Abstract
Traits associated with mating and fertilization success are expected to senesce with age, but limited information is available on their relative rates of senescence. In polyandrous species, male reproductive fitness depends on both mating and fertilization success. Because successful mating is a prerequisite for post-copulatory sexual selection, ejaculate traits are expected to senesce faster than pre-copulatory traits, as pre-copulatory sexual selection is often deemed to be stronger than post-copulatory sexual selection. This pattern has generally been found in the few empirical studies conducted so far. We tested this prediction in the guppy (Poecilia reticulata), a livebearing fish characterized by intense sperm competition, by comparing the expression of male sexual traits at two ages (four and nine months). Contrary to prediction, we found that post-copulatory traits senesced at a significantly slower rate than pre-copulatory traits. We also looked at whether early investment in those sexual traits affects longevity, and the interaction between sperm age (duration of sperm storage inside the male) and male age. Our results suggest that the relative senescence rate of pre- and post-copulatory sexual traits may vary among species with different mating systems and ecology.
Keywords: mosaic ageing, lifespan, life history, sperm ageing, trade-offs, male fertility
1. Introduction
Senescence is usually defined as the decline in physiological function of an organism, accompanied by decreasing fertility and increasing mortality. Despite the growing interest in understanding the proximate and ultimate causes of senescence in reproductive performance, the majority of studies have focused on female fertility only, which is often assumed to be the limiting factor when it comes to producing viable offspring, overlooking the interplay between senescence and fertility in males (reviewed in [1]). Age-dependent patterns of reproductive investment in early adulthood are central to evolutionary theories of ageing which predict that the more a male invests into sexually selected traits and, generally, into reproduction, the sooner he will senesce [2]. The available experimental evidence is in line with this prediction. For example, in the cricket Teleogryllus commodus, males that invest more in sexual advertisement (calls) early in adulthood have a shorter lifespan [3]. Similarly, in the antler flies Protopiophila litigata, high mating rate is associated with a shorter lifespan [4]. Evolutionary theories suggest that the observed trade-off between investment in early reproduction and ageing rate is most likely owing to within-population variation in life-history strategies: individuals that invest more into reproduction early in life, do so at the expense of their somatic maintenance, resulting in the earlier onset of senescence and/or a faster senescence rate, ultimately resulting in a shorter lifespan (‘disposable soma’ theory, [5]). For example, a recent study in which the early condition of neriid flies Telostylinus angusticollis was experimentally manipulated, revealed that high-condition males developed fast with an earlier reproductive peak, but experienced faster reproductive senescence and a shorter lifespan [6].
To date, studies investigating the interplay between reproduction and ageing in males have focused mostly on investment in secondary sexual traits associated with mating acquisition, such as ornaments, armaments or courtship. By contrast, the role of sperm production and germline maintenance in determining the rate of ageing has only recently started to be recognized [7]. In monogamous species the variance in male reproductive success depends primarily on mate acquisition (pre-copulatory sexual selection), but in polyandrous species competitive fertilization success (post-copulatory sexual selection) can explain much of the variance in male reproductive success [8]. Since traits associated with access to mates and competitive fertilization success are costly, a trade-off between ejaculate production and pre-copulatory traits is predicted [9]. Therefore, for polyandrous species, evolutionary ageing theories centred on trade-offs between somatic and reproductive investment in males should account for a more complex scenario in which investment in reproduction includes both pre- and post-copulatory traits. Evidence is growing that ejaculate quality is often compromised in older males (e.g. [10–14]), which may have negative consequences for offspring fitness (but see [14–17]), but it is still unclear how pre- and post-copulatory traits senesce relative to each other. Studies of covariation between pre-and post-copulatory traits are increasing, and there is an increasing awareness of the role of other factors, such as ecological and mating systems conditions, in influencing the balance between pre- and post-copulatory investment [8,9]. Even so, the effect of age on the direction and strength of this covariation has been overlooked by theoreticians and empiricists [1]. This is unfortunate as the relative fitness return of allocating energy to pre- or post-copulatory traits is likely to be age-dependent, shaping a different and asynchronous pattern of senescence of traits in response to different selection pressures.
Another factor which is likely to be age-dependent is the rate of sperm senescence during sperm storage. ‘Post-meiotic’ sperm senescence (sensu [18]), occurs when mature sperm cells are stored by the male before release. Sperm storage (whose duration depends on a male's mating rate), can lead to post-meiotic sperm senescence which has well known negative consequences for a male's fertilization success ([19,20], but see [21]) and offspring fitness (reviewed in [18,22]). Since sperm senescence is driven mainly by oxidative stress during storage within the male, post-meiotic patterns of senescence are expected to follow those predicted at the organismal level, with sperm senescence being exacerbated by increased oxidative stress at the organismal level and therefore expected to be more pronounced in older males [18,22]. In support of this, in Brown Norway rats, sperm from old males are more susceptible to oxidative stress than sperm from younger males [23], whereas in the jungle fowl older males sperm show higher DNA damage possibly owing to a reduced level of antioxidants in seminal fluid [24].
Here, we use a longitudinal approach to provide a comprehensive investigation into the relative ageing of pre- and post-copulatory traits, and between organismal (male) and post-meiotic sperm ageing, in a freshwater fish, the guppy (Poecilia reticulata). Although laborious, a longitudinal approach is necessary to account for selective mortality, to separate within- and between-individual effects of age, and to unravel individual life-history strategies [25]. We assessed traits associated with pre- and post-copulatory success in individual males at two different ages and we recorded their lifespan. Furthermore, we assessed sperm quality at two different post-meiotic ages for both young and old males, by experimentally controlling the duration of male sperm storage [20]. Our design takes advantage of the extensive previous knowledge of male traits involved in pre- and post-copulatory selection and their genetic architecture in guppies. Males exhibit different colour spots (namely, orange and iridescent) that attract females, with more colourful males being more successful [26–28]. Among these ornaments, the area and brightness of orange spots are particularly important in mate attraction ([28–30], for specific studies in the population used here see [31]) probably owing to their association with carotenoid availability [32,33]. Moreover, male colour area at sexual maturation has high heritability [34–36]. Males vary in their ejaculate quality, with large genetic variation among males for both sperm number [37,38] and swimming velocity [35], traits that are positively associated with fertilization success [39].
In our study, we first examined how the expression of traits associated with pre- and post-copulatory reproductive success change with male age and relative to each other. Second, we test how the post-meiotic age of sperm cells interacts with male age to affect sperm performance. Third, we investigated whether reproductive investment in early adulthood (pre- and post-copulatory traits) is associated with lifespan.
