmental systems drift. Furthermore, crocodylians demonstrate how vastly different ontogenetic strategies can produce rather similar morphological (and likely biomechanical) outcomes and how relatively simple modifications to developmental trajectories can generate a full range of cranial morphologies and associated ecologies.
Supplementary Material
Supplementary Material
Acknowledgements
For assistance with collections access we thank: J. Cundiff and J. Rosado (Museum of Comparative Zoology); M. Brown and J. Sagebiel (Texas Memorial Museum, Vertebrate Paleontology Laboratory); P. Holroyd (University of California Museum of Paleontology); D. Brinkman and M. Fox (Yale Peabody Museum of Natural History); S. Miller (Campbell Geology Museum, Clemson University); A. Millhouse (National Museum of Natural History); and P. Campbell (British Museum of Natural History). J. Luna, M. Kerr and M. Whitlock scanned and landmarked several embryonic specimens, and N. Vitek photographed specimens used in this analysis. We also thank J. Hanken, C. Tabin, K. Jones, the Pierce lab, Hans Larsson and three anonymous reviewers for feedback on this work.
Ethics
The majority of specimens used in this study are reposited in accredited museums. Additional embryos were collected following approved methods for reptilian embryos at Harvard University and Imperial College.
Data accessibility
All data, code and results needed to replicate this study are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.6cv82g1 [62]. Additional information to support this study has been uploaded as part of the electronic supplementary material. CT data have been reposited with the museums that hold copyright to the original specimens; requests to use scan data should be made directly to those museums.
Authors' contributions
Z.S.M., conceived/designed the study, collected data, analysed data, interpreted data and drafted the manuscript. K.A.V. collected specimens for use in this study. A.A. conceived/designed the study, interpreted data and drafted the manuscript. S.E.P. designed the study, collected data, interpreted data and drafted the manuscript. All authors gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
Funding was provided by the Department of Organismic and Evolutionary Biology at Harvard University (Z.S.M.), the Society of Vertebrate Paleontology Wood's Award (Z.S.M.), and NSF DDIG Award #1701745 (Z.S.M. and S.E.P.).
References
- 1.Busbey AB. 1995. The structural consequences of skull flattening in crocodilians. In Functional morphology in vertebrate paleontology (ed. Thomason JJ.), pp. 173–192. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.McHenry CR, Clausen PD, Daniel WJT, Meers MB, Pendharkar A. 2006. Biomechanics of the rostrum in crocodilians: a comparative analysis using finite-element modeling. Anat. Rec. 288A, 827–849. ( 10.1002/ar.a.20360) [DOI] [PubMed] [Google Scholar]
- 3.Cleuren J, de Vree F. 2000. Feeding in crocodylians. In Feeding (ed. Schwenk K.), pp. 337–358. San Diego, CA: Academic Press. [Google Scholar]
- 4.Brochu CA. 2001. Crocodylian snouts in space and time: phylogenetic approaches toward adaptive radiation. Am. Zool. 41, 564–585. [Google Scholar]
- 5.Pierce SE, Angielczyk KD, Rayfield EJ. 2008. Patterns of morphospace occupation and mechanical performance in extant crocodilian skulls: a combined geometric morphometric and finite element modeling approach. J. Morphol. 269, 840–864. ( 10.1002/jmor.10627) [DOI] [PubMed] [Google Scholar]
- 6.Erickson GM, Gignac PM, Steppan SJ, Lappin AK, Vliet KA, Brueggen JD, Inouye BD, Kledzik D, Webb GJW. 2012. Insights into the ecology and evolutionary success of crocodilians revealed through bite-force and tooth-pressure experimentation. PLoS ONE 7, e31781-12 ( 10.1371/journal.pone.0031781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langston W., Jr 1973. The crocodilian skull in historical perspective. In Biology of the Reptilia, pt. 4 (eds Gans C, Billet F, Madison PFA), pp. 263–289. London, UK: Academic Press. [Google Scholar]
