Abstract
Thousands of species have been introduced to new ranges worldwide. These introductions provide opportunities for researchers to study evolutionary changes in form and function in response to new environmental conditions. However, almost all previous studies of morphological change in introduced species have compared introduced populations to populations from across the species' native range, so variation within native ranges probably confounds estimates of evolutionary change. In this study, we used microsatellites to locate the source population for the beach daisy Arctotheca populifolia that had been introduced to eastern Australia. We then compared four introduced populations from Australia with their original South African source population in a common-environment experiment. Despite being separated for less than 100 years, source and introduced populations of A. populifolia display substantial heritable morphological differences. Contrary to the evolution of increased competitive ability hypothesis, introduced plants were shorter than source plants, and introduced and source plants did not differ in total biomass. Contrary to predictions based on higher rainfall in the introduced range, introduced plants had smaller, thicker leaves than source plants. Finally, while source plants develop lobed adult leaves, introduced plants retain their spathulate juvenile leaf shape into adulthood. These changes indicate that rapid evolution in introduced species happens, but not always in the direction predicted by theory.
Keywords: common-garden experiment, evolution of increased competitive ability, neoteny, paedomorphosis, plant traits, rapid evolution
1. Introduction
Over 13 000 vascular plant species have become naturalized in new ranges worldwide [1]. These introductions have resulted in many ecological impacts on resident species and ecosystem processes [2]. Increasingly, the evolutionary impacts of introductions are also becoming apparent: introduced species can promote evolutionary diversification in native species, and undergo evolutionary changes themselves [3]. These evolutionary changes can occur rapidly (often within tens to hundreds of generations [4]) and have been demonstrated in a wide range of introduced plants and animals in terrestrial, aquatic and marine environments. Some well-known examples include cane toads in Australia [5], zebra mussels in the USA and Europe [6], and smooth cordgrass on the West Coast of the USA [7].
Common-environment experiments are often used to test for evolutionary changes in introduced species [8]. However, in all but one [9] previous study of morphological change between introduced and native populations of plants, the actual source population for the introduction has been unknown. Without knowing the original source of an introduction, we cannot accurately assess what evolutionary changes have taken place, because the use of native plants from a broad range introduces variation that may obscure differences between native and introduced populations (figure 1). This affects how we interpret differences found between native and introduced plants, and is likely to be especially problematic in cases where introduced plants have large native distributions and thus span wide environmental and/or biotic gradients. To overcome these limitations, we located the source population for Arctotheca populifolia, a beach daisy that was introduced to Australia from South Africa in the 1930s [10]. We set up a common-environment experiment to compare plants from the known source population in South Africa with plants from four introduced populations in Australia. We examined the evidence of evolutionary divergence after introduction using a suite of six plant growth traits and six leaf traits, to capture information about resource acquisition strategy and life-history strategy [11,12].
Figure 1.
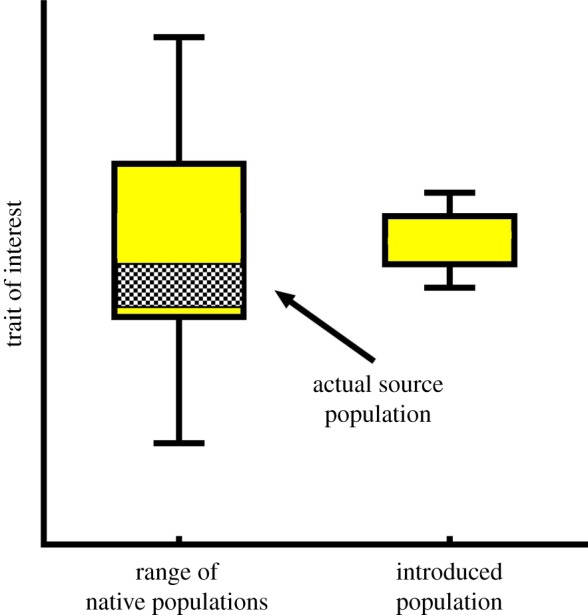
Sampling widely across the range of native populations can add variation that could lead to differences between the actual source population and the introduced population becoming obscured or underestimated. (Online version in colour.)
Introduced species can undergo evolutionary changes via three mechanisms: hybridization, natural selection or genetic drift [3]. Hybridization between introduced and native species is an important process that has been demonstrated in several previous studies (e.g. [7]), but is not occurring in A. populifolia in Australia (there are no native Arctotheca in Australia [13]). Genetic drift can cause evolutionary changes in introduced populations [14], and may be a significant factor for A. populifolia because it has been shown to have one of the lowest levels of genetic diversity for an introduced species [15], but it is usually not possible to predict the direction of these changes. However, we can arrive at several hypotheses for the ways in which natural selection might lead to morphological change in A. populifolia.
