Abstract
The exponential increase in species introductions during the Anthropocene has brought about a major loss of biodiversity. Amphibians have suffered large declines, with more than 16% considered to be threatened by invasive species. We conducted a global meta-analysis of the impacts of alien species on native amphibians to determine which aspects of amphibian ecology are most affected by plant, invertebrate, fish, amphibian, reptile, or mammal introductions. Measures of fitness were most strongly affected; amphibian performance was consistently lower in the presence of alien species. While exposure to alien species caused a significant decrease in amphibian behavioural activity when compared with a no species control, this response was stronger towards a control of native impacting species. This indicates a high degree of prey naiveté towards alien species and highlights the importance of using different types of controls in empirical studies. Alien invertebrates had the greatest overall impact on amphibians. This study sets a new agenda for research on biological invasions, highlighting the lack of studies investigating the impacts of alien species on amphibian terrestrial life-history stages. It also emphasizes the strong ecological impacts that alien species have on amphibian fitness and suggests that future introductions or global spread of alien invertebrates could strongly exacerbate current amphibian declines.
Keywords: amphibian decline, alien species, literature review, invertebrates, prey naiveté, fitness
1. Introduction
It is widely accepted that amphibians are threatened and in decline, to a greater degree than reptiles, birds, or mammals [1–3]. Many reasons have been highlighted as contributing factors, such as habitat loss and alteration, over-exploitation, alien species introductions, emerging infectious diseases, climate change, and chemical contamination [3–6]. Although each of these factors independently poses serious risks to amphibian populations, complex synergistic interactions among them likely exacerbate declines [3–6].
Alien species introductions and establishment have been highlighted as one of the major factors contributing to worldwide amphibian declines and extinctions [7–9]. They can have detrimental effects on native amphibians directly through predation, competition, hybridization, and transmission of parasites and diseases, or indirectly through habitat alteration [5,7,10,11]. Numerous studies have documented how these processes have led to reduced native amphibian survival, decreased abundances, and eventual population decline, displacement, or local extinction [11].
According to the International Union for Conservation of Nature (IUCN), out of 6682 amphibian species listed on their Red List, currently over 16% are considered to be threatened by invasive alien species, and 11% have been categorized as threatened, i.e. considered Vulnerable, Endangered, or Critically Endangered [9,12]. When compared with other vertebrate groups, such as mammals, birds, reptiles, and fish, amphibians appear to be one of the most affected groups, with 41% of their species being threatened, although many species from these vertebrate groups still need to be assessed [12]. A 2010 assessment showed that conservation actions have been relatively successful at mitigating the threat posed by invasive alien species for birds and mammals, but this does not seem to be the case for amphibians [2]. Given that the rate at which alien species are introduced into new environments has reached unprecedented levels and continues to increase worldwide [13], it is important to understand their impacts on native amphibians. Even so, to our knowledge, only three studies have reviewed information on the impacts of alien species on native amphibians [7,10,11].
One of the reasons for amphibians being so susceptible to alien species impacts is that freshwater ecosystems are particularly vulnerable to invasions [14,15]. Most amphibians have complex life histories, with facultative freshwater primary consumer and terrestrial predatory stages. This vulnerability is also related to the intensive human use of water resources for recreation, food, commerce, and transportation, the natural linkages among streams and lakes, and the high dispersal ability of aquatic organisms [15]. Furthermore, freshwater species, including amphibians, seem to be particularly vulnerable to alien aquatic predators because freshwater habitats have quite heterogeneous predation regimes, often with few or no predators, which results in increased prey evolutionary naiveté. This is in comparison to the relatively homogenous regimes found in terrestrial and marine ecosystems [7,14]. Given that most amphibians are exposed to both aquatic and terrestrial habitats at different stages of their life cycles, their vulnerability to alien taxa might change as they progress through these life stages.
In the presence of predators and competitors, amphibians often develop defensive strategies, usually through plastic phenotypic alterations in their behaviour, morphology, life history, or physiology [16]. Defensive behavioural strategies include shifts to safer microhabitats, spatial avoidance behaviours, or reductions in activity level, while plastic morphological defences include increased tail depth, an enhancement of tail colouration, and the development of smaller heads/bodies and shorter limbs (e.g. [17,18]). Either as a direct response to risk, or as a result of induced behavioural or morphological alterations, amphibians can also modify their life-history, by reducing growth and development rates [16,19]. Several studies have shown that these defensive responses can also be triggered when native amphibians are exposed to alien species [7,10,11]. However, due to prey naiveté caused by the lack of a coevolutionary history with alien predators or competitors, responses might be weak, ineffective, or even non-existent (no recognition leading to a weak or no response) [14,20]. A lack of, weak or maladaptive response can be extremely detrimental to native amphibians, often causing decreased abundances and reduced fitness and survival in the presence of invasive alien species (e.g. [21]), which might ultimately result in local extinctions.
According to Bucciarelli et al. [11], the taxonomic groups of alien species that seem to more strongly negatively affect native amphibians are fishes, plants, and amphibians. Indeed, alien fishes, which generally become dominant species when introduced to novel aquatic systems, have had devastating consequences for amphibian species, especially for those amphibians that have not been evolutionarily exposed to fish predators [3]. Alien aquatic invertebrates, especially freshwater crayfish species, are also considered damaging to amphibians' fitness and survival [22]. Furthermore, although some taxonomic groups of alien species clearly show a large impact on amphibians, it is still uncertain if others have equivalent impacts or have simply been less well studied. It is, therefore, of fundamental relevance to investigate the impacts of different taxonomic groups of alien species on native amphibians and to identify the general patterns resulting from those is critical for directing future research and conservation actions.
Several meta-analyses have investigated the impacts of specific groups of alien species (e.g. plants, crayfish [22,23]) on ecosystems in general, but few have focused on the impacts of different alien species groups on a specific group of native species. The aim of this study was to quantitatively determine the ecological impacts of different taxonomic groups of alien species on native amphibians. Specifically, we endeavoured to answer the following questions:
-
1)
Which native amphibians’ ecological response variables are most affected by alien species introductions?
-
2)
Do the extent and direction of alien species impacts differ when compared to a native impacting species or a no species (blank) control?
-
3)
Do the effects of alien species on native amphibians differ between amphibian development stages (freshwater larval stage or terrestrial adult stage)?
-
4)
Does taxonomic identity of the alien species affect the mechanism and magnitude of their ecological effects?
2. Methods
(a). Literature search
Relevant published articles containing quantitative evidence of ecological impacts of alien species on native amphibians were searched for by performing a systematic literature search on ISI Web of Knowledge on 30 March 2016, an additional search on Google Scholar, and a further inspection of the literature cited in initially selected articles (see electronic supplementary material, Appendix S1 for details). The combined searches resulted in a set of 380 articles (260 from ISI Web of Knowledge, 16 from Google Scholar, and 104 from the literature cited sections) selected for initial inclusion in the meta-analysis.
