Abstract
Increased eye size in animals results in a larger retinal image and thus improves visual acuity. Thus, larger eyes should aid both in finding food as well as detecting predators. On the other hand, eyes are usually very conspicuous and several studies have suggested that eye size is associated with predation risk. However, experimental evidence is scant. In this study, we address how predation affects variation in eye size by performing two experiments using Eurasian perch juveniles as prey and either larger perch or pike as predators. First, we used large outdoor tanks to compare selection due to predators on relative eye size in open and artificial vegetated habitats. Second, we studied the effects of both predation risk and resource levels on phenotypic plasticity in relative eye size in indoor aquaria experiments. In the first experiment, we found that habitat altered selection due to predators, since predators selected for smaller eye size in a non-vegetated habitat, but not in a vegetated habitat. In the plasticity experiment, we found that fish predators induced smaller eye size in males, but not in females, while resource levels had no effect on eye size plasticity. Our experiments provide evidence that predation risk could be one of the driving factors behind variation in eye size within species.
Keywords: predation, natural selection, eye size, Perca fluviatilis, phenotypic plasticity, selection gradients
1. Introduction
Animals show large variation in relative eye size both within and among species [1]. Both predation risk and foraging efficiency have been suggested to be two major biological drivers of this variation [2–5]. In addition, there is strong support that abiotic factors such as light availability also affect eye size evolution [6,7]. While the observed variation in eye size among and within species has been suggested to be a result of natural selection [2,5,8,9], few studies have used an experimental approach to demonstrate that natural selection can cause shifts in eye size (but see [4]).
At the moment, there is no consensus regarding how predation risk affects eye size. For example, Zaret & Kerfoot [10] and Beston et al. [5] found a decrease in eye size in high predation environments while Glazier & Deptola [11] found the opposite pattern. In the former case, the explanation was that smaller eyes are less conspicuous and might reduce predation risk, and the latter case larger eyes were suggested to facilitate detection of potential predators. The difference between these conclusions could be due to differences in eye morphology among the study organism used, where for example, compound eyes and camera eyes have different morphology. However, it could also be due to trade-offs with foraging efficiency, predator avoidance efficiency, or size difference between predator and prey. In addition, the majority of studies investigating the drivers of eye size evolution have used a comparative approach (e.g. [11–13]) providing only indirect evidence for selection on eye size. Studies at the micro-evolutionary scale such as selection experiments are therefore needed to obtain a mechanistic understanding of eye size evolution.
In addition to the direct predation effects on eye size, the presence of predators has been shown to alter the food available to prey, which can have indirect consequences on prey eye size. In general, the presence of predators causes an increase in food availability through a trophic cascade for the surviving prey individuals, and thus an increase in per capita food availability [14]. Resource availability has been shown to affect eye size in animals: because eyes are costly to produce and maintain they are usually smaller when food availability is low [3,15]. Since food availability and predators covary in nature it is important to examine the interaction between these two factors, but unfortunately few such studies have been performed, although see Beston et al. [5] who studied this interaction.
Organisms that live under low light intensity generally have larger eyes [6,9], which may be due to the fact that large eyes are usually more effective in absorbing light [1]. However, predation risk and light intensity might interact. In a recent study, Beston et al. [5] found that the relative eye size of fish was significantly smaller in high predation sites compared to low predation sites. Interestingly, at sites without predators that differed in light intensity there was no difference in eye size suggesting that predation, rather than light intensity per se, drives the difference in eye size [5]. However, their study did not use an experimental approach such as a selection experiment on eye size. In Beston et al. [5] low light intensity was caused by canopy cover. However, light intensity might also differ between open and vegetated habitats in the water. Currently, we do not know how light intensity differences across habitats affect the evolution of eye size or interact with predation risk. Admittedly, open and vegetated habitats in the water also differ in structure and complexity, which may also affect eye size. For example, in a comparative study on ray-finned fishes, Caves et al. [16] found that eye size and visual acuity were strongly correlated with structural complexity in the water.
The variation in eye size observed may be expressed either constitutively or through phenotypic plasticity (e.g. [5,17]), although they are not mutually exclusive. Phenotypic plasticity is the ability of an individual genotype to produce different phenotypes when exposed to different environmental conditions [18]. Ultimately, traits that show phenotypic plasticity can become genetically assimilated [19], i.e. they become constitutive through genetic canalization [20]. Hence, studying traits that show adaptive phenotypic plasticity might help us understand trait evolution at a macro-evolutionary scale. If, for example, eye morphological diversity among species or populations exposed to divergent predatory environment mirrors phenotypic plasticity within a species, this would suggest adaptive eye size evolution at the macro-evolutionary scale. Some organisms do show phenotypic plasticity in eye size in response to light and food conditions [3,4,21]. However, few studies have explored how predation risk affects phenotypic plasticity of eye size (but see [17]).
In this study we used a freshwater fish, the Eurasian perch (Perca fluviatilis), to examine how predation, resource level, and aquatic vegetation affect eye size. Eurasian perch is common in freshwaters across northern Eurasia, where it occurs in both open and vegetated habitats [22,23]. Predation vulnerability and mortality are usually high during the first years of life [24,25], thus selection for detecting and avoiding predators should be high. To investigate the effect of predators on eye size in perch we first studied how predation selected on relative eye size in an outdoor tank experiment simulating an open or a vegetated habitat. This experiment estimated selection gradients due to predators on relative eye size in both habitats. In a second experiment we raised perch in aquaria for 10 weeks either in the presence or absence of a non-lethal predator (i.e. the perch can see and smell the predator, but it cannot get to them) at three food levels. This allowed us to examine phenotypic plasticity in eye size in response to predation risk and food levels. Finally, since many organisms, including perch [26], often show sexual size dimorphism we examined differences in eye size between males and females in the plasticity experiment.
If larger eyes are more visible to predators we predict that predators should select for smaller eyes in the outdoor tank experiment where we manipulated habitat complexity. Because eyes are probably less visible in the vegetated environment with less light intensity, we also predict that selection against larger eyes should be stronger in the open water environment compared to the simulated vegetated environment in this tank experiment. In the plasticity experiment in which we manipulate predator presence/absence and resource levels, we predict individuals would develop smaller eyes in the presence of predators since smaller eyes should be less visible, thereby resulting in lower predation risk. As higher resource levels would provide more energy and nutrients for building and maintaining larger eyes, we also predict relatively larger eyes at higher resource levels in the plasticity experiment.