2. Methods
(a). Fish maintenance
Fish used for this experiment were descendants of wild guppies captured from the Tacarigua River in Trinidad. Water temperature was maintained at 26 ± 1°C and illumination was provided with a 12 L : 12 D cycle. All fish were fed a mix of Artemia salina nauplii and commercial dry food (Duplarin). To obtain experimental males of known age reared under the same developmental conditions, more than 50 gravid females from several different tanks were isolated from the stock population, and up to three babies from each female born within a period of 4 days were collected and moved to a larger tank. The age of experimental fish is therefore known ±2 days. Juveniles were maintained under the same conditions as the laboratory stock until sexual maturity (three months). Upon sexual maturation males were isolated in 3 l tanks in light- and temperature-controlled conditions in a recirculating water system (Tecniplast). This system guaranteed the same water quality (e.g. temperature and pH) across all tanks. Every second week throughout the duration of the experiment, males were provided with one non-virgin female for 7 days. This enabled us to standardize mating opportunities and social history among males and, at the same time, to naturally stimulate sperm production (without females males tend to slow down sperm production, [40,41]).
(b). Overview of the experimental design
A total of 68 males were assessed for body size, pre- and post-copulatory traits when four months old (‘young’) and nine months old (‘old’), and lifespan. These selected ages effectively reflect young and old males for this species, as male life expectancy rarely exceed one year of age in the wild or in laboratory conditions [10,42–44]. At each age, sperm traits were assessed as fresh (recently produced, 3 days) or stored (12 days). Tanks were checked daily throughout the experiment and any death recorded to estimate lifespan (±24 h).
(c). Male body size and coloration
Each male was photographed to measure coloration (pre-copulatory reproductive investment) and body size (see the electronic supplementary material for details). Body size (standard length, SL) and the area of coloured spots were measured from digital photographs using ImageJ software (http://rsbweb.nih.gov/ij/download.html), while brightness of orange spots was estimated on colour spectra obtained using the software ColourWorker (http://colourworker.com/index.html).
(d). Ejaculate analysis
Sperm number and sperm swimming velocity were measured for each male at two sperm ages following standard procedures (see [19]). At each age, sperm were assessed fresh and after a period of storage inside the male. Thus, males were initially stripped of ejaculate to empty their sperm reserves (sperm discarded) and then sperm sampled at 3 days (‘fresh’) and at 12 days (‘stored’) after initial stripping (for detailed methods see [19,20]). During this period males were not given any access to females, and the order in which the two tests (3 or 12 days) were performed was randomized (i.e. in half of the males the fresh sperm assay was performed first and in the other half the stored sperm was performed first). Briefly, each male was anaesthetized in a water bath with the anaesthetic MS-222 and placed on a slide under a dissecting microscope with a drop of saline solution (0.9% NaCl). Ejaculate was obtained by applying a gentle pressure to the side of the fish abdomen. Sperm number was estimated following the method described in [41] and sperm velocity (curvilinear sperm velocity, VCL, µm s−1) was assessed using a computer-assisted sperm analysis (CEROS, Hamilton-Thorne Research, Beverly, MA, USA) following previous protocols (for details see [20]). Sperm velocity measurements were based on an average of 121.1 ± 3.9 s.e. sperm tracks per sample.
(e). Statistical analyses
All analyses were performed in R, version 3.3.2 [45]. Means are presented ± standard error (s.e.). The significance of fixed effects in linear mixed models was calculated from F statistics with the ‘lmerTest’ package and Satterthwaite's approximation to calculate the denominator degrees of freedom. The distribution of residuals from the models was checked to ensure model assumptions were met. In the male age × sperm age analysis, values for fresh and stored sperm assays were used, while in the other analyses, to avoid collinearity (see correlation matrix in the electronic supplementary material, table S1), only one measure (average between fresh and stored) was used for sperm velocity and number.
(i). Male age × sperm age
To analyse how sperm number and sperm velocity were affected by male age and sperm age, we carried out a linear mixed model that included male age (young or old), sperm cell age (fresh or stored), and their interaction. Male identity (ID) was included as random factor to account for the multiple data collected from the same male.
(ii). Relative senescence in pre- and post-copulatory traits
To compare traits with different scales we calculate the relative senescence in pre- and post-copulatory traits as the percentage difference between the trait measured at nine months (old males) and that measured at four months (young). We then conducted one sample t-tests for each variable to test whether the decline in pre- and post-copulatory traits differed significantly from zero. Senescence in sperm production and sperm velocity was calculated as stored minus fresh sperm value (sperm cell senescence), and the difference in old minus young male value (male senescence). The difference in the overall decline of pre- versus post-copulatory traits was tested using a linear mixed model, where pre- or post-copulatory was included as fixed effect, and trait and male ID as random factors.
(iii). Longevity and reproductive investment
The effect of early investment in reproductive traits on lifespan was analysed with a model selection approach using the ‘glmulti’ package. Standardized factors (values minus the mean and then divided by the standard deviation) included in the possible models were: body size (SL), pre-copulatory traits (area of orange and iridescent and orange brightness), and post-copulatory traits (sperm velocity and number), all measured at young age. All possible models were ranked based on Akaike's information criterion modified for small sample sizes (AICc), and the best models (within 2 units) are reported along with their Akaike weight. The model-averaged parameter estimates (‘unconditional’) are reported for each factor. These estimates provide an indication of the importance of each predictor, and it is equal to the sum of the weights/probabilities for the models in which it appears.