- 8.Brochu CA. 2003. Phylogenetic approaches toward crocodylian history. Annu. Rev. Earth Planet. Sci. 31, 357–397. ( 10.1146/annurev.earth.31.100901.141308) [DOI] [Google Scholar]
- 9.Gignac PM, Erickson GM. 2016. Ontogenetic bite-force modeling of Alligator mississippiensis: implications for dietary transitions in a large-bodied vertebrate and the evolution of crocodylian feeding. J. Zool. 299, 229–238. ( 10.1111/jzo.12349) [DOI] [Google Scholar]
- 10.Gold MEL, Brochu CA, Norell MA. 2014. An expanded combined evidence approach to the Gavialis problem using geometric morphometric data from crocodylian braincases and eustachian systems. PLoS ONE 9, e105793 ( 10.1371/journal.pone.0105793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe A, Slice DE. 2014. The utility of cranial ontogeny for phylogenetic inference: a case study in crocodylians using geometric morphometrics. J. Evol. Biol. 27, 1078–1092. ( 10.1111/jeb.12382) [DOI] [PubMed] [Google Scholar]
- 12.Sadleir RW. 2009. A morphometric study of crocodylian ecomorphology through ontogeny and phylogeny. Unpublished PhD dissertation, University of Chicago, IL. [Google Scholar]
- 13.Piras P, Colangelo P, Adams DC, Buscalioni A, Cubo J, Kotsakis T, Meloro C, Raia P. 2010. The Gavialis–Tomistoma debate: the contribution of skull ontogenetic allometry and growth trajectories to the study of crocodylian relationships. Evol. Dev. 12, 568–579. ( 10.1111/j.1525-142X.2010.00442.x) [DOI] [PubMed] [Google Scholar]
- 14.Tokita M, Chaeychomsri W, Siruntawineti J. 2013. Developmental basis of toothlessness in turtles: insight into convergent evolution of vertebrate morphology. Evolution 67, 260–273. ( 10.1111/j.1558-5646.2012.01752.x) [DOI] [PubMed] [Google Scholar]
- 15.Bhullar B-AS, Morris ZS, Sefton EM, Tok A, Tokita M, Namkoong B, Camacho J, Burnham DA, Abzhanov A. 2015. A molecular mechanism for the origin of a key evolutionary innovation, the bird beak and palate, revealed by an integrative approach to major transitions in vertebrate history. Evolution 69, 1665–1677. ( 10.1111/evo.12684) [DOI] [PubMed] [Google Scholar]
- 16.Sander K. 1983. The evolution of patterning mechanisms: gleanings from insect embryogenesis and spermatogenesis. In Development and evolution (eds Goodwin BC, Holder N, Wylie CC), pp. 137–159. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Abzhanov A. 2013. von Baer's law for the ages: lost and found principles of developmental evolution. Trends Genet. 29, 712–722. ( 10.1016/j.tig.2013.09.004) [DOI] [PubMed] [Google Scholar]
- 18.Abzhanov A, Kuo WP, Hartmann C, Grant BR, Grant PR, Tabin CJ. 2006. The calmodulin pathway and evolution of elongated beak morphology in Darwin's finches. Development 442, 563–567. [DOI] [PubMed] [Google Scholar]
- 19.Carroll SB. 2008. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134, 25–36. ( 10.1016/j.cell.2008.06.030) [DOI] [PubMed] [Google Scholar]
- 20.Gould SJ. 1977. Ontogeny and phylogeny. Cambridge, MA: Harvard University Press. [Google Scholar]
- 21.Alberch P, Gould SJ, Oster GF, Wake DB. 1979. Size and shape in ontogeny and phylogeny. Paleobiology 5, 296–317. ( 10.1017/S0094837300006588) [DOI] [Google Scholar]
- 22.Smith KK. 2003. Time's arrow: heterochrony and the evolution of development. Int. J. Dev. Biol. 47, 613–621. [PubMed] [Google Scholar]
- 23.McNamara KJ. 2012. Heterochrony: the evolution of development. Evo. Edu. Outreach 5, 203–218. ( 10.1007/s12052-012-0420-3) [DOI] [Google Scholar]
- 24.Powder KE, Milch K, Asselin G, Albertson RC. 2015. Constraint and diversification of developmental trajectories in cichlid facial morphologies. Evodevo 6, 1–14. ( 10.1186/s13227-015-0020-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman DE, Carlo J, de Leon MP, Zollikofer CPE. 2007. A geometric morphometric analysis of heterochrony in the cranium of chimpanzees and bonobos. J. Hum. Evol. 52, 647–662. ( 10.1016/j.jhevol.2006.12.005) [DOI] [PubMed] [Google Scholar]
- 26.Bhullar B-AS, Marugán-Lobón J, Racimo F, Bever GS, Rowe TB, Norell MA, Abzhanov A. 2012. Birds have paedomorphic dinosaur skulls. Nature 487, 223–226. ( 10.1038/nature11146) [DOI] [PubMed] [Google Scholar]