(b). Selection criteria and data extraction
We subsequently inspected the potential of each study to contribute quantitative data to our analysis. Each article to be used in the meta-analysis was required to include quantitative data from the same ecological variable in both invaded and uninvaded environments. Criteria for extracting data from a study and including it in the meta-analysis were: (1) the impacting species had to be alien at the study location, (2) the native amphibian species had to be native to the study location, (3) values of mean, sample size, and standard error/standard deviation/confidence intervals had to be reported for both invaded and uninvaded treatments for at least one response variable of interest, and (4) the study design had to include replicated treatments.
Different types of quantitative evidence of ecological impacts of alien species on native amphibians were extracted from articles and categorized into nine different general response variable categories (table 1). The variables ‘diversity’ and ‘abundance’ were pooled into the same category, given the low number of cases and the expectation that their responses to impact would be in the same general direction (lower diversity/abundance; table 1). The variables ‘behaviour’ and ‘morphology’ were each subdivided into two subcategories, due to the opposite nature of the expected effect sizes of these subcategories (table 1). Data on native amphibians' physiological responses (stress and fear index, electronic supplementary material, table S1) were omitted due to the low number of studies with such data.
Table 1.
General and specific response variables extracted from studies, describing ecological impacts of alien species on native amphibians.
| general response variable category | specific response variable |
|---|---|
| diversity/abundance | richness |
| number of individuals | |
| density | |
| catch per unit effort | |
| fitness/performance | survival |
| mortality (−)a | |
| sexual activity | |
| reproductive/hatching success | |
| growth/mass | growth rate |
| mass (dry weight) | |
| size | |
| volume | |
| biomass | |
| development | developmental stage [24,25] |
| time to hatching | |
| time to metamorphosis | |
| behaviour (activity) | activity level |
| feeding activity | |
| exposure to impacting species | |
| behaviour (avoidance) | avoidance behaviour |
| refuge use | |
| repulsion | |
| morphology (body) | body measurements (length, width, depth) |
| limb measurements (femur, tibiofibula, foot) | |
| morphology (tail) | pigmentation |
| tail muscle and fin measurements (length, depth) |
aThe sign of this trait's effect size was reversed because of the opposite meaning of this variable to the others and so that responses in the same general category were all in the same direction.
The following data were also extracted from each article:
-
(1)
Taxonomic group of the alien species. Alien species were categorized as plants, invertebrates, fishes, amphibians, reptiles, or mammals. We did not include studies involving alien pathogens, as it is often difficult to ascertain their native/alien status in a specific location and due to the low number of studies found with appropriate data. Studies or treatments that reported combined effects of multiple alien species simultaneously were not considered.
-
(2)
Characteristics of the native amphibian species. The taxonomy (following [26]), IUCN Red List status, and amphibian development stage (eggs, larvae, metamorphs, and adults) of native amphibians were recorded.
-
(3)
Type of study. Studies were categorized as either observational, if they consisted of field surveys, or as experimental, if they reported field or in situ, mesocosm, or laboratory experiments. Observational and experimental studies might differ in their methodology and in the variance of the response variables, although it has been shown that differences in data variation between these two types of studies are usually minor and unlikely to affect the outcomes of the meta-analysis [27,28].
-
(4)
Type of control. Native amphibian responses to alien species were compared to either no species (blank control) or an impacting native species (e.g. native predatory fish species).
Relevant data for calculating effect sizes were extracted from each study either directly from the results text or tables (16% of the cases), from graphs using the software DataThief (http://datathief.org/) (76% of the cases), or by contacting and requesting data from corresponding authors (8% of the cases).
One study can provide multiple observation pairs for the meta-analysis if independent experiments are conducted using different species, or if a single experiment measures the effect of an alien species on multiple amphibian species or on different response variables. As such, when a study reported data for different impacting alien species, different native amphibian species, different control types (no species versus native impacting species), or different response variables (e.g. growth, behaviour), each of these was considered a different case, as has been done in many other meta-analysis studies (e.g. [23,28]). In cases where pseudoreplication could be a concern, we took specific actions to ensure independence (see electronic supplementary material, Appendix S2 for details).
Our final dataset included information from 110 studies (electronic supplementary material, table S2), from which 1062 cases evaluating the impact of alien species on native amphibians were extracted (electronic supplementary material, table S1).
(c). Effect size calculation
Effect sizes for the ecological responses of native amphibians to alien species were calculated in relation to each type of control: a blank, no species control, or a control with a native impacting species. Hedges’ d [29], a metric commonly used to measure effect sizes in ecological meta-analyses (e.g. [28,30]) due to a low Type I error rate and high within-study precision [31], was used here.
Hedges’ d calculates effect size as the standardized mean difference between treatment and control groups, including a weighting factor to correct for small sample sizes [29]. Hedges' d was calculated as:
where XI corresponds to the mean of the invaded treatment group, XC the mean of the control group (blank or native species), S corresponds to the pooled standard deviation, and J the weighting factor, which is calculated based on the sample sizes of the treatment and control groups. S was calculated as
where NI and NC correspond to the sample sizes of the invaded treatment and control groups, respectively and, similarly, SI and SC correspond to the standard deviations for the invaded treatment and control groups. J, the weighting factor, was calculated as
The variance of Hedges’ d was calculated as
Large values of Hedges' d are generated by large differences and low variability between the invaded and uninvaded (control) treatments. A negative value of Hedges’ d indicates that the value of the examined response variable is lower in the invaded than in the uninvaded treatment, whereas a positive Hedges' d value indicates an increase in the response variable in the invaded in relation to the uninvaded treatment. It is important to note that, in our analysis, negative numerical values of Hedges’ d do not always reflect ecologically negative effects of alien species on native amphibians. For example, for the general response variable ‘behaviour’, activity level has been shown to often decrease when amphibians are exposed to predator species (e.g. [17]), hence a negative effect size is expected; however, avoidance behaviour or refuge use are expected to be higher in response to predators (e.g. [32]), in which case we would expect a resulting positive effect size. For this same reason, the general variable ‘morphology’ was subdivided into two different subcategories (body and tail). Similarly, for the response variable ‘fitness/performance’, the sign of the effect size calculated for studies documenting the proportion/number of consumed or killed native amphibians (mortality) was reversed (table 1), in order to ensure that all the specific response variables within a general category were predicted to have an effect size with the same sign, i.e. a response in the same direction.