3. Results
(a). Male age × sperm cell age
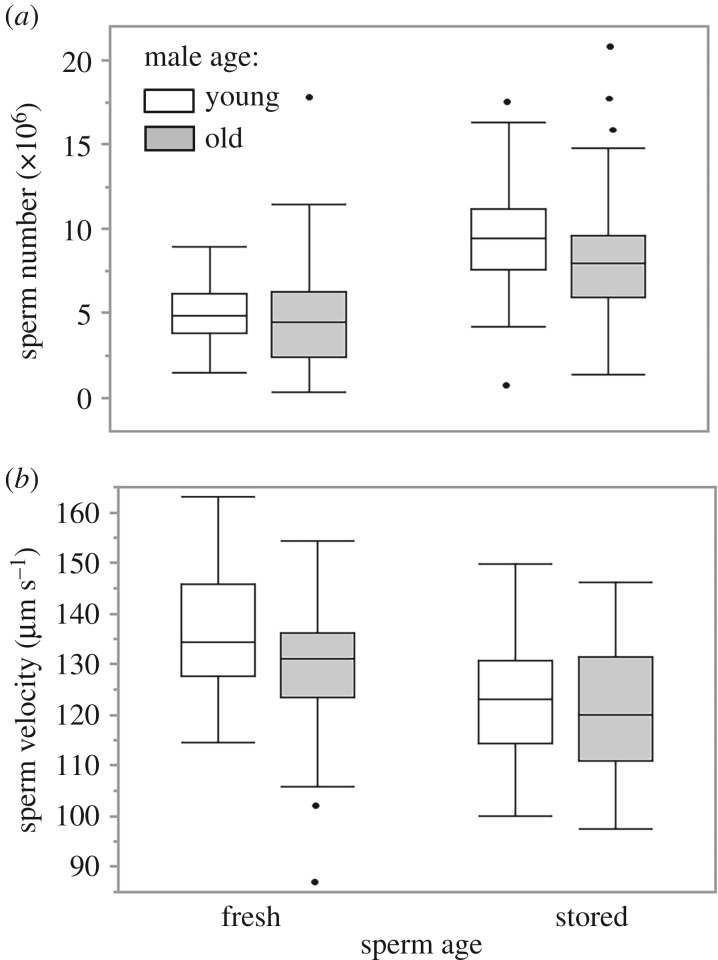
Sperm production and velocity were negatively affected by both male age and sperm age (table 1). There was a trend (p = 0.052) for fresh, but not stored, sperm to swim more slowly in old than young males (figure 1). The mean decline of each trait considered and corresponding t-tests are reported in the electronic supplementary material, table S2.
Table 1.
Results from mixed model investigating the effect of male and sperm age on sperm number and sperm swimming velocity. (Young male and fresh sperm are reference groups for the estimates. Significant p-values are reported in italics.)
| sperm number |
sperm velocity |
|||||
|---|---|---|---|---|---|---|
| estimate (s.e.) | F | p | estimate (s.e.) | F | p | |
| male age | −0.3279 (0.492) | 6.162 | 0.014 | −7.778 (2.207) | 9.221 | 0.003 |
| sperm age | 4.6650 (0.423) | 163.918 | <0.001 | −13.890 (1.933) | 49.460 | <0.001 |
| male age × sperm age | −1.0790 (0.678) | 2.519 | 0.115 | 6.053 (3.089) | 3.839 | 0.052 |
Figure 1.
Sperm number (a) and velocity (b) at different sperm age (fresh and stored) and male age (young and old).
(b). Relative senescence in pre- and post-copulatory traits
On average, the pattern of senescence was more marked in pre-copulatory traits than post-copulatory traits (figure 2). Specifically, the relative decline was significantly different from zero in all pre-copulatory traits (p < 0.001 for all traits). By contrast, for post-copulatory traits (sperm velocity and sperm number, averaged between fresh and stored sperm values) the relative decline was not significantly different from zero (p = 0.081 and 0.067, respectively, see also the electronic supplementary material, table S2). The relative decline for pre-copulatory traits was on average four times larger than the decline in post-copulatory traits (23% versus 6%, respectively), with this difference being highly significant (F = 13.758, p < 0.001). Effect sizes (Cohen's d, [46]) of the difference between old and young trait values are reported in the electronic supplementary material, table S3, and are depicted in figure 2b.
Figure 2.
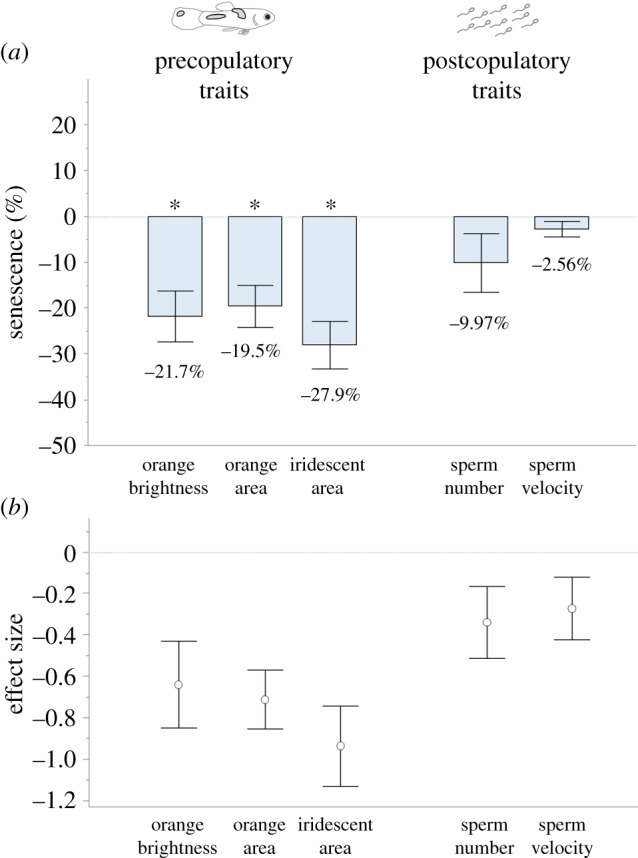
(a) Relative decline of pre- and post-copulatory traits, expressed as percentages. Asterisks indicate that the change between an old and young age is significantly different from zero. The average senescence of pre-copulatory traits was on average four times larger than the senescence in post-copulatory traits (see text). Numbers indicate the mean values of the decline between the two male ages (young and old); bars represent ± s.e. (b) Decline of pre- and post-copulatory traits, expressed as effect size (Cohen's d). (Online version in colour.)
(c). Longevity and reproductive investment
Our analysis on the association between reproductive trait expression at four months and longevity returned five models with an AICc value less than two units above that of the best model (table 2a). The relative importance of the predictors, considering all possible combinations, is reported in table 2b. A plot of the relative importance of the model terms across all possible models is reported in the electronic supplementary material, figure S1.
Table 2.