Selection for morphological change could arise from differences in the abiotic environment between the source and introduced ranges. The source and introduced populations of A. populifolia are at similar latitudes and experience similar temperatures, but rainfall in the introduced range is two to three times higher than in the source location (electronic supplementary material, appendix S1: table S1). There is generally a positive relationship between rainfall and plant size [16], so we predicted that the higher rainfall in Australia would lead plants from the introduced populations of A. populifolia to be larger than plants from the source population. Specifically, we predicted that the Australian plants would have greater total and above-ground biomass, and be taller and longer than their South African counterparts. The one size trait that we did not expect to be greater in Australia was below-ground biomass. We predicted that the introduced plants would allocate a greater percentage of their resources above-ground than below-ground in response to higher water availability [17]. Wetter conditions might also favour the evolution of larger, thinner, higher specific leaf area (SLA) leaves with lower dry matter content and low tissue density in the introduced range [11,12,18]. However, we did not predict a difference in leaf shape. Leaf shape affects leaf thermal properties [19], but there is little difference in temperature between the source and introduced range.
Selection for morphological change could also arise as a result of differences in biotic interactions between the source and introduced range. The evolution of increased competitive ability (EICA) hypothesis proposes that release from specialist herbivores allows introduced plants to shift resources from defence into growth and reproduction, and so evolve increased competitive ability and larger size [20]. We do not yet have data on the degree of herbivory experienced by A. populifolia in its native versus source ranges, but if enemy release is occurring, we would expect the plants to grow larger in Australia (taller and longer, with greater total, above-ground and below-ground biomass). While leaf shape can affect apparency to herbivores [21], we have no specific predictions as to how leaf shape might change in response to decreased herbivory. However, smaller, tougher leaves are less vulnerable to herbivory [18,22], so reduced herbivory in the introduced range might allow the evolution of larger, thinner, higher SLA leaves with lower dry matter content and low tissue density [12,18].
Biotic and abiotic selective pressures are not mutually exclusive. In the case of A. populifolia in Australia, selective pressures from enemy release and increased rainfall are predicted to act in the same direction, favouring increases in plant size and larger, higher SLA leaves (predictions summarized in table 1). The only trait considered here for which biotic and abiotic selective pressures are predicted to have opposing effects is below-ground biomass (increasing under EICA, decreasing in response to higher rainfall; table 1). Further, there are other mechanisms through which plant traits might change, including genetic drift and differences in other, unmeasured selective pressures between the native and introduced range. Thus, we cannot definitively prove which mechanisms underpin any observed changes. However, we can determine whether there have been evolutionary changes in plant traits between the source and introduced populations of A. populifolia, and observe whether any trait changes are broadly consistent with the collective predictions based on EICA and rainfall.
Table 1.
Summary of predictions and outcomes for each trait. Predictions are based on (1) EICA theory and (2) higher rainfall in Australia. ‘+’ indicates a prediction for greater values of the trait in Australia, ‘−’ indicates a prediction for lower values of the trait in Australia and ‘.’ indicates no difference (or no particular prediction). The fourth column gives the observed results for each trait (details in figure 1 and figure 2). Only five of the 24 predictions (indicated by asterisks) were consistent with observed results.
| trait | prediction under EICA | prediction based on higher rainfall in Australia | observed |
|---|---|---|---|
| total biomass | + | + | . |
| plant length | + * | + * | + |
| plant height | + | + | − |
| plant length/height | . | . | − |
| above-ground biomass | + | + | . |
| below-ground biomass | + | − * | − |
| SLA | + | + | . |
| leaf dry matter content | − | − | . |
| leaf area | + | + | − |
| leaf density | − * | − * | − |
| leaf thickness | − | − | + |
| leaf shape | . | . | − |
2. Methods
(a). Study species
Arcthotheca populifolia (P.J. Bergius) Norlindh is a perennial, semi-succulent herb in the Asteraceae. It is a coastal pioneer species native to South Africa where it is grows on the foredunes of sandy beaches and is common along the coastline [23]. The earliest records of this species in Australia date back to the 1930s, and it is now present in two separate regions: the east coast and southwest Western Australia [10].
Location of the source population for the Arctotheca populifolia populations in eastern Australia was based on microsatellite data collected by Rollins et al. [15], complemented by new analyses (details in electronic supplementary material, appendix S2). Briefly, these data give four indications that the east Australian populations of A. populifolia are most closely aligned with a population from Arniston in southwest South Africa: STRUCTURE analysis; principal component analysis; differentiation measured by RST (electronic supplementary material, appendix S2); and the fact that Arniston is the only South African population that includes all of the rare alleles found in the east Australian plants. These indications that Arniston is the source demand formal statistical comparison of two hypotheses: that Arniston is, or is not, the source of the east Australian plants. Therefore, we performed log odds analyses (LOD, electronic supplementary material, appendix S2) which indicate that Arniston is at least 1099 times more likely to be the source population than any other South African population (despite our making the tests conservative, i.e. biased against making such a conclusion; see electronic supplementary material, appendix S2). Thus, it seems reasonable to conclude that Arniston is the source for the east Australian invasion.
(b). Collection locations
We collected seeds from four locations spanning approximately 600 km on the east coast of Australia (Mallacoota, Narooma, Wairo Beach and Treachery Beach; electronic supplementary material, appendix S1) in February 2011, and from the source population in Arniston, South Africa in April 2011. These locations correspond to those used in the microsatellite study [15]. At each location, seeds from multiple seed heads on individual plants were collected. The number of individuals sampled at each location ranged from 17 to 46 (electronic supplementary material, appendix S3, table S3a).