(d). Data analysis
All statistical analyses were performed using the ‘metafor’ package (v. 1.9–9 [33]) in the R software environment (v. 3.5.1; R Core Team 2018). Data were analysed using multi-level random effects models (rma.mv function). Random effects models are useful because they assume that heterogeneity in effect sizes occurs not only due to sampling error, but also due to random sources of variation, as is the case in ecological datasets.
Meta-regression random effects models were run separately for each response variable category and type of control (native impacting species or no species). They were further run separately by (1) native amphibian development stage (eggs/larvae and metamorphs/adults) and (2) alien species taxonomic group (plants, invertebrates, fishes, amphibians, reptiles, and mammals). To further control for potential pseudoreplication among effect sizes, type of study (observational or experimental), nested within publication ID, was included as a random effect in every model [33], and models were only run for datasets having at least three or more effect sizes. Nevertheless, to encourage caution in interpreting results from a small number of effect sizes or publications, we explicitly present both sample size and number of unique publications used for each model (figures 1, 2, and S4).
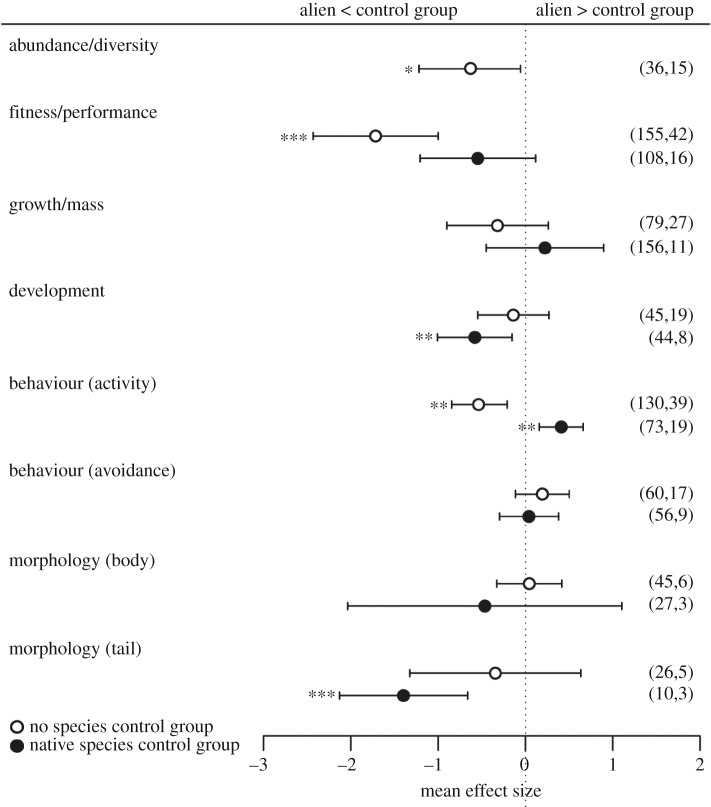
Figure 1.
Effect sizes of response variables describing ecological impacts of alien species on native amphibians, considering different control types (no species or native species). Error bars represent 95% confidence intervals (CI) and effects are considered significant when CIs do not overlap zero. Sample size and number of publications are shown in parentheses. *p < 0.05, **p ≤ 0.01, ***p ≤ 0.001.
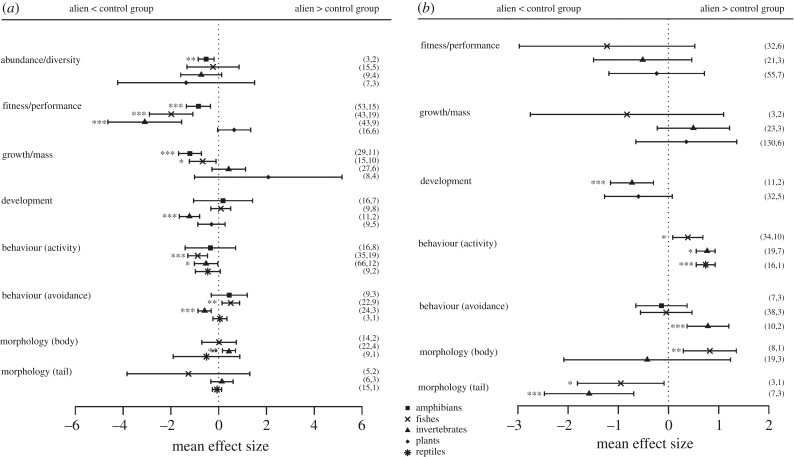
Figure 2.
Effect sizes of response variables describing ecological impacts of various taxonomic groups of alien species (amphibians, fishes, invertebrates, plants, reptiles) on native amphibians, considering (a) no species or (b) a native species as a control. Error bars represent 95% confidence intervals (CI) and effects are considered significant when CIs do not overlap zero. Sample size and number of publications are shown in parentheses. Sample size was too small for the taxonomic group ‘Mammals’ to be included. *p < 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Bias-corrected 95% bootstrap confidence intervals (CI) of effect sizes were calculated for each individual response variable category, in order to test if effect size estimates were significantly different from zero. An effect size is considered statistically significant if its CI do not overlap with zero [34]. Large CIs indicate a large amount of unexplained variance, while small CIs usually indicate small variance, hence, similar effect sizes across different studies.
(e). Heterogeneity, publication bias, and sensitivity analysis
To examine the amount of residual heterogeneity of effect sizes, i.e. whether variance among effect sizes was significantly larger than would be expected from sampling error alone [34], Q statistics were calculated for each of the meta-regression models.
Because funnel plots have been shown to be unreliable indicators of publication bias (the tendency of journals to publish studies with significant results [35]), Egger's regression test was used here [36]. This test examines whether the y-intercept in a linear regression between normalized effect size (effect size/SE) and precision (1/SE) is different from zero [36]. When this y-intercept differs significantly from zero, the relationship between effect size and the precision of studies is considered asymmetrical and therefore biased [37]. To test for this, the variance of effect sizes was included as a moderator in our models in the initial analysis. Alpha = 0.10 was used for this test [36]. Furthermore, publication bias was also tested by calculating Rosenberg's fail-safe number [38], which indicates the number of studies that would need to be added to the meta-analysis to change its results from significant to non-significant. If the fail-safe number is larger than 5N + 10, in which N represents the total number of cases in the dataset, the analysis is considered robust.
Sensitivity of all meta-regression models was examined by fitting models with and without influential outliers. Hat values and standardized residuals were examined for each model, and effect sizes with hat values greater than two times the average hat value (i.e. influential) and with standardized residual values exceeding 3.0 (outliers), were removed, following [39].