(a) Summary of the model selection statistics for models predicting lifespan in males. The best five models are reported. Models are ranked by increasing order of their AICc values, which is the bias-corrected modified Akaike information criterion. Wi is the Akaike weight of the i model. (b) The estimates and relative importance of the various predictors across all models in the candidate set.
| model no. | predictors | AICc | Wi | |
|---|---|---|---|---|
| (a) | ||||
| 1 | sperm number + orange area + iridescent area | 397.2066 | 0.11696737 | |
| 2 | orange area + iridescent area | 397.5386 | 0.09907569 | |
| 3 | sperm number + orange area | 397.7287 | 0.09008931 | |
| 4 | sperm number | 398.2882 | 0.06810509 | |
| 5 | body size (SL) + orange area + iridescent area | 398.9528 | 0.04885005 |
| trait | unconditional estimate | unconditional variance | numbers of models | importance |
|---|---|---|---|---|
| (b) | ||||
| orange area | −19.6977 | 281.4711 | 32 | 0.7142 |
| sperm number | 14.8834 | 239.1942 | 32 | 0.6152 |
| iridescent area | −15.4484 | 298.5260 | 32 | 0.5832 |
| body size (SL) | 1.9699 | 25.3205 | 32 | 0.2483 |
| sperm velocity | 1.4828 | 16.9268 | 32 | 0.2300 |
| brightness | −0.2232 | 9.4732 | 32 | 0.2164 |
| (intercept) | 390.6857 | 124.3882 | 64 | 1.0000 |
4. Discussion
Our longitudinal study on reproductive investment in male guppies revealed senescence in a number of pre- and post-copulatory traits as males aged. Differently from previous work on other species [11,47,48] however, our comparison of senescence rate between pre- and post-copulatory traits shows that the senescence was more pronounced in male pre-copulatory traits (ornaments) than in post-copulatory traits (sperm traits). Furthermore, lifespan was positively correlated with sperm production but negatively with orange coloration, suggesting different ageing trajectories associated with pre- and post-copulatory investment in early adulthood or different pattern of life-history trade-offs.
(a). Senescence of pre- and post-copulatory traits
We found evidence of senescence (within-individual decline of values with male age) for a number of pre- and the post-copulatory sexual traits. A previous study on another guppy population showed an increase in orange and iridescent coloration with age [43], while in our study the area (and brightness) of orange and iridescent spots was reduced in older males. These contrasting results may be owing to differences among populations in the strength of selection on pre- and post-copulatory traits. We also found a small increase in body size with age, in agreement with previous studies, showing that male guppies continue to grow throughout their life albeit at a very low rate [43,49]. Sperm quality declined with male age, confirming previous work [10], and in line with evidence from many taxa that older males produce lower quality sperm owing to senescence of the germline [14,17]. In our study, the number of sperm produced decreased with male age, suggesting a decrease in the amount of spermatogenic tissue and/or its efficiency in older males. This finding is, however, different to that of [10,49], which is most likely owing to the methods used. In the present study, we controlled for the number of days left to replenish sperm reserve and male mating history, while in earlier studies males were kept in large mixed groups. The increase in sperm count in old males reported by these studies [10,49] may therefore be explained by the lower mating rate of old males. A slower mating rate is expected to increase male sperm reserves [50], which is confirmed by the positive association between the number of sperm and the time since the last stripping (i.e. stored sperm > fresh sperm) that we found in the present study. Consistent with recent work, we also found that male sperm storage negatively affected sperm quality [19,20]. Together, these results highlight the importance of controlling mating rate and sperm age to disentangle pre- from post-meiotic effects in studying ageing of reproductive function. Sperm ageing is indeed seldom considered in ageing studies, despite evidence mounting that many post-copulatory traits are affected by ageing inside the male body across different taxa (e.g. [19,20,51,52]), and therefore urging researchers to control the length of sperm storage in ageing experiments [18,22].
(b). Male age × sperm cell age
Sperm reserves declined with male age, and, as expected, increased with the length of storage (the longer from the initial stripping, the more sperm accumulate). There was no significant interaction between sperm storage and male age. Sperm velocity declined with both male and sperm age, and despite the non-significant interaction between the two, the trend suggests that post-meiotic sperm senescence was more pronounced in young than old males (figure 1b). This trend may suggest a differential investment in stored sperm protection by old males, but a larger sample size is needed to confirm this result.
(c). Relative senescence between pre- and post-copulatory traits
Patterns of covariation between pre- and post-copulatory traits have been studied extensively [8,9,53]. However, covariation of these traits with age has seldom been considered, and even more rarely empirically measured [1]. Only few studies, as far as we know, have looked at this and both found that in birds, sperm quality declines more rapidly with age than traits associated with mate attraction [11,47,48]. In particular, Preston et al. [11] found that ‘showy’ males (that displayed more) continue displaying at higher rates at old age, when their sperm quality declines markedly. A similar pattern was shown in Drosophila melanogaster, in which mating success shows a strong decline when males are six weeks old, whereas sperm competitiveness is already reduced at four weeks age [48], and in the mite Rhizoglyphus robini, in which the negative effect of age was stronger for post-copulatory than for pre-copulatory success [12]. Interestingly, we found a similar pattern but in the opposite direction, with males continuing to invest highly in sperm traits at old age when their pre-copulatory traits decline markedly (but see [43] for a contrasting senescence pattern of precopulatory traits in a different guppy population). The rate of senescence we found in guppies markedly differed between pre- and post-copulatory traits, with a relative decline of pre-copulatory traits being, on average, four times larger than that of post-copulatory traits. One exception may be Caenorhabditis elegans, in which male mating success has been reported to decline with age more rapidly than sperm quality and number [54]. In the C. elegans mating system however, most of the individuals in natural populations are hermaphrodites and male mating success is high only when hermaphrodites are sperm-depleted [55], making it difficult to compare findings with gonochoristic species.
There are several non-mutually exclusive explanations for this pattern. We suggest that maintaining competitive ejaculates is more crucial for male guppy reproductive success than maintaining competitive attractive traits. This conclusion may seem counterintuitive, as the advantage of producing competitive ejaculates is only realized upon mating, but, as we will discuss, different fitness benefits associated with pre- and post-copulatory traits could explain the pattern we observed. As stressed by Lüpold et al. [56] when looking at patterns of (co)variance it is important to consider the marginal benefit associated with the relative investment into pre- and post-copulatory traits. In the guppy, both episodes of sexual selection are important [28]. Direct male–male competition before mating is probably less important and the acquisition of matings mainly depends on a male's relative attractiveness (size of colour spots) and courtship display rate [26]. Males are unable to monopolize females before or after mating, and under this scenario it is hypothesized that sexual selection should equally favour investment in pre- and post-copulatory traits [56]. However, the decline we observed was far more pronounced for pre-copulatory traits, suggesting that selection has instead favoured the evolution of greater investment in maintaining post-copulatory traits, possibly at the expense of maintenance of pre-copulatory traits. This pattern is likely to be adaptive for a male guppy, as males face very high levels of sperm competition in this species, with more than 95% multiple paternity in the wild [57]. Most importantly, females store sperm for months and stored sperm significantly contributes to a male's reproductive success [58] even well beyond his death [59]. The fertilization success of stored sperm is positively correlated with sperm velocity [60], indicating that a sustained investment in sperm quality may be advantageous also when precopulatory investment declines.