(c). Minimizing possible maternal effects
To avoid confounding maternal effects with genetic effects when comparing our populations, we used the seeds collected in the field to grow parent plants which then produced a standardized generation of offspring for our main experiment. The maternal environment in which seeds are produced can affect several plant traits in the offspring, independent of the genetic make-up of the mother plant [24]. This occurs mostly in early stage traits of plant development (e.g. seed mass and germination) but has also been documented for traits over the whole life cycle of the plant (e.g. growth and flowering) [25].
We randomly selected between three and eight seeds from each plant that was collected in the field, and removed the tough outer seed coats by hand. We placed seeds on filter paper in lidded plastic Petri dishes and on the 21 and 22 October 2012, we added MilliQ water to each Petri dish which we then sealed with Parafilm. The seeds began to germinate after a few days. In the weeks that followed, germinating seeds were removed and potted in soil made up with river sand, cocopeat and fertilizer (details in electronic supplementary material, appendix S3, table S3b). Pots were haphazardly placed on the greenhouse benches, and positions were randomly rotated every four to six weeks. Glasshouse temperatures were controlled between 10°C and 25°C and plants were watered every evening at 17.00 by automatic drippers (also at 9.00 in the early stages of establishment). As germination progressed, it became apparent that in some groups more seeds would need to be germinated in order to have enough parent plants. From 5 to 7 December, water was added to a second round of seeds from the South African population. On 14 December, water was added to a second round of seeds from two of the Australian populations (Narooma and Treachery Beach). Seeds from the first round were still germinating at this point and continued to do so up until 14 January.
Plants began to flower in February 2013 and we pollinated them every 3–4 days until senescence in November. For each one of the five populations, we collected pollen from all flowering individuals using a paintbrush and a Petri dish, mixed the pollen and then distributed it back to all available flowers in that population. Flowers that had been pollinated were marked with small tags. In between pollination events, all buds and flowers were covered with small drawstring organza bags. This was both to exclude any possible pollinators, and to collect seeds. In total, we planted 356 parent plants, of which 215 flowered and 186 produced seeds for the next generation of experimental plants.
(d). Experimental plants
We germinated and planted the standardized generation of experimental plants in the same way as the parent plants in the previous year, with water being first added on 3 and 4 December 2013. For South African plants, we randomly selected 15 seeds for germination (if there were less than 15 seeds available we used them all). For Australian plants, we randomly selected 10 seeds per plant for germination. We planted 340 plants for a year-long experiment, and 91 plants for harvesting at 12 weeks to assess above- and below-ground biomass (details in electronic supplementary material, appendix S3, table S3c). After four weeks, we stopped planting seedlings so that the age of all the experimental plants would be within one month of each other. Pot randomization and glasshouse controls were identical to the previous year.
(e). Trait measurements
We marked plants with their week of germination. All plant traits were measured in weekly batches for four consecutive weeks so that all plants in each batch were aged within a week of one another for measurements. Data were collected according to standardized protocols [26].
(i). Plant size and growth form
Because A. populifolia is a spreading plant, we measured a range of size traits, including total biomass (a widely accepted indicator of plant performance that is directly related to plant fitness [27]), plant length (an indicator of plant size in spreading plants), height (an indicator of the ability to compete for light) and the ratio of length to height (indicative of the overall shape of the plant). We collected data on plant height and length at nine weeks of age. After this time, the plants required staking and this affected their natural growth form. We measured plant height using a ruler from the base of the stem to the tallest part of the plant. We measured plant length by tracing along the stem with a piece of string and then measuring the string. For growth form, we calculated an index of plant height to length, where plants with an upright growth form would have values closer to one, and plants with a horizontal growth form would have values closer to zero. We measured biomass at 12 weeks by harvesting the subset of plants in the small pots, removing soil, separating above- and below-ground biomass, and drying the material at 60°C for 72 h before weighing. When the plants in the big pots were beginning to senesce (approx. 11 months after planting), we harvested above-ground biomass and dried it at 60°C for 72 h. It was not practical to measure below-ground biomass at this stage.
(ii). Leaf traits
We measured leaf traits when plants were at nine weeks of age. For each plant, we counted down from the top of the plant and removed the leaf and petiole of the second fully formed adult leaf. We immediately measured fresh weight (g) using a Mettler Toledo XS analytical balance, and then scanned an image of the leaf using a Canon flatbed scanner (CanoScan LiDE 200). We used ImageJ [28] to obtain area and perimeter values. Using a Mercer dial gauge, we took three measurements of leaf thickness (midway between the margin and the midrib at the widest part of the leaf on each side, and then at a similar distance from the top of the leaf, avoiding veins where possible) and then calculated average leaf thickness. We then dried the leaves at 60°C for 48 h and measured dry weight. Using these data, we calculated the following traits: SLA—the one sided area of a fresh leaf divided by its oven-dry mass; leaf dry-matter content (LDMC)—the oven-dry mass of a leaf divided by its water-saturated fresh mass; leaf density—the dry mass of a leaf divided by its volume (thickness × area) and leaf shape (excluding petioles) using a leaf dissection index: (perimeter/[√(area)] (following [29])).