3. Results
The majority of studies used in our analysis were experimental (88.2%, N = 97 articles); only 13.6% of the articles included field surveys (N = 15 articles). Most studies were performed in North America (58%) and all continents were represented, except Africa (electronic supplementary material, figure S1). Most studies were performed in temperate climates (88.7%), while the tropics were poorly represented (11.3%, with 5.1% coming from the Australian tropics). The dataset included a total of 53 alien species, largely represented by fishes, followed by plants, invertebrates, amphibians, reptiles, and finally mammals (electronic supplementary material, table S3). The alien species most used in studies were Oncorynchus mykiss (rainbow trout) for fishes, Phragmites australis (common reed) for plants, and Procambarus clarkii (red swamp crayfish) for invertebrates (electronic supplementary material, figure S2). Among the 107 native amphibian species investigated (electronic supplementary material, table S4), most of them were from three families: Hylidae, Ranidae, and Bufonidae, with North American Anaxyrus americanus (American toad, N = 114 cases), Pseudacris regilla (Pacific tree frog, N = 46 cases), and European Bufo bufo (common toad, N = 33 cases) being the most commonly studied species (electronic supplementary material, figure S3). No studies were found reporting the effects of alien species on caecilian amphibians (Gymnophiona). Out of 1062 cases, fitness/performance was the general response variable most frequently represented (N = 263 cases), followed by growth/mass (N = 235 cases), and behaviour (activity) (N = 203 cases).
(a). Effects of alien species
(i). Ecological response variables
Amphibian diversity/abundance, fitness/performance, and behaviour (activity) were significantly affected by alien species, compared to a blank control (no species) (table 2). In the presence of alien species, amphibian diversity and abundance were reduced, and their fitness and activity levels were significantly lower (figure 1). When compared to native impacting species, alien species only significantly affected amphibian behaviour (activity) and development (table 2 and figure 1). Amphibian activity was significantly higher in the presence of alien species than of native impacting species. On the other hand, amphibian development time was significantly shorter in the presence of alien than of native species (figure 1).
Table 2.
Meta-regression models of the impacts of alien species on native amphibians, considering different control types (no species or impacting native species). Significant differences (p < 0.05) are highlighted in italics.
| control type | response variable | mean effect | 95% CI | p | heterogeneity statistics | random variables (σ) |
|---|---|---|---|---|---|---|
| no species | diversity/abundance | −0.64 | −1.22, −0.06 | 0.03 | Q = 110.31, d.f. = 35, p < 0.0001 | ID = 0.55, ID(Type) = 0.55 |
| fitness/performance | −1.72 | −2.44, −1.00 | <0.001 | Q = 1237.27, d.f. = 154, p < 0.0001 | ID = 2.51, ID(Type) = 2.51 | |
| growth/mass | −0.32 | −0.90, 0.26 | 0.28 | Q = 457.81, d.f. = 78, p < 0.0001 | ID = 1.03, ID(Type) = 1.03 | |
| development | −0.14 | −0.55, 0.27 | 0.50 | Q = 239.59, d.f. = 44, p < 0.0001 | ID = 0.28, ID(Type) = 0.28 | |
| behaviour (activity) | −0.53 | −0.85, −0.21 | 0.001 | Q = 544.30, d.f. = 129, p < 0.0001 | ID = 0.44, ID(Type) = 0.44 | |
| behaviour (avoidance) | 0.19 | −0.11, 0.50 | 0.22 | Q = 221.38, d.f. = 59, p < 0.0001 | ID = 0.16, ID(Type) = 0.16 | |
| morphology (body) | 0.05 | −0.33, 0.42 | 0.81 | Q = 128.06, d.f. = 44, p < 0.0001 | ID = 0, ID(Type) = 0.19 | |
| morphology (tail) | −0.34 | −1.32, 0.63 | 0.49 | Q = 129.71, d.f. = 25, p < 0.0001 | ID = 1.13, ID(Type) = 0 | |
| native species | fitness/performance | −0.54 | −1.21, 0.12 | 0.11 | Q = 830.79, d.f. = 107, p < 0.0001 | ID = 0.83, ID(Type) = 0.83 |
| growth/mass | 0.22 | −0.45, 0.90 | 0.51 | Q = 972.05, d.f. = 155, p < 0.0001 | ID = 0.59, ID(Type) = 0.59 | |
| development | −0.58 | −1.01, −0.15 | 0.01 | Q = 212.08, d.f. = 43, p < 0.0001 | ID = 0.13, ID(Type) = 0.13 | |
| behaviour (activity) | 0.41 | 0.16, 0.66 | 0.001 | Q = 214.55, d.f. = 72, p < 0.0001 | ID = 0.11, ID(Type) = 0.11 | |
| behaviour (avoidance) | 0.04 | −0.30, 0.38 | 0.81 | Q = 213.93, d.f. = 55, p < 0.0001 | ID = 0.10, ID(Type) = 0.10 | |
| morphology (body) | −0.46 | −2.04, 1.11 | 0.56 | Q = 205.51, d.f. = 26, p < 0.0001 | ID = 0.93, ID(Type) = 0.93 | |
| morphology (tail) | −1.40 | −2.13, −0.66 | 0.0002 | Q = 28.27, d.f. = 9, p = 0.001 | ID = 0.14, ID(Type) = 0.14 |
(ii). Development stages
The magnitude and direction of alien species effects on eggs and larvae were extremely similar to those observed for the dataset with all development stages together (figure 1 and electronic supplementary material, table S5 and figure S4). However, there was a significant reduction in larval tail measurements in the presence of alien compared with native impacting species (electronic supplementary material, figure S4), indicating that tails of amphibian larvae are longer and/or deeper when exposed to native than to alien species.
Alien species significantly affected behavioural avoidance and body morphology of amphibian metamorphs and adults (electronic supplementary material, table S5 and figure S4). Adult amphibians showed greater avoidance of alien species and developed longer limbs or bulkier bodies, regardless of control type. Even though the latter results are derived from one single study (electronic supplementary material, figure S4), it is important to note that this study refers to the effect of two different taxa of alien species on a native amphibian species [40].
(iii). Taxonomic identity of alien species
Different taxonomic groups of alien species differed in their effects on native amphibians, relative to blank controls. Mean effect sizes showed that alien plants appeared to significantly induce higher amphibian fitness (electronic supplementary material, table S6, figure 2a). Conversely, in the presence of alien invertebrates, native amphibians had significantly decreased fitness, shorter development times (only two studies, both performed on the impacts of an invasive crayfish, Procambarus clarkii, on the community of native amphibians in Portugal [19,41]), and reduced activity and avoidance behaviour. Alien invertebrates also induced longer bodies in native amphibians, although this was based on a single study looking at impacts of Gambusia holbrooki (among other predators) on larvae of Pelodytes punctatus in Spain [40] (electronic supplementary material, table S6, figure 2a). The presence of exotic fish species caused a large reduction in amphibians' fitness, as well as significantly lower growth, behavioural activity, and greater avoidance behaviour (electronic supplementary material, table S6, figure 2a). In the presence of alien amphibians, native amphibian abundance and diversity, fitness, and growth were significantly lower. For abundance and diversity, this was based on two studies, one on the impact of Xenopus laevis on the native amphibian community in Sicily [42] and another on the effects of a Lithobates catesbeianus invasion on native frog communities in China [43]. There was no evidence of significant effects of alien reptiles on native amphibians, when compared with blank controls (electronic supplementary material, table S6, figure 2a).