2. Material and methods
(a). Predator selection experiment
To investigate selection on eye size in juvenile perch due to predation we used data from the predator selection study in Svanbäck & Eklöv [27]. In this experiment large perch (194.5 ± 7.3 mm, average ± s.e.) were used as predators and young-of-the-year (YOY) perch (51.2 ± 4.1 mm) as prey. Both predators (N = 38) and prey were caught in Lake Erken (59°50′ N 18°35′ E), using a seine. The seine was pulled through open and vegetated water and therefore caught pelagic as well as littoral perch. Perch from these two habitats differ in pigmentation such that perch from the open habitat had light and perch from the vegetated habitat had dark pigmentation [27]. However, there was no correlation between relative eye size and the level of pigmentation in the fish used in this experiment (N = 587, r = 0.037, p = 0.373). Perch were also added haphazardly into the experimental tanks and thus, the perch in each tank varied in pigmentation. Under the assumption that the reflection of the eye is most easily visible against dark body pigmentation in perch, adding a mix of pigmented perch would be predicted to increase variation in selection on eye size. The study was conducted in 7 m3 outdoor tanks (3 m in diameter, 1.2 m water depth) with (artificial aquatic vegetation trials) or without (open water trials) artificial aquatic vegetation. This design enabled us to investigate whether predator selection on eye size differs between two common lake environments. The artificial vegetation consisted of white polypropylene strings attached to a plastic net (mesh size 10 mm, 200 strings m−2), which were held to the bottom of the tank by reinforcement bars. The tanks were filled with water about six weeks before the experiment started, allowing algae to colonize the artificial vegetation. The day before the start of the experiment, 36 prey fish per trial were measured for total length and digitally photographed for eye size measurements (see below). Each prey individual was then individually marked using colour dye (Visible Implant Elastomer, NMT Inc.) that was injected under the skin. After photographing and labelling, the prey were put into a container within the experimental tank to recover and acclimatize to the tank water. At the same time, three predatory perch were put into the tank (outside the container with the prey) to acclimatize to the experimental conditions. The predators for each replicate were haphazardly chosen from the pool of 38 predators. The experiment started the next morning by releasing the prey fish from the container so that they were exposed to the predators. Before releasing the fish, the container was screened and dead prey fish were removed. Thus, between 32 and 36 prey fish were used in each replicate. The experimental tanks were then surveyed every 30 min and the experiment was terminated when approximately 50% of the prey fish had been eaten, which took 2–4 h. To terminate the experiment, predators were removed and remaining prey individuals were collected for later analysis. After each trial, all predators were returned to the pool of predators, whereas the prey fish were only used once. We only analysed trials where we had 40–60% predation mortality (54 ± 3% mortality, average ± s.e. for the trials included in this study: N = 11 for open and N = 6 for vegetated habitat, respectively). This cut-off excluded 13 trials with unmotivated predators (3 trials with less than 4 prey taken) and trials where only a few or no prey were still alive (10 trials with less than 4 prey still alive). Because sex determination of perch requires killing the fish to inspect their gonads, we could not examine sex difference in selection on eye size.
Because we did not measure light levels in the original experiment [27], we rebuilt two cylindrical tanks (diameter 100 cm, height 100 cm) in a large indoor arena, and added the artificial vegetation from the original experiment into one of the tanks. Light was provided by fluorescent lamps 1.5 m above the water surface. We let the water sit in the tank for three weeks after which we measured the light levels (lux) at the surface, in the middle of the tanks, and at the bottom of the tanks. We found no difference in light levels at the surface of the tanks (280 lux in both tanks). Light level as a proportion to surface level was much lower in the tank with vegetation (53.6% and 30.3% in the middle of the tank and at the bottom, respectively) compared to the open water tank (73.2% and 48.2% in the middle of the tank and at the bottom, respectively). Thus, light level was 26.8% lower in the tank with vegetation in the middle of the tank and 37.0% lower at the bottom showing that our artificial vegetation reduced light levels.
(b). Phenotypic plasticity experiment
To investigate the effects of predator presence and food ration on eye size plasticity in perch we used data from Svanbäck et al. [28]. In this experiment we used 1-year-old perch (79.3 ± 4.5 mm, 4.4 ± 1.0 g, mean fish length and weight ± s.d.) as prey and northern pike (Esox lucius; 341.6 ± 49.2 mm, 207.3 ± 76.7 g) as predators. Perch were collected from Lake Mälaren (59°20′ N 17°52′ E) and pike from Lake Messormen (59°33′ N 18°26′ E) and Lake Hersen (59°34‴ N 18°24′ E). Perch co-occur with pike in Lake Mälaren [29] as well as in Lake Messormen and Lake Hersen (K Karlsson 2013, personal observations). After collection both perch and pike were allowed to acclimatize to laboratory conditions for at least six weeks before being used in the experiment. The experiment was carried out in 36 different 105 l aquaria (75 × 40 × 35 cm) containing a 3 cm layer of fine sand on the bottom. The aquaria were visually isolated from each other. Each aquarium was separated into two equal parts with a transparent plastic wall with holes allowing water circulation. This set-up allowed the perch to be affected by both visual and olfactory predator cues. Fifty per cent of the water was replaced once a week. The photoperiod was 12 h light/12 h dark and the temperature was kept between 19–20°C using a thermostat heater in each aquarium.
The experiment was set up as a 3 × 2 fully factorial experiment with three different levels of food ration (high, medium, low), two predator treatments (presence, absence), and six replicates per treatment combination, allowing us to investigate the effects of food level and predator presence on eye size plasticity. Perch were fed frozen chironomids every day. The high food ration treatment tanks received food equalling 15% of the initial total fish body weight per day, which is close to maximum food conversion of perch at that specific size and temperature [30]. The medium and low food treatments received 10% and 5% of the initial body weight, respectively. In the predator treatment, one pike was placed in the left compartment of the divided aquaria. Pike were fed juvenile perch twice every week and all individual predators actively fed during the experiment.