Our raw data are available in electronic supplementary material, appendix S6.
(f). Statistical analyses
We compared trait values among the five populations using one-way analyses of variance (ANOVAs, performed in SPSS version 22.0) with a planned contrast between the South African source population and the four Australian introduced populations. Data for SLA were log10-transformed before analysis. To account for multiple tests (one per trait), we applied a Holm–Bonferroni sequential correction [30]. This did not change the significance of any of these results. Our experimental plants either came from separate maternal lines or were half-sibs. The effect of maternal line made almost no difference to our analyses (electronic supplementary material, appendix S3, table S3d).
Given that the Australian populations experience a range of environments across 5° of latitude, we contrasted all traits among the Australian populations with one-way ANOVAs, followed by a Holm–Bonferroni sequential correction. Only one of the 12 traits showed significant differences among Australian populations (electronic supplementary material, appendix S4, table S4, figure S4).
Our hypotheses were about individual traits, so we began with univariate analyses. However, different plant traits are often correlated [11], so we also used a multivariate analysis of variance (MANOVA) to test for differences between source and introduced plants in the expression of the nine plant traits that were measured on the same individuals. This MANOVA was run using the manylm() function of the mvabund package in R [31].
3. Results
Introduced A. populifolia arrived in Australia less than 100 years ago and yet the plants display striking morphological differences from their South African source population (figure 2).
Figure 2.

A representative source plant and a representative introduced plant showing some of the key morphological changes that have evolved since introduction.
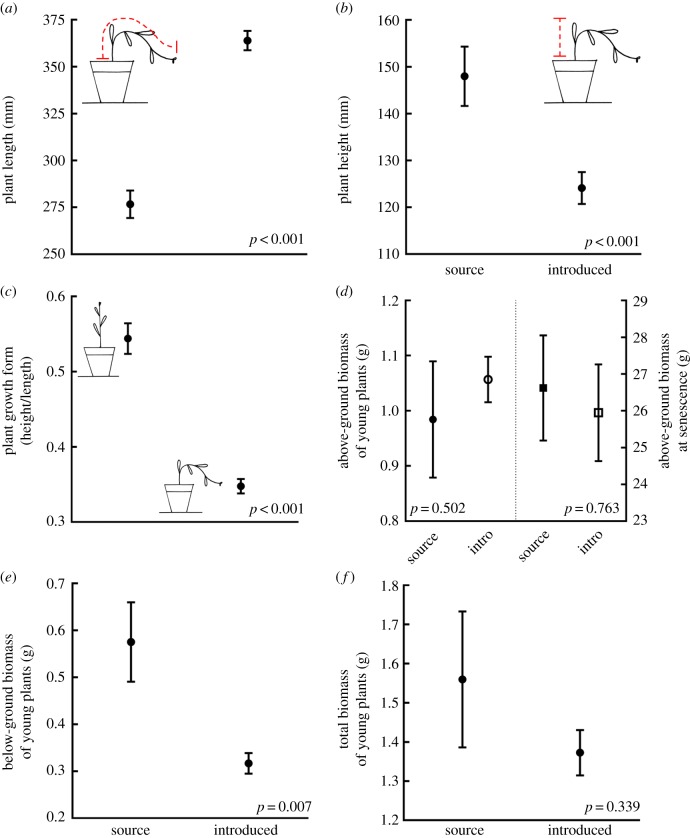
Consistent with our predictions based on EICA and the higher rainfall in Australia (table 1), introduced plants were 31% longer than were plants from the source population (p < 0.001; figure 3a). However, they were significantly less tall (p < 0.001; figure 3b), exhibiting a sprawling, horizontal growth form, as opposed to the more upright source plants (p < 0.001; figure 3c). Counter to our predictions, there was no difference in the amount of above-ground biomass produced by introduced and source plants at either the 12-week harvest (p = 0.502; figure 3d), or the end-of-life harvest (p = 0.763; figure 3d). There was also no significant difference in total biomass between introduced and source plants (p = 0.339; figure 3f). However, the introduced plants had 45% less below-ground biomass than did the source plants (p = 0.007; figure 3e), resulting in a higher percentage of overall biomass being allocated above-ground for introduced (78%) compared to source (64%) plants (p < 0.001). This result is consistent with our predictions based on rainfall, but counter to the predictions from EICA (table 1).
Figure 3.
Six plant growth traits comparing source plants and introduced plants showing mean values (±s.e.): plant length measured along the stem (a); plant height measured from the base of the stem to the tallest part of the plant (b); plant growth form as an index of plant height to length, where plants with an upright growth form would have values closer to one, and plants with a horizontal growth form would have values closer to zero (c); above-ground biomass of young plants (left-hand side of graph) and of plants at senescence (right-hand side of graph) (d); below-ground biomass (e); and total biomass (f) of young plants. The p-values for each trait are from a planned contrast between the South African source population and the four Australian introduced populations following a one-way ANOVA. y-axes have been truncated. (Online version in colour.)