Regarding the impacts of different taxonomic groups of alien species relative to those of native impacting species, results are somewhat limited by small sample sizes for some response variables and should be interpreted with caution (electronic supplementary material, table S6, figure 2b). However, native amphibians exhibited faster development (the same two studies mentioned above performed in Portugal [19,41]), higher activity, higher avoidance behaviours (only [32,41]), and shorter tails in the presence of alien invertebrates than of native impacting species. A higher amphibian activity was also observed in the presence of alien fishes and reptiles (only one study in the case of reptiles, concerning the impact of an invasive turtle species, Trachemys scripta elegans, on a native anuran community in Spain [21]), indicating that activity was generally lower in the presence of native compared with alien impacting species. Amphibians also developed significantly bulkier bodies and shorter tails when exposed to alien fishes compared to native impacting species, although this was based on the single study looking at impacts of Gambusia holbrooki mentioned earlier [40] (figure 2b).
(b). Heterogeneity, publication bias, and sensitivity analysis
A significant amount of heterogeneity was detected in many of the meta-regression models (table 1; electronic supplementary material, tables S5 and S6), suggesting that there was a considerable amount of variance not accounted for by the models.
Publication bias using Egger's test, which was tested for the initial random effects models (considered in table 1), was found for some response variables in the ‘no species control’ dataset: fitness/performance (p < 0.0001), development (p = 0.0012), avoidance behaviour (p < 0.0001), and tail morphology (p = 0.0015). For the ‘native species control’ dataset, publication bias was only found for body morphology (p = 0.0012). Egger's test was not significant for any other variable (all tests p > 0.1), indicating that there was only mild publication bias in this study, unlikely to influence our general results. Rosenberg's fail-safe number, which was estimated to be 21 368 reinforces this result, given that it is much larger than 5N + 10 = 5320.
Regarding sensitivity analysis, no influential outliers were found.
4. Discussion
Our study is the first meta-analysis to explore global trends of ecological impacts of a wide range of taxonomic groups of alien species (plants, invertebrates, fishes, amphibians, reptiles, and mammals) on native amphibians. We found that alien species have significant effects on native amphibians, usually related to a reduction in fitness/performance components, and that invertebrates had impacts on the most aspects of native amphibian ecology. This significant decrease in fitness may be an indirect consequence of a weaker behavioural defensive response shown by native amphibians towards alien than native impacting species, probably in turn a consequence of a lack of, or short, coevolutionary history, resulting in increased prey naiveté and reducing the development of adaptive prey responses.
Amphibian fitness and performance were significantly reduced in the presence of alien species compared with a blank control, suggesting a strong negative effect of alien species on components such as survival, and reproductive or hatching success, of native amphibians. Other response variables that significantly and consistently decreased in the presence of alien species relative to a blank control were diversity and/or abundance measures, namely richness, number of individuals, and density. Similarly, a previous meta-analysis on the overall effects of invasive species on aquatic ecosystems found that aquatic invaders consistently decreased the abundance and diversity of aquatic communities [28]. Our study demonstrates that this trend of reduced diversity found by Gallardo et al. [28] considering fish, invertebrates, plankton, and macrophytes, also appears to hold for native amphibians in aquatic ecosystems. However, it is important to note that such strong responses were not observed when the effects of alien species were compared to those in the presence of a native species control, indicating that native impacting species also exert strong effects on native amphibians.
The studies used in our analysis suggest different mechanisms responsible for the alterations in ecological response variables caused by the presence of alien species. The most commonly suggested mechanism was predation, followed by habitat alteration, competition, and toxicity. This is not surprising, given that predation is a major selective force influencing the dynamics, structure, and evolutionary processes of prey communities [44] and that previous studies have highlighted predation [7,10] and competition [11] as important impact mechanisms of alien species on native amphibians.
Predators affect dynamics of prey populations directly via consumption and indirectly by imposing non-lethal effects upon them [20,45]. In fact, non-lethal predatory effects, such as alterations in behaviour, morphology, or life-history, have been suggested to exert similar or stronger effects than direct consumption on the dynamics of prey populations, and this seems to be particularly evident in aquatic ecosystems [45,46]. In this study, the presence of alien species caused a significant reduction in behavioural activity level, which is one of the most commonly reported behavioural defensive strategies in amphibians, known to decrease prey detectability and increase survival odds (e.g. [47,48]). However, this was only observed when compared with a blank control; activity levels of native amphibians exposed to alien species were higher than when exposed to a native impacting species. This indicates that, although native amphibians decrease activity levels when exposed to alien species, they do it to a lesser extent than when exposed to native impacting species. This probably reflects a lack of, or a short, coevolutionary history between alien and native species, which results in a high degree of prey naiveté and weaker defence responses. When alien species invade new areas, native species might not be able to detect or identify these novel species as a dangerous threat, resulting in a lack of, or the development of weak or inappropriate, defence responses [14,20]. This naiveté effect has long been known and is expressed through prey behaviour, morphology, or life-history traits [20]. Prey naiveté is likely to increase prey mortality and severely impact invaded populations (e.g. [21]). Results of this study indicate that prey naiveté and a consequent weak behavioural response could be one of the causes of the strong decrease in fitness and performance, as well as in diversity and abundance, of native amphibians exposed to alien species.
Another possible example of prey naiveté in our study is related to changes in tail morphology of amphibian larvae. Native amphibians developed shorter tails in the presence of alien than native impacting species (although not against a blank control). In the presence of native predators, morphological defences typically consist of developing deeper, longer, and more pigmented tails, which lure predator strikes away from the vulnerable body and enable larvae to generate faster swimming bursts and improve manoeuvrability, all of which are adaptive responses that increase survival under predation (e.g. [49,50]). As such, a reduction in effect size of tail measurements of native amphibians towards alien predators, when compared to native impacting species, probably indicates a very strong response towards the latter and a lack of response towards the former, or even the development of a maladaptive morphological response towards alien species.