We put 4 haphazardly chosen perch into each aquarium, giving 4 fish per replicate. We ran the experiment for 10 weeks. One week into the experiment, one fish per aquarium was sacrificed for another project, leaving three fish per aquarium. Because of mortality in some of the aquaria, we were left with one to three fish per aquarium (total 82 individuals, average 2.3 fish per replicate) at the end of the experiment. There was no effect of treatment on mortality [28] nor was there an effect of mortality on eye size (p = 0.493). The amount of food supplied was adjusted according to the number of live fish per aquarium. For example, if one fish died the fish were fed 75% of the initial ration given in the aquaria. At the termination of the experiment all fish were killed using an overdose of benzocaine, individually weighed (to the nearest 0.1 g) and measured for length (total length to the nearest mm). After length and weight measurements the fish were placed on a piece of Styrofoam, fins fixed with needles, and photographed with a digital camera for eye size measurements (see below).
(c). Eye size
From the digital photos (Nikon D300S) collected after the selection and plasticity experiments we measured eye size from digitized landmarks (see electronic supplementary material, figure S1). For both experiments we photographed the left side of the fish. In the selection experiment we used the measured eye size and body size as our independent variables. Since we analysed standardized selection gradients [31] we can examine the effect of selection on eye size independently of selection on body length. For the plasticity experiment we analysed plasticity in relative eye size, i.e. eye size/body size (total length).
(d). Statistics
In the predator selection experiment, we estimated and analysed the standardized linear, quadratic, and correlational selection gradients [31,32] for selection on fish total length and eye size. Each trait was standardized to mean zero and variance one. The magnitude and significance of the selection gradient for each trait in each trial was obtained from multiple logistic regressions [33]. We used t-tests to test if the average selection gradient in each environment (open water/simulated vegetation) was different from zero and also to test for differences in selection gradients between the environments. The selection gradients were weighted with the inverse of their standard error (see electronic supplementary material, table S1) in the t-tests (using the package ‘weights’ [34]), so that the more uncertain estimates have less weight in the analyses, though un-weighted t-tests yielded similar results. To confirm the results from the t-tests, we performed mixed effects logistic regressions testing for differences in average selection gradients, modelling trials as a random effect with individuals nested within trials. First, we performed mixed effects logistic regression on trials in open water and vegetation separately to test for effects of eye size and fish length on survival. Second, we tested if the relationships between eye size and length on fish survival differed between open water and vegetation. The results from the mixed effects models are presented in electronic supplementary material, table S2. Eye size and body length are highly correlated (see electronic supplementary material, figures S2 and S3). However, selection gradient analysis controls for the effect of length since length is included in the model. In addition, we also calculated standardized selection differentials on relative eye size (eye size/body size) for all trials as the covariance between relative survival and standardized (mean zero and variance one) relative eye size [35]. t-tests were then used to test if the average selection differential in each environment (open water/vegetation) was different from zero and also to test for differences in selection differential between the environments.
For the phenotypic plasticity experiment on relative eye size in relation to body size (i.e. eye size/body size), we used a nested ANOVA to analyse the effect of our treatments (predator, food ration, and sex) and all two- and three-way interactions. Individuals were nested in tanks. In addition, we also used a nested ANCOVA on the effect of our treatments (predator, food, and sex) on the raw eye size using body length as covariate. Here, we first included all two-, three-, and four-way interactions, however, since the four-way interaction was insignificant (p = 0.362) we dropped this from the model. All statistical analyses were performed in R 3.4.1 [36].
3. Results
(a). Predator selection experiment
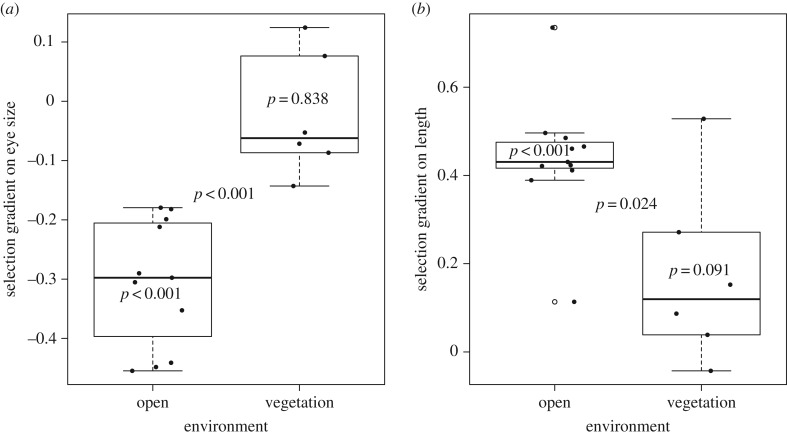
The average selection gradient on eye size differed between trials in open water and in vegetation (figure 1a, table 1). Individuals with a relatively smaller eye survived better in open water trials whereas no selection on relative eye size was found in trials with vegetation (figure 1a, table 1, electronic supplementary material, table S3A; mean (±s.d.)). Relative eye size before selection in open water and vegetation water was 0.0842 ± 0.0015 and 0.0825 ± 0.0026, respectively, and 0.0830 ± 0.0014 and 0.0820 ± 0.002 after selection in open and vegetation water, respectively (test for difference before and after selection: open water; paired t-test, t = −7.02, d.f. = 10, p < 0.001, vegetation; paired t-test, t = −2.43, d.f. = 5, p = 0.060). In addition to the effects of eye size on survival, we found that larger individuals survived better compared to smaller sized individuals both in open water and in vegetation (figure 1b, table 1, electronic supplementary material, table S3B, mean (±s.d.)). Size before selection was 50.17 ± 2.23 mm and 50.87 ± 2.23 mm in open and vegetated water, respectively, and 51.46 ± 2.65 mm and 51.87 ± 2.49 mm after selection in open and vegetated water, respectively (test for difference before and after selection: open water; paired t-test, t = 4.40, d.f. = 10, p = 0.001, vegetation; paired t-test, t = 2.72, d.f. = 5, p = 0.042). We also found that selection on body size was greater in open water compared to in vegetation (figure 1b, table 1). Quadratic and correlational selection gradients were never found significant and they did not differ between open water and vegetation trials (table 1). The significance of the selection gradients within each environment as well as the differences between the environments were corroborated by the mixed effects logistic regressions (electronic supplementary material, table S2). `
Figure 1.