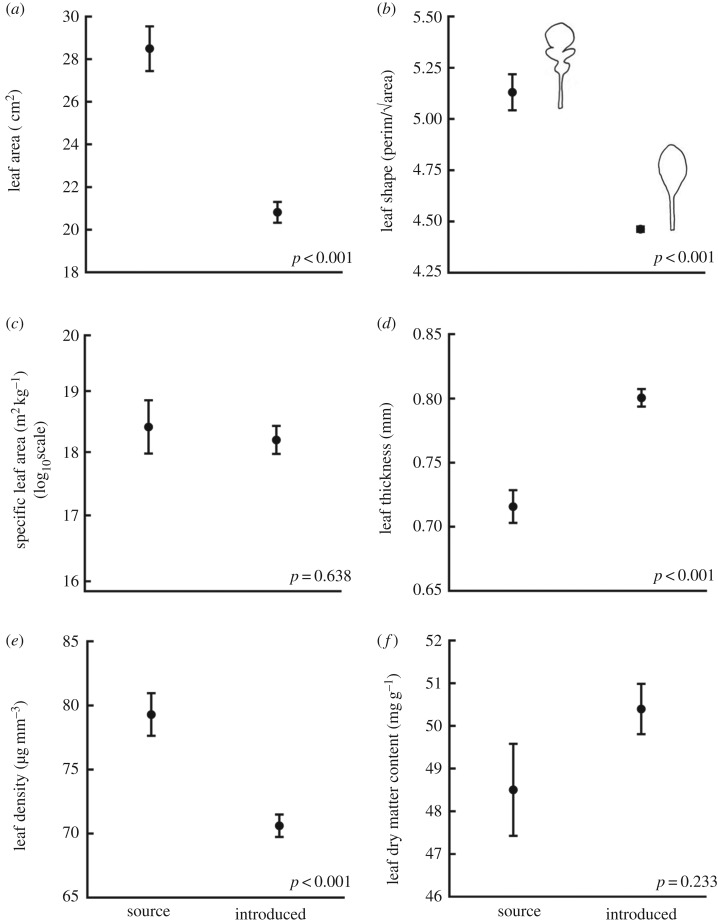
Contrary to what we predicted based on both EICA and differences in rainfall (table 1), the leaves of the introduced plants were 27% smaller than were the leaves of the source plants (p < 0.001; figure 4a). In addition, the source and introduced plants had leaves that were surprisingly different shapes (p < 0.001; figure 4b). In the source plants, juvenile leaves had a simple spathulate shape and adult leaves developed lobes around the perimeter. The introduced plants had lost this lobed adult leaf form and only produced spathulate leaves (figure 5). There was no significant difference between the SLA of the introduced plants and the source plants (p = 0.638; figure 4c). SLA is the area of a fresh leaf divided by its dry mass, and in these two groups of plants these traits scale in the same direction—the leaves of the introduced plants had a smaller area (p < 0.001) and less dry mass (p < 0.001) than did the leaves of the source plants. But leaf thickness and leaf density are also key components of SLA where SLA ≈ 1/(thickness×density) [32]. Differences in leaf thickness and density do not always affect SLA values because they can trend in opposite directions. For example, tough sclerophyllous leaves (thin and dense) and fleshy succulent leaves (thick and wet) can have the same SLA values [33]. It is crucial to separate SLA into these two measures, because they can vary separately and are more responsive to environmental gradients than SLA alone [32]. Indeed, in A. populifolia the leaves of introduced plants were thicker (p < 0.001; figure 4d) and less dense (p < 0.001; figure 4e) than were the leaves of the source plants.
Figure 4.
Mean values (±s.e.) of six leaf traits comparing source plants and introduced plants. The p-values for each trait are from a planned contrast between the South African source population and the four Australian introduced populations following a one-way ANOVA. y-axes have been truncated.
Figure 5.

Adult and juvenile leaf pairs of source and introduced plants. Adult leaves are approximately 15 cm in length.
There was no significant difference between the LDMC of the introduced plants and the source plants in the planned contrast between all four Australian populations and the South African population (p = 0.233; figure 4f). However, LDMC was the one trait for which the Australian populations varied significantly, so we also ran comparisons between each individual Australian population and the population from South Africa. We found a significant (p = 0.004) difference in LDMC between the South African population and plants from Malacoota (our southernmost Australian population), but no significant difference between the South African plants and plants from any other Australian population (all p > 0.5). Notably, our measured levels of LDMC are some of the lowest reported in the literature (we measured mean LDMC of 50 mg g−1 in the introduced range and 49 mg g−1 in the source population; in Hodgson [34], the 5–95% range for 1950 species was 93–387 mg g−1). Since leaf water capacity = 1000 – LDMC, this indicates that the leaves of these plants have a very high water-storing capacity.
Source and introduced plants were found to be significantly different from each other when we tested individuals for differences using a MANOVA (p < 0.001; electronic supplementary material, appendix S5, figure S5, table S5). Ordination plots (electronic supplementary material, figure S5) and the proportion of variation explained by each trait in the analysis (electronic supplementary material, table S5) are included in appendix S5.