Another important factor that may contribute to explaining the observed pattern of senescence of male colour traits and sperm traits is the extrinsic mortality under which our study population has evolved. Lower Tacarigua fish are characterized by high predation levels [27], and fish from high predation populations show a strong decline in swimming performance with age [61], suggesting that old fish may be exposed to higher predation risk. Also, colourful males are prone to an increased predation risk compared to duller males [62,63]. Because of these two factors, in high predation populations a reduction in colour spots size or brightness as males age may be favoured. Regarding post-copulatory traits, ejaculate investment and predation risk tend to covary positively in natural populations [64], indirectly suggesting that ejaculate investment is unlikely to affect extrinsic mortality differently at different ages. If our hypothesis is true, the pattern of senescence of pre- and postcopulatory investment should differ among high and low predation populations (i.e. low predation populations should show a relatively slower senescence of colour spots).
It has to be noted that we measured the expression of sexual traits at different ages and not their effect directly on male reproductive success. Depending on the shape of the fitness function linking trait expression with reproductive success, the decline in the phenotypic expression of one sexual trait may or may not be associated with a proportional effect on reproductive fitness. For example, one may speculate that the relatively small age-related decline in ejaculate quality traits may have a large impact on male fertilization success, or that, on the other hand, the decline in male coloration has a limited effect on male mating success. For several reasons we think this is unlikely. The decline we observed in sperm number and velocity is expected to have a minor effect on a male's sperm competitive ability. Males transfer, on average, 0.5 million sperm per mating [65]. Based on the size of sperm reserves we estimated, a young male may perform on average 14 consecutive matings before running out of sperm, whereas an old male may perform 13 matings. The risk of sperm depletion is therefore very similar between the two ages and relevant only in extreme female biased conditions. This is a rare circumstance considering only 10% of the females in a population are sexually receptive at any time [26]. Sperm velocity can explain, when the full phenotypic variation is considered and sperm number is controlled for, about 10% of the variance in fertilization success [39]. In our sample the ranges of sperm velocity largely overlapped between male ages (108–150 µm s−1 in young males, and 95–154 µm s−1 in old males). The mean sperm velocity difference between young and old males was 4 µm s−1, less than 7% of the observed phenotypic range, a difference which is expected to account for less than 1% of the variance in postcopulatory success. On the other side, the decline in pre-copulatory traits (and in particular the 20% decline in orange area) is expected to have a relevant effect on male mating success, as orange coloration explains about 65% of the variance in female responsiveness to males (=willingness to mate) [66]. However, it is worth noting that a previous study showed female choice was not associated with male age once differences in male coloration among males were taken into account [10], or male size (that increases with age) (e.g. [29,65]). Also, males can probably partially compensate for the lower attractiveness with behavioural strategies, such as increasing courtship and/or selecting the social context in which to court [67], or circumventing female choice by forcing copulations [68]. A direct evaluation of a male's age on mating success in the guppy therefore requires to be confirmed by testing males of different ages in a competitive context [49].
Our results and those of previous studies, highlight the importance of considering different patterns of senescence in sexual traits, in particular between traits associated with mating attraction and ejaculate quality. Clearly, it will be useful to extend our data to include more time points (we measured two ages) to clarify how the senescence pattern changes with time. Sexual traits may show little signs of senescence for most of the male's adult life and subsequently rapidly decrease, or senescence may occur at a constant rate, or a combination of the two, as observed in females [61]. Independently of the pattern of senescence over time, our general (and unexpected) conclusions are important: older males show a stronger age-related reduction in the expression of pre-copulatory traits than in post-copulatory traits. While classical evolutionary theory of ageing predicts a uniform pattern of senescence among traits within populations [69], it seems clear, from both biomedical empirical research in humans and animals [1], that senescence rates are not uniform, but rather reflect a ‘mosaic ageing’ where the decline in different parts of the body affect different functions at different rates [70]. This idea can be applied to sexually selected traits and our findings underline the crucial role of considering both pre- and post-copulatory traits when studying reproductive investment [1].
(d). Longevity and reproductive investment
Despite the huge interest in senescence and its evolutionary consequences, theoretical and empirical research centred on the trade-off between reproduction and lifespan [7] has rarely included post-copulatory traits (but see [47]). In our study, we related early investment in both types of traits with lifespan, predicting that heavy early investment in reproductive traits should negatively impact lifespan. We found that the investment in pre-copulatory traits (namely, orange coloration and iridescent) is negatively associated with lifespan, in agreement with previous studies (e.g. [11,71]) suggesting a trade-off between these two components. Similarly, early investment in post-copulatory traits, has been found to be negatively associated with survival. In the fowl (Gallus gallus), for example, males with high sperm production had a shorter lifespan, suggesting a trade-off between sperm production and longevity [47]. However, in contrast with previous results we found that males producing more sperm lived longer. This may indicate that high sperm production is positively linked with other characteristics that enhance male survival, like, for example, swimming performance under strong condition-dependent selection (see [61]). However, this result is also in agreement with the hypothesis that showy males have a higher extrinsic mortality and may therefore be selected to invest less in somatic and ejaculate maintenance. Despite evidence that sexual selection affects male ageing and that polyandry is ubiquitous [72], integrating post-copulatory traits into ageing research is still in its infancy and theoretical models and empirical investigations are clearly needed. The few longitudinal studies in which the senescence rate of pre- and post-copulatory traits has been compared simultaneously [11,47] suggest that post-copulatory traits may show faster senescence. Our study is, to our knowledge, the first to highlight that the opposite pattern can also occur. Our results align with previous work indicating that asynchrony [69], in senescence rate among sexual traits may be the rule [11,73] and call for more research to understand why species differ so strikingly in the pattern of senescence of their sexual traits.
Supplementary Material
Acknowledgements
We thank Silvia Cattelan for her help with fish maintenance and Michael Jennions and Leigh Simmons for their useful comments on previous versions of the manuscript. We are also grateful to the associate editor and two anonymous reviewers for their useful comments.