Box plots of selection gradients on (a) eye size and (b) body length in open water and in vegetation. Points show the selection gradients in individual trials (experimental tanks). p-values inside a box shows whether the average selection gradient is different from zero and p-values between the boxes shows whether there were differences in selection between the environments.
Table 1.
Logistic regression analyses of natural selection for each trial in the predator selection experiment. Values reported are the logistic regression coefficient and within parentheses, the estimated selection gradient for eye size (E) and length (L), and the quadratic (E × E, and L × L) and correlational (E × L) selection gradients for each experimental replicate for trials performed in open water and vegetation. Estimates of selection gradients in italics indicate that they are significant (p < 0.05). N refers to the number of prey individuals used in each replicate. For both environments, the average was tested (t-test) to see if they differed from zero. t-tests were also used to check if the gradients differed between the environments. See electronic supplementary material, table S1 for standard errors of the slopes for the logistic regression.
| environment | N | E | L | E × E | L × L | E × L |
|---|---|---|---|---|---|---|
| open water | ||||||
| trial #6 | 35 | −0.589 (−0.305) | 0.733 (0.389) | −0.441 (−0.255) | −0.436 (−0.191) | −0.184 (−0.040) |
| trial #8 | 32 | −0.501 (−0.199) | 1.370 (0.461)* | 0.509 (0.126) | 0.360 (0.073) | −0.480 (−0.137) |
| trial #10 | 34 | −2.179 (−0.441)* | 2.064 (0.423)* | −1.563 (−0.321)† | −0.709 (−0.064) | 2.380 (0.449)† |
| trial #12 | 33 | −0.288 (−0.290) | 0.990 (0.485)† | −0.207 (0.036) | 0.582 (0.415) | −1.218 (−0.581) |
| trial #14 | 36 | −1.177 (−0.448) | 1.928 (0.735)* | −0.014 (0.008) | −0.437 (−0.068) | 0.441 (0.103) |
| trial #16 | 34 | −0.863 (−0.353) | 1.084 (0.421) | 0.318 (0.140) | −1.985 (−0.731)* | 0.659 (0.257) |
| trial #21 | 35 | −0.678 (−0.182) | 0.333 (0.114) | 0.397 (0.083) | −0.491 (−0.226) | 0.440 (0.194) |
| trial #23 | 36 | −0.969 (−0.455) | 1.048 (0.496) | 0.416 (0.203) | −0.057 (−0.020) | −0.170 (−0.089) |
| trial #24 | 36 | −0.451 (−0.179) | 1.263 (0.466) | 0.272 (0.077) | 1.107 (0.155) | −0.184 (−0.131) |
| trial #27 | 36 | −1.143 (−0.212) | 1.571 (0.412) | −4.310 (−1.017) | −5.564 (−1.492)† | 9.568 (2.367) |
| trial #28 | 34 | −0.581 (−0.298) | 0.934 (0.431) | 3.512 (1.403)† | 4.104 (1.792)† | −6.968 (−2.921)† |
| average | −0.259 | 0.405 | 0.082 | −0.030 | −0.085 | |
| within treatment t-value | −3.486** | 7.742*** | 0.192 | −0.331 | 0.123 | |
| vegetation | ||||||
| trial #5 | 32 | −0.198 (−0.143) | 0.502 (0.271) | 0.157 (0.095) | 0.878 (0.537)† | −0.037 (−0.042) |
| trial #11 | 35 | −0.308 (0.124) | 1.115 (0.039) | 2.683 (0.239) | 4.576 (0.501)* | −5.817 (−0.524)† |
| trial #13 | 33 | −0.523 (−0.087) | 1.830 (0.528)* | −0.889 (−0.289) | −1.553 (−0.392)† | 2.475 (0.633)† |
| trial #19 | 36 | −0.204 (−0.072) | 0.224 (0.087) | 0.158 (0.041) | −0.630 (−0.235) | 0.671 (0.258) |
| trial #22 | 34 | 0.255 (0.076) | −0.138 (−0.043) | 1.014 (0.356) | 0.259 (0.074) | −1.522 (−0.545) |
| trial #25 | 36 | −0.176 (−0.052) | 0.530 (0.152) | −1.161 (−0.381) | −0.728 (−0.262) | 2.016 (0.671) |
| average | −0.025 | 0.172 | 0.010 | 0.037 | 0.075 | |
| within treatment t-value | −0.215 | 2.09† | 0.062 | 0.558 | 0.161 | |
| between treatment t-value | −2.830** | 2.30* | 0.143 | −0.547 | 0.048 | |
†p < 0.10, *p < 0.05, **p < 0.01, ***p < 0.001.
The selection differentials showed that individuals with smaller relative eyes had a better chance of survival both in open water (one sample t-test, t = −9.117, d.f. = 10, p < 0.001) and in vegetation (one sample t-test, t = −2.614, d.f. = 5, p = 0.0475). However, the selection differentials on relative eye size were larger in open water than in vegetation (electronic supplementary material, figure S4, two sample t-test, t = −3.155, d.f. = 10.052, p = 0.0102).