4. Discussion
Introduced populations of A. populifolia have evolved many remarkable changes in plant and leaf morphology. These evolutionary changes could have been caused by adaptation to biotic and abiotic factors by natural selection, or by genetic drift.
The introduced plants had evolved to be longer than the source plants. This is consistent with previous work on introduced plants in general [35,36], as well changes observed over time in herbarium specimens of A. populifolia [37]. The introduced plants had also evolved a more horizontal growth form than the source plants, which stood more upright. However, even with these differences, there was no difference in the total amount of above-ground biomass produced by introduced and source plants measured at two different life stages. Growing longer but using the same amount of biomass indicates that the introduced plants have evolved cheaper construction costs and a faster growth rate—a strategy consistent with previous studies on other introduced plants [36,38]. In addition, for a coastal dune plant like A. populifolia, an increase in growth (especially stem length) and a shift in biomass allocation from below-ground to above-ground are both mechanisms consistent with a response to burial by sand [39]. These strategies could help plants survive burial events [40,41], and might provide an adaptive explanation for the changes we observed.
Contrary to our predictions based on both EICA and the greater rainfall in Australia, the leaves of the introduced plants had evolved to be smaller and thicker than the leaves of the source plants. In general, succulent plants adapt to water-deficient environments by storing water in their leaves, resulting in thick leaves with high leaf water content [33]. Coastal plants like A. populifolia must also adapt their leaves to a combination of severe stresses like salt spray, sand burial, wind exposure and nutrient deficiency [42]. Changes to these stresses in the new range could be responsible for driving the evolutionary adaptations we observed in the introduced plants—for example, a decrease in leaf size has been shown with increased wind speed [43] and decreased nutrient availability [44].
Surprisingly, the introduced plants had evolved leaves that were a different shape to the leaves of the source plants (figure 5). The retention of juvenile characteristics into adulthood (known as paedomorphosis) can explain historical evolutionary changes in leaf form [45], but this is the first study showing paedomorphosis occurring in contemporary evolution. This remarkable change in leaf shape could result in a number of functionally significant impacts on thermoregulation and hydraulic efficiency [19]. Previous work shows that the switch between complex and simple leaves could evolve via the regulation of the KNOX (Knotted-like homeobox) genes [46]. A reciprocal genetic transplant experiment on two closely related species with different shaped leaves (Arabidopsis thaliana—simple leaves and Cardamine hirsuta—complex leaves) showed that when KNOX genes are turned off, a plant that normally produces complex leaves can switch to producing simple leaves [47]. In the case of A. populifolia with a single introduction on the east coast of Australia and a presumably small founder population, it is possible that genetic drift could have resulted in certain genetic variants becoming fixed in the introduced plants. One of the fixed changes might have affected regulation of morphological genes such as KNOX to produce the observed switch to simple leaves.
Growing native and introduced plants in a common-environment experiment is a fundamental test for evolutionary changes following introduction to a new range. But without careful consideration of how we sample the native range, the results of these experiments can be distorted. A study re-analysing 32 comparisons of native and introduced populations in common-environment experiments showed that among-population variation due to geographical clines was so substantial that when it was included in the analyses (by including the effect of latitude), it not only changed the significance and magnitude of some trait differences between native and introduced populations but even reversed the direction of some changes [8]. This has worrying implications for how we have been interpreting differences between native and introduced populations in previous common-environment experiments. The only precise test of evolutionary change since introduction can be achieved when we can use the known source population as an accurate point of reference against which we can assess what changes have taken place, as we have done in this study.
In conclusion, we have shown that despite low levels of genetic diversity, introduced species can evolve marked morphological changes. However, these changes are not always consistent with what is predicted by theory. This finding aligns with a meta-analysis of invasive plants which showed that evolution happens—just not always in the direction predicted by the EICA hypothesis [36], or with predictions based on rainfall. We have also shown how comparing introduced plants with their source population provides a powerful test for uncovering rapid evolution in action.
Supplementary Material
Acknowledgements
We thank Justin Chan, Casey Gibson, Martin Kim and Geoff McDonnell for help in the glasshouse and laboratory, and Stephen Bonser for advice and support. Gordana Popovic performed the multivariate analysis and created the ordination plots. We thank Nic Beatson for assistance with computing. Thanks also to Brian Husband and two anonymous referees, who gave helpful comments that substantially improved the manuscript.
Data accessibility
Our raw data are available in electronic supplementary material, appendix S6.
Authors' contributions
A.T.M. conceived the idea. A.T.M., W.B.S., R.F. and C.R.B. obtained funding. C.R.B., S.C., P.F. and R.B. collected data. C.R.B. led the analysis and writing with particular input from W.B.S. and A.T.M. W.B.S. and A.G.B.P. contributed to data analysis. All authors contributed to writing and interpretation.
Competing interests
We have no competing interests to declare.
Funding
This research was funded by an Australian Government Research Training Program Scholarship to C.R.B., and ARC grant (DP0984222) to A.T.M., W.B.S. and R.F.