- 27.Koyabu D, et al. 2014. Mammalian skull heterochrony reveals modular evolution and a link between cranial development and brain size. Nat. Commun. 5, 3625 ( 10.1038/ncomms4625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson MWJ. 1985. Reproductive biology and embryology of the crocodilians. In Biology of the Reptilia, pt. 14 (eds Gans C, Billet F, Madison PFA), pp. 329–491. London, UK: Academic Press. [Google Scholar]
- 29.Adams DC, Otárola-Castillo E. 2013. geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 4, 393–399. ( 10.1111/2041-210X.12035) [DOI] [Google Scholar]
- 30.Rohlf FJ. 2015. The tps series of software. Hystrix, the Italian Journal of Mammalogy 26, 9–12. [Google Scholar]
- 31.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 32.Rohlf FJ. 1990. Morphometrics. Annu. Rev. Ecol. Syst. 21, 299–316. ( 10.1146/annurev.es.21.110190.001503) [DOI] [Google Scholar]
- 33.Zelditch ML, Swiderski DL, Sheets HD. 2012. Geometric morphometrics for biologists: a primer. London, UK: Academic Press. [Google Scholar]
- 34.Adams DC, Collyer ML. 2015. Permutation tests for phylogenetic comparative analyses of high-dimensional shape data: what you shuffle matters. Evolution 69, 823–829. ( 10.1111/evo.12596) [DOI] [PubMed] [Google Scholar]
- 35.Reilly SM, Wiley EO, Meinhardt DJ. 1997. An integrative approach to heterochrony: the distinction between interspecific and intraspecific phenomena. Biol. J. Linn. Soc. 60, 119–143. ( 10.1111/j.1095-8312.1997.tb01487.x) [DOI] [Google Scholar]
- 36.Brochu CA. 1997. Morphology, fossils, divergence timing, and the phylogenetic relationships of Gavialis. Syst. Biol. 46, 479–522. ( 10.1093/sysbio/46.3.479) [DOI] [PubMed] [Google Scholar]
- 37.Oaks JR. 2011. A time-calibrated species tree of Crocodylia reveals a recent radiation of the true crocodiles. Evolution 65, 3285–3297. ( 10.1111/j.1558-5646.2011.01373.x) [DOI] [PubMed] [Google Scholar]
- 38.Narváez I, Brochu CA, Escaso F, Pérez-García A, Ortega F. 2016. New Spanish Late Cretaceous eusuchian reveals the synchronic and sympatric presence of two allodaposuchids. Cretac. Res. 65, 112–125. ( 10.1016/j.cretres.2016.04.018) [DOI] [Google Scholar]
- 39.Abzhanov A. 2017. The old and new faces of morphology: the legacy of D'Arcy Thompson's ‘theory of transformations’ and ‘laws of growth’. Development 144, 4284–4297. ( 10.1242/dev.137505) [DOI] [PubMed] [Google Scholar]
- 40.Hanken J. 2014. Is heterochrony still an effective paradigm for contemporary studies of evo-devo? In Conceptual change in biology (ed. Love AC.), pp. 97–110. Dordrecht, Netherlands: Springer. [Google Scholar]
- 41.Young NM, et al. 2014. Embryonic bauplans and the developmental origins of facial diversity and constraint. Development 141, 1059–1063. ( 10.1242/dev.099994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duboule D. 1994. Temporal colinearity and the phylotypic progression: a basis for the stability of a vertebrate Bauplan and the evolution of morphologies through heterochrony. Development 1994, 135–142. [PubMed] [Google Scholar]
- 43.Richardson MK. 2012. A phylotypic stage for all animals? Dev. Cell 22, 903–904. ( 10.1016/j.devcel.2012.05.001) [DOI] [PubMed] [Google Scholar]
- 44.Irie N, Kuratani S. 2011. Comparative transcriptome analysis reveals vertebrate phylotypic period during organogenesis. Nat. Commun. 2, 248–246 ( 10.1038/ncomms1248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalinka AT, Varga KM, Gerrard DT, Preibisch S, Corcoran DL, Jarrells J, Ohler U, Bergman CM, Tomancak P. 2010. Gene expression divergence recapitulates the developmental hourglass model. Nature 468, 811–814. ( 10.1038/nature09634) [DOI] [PubMed] [Google Scholar]
- 46.Sanger TJ, Sherratt E, McGlothlin JW, Brodie ED III, Losos JB, Abzhanov A. 2013. Convergent evolution of sexual dimorphism in skull shape using distinct developmental strategies. Evolution 67, 2180–2193. ( 10.1111/evo.12100) [DOI] [PubMed] [Google Scholar]
- 47.Wilberg EW. 2017. Investigating patterns of crocodyliform cranial disparity through the Mesozoic and Cenozoic. Zool. J. Linn. Soc. 181, 189–208. ( 10.1093/zoolinnean/zlw027) [DOI] [Google Scholar]