On the contrary, results of this meta-analysis showed that native amphibians developed more quickly in the presence of alien species than of native impacting species. A reduction in development time can be a direct response to predation risk, allowing prey to leave risky predacious environments earlier, thereby reducing mortality risk [16,18,51]. This might indicate that alterations in development as responses to impacting species require low energetic expenditure and non-specific predator recognition. However, it more likely means that the very marked reduction in amphibian activity level generally observed in the presence of native impacting species, is having a negative impact on amphibian development time. Indeed, behaviourally defended prey often allocate fewer resources into growth and development, resulting in delayed development [51].
Our results highlight the importance of examining different types of controls in meta-analysis studies. The previous examples show how the extent and direction of the impact of alien species can differ between tests using a blank control and a native impacting species control. These results also have particular importance for researchers designing experimental studies aiming to evaluate the impacts of alien species on native amphibians (and other taxonomic groups), with respect to the choice of control types. The type of control used will affect interpretation of the results. For example, out of the 40 studies that we scored for behavioural activity, only 18 used a native and a blank control; the others only used a blank control (except study [3] that used only a native control). Our results demonstrate the importance of using both types of control in experimental investigations on the effects of alien species on native amphibians to assist with more accurate interpretation of the ecological implications of biological invasions.
The effects of alien species on freshwater larval stages of native amphibians were much stronger than those towards terrestrial adult amphibians, and largely reflected the general trends found for amphibians overall. The effects of alien species on terrestrial life-history stages were generally weak or variable, likely reflecting the very small number of studies published examining the impacts of alien species on native amphibian adults or metamorphs. This is probably because of the increased difficulty of capturing and keeping adult amphibians in the laboratory, especially in long-term experiments. Alternatively, mortality rates are generally much higher in larvae than adult anurans, perhaps causing effects of alien species to be much less pronounced in the latter [52]. Regardless, these weaker impacts are not surprising, taking into account that biological invasions have been shown to have greater impacts in freshwater than terrestrial ecosystems [14,15].
We found that the magnitude and direction of alien species effects on native amphibians differed among alien taxa. A decrease in native amphibian fitness appeared to be a consistent outcome caused by alien amphibians, fishes, and invertebrates. However, alien amphibians were the only group to cause a significant decrease in native amphibian abundance/diversity, while invertebrates were the only taxon causing a decrease in development time and triggering the development of larger body sizes. Alien reptiles and plants seemed to have a weaker impact on native amphibians but, once again, this may reflect the low number of studies examining the quantitative effects of these alien taxonomic groups on amphibians and highlights the pressing need for more research in this area. Interestingly, our results suggest a tendency for alien plants to induce positive effects on amphibian fitness/performance, i.e. increased fitness. This may be related to specific alien plants providing better breeding habitats and oviposition sites, as well as effective refuges from predators to native amphibians (e.g. [53]). This positive association shows that some introduced plants may be beneficial for some amphibian species and is a result that certainly deserves further investigation.
The question of which alien taxonomic group has the strongest impacts on native amphibians is important for the management of threatened amphibian taxa. Here we show that, out of all the taxa examined, alien invertebrates had the most consistently significant negative effects on native amphibians, inferred by causing the largest reduction in amphibian fitness and inducing significant changes in the highest number of ecological traits. This is surprising as, while most reviews have considered effects of invertebrates, alien fish are usually described as having the strongest impacts on amphibians [7,10,11]. Importantly, although studies analysed here only included eight invertebrate species (electronic supplementary material, table S3), this taxon represented the highest number of individual cases examined in our dataset (318 cases out of 1062). Interestingly, most of these derived from studies on two crayfish species, the red swamp crayfish, Procambarus clarkii, and the signal crayfish, Pacifastacus leniusculus, with the former having higher representation. Procambarus clarkii is a crayfish species native to Mexico and the USA that, due to its commercial value and its success as an invader, is now present in all continents except Australia and Antarctica, making it the most cosmopolitan crayfish species in the world [54]. This species has proven to be an extremely successful invader, often exerting wide environmental impacts and affecting the structure and functioning of invaded aquatic ecosystems [55]. Other than being an effective predator of amphibian larvae, P. clarkii can impact amphibians by inducing alterations in larval behaviour, morphology, and life-history, by inflicting serious injury to amphibian prey, by decreasing habitat complexity (refuges and spawning sites), by deteriorating water quality, and by causing the displacement of amphibian populations from their natural breeding habitats [19,32,56,57]. It is notable that very few studies comparing the relative effects of different taxonomic groups on the environment have been made on alien invertebrates and this study suggests that these, and in particular crayfish, may have a very high impact on the environment and would be of particular interest to assess. Nevertheless, a global meta-analysis on alien crayfish impacts suggests consistent and negative effects among introduced crayfish species [22], reinforcing the results found here.
Understanding the impacts that alien species that establish in a new environment can have on native populations is a complex endeavour. Our study highlights the strong and diverse impacts that different alien taxonomic groups have on native amphibians. Understanding the complexity of these impacts is fundamental for outlining priority management and conservation actions needed to preserve native amphibian biodiversity. Given the pivotal roles of amphibians in the functioning of ecosystems, and the alarming number of threatened species in this group, we hope this global synthesis will help highlight the strong impacts that alien species can have on native amphibians and warrant critical attention to this issue.
Supplementary Material
Acknowledgements
We are greatly indebted to Erin Jooste for helping us with data gathering and processing.
Data accessibility
The dataset supporting this article has been uploaded as part of the electronic supplementary material. Additional data available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.b6f4n81 [58].
Authors' contributions
A.L.N. conceived and coordinated the study. All authors participated in the design of the study and extracted data from numerous studies. J.M.F. carried out the statistical analyses. A.L.N. and J.M. drafted the manuscript. All authors revised the manuscript and approved for publication.
Competing interests
The authors declare that they have no competing interests.
Funding
All authors thank the DST-NRF Centre of Excellence for Invasion Biology for financial support. This study was also funded by the South African National Department of Environment Affairs through the South African National Biodiversity Institute.