Ethics
This research was conducted under the approval of the University of Padova's Animal Ethics Committee (approval no: 12/2014).
Data accessibility
Data supporting the results are available as part of the electronic supplementary material.
Authors' contribution
C.G. conceived the study with input from A.D. and A.P., C.G. and A.D. collected the data, C.G. and A.P. ran the analyses. All authors contributed substantially to the writing of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by a Marie Curie International Outgoing fellowship within the 7th European Community Framework Programme (grant no. 272613) to C.G., and by the University of Padova (grant no. CPDA120105) to A.P.
References
- 1.Lemaître JF, Gaillard JM. 2017. Reproductive senescence: new perspectives in the wild. Biol. Rev. 92, 2182–2199. ( 10.1111/brv.12328) [DOI] [PubMed] [Google Scholar]
- 2.Bonduriansky R, Maklakov A, Zajitschek F, Brooks R. 2008. Sexual selection, sexual conflict and the evolution of ageing and life span. Funct. Ecol. 22, 443–453. ( 10.1111/j.1365-2435.2008.01417.x) [DOI] [Google Scholar]
- 3.Hunt J, Brooks R, Jennions MD, Smith MJ, Bentsen CL, Bussiere LF. 2004. High-quality male field crickets invest heavily in sexual display but die young. Nature 432, 1024–1027. ( 10.1038/nature03084) [DOI] [PubMed] [Google Scholar]
- 4.Bonduriansky R, Brassil CE. 2005. Reproductive ageing and sexual selection on male body size in a wild population of antler flies (Protopiophila litigata). J. Evol. Biol. 18, 1332–1340. ( 10.1111/j.1420-9101.2005.00957.x) [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood TB. 1977. Evolution of ageing. Nature 270, 301–304. ( 10.1038/270301a0) [DOI] [PubMed] [Google Scholar]
- 6.Hooper AK, Spagopoulou F, Wylde Z, Maklakov AA, Bonduriansky R. 2017. Ontogenetic timing as a condition-dependent life history trait: high-condition males develop quickly, peak early and age fast. Evolution 71, 671–685. ( 10.1111/evo.13172) [DOI] [PubMed] [Google Scholar]
- 7.Maklakov AA, Immler S. 2016. The expensive germline and the evolution of ageing. Curr. Biol. 26, R577–R586. ( 10.1016/j.cub.2016.04.012) [DOI] [PubMed] [Google Scholar]
- 8.Evans JP, Garcia-Gonzalez F. 2016. The total opportunity for sexual selection and the integration of pre-and post-mating episodes of sexual selection in a complex world. J. Evol. Biol. 29, 2338–2361. ( 10.1111/jeb.12960) [DOI] [PubMed] [Google Scholar]
- 9.Simmons LW, Lüpold S, Fitzpatrick JL. 2017. Evolutionary trade-off between secondary sexual traits and ejaculates. Trends Ecol. Evol. 32, 964–976. ( 10.1016/j.tree.2017.09.011) [DOI] [PubMed] [Google Scholar]
- 10.Gasparini C, Marino IAM, Boschetto C, Pilastro A. 2010. Effect of male age on sperm traits and sperm competition success in the guppy (Poecilia reticulata). J. Evol. Biol. 23, 124–135. ( 10.1111/j.1420-9101.2009.01889.x) [DOI] [PubMed] [Google Scholar]
- 11.Preston BT, Saint Jalme M, Hingrat Y, Lacroix F, Sorci G. 2011. Sexually extravagant males age more rapidly. Ecol. Lett. 14, 1017–1024. ( 10.1111/j.1461-0248.2011.01668.x) [DOI] [PubMed] [Google Scholar]
- 12.Radwan J, Michalczyk L, Prokop Z. 2005. Age dependence of male mating ability and sperm competition success in the bulb mite. Anim. Behav. 69, 1101–1105. ( 10.1016/j.anbehav.2004.09.006) [DOI] [Google Scholar]
- 13.Jones TM, Featherston R, Paris DBBP, Elgar MA. 2006. Age-related sperm transfer and sperm competitive ability in the male hide beetle. Behav. Ecol. 18, 251–258. ( 10.1093/beheco/arl077) [DOI] [Google Scholar]
- 14.Johnson SL, Zellhuber-McMillan S, Gillum J, Dunleavy J, Evans JP, Nakagawa S, Gemmell NJ. 2018. Evidence that fertility trades off with early offspring fitness as males age. Proc. R. Soc. B 285, 20172174 ( 10.1098/rspb.2017.2174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidd SA, Eskenazi B, Wyrobek AJ. 2001. Effects of male age on semen quality and fertility: a review of the literature. Fertil. Steril. 75, 237–248. ( 10.1016/S0015-0282(00)01679-4) [DOI] [PubMed] [Google Scholar]
- 16.Radwan J. 2003. Male age, germline mutations and the benefits of polyandry. Ecol. Lett. 6, 581–586. ( 10.1046/j.1461-0248.2003.00484.x) [DOI] [Google Scholar]
- 17.Johnson SL, Gemmell NJ. 2012. Are old males still good males and can females tell the difference? Bioessays 34, 609–619. ( 10.1002/bies.201100157) [DOI] [PubMed] [Google Scholar]
- 18.Pizzari T, Dean R, Pacey A, Moore H, Bonsall MB. 2008. The evolutionary ecology of pre- and post-meiotic sperm senescence. Trends Ecol. Evol. 23, 131–140. ( 10.1016/j.tree.2007.12.003) [DOI] [PubMed] [Google Scholar]
- 19.Gasparini C, Dosselli R, Evans JP. 2017. Sperm storage by males causes changes in sperm phenotype and influences the reproductive fitness of males and their sons. Evol. Lett. 1, 16–25. ( 10.1002/evl3.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasparini C, Kelley JL, Evans JP. 2014. Male sperm storage compromises sperm motility in guppies. Biol. Lett. 10, 20140681 ( 10.1098/rsbl.2014.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Firman R, Young F, Rowe D, Duong H, Gasparini C. 2015. Sexual rest and post-meiotic sperm ageing in house mice. J. Evol. Biol. 28, 1373–1382. ( 10.1111/jeb.12661) [DOI] [PubMed] [Google Scholar]
- 22.Reinhardt K. 2007. Evolutionary consequences of sperm cell aging. Q. Rev. Biol. 82, 375–393. ( 10.1086/522811) [DOI] [PubMed] [Google Scholar]