References
- 1.Van Kleunen M, et al. 2015. Global exchange and accumulation of non-native plants. Nature 525, 100 ( 10.1038/nature14910) [DOI] [PubMed] [Google Scholar]
- 2.Vilà M, et al. 2011. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 14, 702–708. ( 10.1111/j.1461-0248.2011.01628.x) [DOI] [PubMed] [Google Scholar]
- 3.Vellend M, Harmon LJ, Lockwood JL, Mayfield MM, Hughes AR, Wares JP, Sax DF. 2007. Effects of exotic species on evolutionary diversification. Trends Ecol. Evol. 22, 481–488. ( 10.1016/j.tree.2007.02.017) [DOI] [PubMed] [Google Scholar]
- 4.Cox GW. 2004. Alien species and evolution: the evolutionary ecology of exotic plants, animals, microbes, and interacting native species. Washington, DC: Island Press. [Google Scholar]
- 5.Phillips BL, Brown GP, Webb JK, Shine R. 2006. Invasion and the evolution of speed in toads. Nature 439, 803 ( 10.1038/439803a) [DOI] [PubMed] [Google Scholar]
- 6.Elderkin CL, Klerks PL, Theriot E. 2001. Shifts in allele and genotype frequencies in zebra mussels, Dreissena polymorpha, along the latitudinal gradient formed by the Mississippi River. J. N. Am. Benthol. Soc. 20, 595–605. ( 10.2307/1468090) [DOI] [Google Scholar]
- 7.Daehler CC, Strong DR. 1997. Reduced herbivore resistance in introduced smooth cordgrass (Spartina alterniflora) after a century of herbivore-free growth. Oecologia 110, 99–108. ( 10.1007/s004420050138) [DOI] [PubMed] [Google Scholar]
- 8.Colautti RI, Maron JL, Barrett SC. 2009. Common garden comparisons of native and introduced plant populations: latitudinal clines can obscure evolutionary inferences. Evol. Appl. 2, 187–199. ( 10.1111/j.1752-4571.2008.00053.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dlugosch KM, Parker IM. 2008. Invading populations of an ornamental shrub show rapid life history evolution despite genetic bottlenecks. Ecol. Lett. 11, 701–709. ( 10.1111/j.1461-0248.2008.01181.x) [DOI] [PubMed] [Google Scholar]
- 10.AVH Database. 2016. Australia's Virtual Herbarium, Council of Heads of Australasian Herbaria See http://avh.ala.org.au (accessed on 10 August 2016).
- 11.Moles AT. 2018. Being John Harper: using evolutionary ideas to improve understanding of global patterns in plant traits. J. Ecol. 106, 1–18. ( 10.1111/1365-2745.12887) [DOI] [Google Scholar]
- 12.Wright IJ, et al. 2004. The world-wide leaf economics spectrum. Nature 428, 821–827. ( 10.1038/nature02403) [DOI] [PubMed] [Google Scholar]
- 13.Australian National Herbarium. 2018. Australian Plant Name Index, version 1.0213 See http://www.anbg.gov.au/apni/ (accessed 18 December 2018).
- 14.Sakai AK, et al. 2001. The population biology of invasive species. Annu. Rev. Ecol. Syst. 32, 305–332. ( 10.1146/annurev.ecolsys.32.081501.114037) [DOI] [Google Scholar]
- 15.Rollins LA, et al. 2013. High genetic diversity is not essential for successful introduction. Ecol. Evol. 3, 4501–4517. ( 10.1002/ece3.824/pdf) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moles AT, Warton DI, Warman L, Swenson NG, Laffan SW, Zanne AE, Pitman A, Hemmings FA, Leishman MR. 2009. Global patterns in plant height. J. Ecol. 97, 923–932. ( 10.1111/j.1365-2745.2009.01526.x) [DOI] [Google Scholar]
- 17.Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. 2012. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol. 193, 30–50. ( 10.1111/j.1469-8137.2011.03952.x) [DOI] [PubMed] [Google Scholar]
- 18.Moles AT, Westoby M. 2000. Do small leaves expand faster than large leaves, and do shorter expansion times reduce herbivore damage? Oikos 90, 517–524. ( 10.1034/j.1600-0706.2000.900310.x) [DOI] [Google Scholar]
- 19.Nicotra AB, Leigh A, Boyce CK, Jones CS, Niklas KJ, Royer DL, Tsukaya H. 2011. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 38, 535–552. ( 10.1071/FP11057) [DOI] [PubMed] [Google Scholar]
- 20.Blossey B, Nötzold R. 1995. Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J. Ecol. 83, 887–889. ( 10.2307/2261425) [DOI] [Google Scholar]
- 21.Feeny P. 1976. Plant apparency and chemical defense. Rec. Adv. Phytochem. 10, 1–40. [Google Scholar]
- 22.Coley PD. 1983. Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol. Monogr. 53, 209–229. ( 10.2307/1942495) [DOI] [Google Scholar]
- 23.SANBI database. 2016. South African National Biodiversity Institute See http://pza.sanbi.org (accessed 10 August 2016).