References
- 1.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fishman DL, Waller RW. 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786. ( 10.1126/science.1103538) [DOI] [PubMed] [Google Scholar]
- 2.Hoffman M, et al. 2010. The impact of conservation on the status of the world's vertebrates. Science 330, 1503–1509. ( 10.1126/science.1194442) [DOI] [PubMed] [Google Scholar]
- 3.Whittaker K, Koo MS, Wake DB, Vredenburg VT. 2013. Global declines of amphibians. In Encyclopedia of biodiversity, 2nd edn, vol. 3 (ed. Levin SA.), pp. 691–699. Waltham, MA: Academic Press. [Google Scholar]
- 4.Alford RA, Richards SJ. 1999. Global amphibian declines: a problem in applied ecology. Annu. Rev. Ecol. Evol. Syst. 30, 133–165. ( 10.1146/annurev.ecolsys.30.1.133) [DOI] [Google Scholar]
- 5.Collins JP, Storfer A. 2003. Global amphibian declines: sorting the hypotheses. Divers. Distrib. 9, 89–98. ( 10.1046/j.1472-4642.2003.00012.x) [DOI] [Google Scholar]
- 6.Blaustein AR, Han BA, Relyea RA, Johnson PTJ, Buck JC, Gervasi SS, Kats LB. 2011. The complexity of amphibian population declines: understanding the role of cofactors in driving amphibian losses. Ann. NY Acad. Sci. 1223, 108–119. ( 10.1111/j.1749-6632.2010.05909.x) [DOI] [PubMed] [Google Scholar]
- 7.Kats LB, Ferrer RP. 2003. Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers. Distrib. 9, 99–110. ( 10.1046/j.1472-4642.2003.00013.x) [DOI] [Google Scholar]
- 8.Bellard C, Cassey P, Blackburn TM. 2016. Alien species as a driver of recent extinctions. Biol. Lett. 12, 20150623 ( 10.1098/rsbl.2015.0623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellard C, Genovesi P, Jeschke JM. 2016. Global patterns in threats to vertebrates by biological invasions. Proc. R. Soc. B 283, 20152454 ( 10.1098/rspb.2015.2454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilliod DS, Griffiths RA, Kuzmin SL. 2012. Ecological impacts of non-native species. In Conservation and decline of amphibians: ecological aspects, effect of humans, and management (eds Heatwole H, Wilkinson JW), vol. 10, pp. 3343–3382. Chipping Norton, NSW: Surrey Beatty & Sons. [Google Scholar]
- 11.Bucciarelli GM, Blaustein AR, Garcia TS, Kats LB. 2014. Invasion complexities: the diverse impacts of nonnative species on amphibians. Copeia 2014, 611–632. ( 10.1643/OT-14-014) [DOI] [Google Scholar]
- 12.IUCN. 2018. The IUCN Red List of Threatened Species. Version 2018–1. http://www.iucnredlist.org (Retrieved on 05 July 2018).
- 13.Seebens H, et al. 2017. No saturation in the accumulation of alien species worldwide. Nat. Commun. 8, 14435 ( 10.1038/ncomms14435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox JG, Lima SL. 2006. Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends Ecol. Evol. 21, 674–680. ( 10.1016/j.tree.2006.07.011) [DOI] [PubMed] [Google Scholar]
- 15.Gherardi F. 2007. Biological invaders in inland waters: profiles, distribution, and threats, p. 734 Dordrecht, The Netherlands: Springer. [Google Scholar]
- 16.Benard MF. 2004. Predator-induced phenotypic plasticity in organisms with complex life cycles. Annu. Rev. Ecol. Evol. Syst. 35, 651–673. ( 10.1146/annurev.ecolsys.35.021004.112426) [DOI] [Google Scholar]
- 17.Relyea RA. 2001. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82, 523–540. ( 10.1890/0012-9658(2001)082[0523:MABPOL]2.0.CO;2) [DOI] [Google Scholar]
- 18.Nicieza AG, Álvarez DA, Atienza EMS. 2006. Delayed effects of larval predation risk and food quality on anuran juvenile performance. J. Evol. Biol. 19, 1092–1103. ( 10.1111/j.1420-9101.2006.01100.x) [DOI] [PubMed] [Google Scholar]
- 19.Nunes AL, Orizaola G, Laurila A, Rebelo R. 2014. Morphological and life-history responses of anurans to predation by an invasive crayfish: an integrative approach. Ecol. Evol. 4, 1491–1503. ( 10.1002/ece3.979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR. 2010. Predator-prey naiveté, antipredator behavior, and the ecology of predator invasions. Oikos 119, 610–621. ( 10.1111/j.1600-0706.2009.18039.x) [DOI] [Google Scholar]
- 21.Polo-Cavia N, Gonzalo A, Lopez P, Martin J. 2010. Predator recognition of native but not invasive turtle predators by naive anuran tadpoles. Anim. Behav. 80, 461–466. ( 10.1016/j.anbehav.2010.06.004) [DOI] [Google Scholar]
- 22.Twardochleb LA, Olden JD, Larson ER. 2013. A global meta-analysis of the ecological impacts of nonnative crayfish. Freshw Sci. 32, 1367–1382. ( 10.1899/12-203.1) [DOI] [Google Scholar]
- 23.Vilà M, et al. 2011. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 14, 702–708. ( 10.1111/j.1461-0248.2011.01628.x) [DOI] [PubMed] [Google Scholar]
- 24.Gosner KL. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190. [Google Scholar]
- 25.Donavan LA. 1980. Morphological features of the stages in the development of Ambystoma talpoideum (Holbrook) from the fertilized egg to the adult. PhD Dissertation, University of Southern Mississippi, Hattiesburg, MS, USA. [Google Scholar]
- 26.Frost DR. 2018. Amphibian species of the world: an online reference. Version 6.0. New York, NY: American Museum of Natural History; (http://research.amnh.org/herpetology/amphibia/index.html) [Google Scholar]
- 27.Hillebrand H, Gurevitch J. 2014. Meta-analysis results are unlikely to be biased by differences in variance and replication between ecological lab and field studies. Oikos 123, 794–799. ( 10.1111/oik.01288) [DOI] [Google Scholar]
- 28.Gallardo B, Clavero M, Sánchez MI, Vilà M. 2016. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Change Biol. 22, 151–163. ( 10.1111/gcb.13004) [DOI] [PubMed] [Google Scholar]
- 29.Gurevitch J, Curtis PS, Jones MH. 2001. Meta-analysis in ecology. Adv. Ecol. Res. 32, 199–247. ( 10.1016/S0065-2504(01)32013-5) [DOI] [Google Scholar]
- 30.van Kleunen M, Weber E, Fischer M. 2010. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 13, 235–245. ( 10.1111/j.1461-0248.2009.01418.x) [DOI] [PubMed] [Google Scholar]
- 31.Lajeunesse MJ, Forbes MR. 2003. Variable reporting and quantitative reviews: a comparison of three meta-analytical techniques. Ecol. Lett. 6, 448–454. ( 10.1046/j.1461-0248.2003.00448.x) [DOI] [Google Scholar]