- 23.Zubkova EV, Robaire B. 2006. Effects of ageing on spermatozoal chromatin and its sensitivity to in vivo and in vitro oxidative challenge in the brown Norway rat. Hum. Reprod. 21, 2901–2910. ( 10.1093/humrep/del193) [DOI] [PubMed] [Google Scholar]
- 24.Noguera JC, Dean R, Isaksson C, Velando A, Pizzari T. 2012. Age-specific oxidative status and the expression of pre-and postcopulatory sexually selected traits in male red junglefowl, Gallus gallus. Ecol. Evol. 2, 2155–2167. ( 10.1002/ece3.300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clutton-Brock T, Sheldon BC. 2010. Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 25, 562–573. ( 10.1016/j.tree.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 26.Houde AE. 1997. Sex, color and mate choice in guppies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 27.Magurran AE. 2005. Evolutionary ecology: the Trinidadian guppy. Oxford, UK: Oxford University Press. [Google Scholar]
- 28.Devigili A, Evans JP, Di Nisio A, Pilastro A. 2015. Multivariate selection drives concordant patterns of pre-and postcopulatory sexual selection in a livebearing fish. Nat. Commun. 6, 8291 ( 10.1038/ncomms9291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kodric-Brown A. 1993. Female choice of multiple male criteria in guppies: interacting effects of dominance, coloration and courtship. Behav. Ecol. Sociobiol. 32, 415–420. ( 10.1007/BF00168825) [DOI] [Google Scholar]
- 30.Brooks R, Endler JA. 2001. Female guppies agree to differ: phenotypic and genetic variation in mate-choice behavior and the consequences for sexual selection. Evolution 55, 1644–1655. ( 10.1111/j.0014-3820.2001.tb00684.x) [DOI] [PubMed] [Google Scholar]
- 31.Evans JP, Bisazza A, Pilastro A. 2004. Female mating preferences for colourful males in a population of guppies subject to high predation. J. Fish Biol. 65, 1154–1159. ( 10.1111/j.0022-1112.2004.00502.x) [DOI] [Google Scholar]
- 32.Endler JA. 1980. Natural selection on color patterns in Poecilia reticulata. Evolution 34, 76–91. ( 10.1111/j.1558-5646.1980.tb04790.x) [DOI] [PubMed] [Google Scholar]
- 33.Kodric-Brown A. 1989. Dietary carotenoids and male mating success in the guppy: an environmental component to female choice. Behav. Ecol. Sociobiol. 25, 393–401. ( 10.1007/BF00300185) [DOI] [Google Scholar]
- 34.Postma E, Spyrou N, Rollins LA, Brooks RC. 2011. Sex-dependent selection differentially shapes genetic variation on and off the guppy Y chromosome. Evolution 65, 2145–2156. ( 10.1111/j.1558-5646.2011.01314.x) [DOI] [PubMed] [Google Scholar]
- 35.Evans JP. 2010. Quantitative genetic evidence that males trade attractiveness for ejaculate quality in guppies. Proc. R. Soc. B 277, 3195–3201. ( 10.1098/rspb.2010.0826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houde AE. 1992. Sex-linked heritability of a sexually selected character in a natural population of Poecilia reticulata (Pisces: Poeciliidae) (guppies). Heredity 69, 229–235. ( 10.1038/hdy.1992.120) [DOI] [Google Scholar]
- 37.Gasparini C, Devigili A, Dosselli R, Pilastro A. 2013. Pattern of inbreeding depression, condition dependence, and additive genetic variance in Trinidadian guppy ejaculate traits. Ecol. Evol. 3, 4940–4953. ( 10.1002/ece3.870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cattelan S, Nisio AD, Pilastro A. 2018. Stabilizing selection on sperm number revealed by artificial selection and experimental evolution. Evolution 72, 698–706. ( 10.1111/evo.13425) [DOI] [PubMed] [Google Scholar]
- 39.Boschetto C, Gasparini C, Pilastro A. 2011. Sperm number and velocity affect sperm competition success in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 65, 813–821. ( 10.1007/s00265-010-1085-y) [DOI] [Google Scholar]
- 40.Bozynski CC, Liley NR. 2003. The effect of female presence on spermiation, and of male sexual activity on ‘ready’ sperm in the male guppy. Anim. Behav. 65, 53–58. ( 10.1006/anbe.2002.2024) [DOI] [Google Scholar]
- 41.Cattelan S, Evans JP, Pilastro A, Gasparini C. 2016. The effect of sperm production and mate availability on patterns of alternative mating tactics in the guppy. Anim. Behav. 112, 105–110. ( 10.1016/j.anbehav.2015.11.024) [DOI] [Google Scholar]
- 42.Olendorf R, Rodd FH, Punzalan D, Houde AE, Hurt C, Reznick DN, Hughes KA. 2006. Frequency-dependent survival in natural guppy populations. Nature 441, 633–636. ( 10.1038/nature04646) [DOI] [PubMed] [Google Scholar]
- 43.Miller LK, Brooks R. 2005. The effects of genotype, age, and social environment on male ornamentation, mating behavior, and attractiveness. Evolution 59, 2414–2425. ( 10.1111/j.0014-3820.2005.tb00951.x) [DOI] [PubMed] [Google Scholar]
- 44.Magris M, Chimetto G, Rizzi S, Pilastro A. 2018. Quick-change artists: male guppies pay no cost to repeatedly adjust their sexual strategies. Behav. Ecol. 29, 1113–1123. ( 10.1093/beheco/ary087) [DOI] [Google Scholar]
- 45.R Development Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 46.Cohen J. 1988. Statistical power analysis for the behavioral sciences, 2nd edn Hillsdale, NJ: Lawrence Earlbaum Associates. [Google Scholar]
- 47.Cornwallis CK, Dean R, Pizzari T. 2014. Sex-specific patterns of aging in sexual ornaments and gametes. Am. Nat. 184, E66–E78. ( 10.1086/677385) [DOI] [PubMed] [Google Scholar]
- 48.Koppik M, Fricke C. 2017. Gene expression changes in male accessory glands during ageing are accompanied by reproductive decline in Drosophila melanogaster. Mol. Ecol. 26, 6704–6716. ( 10.1111/mec.14384) [DOI] [PubMed] [Google Scholar]