(b). Phenotypic plasticity experiment
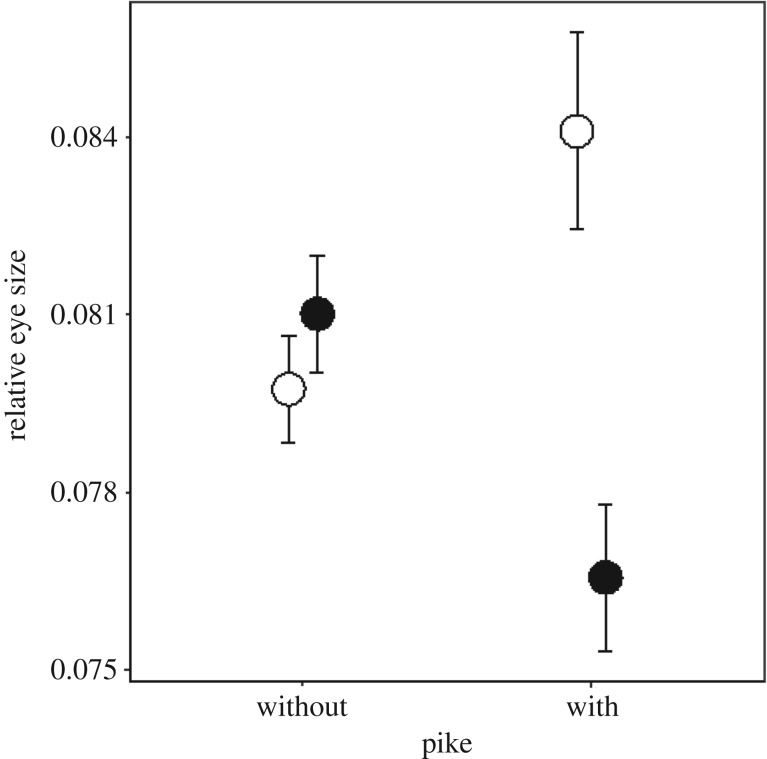
The relative eye size was influenced by the interaction between the presence of pike and the sex of the individual (table 2). The interaction between predator presence and sex suggest that the effect of sex and predators was because males reduced the relative size of their eyes by 5.50% in the presence of pike whereas females increased the relative size of their eyes by 5.46% (figure 2, table 2). No significant effect of food ration was found (table 2).
Table 2.
Results from Nested ANOVA on relative eye size (in relation to body size) from the plasticity experiment.
| treatment | d.f. | F-value | p-value |
|---|---|---|---|
| pike | 1,30 | 0.044 | 0.8355 |
| sex | 1,38 | 5.594 | 0.0232 |
| ration | 2,30 | 1.715 | 0.1971 |
| pike × sex | 1,38 | 14.251 | 0.0005 |
| pike × ration | 2,30 | 1.065 | 0.3573 |
| sex × ration | 2,38 | 1.269 | 0.2926 |
| pike × sex × ration | 2,38 | 0.735 | 0.4863 |
Figure 2.
Relative eye size (eye size/body length: mean ± s.e.) at the end of the plasticity experiment in the presence and absence of pike for males (filled symbols) and females (open symbols).
When analysing the effects on raw eye size with body length as a covariate, we found that the three-way interaction sex × food ration × length was significant and the three-way interaction pike × food ration × length was marginally insignificant (electronic supplementary material, table S4 and figure S5). In this analysis we found no effect of sex, however, the marginally insignificant interaction between pike and sex suggests that, in the presence of pike, females get larger eyes whereas males get smaller eyes compared to the absence of pike (electronic supplementary material, table S4 and figure S6) corroborating the analysis on relative eye size.
4. Discussion
In this study we manipulated habitat structure and found that habitat altered predatory selection on eye size. Selection for smaller eye size was observed only in the open water environment and not in the vegetation environment. We suggest that the greater effect of predators in open water is due to the reflectance of perch's eyes. The majority of fish, including Percidae, have a reflective layer behind the photoreceptors of the retina, the retinal tapetum lucidum, and, as a result, their eyes show reflectance in the water [37,38]. Such reflection has been shown to increase predation risk [39]. In addition, studies have shown that fish predators attack at eye region of the fish or eye spot on the body [40]. Hence, we suggest that large eyes, which should reflect more light and make perch more conspicuous, results in lower survival in low habitat complexity environments. Similar associations between predator presence and eye size have been found in field studies on killifish and daphnids [5,10]. However, our study used an experimental approach focusing on the selection on eye size per se, which has the advantage of fewer confounding environmental variables compared to correlative field studies.
As predicted, selection against large eyes was stronger in the open environment compared to in the vegetated environment: in fact, there was no significant selection from predation on eye size in the artificial vegetation. Since perch occur in open and vegetated habitats under natural conditions [41,42] we find it unlikely that the bright conditions in our open environment treatment are a disadvantage to the prey, e.g. that they are effectively ‘blinded by the light’. Instead, there might be several explanations for why we did not find selection on eye size in the vegetation treatment. First, the eyes of prey might be less visible to predators in the vegetated environment. For example, under the assumption that there is less light in vegetation (e.g. [43]), there might be a threshold light level below which eyes are difficult or easy to distinguish against a certain background. We find this explanation unlikely since a flash of light in an otherwise dark environment might be particularly salient to a predator. Second, prey with large eyes might more effectively detect potential predators compared to prey with small eyes in the vegetated habitat, thereby offsetting any disadvantage of large eyes. In support of this hypothesis, Glazier & Deptola [11] found that an amphipod had larger eyes in the presence of predators, which they suggested was because large eyes enable the amphipods to better detect and avoid fish predators. Finally, it might be that the observed difference in selection due to predators between the open and the vegetated habitat was a result of the physical structure in these two habitats rather than light level per se. Although our additional tests of light level showed reduced light levels in vegetation (see also [43]) we cannot tell for certain whether the lack of selection on eye size in our vegetation trials was due to reduced light, an effect of the structure per se, or both. Further experiments manipulating only light level will be needed to look at the effects of light.
If eye plasticity in perch is adaptive, the results of our phenotypic plasticity experiment should match the results of our selection experiment where individuals with smaller eyes had better survival. The plasticity experiment showed that eye size is plastic and predators induced relatively smaller eyes in our prey fish, thereby supporting the findings of the selection experiment. A similar decrease in eye size in the presence of a potential predator has been found in damselfish [17]. Interestingly Lönnstedt et al. [17] found that an increase in false eyespots at the end of the dorsal fin traded off against eye size in a damselfly fish, which the authors suggested is an adaptation to predation risk. False eyes have been shown to direct predator attacks towards the eye spot [44,45], providing further support that eyes are targets for selection by predators. Past studies have also shown that organisms show phenotypic plasticity in eye size in response to light and food conditions [3,21], but our study together with that of Lönnstedt et al. [17] is one of the few showing plastic responses in eye size due to predators.