- 48.Mallarino R, Grant PR, Grant BR, Herrel A, Kuo WP, Abzhanov A. 2011. Two developmental modules establish 3D beak-shape variation in Darwin's finches. Proc. Natl Acad. Sci. USA 108, 4057–4062. ( 10.1073/pnas.1011480108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tokita M, Yano W, James HF, Abzhanov A. 2016. Cranial shape evolution in adaptive radiations of birds: comparative morphometrics of Darwin's finches and Hawaiian honeycreepers. Phil. Trans. R. Soc. B 372, 20150481 ( 10.1098/rstb.2015.0481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Felice RN, Goswami A. 2017. Developmental origins of mosaic evolution in the avian cranium. Proc. Natl Acad. Sci. USA 115, 555–560. ( 10.1073/pnas.1716437115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanger TJ, Seav SM, Tokita M, Langerhans RB, Ross LM, Losos JB, Abzhanov A. 2014. The oestrogen pathway underlies the evolution of exaggerated male cranial shapes in Anolis lizards. Proc. R. Soc. B 281, 20140329 ( 10.1098/rspb.2014.0329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.True JR, Haag ES. 2001. Developmental system drift and flexibility in evolutionary trajectories. Evol. Dev. 3, 109–119. ( 10.1046/j.1525-142x.2001.003002109.x) [DOI] [PubMed] [Google Scholar]
- 53.Gardiner DM, Torok MA, Mullen LM, Bryant SV. 1998. Evolution of vertebrate limbs: robust morphology and flexible development. Am. Zool. 38, 659–671. ( 10.1093/icb/38.4.659) [DOI] [Google Scholar]
- 54.Ludwig MZ, Bergman C, Patel NH, Kreitman M. 2000. Evidence for stabilizing selection in a eukaryotic enhancer element. Nature 403, 564–567. ( 10.1038/35000615) [DOI] [PubMed] [Google Scholar]
- 55.Robinson R. 2011. Different paths, same structure: ‘developmental systems drift’ at work. PLoS Biol. 9, e1001113 ( 10.1371/journal.pbio.1001113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haag ES. 2014. The same but different: worms reveal the pervasiveness of developmental system drift. PLoS Genet. 10, e1004150 ( 10.1371/journal.pgen.1004150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brugmann SA, Kim J, Helms JA. 2006. Looking different: understanding diversity in facial form. Am. J. Med. Genet. A 140A, 2521–2529. ( 10.1002/ajmg.a.31361) [DOI] [PubMed] [Google Scholar]
- 58.Ahi EP. 2016. Signalling pathways in trophic skeletal development and morphogenesis: insights from studies on teleost fish. Dev. Biol. 420, 11–31. ( 10.1016/j.ydbio.2016.10.003) [DOI] [PubMed] [Google Scholar]
- 59.Wu P, Jiang T-X, Suksaweang S, Widelitz RB, Chuong C-M. 2004. Molecular shaping of the beak. Science 305, 1465–1466. ( 10.1126/science.1098109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu D, Marcucio RS. 2009. Unique organization of the frontonasal ectodermal zone in birds and mammals. Dev. Biol. 325, 200–210. ( 10.1016/j.ydbio.2008.10.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gunter HM, Koppermann C, Meyer A. 2014. Revisiting de Beer's textbook example of heterochrony and jaw elongation in fish: calmodulin expression reflects heterochronic growth, and underlies morphological innovation in the jaws of belonoid fishes. Evodevo 5, 8 ( 10.1186/2041-9139-5-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morris ZS, Vliet KA, Abzhanov A, Pierce SE. 2019. Data from: Heterochronic shifts and conserved embryonic shape underlie crocodylian craniofacial disparity and convergence Dryad Digital Repository. ( 10.5061/dryad.6cv82g1) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Morris ZS, Vliet KA, Abzhanov A, Pierce SE. 2019. Data from: Heterochronic shifts and conserved embryonic shape underlie crocodylian craniofacial disparity and convergence Dryad Digital Repository. ( 10.5061/dryad.6cv82g1) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data, code and results needed to replicate this study are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.6cv82g1 [62]. Additional information to support this study has been uploaded as part of the electronic supplementary material. CT data have been reposited with the museums that hold copyright to the original specimens; requests to use scan data should be made directly to those museums.