- 32.Nunes AL, Richter-Boix A, Laurila A, Rebelo R. 2013. Do anuran larvae respond behaviourally to chemical cues from an invasive crayfish predator? A community-wide study. Oecologia 171, 115–127. ( 10.1007/s00442-012-2389-6) [DOI] [PubMed] [Google Scholar]
- 33.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 34.Adams DC, Gurevitch J, Rosenberg MS. 1997. Resampling tests for meta-analysis of ecological data. Ecology 78, 1277–1283. ( 10.1890/0012-9658(1997)078[1277:RTFMAO]2.0.CO;2) [DOI] [Google Scholar]
- 35.Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. 2006. The case of the misleading funnel plot. Br. Med. J. 333, 597–600. ( 10.1136/bmj.333.7568.597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egger M, Smith GD, Schneider M, Minder C. 1997. Bias in meta-analysis detected by a simple, graphical test. Br. Med J. 315, 629–634. ( 10.1136/bmj.315.7109.629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sterne JAC, Egger M. 2005. Regression methods to detect publication and other bias in meta-analysis. In Publication bias in meta-analysis: prevention, assessment and adjustments (eds Rothstein HR, Sutton AJ, Borenstein M). Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- 38.Rosenberg MS. 2005. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution 59, 464–468. ( 10.1111/j.0014-3820.2005.tb01004.x) [DOI] [PubMed] [Google Scholar]
- 39.Habeck CW, Schultz AK. 2015. Community-level impacts of white-tailed deer on understorey plants in North American forests: a meta-analysis. AoB Plants 7, plv119 ( 10.1093/aobpla/plv119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pujol-Buxó E, San Sebastián O, Garriga N, Llorente GA. 2013. How does the invasive/native nature of species influence tadpoles' plastic responses to predators? Oikos 122, 19–29. ( 10.1111/j.1600-0706.2012.20617.x) [DOI] [Google Scholar]
- 41.Almeida E, Nunes A, Andrade P, Alves S, Guerreiro C, Rebelo R. 2011. Antipredator responses of two anurans towards native and exotic predators. Amph-Rept. 32, 341–350. ( 10.1163/017353711X579849) [DOI] [Google Scholar]
- 42.Lillo F, Faraone FP, Valvo ML. 2011. Can the introduction of Xenopus laevis affect native amphibian populations? Reduction of reproductive occurrence in presence of the invasive species. Biol. Inv. 13, 1533–1541. ( 10.1007/s10530-010-9911-8) [DOI] [Google Scholar]
- 43.Li Y, Ke Z, Wang Y, Blackburn TM. 2011. Frog community responses to recent American bullfrog invasions. Curr. Zool. 57, 83–92. ( 10.1093/czoolo/57.1.83) [DOI] [Google Scholar]
- 44.Johnson MTJ, Agrawal AA. 2003. The ecological play of predator-prey dynamics in an evolutionary theatre. Trends Ecol. Evol. 18, 549–551. ( 10.1016/j.tree.2003.09.001) [DOI] [Google Scholar]
- 45.Preisser EL, Bolnick DI, Benard MF. 2005. Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 86, 501–509. ( 10.1890/04-0719) [DOI] [Google Scholar]
- 46.Werner EE, Peacor SD. 2006. Lethal and nonlethal predator effects on an herbivore guild mediated by system productivity. Ecology 87, 347–361. ( 10.1890/05-0091) [DOI] [PubMed] [Google Scholar]
- 47.Van Buskirk J. 2002. A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in anuran larvae. Am. Nat. 160, 87–102. ( 10.1086/340599) [DOI] [PubMed] [Google Scholar]
- 48.Laurila A, Lindgren B, Laugen AT. 2008. Antipredator defenses along a latitudinal gradient in Rana temporaria. Ecology 89, 1399–1413. ( 10.1890/07-1521.1) [DOI] [PubMed] [Google Scholar]
- 49.Van Buskirk J, Anderwald P, Lüpold S, Reinhardt L, Schuler H. 2003. The lure effect, tadpole tail shape, and the target of dragonfly strikes. J. Herpetol. 37, 420–424. ( 10.1670/0022-1511(2003)037[0420:TLETTS]2.0.CO;2) [DOI] [Google Scholar]
- 50.Dayton GH, Saenz D, Baum KA, Langerhans RB, DeWitt TJ. 2005. Body shape, burst speed and escape behavior of larval anurans. Oikos 111, 582–591. ( 10.1111/j.1600-0706.2005.14340.x) [DOI] [Google Scholar]
- 51.Higginson AD, Ruxton GD. 2010. Adaptive changes in size and age at metamorphosis can qualitatively vary with predator type and available defenses. Ecology 91, 2756–2768. ( 10.1890/08-2269.1) [DOI] [PubMed] [Google Scholar]
- 52.Licht LE. 1974. Survival of embryos, tadpoles, and adults of the frogs Rana aurora aurora and Rana pretiosa pretiosa sympatric in southwestern British Columbia. Can. J. Zool. 52, 613–627. ( 10.1139/z74-079) [DOI] [PubMed] [Google Scholar]
- 53.Holzer KA, Lawler SP. 2015. Introduced reed canary grass attracts and supports a common native amphibian. J. Wildlife Manage. 79, 1081–1090. ( 10.1002/jwmg.930) [DOI] [Google Scholar]
- 54.Holdich DM, Reynolds JD, Souty-Grosset C, Sibley PJ. 2009. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Manag. Aquat. Ecol. 11, 394–395. ( 10.1051/kmae/2009025) [DOI] [Google Scholar]
- 55.Souty-Grosset C, Anastácio PM, Aquiloni L, Banha F, Choquer J, Chucholl C, Tricarico E. 2016. The red swamp crayfish Procambarus clarkii in Europe: impacts on aquatic ecosystems and human well-being. Limnologica 58, 78–93. ( 10.1016/j.limno.2016.03.003) [DOI] [Google Scholar]
- 56.Nunes AL, Cruz MJ, Tejedo M, Laurila L, Rebelo R. 2010. Nonlethal injury caused by an invasive alien predator and its consequences for an anuran tadpole. Basic Appl. Ecol. 11, 645–654. ( 10.1016/j.baae.2010.09.003) [DOI] [Google Scholar]
- 57.Cruz MJ, Rebelo R, Crespo EG. 2006. Effects of an introduced crayfish, Procambarus clarkii, on the distribution of south-western Iberian amphibians in their breeding habitats. Ecography 29, 329–338. ( 10.1111/j.2006.0906-7590.04333.x) [DOI] [Google Scholar]
- 58.Nunes AL, Fill JM, Davies SJ, Louw M, Rebelo AD, Thorp CJ, Vimercati G, Measey J. 2019. Data from: A global meta-analysis of the ecological impacts of alien species on native amphibians Dryad Digital Repository. ( 10.5061/dryad.b6f4n81) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Nunes AL, Fill JM, Davies SJ, Louw M, Rebelo AD, Thorp CJ, Vimercati G, Measey J. 2019. Data from: A global meta-analysis of the ecological impacts of alien species on native amphibians Dryad Digital Repository. ( 10.5061/dryad.b6f4n81) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The dataset supporting this article has been uploaded as part of the electronic supplementary material. Additional data available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.b6f4n81 [58].