Since perch undergo an ontogenetic shift in habitat, e.g. there is a switch from the open pelagic habitat to the benthic vegetated habit in the juvenile stage [46], the maintenance of plasticity in populations is important for this habitat shift. These two habitats might select for differences in eye size with regard to prey capture of the perch as well as with regard to escape from predators. For example, the vegetated habitat might require relatively larger eyes due to a lower light level compared to the open habitat [8]. Similarly, we found that selection on eye size was higher in the open habitat suggesting different relative eye size optima in these two habitats with regard to escape from predators. Hence, phenotypic plasticity in eye size might facilitate survival and growth when switching from one habitat to another.
In the plasticity experiment, we found that exposure to predators induced a decrease in relative eye size only in male perch whereas relative eye size increased in females. Similarly, in a recent study on cichlids, reduced plasticity in female body morphology and eye size compared to males was found when individuals were exposed to predator risk alarm cues [47]. A reduced eye size in the presence of predators might be advantageous in both males and females, but males and females might trade-off eye size differently against other costly traits, resulting in differing optimal levels of plasticity of eye size in each sex. Perch show size dimorphism with females being about 20% longer than males [26]. We suggest two explanations for the increase in relative eye size in females. First, the increase in relative eye size in females might be due to the fact that females need to gather more energy than males since females are larger. The need to gain more energy might be facilitated by larger eyes since larger eyes increase visual capacity and therefore foraging efficiency [1]. Hence, although larger eyes result in a higher predation risk the need for extra energy gain in females might offset the risk of predation, since females might invest in larger eyes in low resource habitats to find food more easily. However, we did not find an effect of resource level in the plasticity experiment, which suggests that at least under the resource levels we used this explanation is not valid. Perch did however grow in our experiment since an increase in body condition and plasticity in body shape as an effect of food in our 10-week experiment was found [28]. A second explanation could be that females experience other types of benefits of having large eyes, e.g. due to the need to locate and assess mates. As a result of these benefits, eye size plasticity may differ in females. Additional experiments or comparative studies using other species with sexual size dimorphism are needed to explore this interesting result further.
Our estimates of selection gradients (x̄ = 0.26 and x̄ = 0.41 in open water for eye size and body size, respectively) are higher than the averages reported in reviews of selection gradients for morphological traits [48,49]. We have no obvious explanation for these high values, but we note that we have a fairly simple experimental design with few confounding environmental variables and a relatively short experimental time period. Body size showed a higher selection gradient than eye size. Within certain size ranges, fish show size selective predation [50,51] acting on body size and the observed positive selection gradient suggests that the predators selected strongly for a small body size. But despite this strong selection on body size, we found a significant effect of relative eye size.
Eyes are costly to produce and maintain, which results in individuals having larger eyes at higher resource levels [3,15]. Hence, we predicted relatively larger eyes in the plasticity experiment at higher resource levels. However, we found no effect of resource levels on relative eye size in our experiment, and thus no support for our prediction. In contrast, a study on several species of daphnids showed an increase in absolute and relative eye size at high compared to low resource levels [3]. On the other hand, Merry et al. [52] showed that a butterfly had relatively larger eyes at low compared to high resource levels. Merry et al. [52] suggest that low resource densities require individuals to invest more in traits beneficial to foraging when they are smaller. One explanation for these contrasting results is differences in how cryptic versus conspicuous prey might respond to changes in resources. However, this hypothesis remains untested and requires additional experiments.
We used two different predator species in the experiments, pike and perch. Pike and larger sizes of perch are both piscivorous and feed to a large extent on juvenile perch [25,53]. One difference between them is that while pike is a sit-and-wait predator, piscivorous perch can also be an active hunting predator [54]. The difference in behaviour between the two predator species (sit-and-wait versus actively hunting) may result in different strength of selection on eye size. However, both perch and pike are visually hunting predators, thus they will both likely have a greater chance to detect conspicuous prey (in this study; prey with larger eyes). Similarly, the prey populations of perch differed between the two studies. While the magnitude of plastic responses may differ among populations [55,56] we believe the direction would still be the same and, thus, the results of the two experiments are comparable.
In summary, we found that predation affects eye size in perch in terms of survival and phenotypic plasticity. The combined results of the selection and the phenotypic plasticity experiments provide evidence for selection against large eyes under habitat conditions that are associated with light intensity. Since we found differences between environmental conditions and between males and females, an interesting avenue of further experimental work would be to manipulate light intensity and to study how selection from predators affects eye size in males and females.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Martin Lind, Anssi Laurila, David Wheatcroft, and two anonymous reviewers for helpful comments on the earlier version of the manuscript.
Ethics
This research was performed in accordance with the laws, guide lines, and ethical standards of Sweden, where the research was performed.
Data accessibility
Data and R codes are available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.475bv7s [57].
Authors' contributions
R.S. and F.J. designed the research. R.S. performed the fieldwork and performed the analysis. R.S. and F.J. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This project was funded by the Swedish Research Council to R.S. and F.J.