- 24.Roach DA, Wulff RD. 1987. Maternal effects in plants. Annu. Rev. Ecol. Syst. 18, 209–235. ( 10.1146/annurev.es.18.110187.001233) [DOI] [Google Scholar]
- 25.Helenurm K, Schaal BA. 1996. Genetic and maternal effects on offspring fitness in Lupinus texensis (Fabaceae). Am. J. Bot. 83, 1596–1608. ( 10.1002/j.1537-2197.1996.tb12818.x) [DOI] [Google Scholar]
- 26.Pérez-Harguindeguy N, et al. 2013. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234. ( 10.1071/BT12225) [DOI] [Google Scholar]
- 27.Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E. 2007. Let the concept of trait be functional! Oikos 116, 882–892. ( 10.1111/j.2007.0030-1299.15559.x) [DOI] [Google Scholar]
- 28.Rasband WS. 1997–2016 ImageJ. Bethesda, MD: US National Institutes of Health. See http://imagej.nih.gov/ij/.
- 29.McLellan T, Endler JA. 1998. The relative success of some methods for measuring and describing the shape of complex objects. Syst. Biol. 47, 264–281. ( 10.1080/106351598260914) [DOI] [Google Scholar]
- 30.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70. [Google Scholar]
- 31.Wang Y, Naumann U, Wright ST, Warton DI. 2012. mvabund–an R package for model-based analysis of multivariate abundance data. Methods Ecol. Evol. 3, 471–474. ( 10.1111/j.2041-210X.2012.00190.x) [DOI] [Google Scholar]
- 32.Witkowski ETF, Lamont BB. 1991. Leaf specific mass confounds leaf density and thickness. Oecologia 88, 486–493. ( 10.1007/BF00317710) [DOI] [PubMed] [Google Scholar]
- 33.Vendramini F, Díaz S, Gurvich DE, Wilson PJ, Thompson K, Hodgson JG. 2002. Leaf traits as indicators of resource-use strategy in floras with succulent species. New Phytol. 154, 147–157. ( 10.1046/j.1469-8137.2002.00357.x) [DOI] [Google Scholar]
- 34.Hodgson J, et al. 2011. Is leaf dry matter content a better predictor of soil fertility than specific leaf area? Ann. Bot. 108, 1337–1345. ( 10.1093/aob/mcr225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D. 2005. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144, 1–11. ( 10.1007/s00442-005-0070-z) [DOI] [PubMed] [Google Scholar]
- 36.Felker-Quinn E, Schweitzer JA, Bailey JK. 2013. Meta-analysis reveals evolution in invasive plant species but little support for Evolution of Increased Competitive Ability (EICA). Ecol. Evol. 3, 739–751. ( 10.1002/ece3.488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buswell JM, Moles AT, Hartley S. 2011. Is rapid evolution common in introduced plant species? J. Ecol. 99, 214–224. ( 10.1111/j.1365-2745.2010.01759.x) [DOI] [Google Scholar]
- 38.Kumschick S, Hufbauer RA, Alba C, Blumenthal DM. 2013. Evolution of fast-growing and more resistant phenotypes in introduced common mullein (Verbascum thapsus). J. Ecol. 101, 378–387. ( 10.1111/1365-2745.12044) [DOI] [Google Scholar]
- 39.Gilbert ME, Ripley BS. 2008. Biomass reallocation and the mobilization of leaf resources support dune plant growth after sand burial. Physiol. Plant. 134, 464–472. ( 10.1111/j.1399-3054.2008.01153.x) [DOI] [PubMed] [Google Scholar]
- 40.Maun MA. 1998. Adaptations of plants to burial in coastal sand dunes. Can. J. Bot. 76, 713–738. ( 10.1139/cjb-76-5-713) [DOI] [Google Scholar]
- 41.Gilbert ME, Ripley BS. 2010. Resolving the differences in plant burial responses. Austral. Ecol. 35, 53–59. ( 10.1111/j.1442-9993.2009.02011.x) [DOI] [Google Scholar]
- 42.Hesp PA. 1991. Ecological processes and plant adaptations on coastal dunes. J. Arid Environ. 21, 165–191. ( 10.1016/S0140-1963(18)30681-5) [DOI] [Google Scholar]
- 43.Retuerto R, Woodward FI. 1993. The influences of increased CO2 and water supply on growth, biomass allocation and water use efficiency of Sinapis alba L. grown under different wind speeds. Oecologia 94, 415–427. ( 10.1007/bf00317118) [DOI] [PubMed] [Google Scholar]
- 44.Givnish TJ. 1987. Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytol. 106, 131–160. ( 10.1111/j.1469-8137.1987.tb04687.x) [DOI] [Google Scholar]
- 45.Pryer KM, Hearn DJ. 2009. Evolution of leaf form in marsileaceous ferns: evidence for heterochrony. Evolution 63, 498–513. ( 10.1111/j.1558-5646.2008.00562.x) [DOI] [PubMed] [Google Scholar]
- 46.Bharathan G, Goliber TE, Moore C, Kessler S, Pham T, Sinha NR. 2002. Homologies in leaf form inferred from KNOXI gene expression during development. Science 296, 1858–1860. ( 10.1126/science.1070343) [DOI] [PubMed] [Google Scholar]
- 47.Hay A, Tsiantis M. 2006. The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat. Genet. 38, 942 ( 10.1038/ng1835) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our raw data are available in electronic supplementary material, appendix S6.