References
- 1.Land MF, Nilsson DE. 2012. Animal eyes. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Garamszegi LZ, Moller AP, Erritzoe J. 2002. Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proc. R. Soc. Lond. B 269, 961–967. ( 10.1098/rspb.2002.1967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandon CS, Dudycha JL. 2014. Ecological constraints on sensory systems: compound eye size in Daphnia is reduced by resource limitation. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 200, 749–758. ( 10.1007/s00359-014-0918-y) [DOI] [PubMed] [Google Scholar]
- 4.Brandon CS, James T, Dudycha JL. 2015. Selection on incremental variation of eye size in a wild population of Daphnia. J. Evol. Biol. 28, 2112–2118. ( 10.1111/jeb.12711) [DOI] [PubMed] [Google Scholar]
- 5.Beston SM, Wostl E, Walsh MR. 2017. The evolution of vertebrate eye size across an environmental gradient: phenotype does not predict genotype in a Trinidadian killifish. Evolution 71, 2037–2049. ( 10.1111/evo.13283) [DOI] [PubMed] [Google Scholar]
- 6.Hiller-Adams P, Case JF. 1985. Optical parameters of the eyes of some benthic decapods as a function of habitat depth (Crustacea, Decapoda). Zoomorphology 105, 108–113. ( 10.1007/bf00312145) [DOI] [Google Scholar]
- 7.McGaugh SE, et al. 2014. The cavefish genome reveals candidate genes for eye loss. Nat. Commun. 5, 5307 ( 10.1038/ncomms6307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer T, Desender K, Morwinsky T, Betz O. 1998. Eye morphology reflects habitat demands in three closely related ground beetle species (Coleoptera: Carabidae). J. Zool. 245, 467–472. ( 10.1111/j.1469-7998.1998.tb00121.x) [DOI] [Google Scholar]
- 9.Veilleux CC, Lewis RJ. 2011. Effects of habitat light intensity on mammalian eye shape. Anat. Rec. 294, 905–914. ( 10.1002/ar.21368) [DOI] [PubMed] [Google Scholar]
- 10.Zaret TM, Kerfoot WC. 1975. Fish predation on Bosmina longirostris—body-size selection versus visibility selection. Ecology 56, 232–237. ( 10.2307/1935317) [DOI] [Google Scholar]
- 11.Glazier DS, Deptola TJ. 2011. The amphipod Gammarus minus has larger eyes in freshwater springs with numerous fish predators. Invertebr. Biol. 130, 60–67. ( 10.1111/j.1744-7410.2010.00220.x) [DOI] [Google Scholar]
- 12.Liu Y, Ding L, Lei J, Zhao E, Tang YZ. 2012. Eye size variation reflects habitat and daily activity patterns in colubrid snakes. J. Morphol. 273, 883–893. ( 10.1002/jmor.20028) [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Ortega C, Santos ESA, Gil D. 2014. Species-specific differences in relative eye size are related to patterns of edge avoidance in an Amazonian rainforest bird community. Ecol. Evol. 4, 3736–3745. ( 10.1002/ece3.1194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abrams PA. 1995. Implications of dynamically variable traits for identifying, classifying, and measuring direct and indirect effects in ecological communities. Am. Nat. 146, 112–134. ( 10.1086/285789) [DOI] [Google Scholar]
- 15.Niven JE, Laughlin SB. 2008. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804. ( 10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- 16.Caves EM, Sutton TT, Johnsen S. 2017. Visual acuity in ray-finned fishes correlates with eye size and habitat. J. Exp. Biol. 220, 1586–1596. ( 10.1242/jeb.151183) [DOI] [PubMed] [Google Scholar]
- 17.Lönnstedt OM, McCormick MI, Chivers DP. 2013. Predator-induced changes in the growth of eyes and false eyespots. Sci. Rep. 3, 2259 ( 10.1038/srep02259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pigliucci M, Murren CJ, Schlichting CD. 2006. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 209, 2362–2367. ( 10.1242/jeb.02070) [DOI] [PubMed] [Google Scholar]
- 19.West-Eberhard MJ. 2005. Developmental plasticity and the origin of species differences. Proc. Natl Acad. Sci. USA 102, 6543–6549. ( 10.1073/pnas.0501844102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waddington CH. 1942. Canalization of development and the inheritance of acquired characters. Nature 150, 563–565. ( 10.1038/150563a0) [DOI] [PubMed] [Google Scholar]
- 21.Rohner N, Jarosz DF, Kowalko JE, Yoshizawa M, Jeffery WR, Borowsky RL, Lindquist S, Tabin CJ. 2013. Cryptic variation in morphological evolution: HSP90 as a capacitor for loss of eyes in cavefish. Science 342, 1372–1375. ( 10.1126/science.1240276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persson L. 1986. Effects of reduced interspecific competition on resource utilization in perch (Perca fluviatilis). Ecology 67, 355–364. ( 10.2307/1938578) [DOI] [Google Scholar]
- 23.Svanbäck R, Eklöv P. 2003. Morphology dependent foraging efficiency in perch: a trade-off for ecological specialization? Oikos 102, 273–284. ( 10.1034/j.1600-0706.2003.12657.x) [DOI] [Google Scholar]
- 24.Lundvall D, Svanbäck R, Persson L, Byström P. 1999. Size-dependent predation in piscivores: interactions between predator foraging and prey avoidance abilities. Can. J. Fish. Aquat. Sci. 56, 1285–1292. ( 10.1139/f99-058) [DOI] [Google Scholar]
- 25.Persson L, Byström P, Wahlström E. 2000. Cannibalism and competition in Eurasian perch: population dynamics of an ontogenetic omnivore. Ecology 81, 1058–1071. ( 10.1890/0012-9658(2000)081[1058:CACIEP]2.0.CO;2) [DOI] [Google Scholar]
- 26.Estlander S, et al. 2017. Latitudinal variation in sexual dimorphism in life-history traits of a freshwater fish. Ecol. Evol. 7, 665–673. ( 10.1002/ece3.2658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svanbäck R, Eklöv P. 2011. Catch me if you can—predation affects divergence in a polyphenic species. Evolution 65, 3515–3526. ( 10.1111/j.1558-5646.2011.01398.x) [DOI] [PubMed] [Google Scholar]
- 28.Svanbäck R, Zha YH, Brönmark C, Johansson F. 2017. The interaction between predation risk and food ration on behavior and morphology of Eurasian perch. Ecol. Evol. 7, 8567–8577. ( 10.1002/ece3.3330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Degerman E, Hammar J, Nyberg P, Svardson G. 2001. Human impact on the fish diversity in the four largest lakes of Sweden. Ambio 30, 522–528. ( 10.1579/0044-7447-30.8.522) [DOI] [PubMed] [Google Scholar]
- 30.Lessmark O. 1983. Competition between perch (Perca fluviatilis) and roach (Rutilus rutilus) in south Swedish lakes. PhD thesis, University of Lund, Lund, Sweden. [Google Scholar]
- 31.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.1111/j.1558-5646.1983.tb00236.x) [DOI] [PubMed] [Google Scholar]
- 32.Arnold SJ, Wade MJ. 1984. On the measurement of natural and sexual selection—theory. Evolution 38, 709–719. ( 10.1111/j.1558-5646.1984.tb00344.x) [DOI] [PubMed] [Google Scholar]
- 33.Janzen FJ, Stern HS. 1998. Logistic regression for empirical studies of multivariate selection. Evolution 52, 1564–1571. ( 10.1111/j.1558-5646.1998.tb02237.x) [DOI] [PubMed] [Google Scholar]
- 34.Pasek J. 2016. weights: Weighting and Weighted Statistics. R package version 0.85. https://CRAN.R-project.org/package=weights (written with some assistance from Alex Tahk, some code modified from R-core; Additional contributions by Gene Culter and Marcus Schwemmle)
- 35.Brodie ED, Moore AJ, Janzen FJ. 1995. Visualizing and quantifying natural selection. Trends Ecol. Evol. 10, 313–318. ( 10.1016/s0169-5347(00)89117-x) [DOI] [PubMed] [Google Scholar]
- 36.R Development Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 37.Braekevelt CR, McIntyre DB, Ward FJ. 1989. Development of the retinal tapetum lucidum of the Walleye (Stizostedion vitreum vitreum). Histol. Histopathol. 4, 63–70. [PubMed] [Google Scholar]
- 38.Ollivier FJ, Samuelson DA, Brooks DE, Lewis PA, Kallberg ME, Komaromy AM. 2004. Comparative morphology of the tapetum lucidum (among selected species). Vet. Ophthalmol. 7, 11–22. ( 10.1111/j.1463-5224.2004.00318.x) [DOI] [PubMed] [Google Scholar]
- 39.Santon M, Bitton P-P, Dehm J, Fritsch R, Harant UK, Anthes N, Michiels NK.2018. Predator detection through active photolocation in a diurnal fish. bioRxiv. ( ) [DOI] [PMC free article] [PubMed]
- 40.McPhail JD. 1977. A possible function of the caudal spot in Characid fishes. Can. J. Zool. Rev. Can. Zool. 55, 1063–1066. ( 10.1139/z77-136) [DOI] [Google Scholar]
- 41.Svanbäck R, Eklöv P. 2002. Effects of habitat and food resources on morphology and ontogenetic growth trajectories in perch. Oecologia 131, 61–70. ( 10.1007/s00442-001-0861-9) [DOI] [PubMed] [Google Scholar]
- 42.Svanbäck R, Eklöv P, Fransson R, Holmgren K. 2008. Intraspecific competition drives multiple species resource polymorphism in fish communities. Oikos 117, 114–124. ( 10.1111/j.2007.0030-1299.16267.x) [DOI] [Google Scholar]
- 43.Unni KS. 1971. An ecological study of the macrophytic vegetation of the Doodhadhari Lake, Raipur, MP, India. 2. Physical factors. Hydrobiologia 38, 479–487. ( 10.1007/BF00036554) [DOI] [Google Scholar]
- 44.Blest AD. 1957. The function of eyespot patterns in the Lepidoptera. Behavoiur 11, 209–256. ( 10.1163/156853956X00048) [DOI] [Google Scholar]
- 45.Smith SM. 1976. Predatory behaviour of young turquoise-browed motmots, Eumomota superciliosa. Behaviour 56, 309–320. ( 10.1163/156853976X00082) [DOI] [Google Scholar]
- 46.Byström P, Persson L, Wahlstrom E, Westman E. 2003. Size- and density-dependent habitat use in predators: consequences for habitat shifts in young fish. J. Anim. Ecol. 72, 156–168. ( 10.1046/j.1365-2656.2003.00681.x) [DOI] [Google Scholar]
- 47.Meuthen D, Baldauf SA, Bakker TCM, Thunken T. 2018. Neglected patterns of variation in phenotypic plasticity: age- and sex-specific antipredator plasticity in a cichlid fish. Am. Nat. 191, 475–490. ( 10.1086/696264) [DOI] [PubMed] [Google Scholar]
- 48.Kingsolver JG, Diamond SE. 2011. Phenotypic selection in natural populations: what limits directional selection? Am. Nat. 177, 346–357. ( 10.1086/658341) [DOI] [PubMed] [Google Scholar]
- 49.Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gibert P, Beerli P. 2001. The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261. ( 10.1086/319193) [DOI] [PubMed] [Google Scholar]
- 50.Mittelbach GG, Persson L. 1998. The ontogeny of piscivory and its ecological consequences. Can. J. Fish. Aquat. Sci. 55, 1454–1465. ( 10.1139/cjfas-55-6-1454) [DOI] [Google Scholar]
- 51.Nilsson PA, Brönmark C. 2000. Prey vulnerability to a gape-size limited predator: behavioural and morphological impacts on northern pike piscivory. Oikos 88, 539–546. ( 10.1034/j.1600-0706.2000.880310.x) [DOI] [Google Scholar]
- 52.Merry JW, Kemp DJ, Rutowski RL. 2011. Variation in compound eye structure: effects of diet and family. Evolution 65, 2098–2110. ( 10.1111/j.1558-5646.2011.01285.x) [DOI] [PubMed] [Google Scholar]
- 53.Winfield IJ, Fletcher JM, Ben James J. 2012. Long-term changes in the diet of pike (Esox lucius), the top aquatic predator in a changing Windermere. Freshw. Biol. 57, 373–383. ( 10.1111/j.1365-2427.2011.02607.x) [DOI] [Google Scholar]
- 54.Eklov P, Diehl S. 1994. Piscivore efficiency and refuging prey—the importance of predator search mode. Oecologia 98, 344–353. ( 10.1007/BF00324223) [DOI] [PubMed] [Google Scholar]
- 55.Svanbäck R, Schluter D. 2012. Niche specialization influences adaptive phenotypic plasticity in the threespine stickleback. Am. Nat. 180, 50–59. ( 10.1086/666000) [DOI] [PubMed] [Google Scholar]
- 56.Lind MI, Johansson F. 2007. The degree of adaptive phenotypic plasticity is correlated with the spatial environmental heterogeneity experienced by island populations of Rana temporaria. J. Evol. Biol. 20, 1288–1297. ( 10.1111/j.1420-9101.2007.01353.x) [DOI] [PubMed] [Google Scholar]
- 57.Svanbäck R, Johansson F. 2019. Data from: Predation selects for smaller eye size in a vertebrate: effects of environmental conditions and sex Dryad Digital Repository. ( 10.5061/dryad.475bv7s) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Svanbäck R, Johansson F. 2019. Data from: Predation selects for smaller eye size in a vertebrate: effects of environmental conditions and sex Dryad Digital Repository. ( 10.5061/dryad.475bv7s) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data and R codes are available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.475bv7s [57].