- 49.Devigili A, Doldán-Martelli V, Pilastro A. 2015. Exploring simultaneous allocation to mating effort, sperm production, and body growth in male guppies. Behav. Ecol. 26, 1203–1211. ( 10.1093/beheco/arv067) [DOI] [Google Scholar]
- 50.Evans JP, Magurran AE. 1999. Male mating behaviour and sperm production characteristics under varying sperm competition risk in guppies. Anim. Behav. 58, 1001–1006. ( 10.1006/anbe.1999.1212) [DOI] [PubMed] [Google Scholar]
- 51.Froman DP, Bernier PE. 1987. Identification of heritable spermatozoal degeneration within the ductus deferens of the chicken (Gallus domesticus). Biol. Reprod. 37, 969–977. ( 10.1095/biolreprod37.4.969) [DOI] [PubMed] [Google Scholar]
- 52.Tarin JJ, Perez-Albala S, Cano A. 2000. Consequences on offspring of abnormal function in ageing gametes. Hum. Reprod. Update 6, 532–549. ( 10.1093/humupd/6.6.532) [DOI] [PubMed] [Google Scholar]
- 53.Andersson M, Simmons LW. 2006. Sexual selection and mate choice. Trends Ecol. Evol. 21, 296–302. ( 10.1016/j.tree.2006.03.015) [DOI] [PubMed] [Google Scholar]
- 54.Chatterjee I, et al. 2013. Dramatic fertility decline in aging C. elegans males is associated with mating execution deficits rather than diminished sperm quality. Exp. Gerontol. 48, 1156–1166. ( 10.1016/j.exger.2013.07.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleemann GA, Basolo AL. 2007. Facultative decrease in mating resistance in hermaphroditic Caenorhabditis elegans with self-sperm depletion. Anim. Behav. 74, 1339–1347. ( 10.1016/j.anbehav.2007.02.031) [DOI] [Google Scholar]
- 56.Lüpold S, Tomkins JL, Simmons LW, Fitzpatrick JL. 2014. Female monopolization mediates the relationship between pre-and postcopulatory sexual traits. Nat. Commun. 5, 3184 ( 10.1038/ncomms4184) [DOI] [PubMed] [Google Scholar]
- 57.Neff BD, Pitcher TE, Ramnarine IW. 2008. Inter-population variation in multiple paternity and reproductive skew in the guppy. Mol. Ecol. 17, 2975–2984. ( 10.1111/j.1365-294X.2008.03816.x) [DOI] [PubMed] [Google Scholar]
- 58.Gasparini C, Evans JP. 2018. Female control over multiple matings increases the opportunity for postcopulatory sexual selection. Proc. R. Soc. B 285, 20181505 ( 10.1098/rspb.2018.1505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.López-Sepulcre A, Gordon SP, Paterson IG, Bentzen P, Reznick DN. 2013. Beyond lifetime reproductive success: the posthumous reproductive dynamics of male Trinidadian guppies. Proc. R. Soc. B 280, 20131116 ( 10.1098/rspb.2013.1116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Devigili A, Di Nisio A, Grapputo A, Pilastro A. 2016. Directional postcopulatory sexual selection is associated with female sperm storage in Trinidadian guppies. Evolution 70, 1829–1843. ( 10.1111/evo.12989) [DOI] [PubMed] [Google Scholar]
- 61.Reznick DN, Bryant MJ, Roff D, Ghalambor CK, Ghalambor DE. 2004. Effect of extrinsic mortality on the evolution of senescence in guppies. Nature 431, 1095–1099. ( 10.1038/nature02936) [DOI] [PubMed] [Google Scholar]
- 62.Millar NP, Reznick DN, Kinnison MT, Hendry AP. 2006. Disentangling the selective factors that act on male colour in wild guppies. Oikos 113, 1–12. ( 10.1111/j.0030-1299.2006.14038.x) [DOI] [Google Scholar]
- 63.Gordon SP, Reznick D, Arendt JD, Roughton A, Ontiveros Hernandez MN, Bentzen P, López-Sepulcre A. 2015. Selection analysis on the rapid evolution of a secondary sexual trait. Proc. R. Soc. B 282, 20151244 ( 10.1098/rspb.2015.1244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elgee KE, Evans JP, Ramnarine IW, Rush SA, Pitcher TE. 2010. Geographic variation in sperm traits reflects predation risk and natural rates of multiple paternity in the guppy. J. Evol. Biol. 23, 1331–1338. ( 10.1111/j.1420-9101.2010.01996.x) [DOI] [PubMed] [Google Scholar]
- 65.Pilastro A, Mandelli M, Gasparini C, Dadda M, Bisazza A. 2007. Copulation duration, insemination efficiency and male attractiveness in guppies. Anim. Behav. 74, 321–328. ( 10.1016/j.anbehav.2006.09.016) [DOI] [Google Scholar]
- 66.Houde AE. 1987. Mate choice based upon naturally occurring color-pattern variation in a guppy population. Evolution 41, 1–10. ( 10.1111/j.1558-5646.1987.tb05766.x) [DOI] [PubMed] [Google Scholar]
- 67.Gasparini C, Serena G, Pilastro A. 2013. Do unattractive friends make you look better? Context-dependent male mating preferences in the guppy. Proc. R. Soc. B 280, 20123072 ( 10.1098/rspb.2012.3072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liley NR. 1966. Ethological isolating mechanisms in four sympatric species of Poeciliid fishes. Behav. Suppl. 13, 1–197. [Google Scholar]
- 69.Maynard Smith J. 1962. Review lectures on senescence: I. The causes of aging. Proc. R. Soc. Lond. B 157, 115–127. ( 10.1098/rspb.1962.0065) [DOI] [Google Scholar]
- 70.Walker LC, Herndon JG. 2010. Mosaic aging. Med. Hypotheses 74, 1048–1051. ( 10.1016/j.mehy.2009.12.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hunt J, Jennions MD, Spyrou N, Brooks R. 2006. Artificial selection on male longevity influences age-dependent reproductive effort in the black field cricket Teleogryllus commodus. Am. Nat. 168, E72–E86. ( 10.1086/506918) [DOI] [PubMed] [Google Scholar]
- 72.Taylor ML, Price TA, Wedell N. 2014. Polyandry in nature: a global analysis. Trends Ecol. Evol. 29, 376–383. ( 10.1016/j.tree.2014.04.005) [DOI] [PubMed] [Google Scholar]
- 73.Hayward AD, Moorad J, Regan CE, Berenos C, Pilkington JG, Pemberton JM, Nussey DH. 2015. Asynchrony of senescence among phenotypic traits in a wild mammal population. Exp. Gerontol. 71, 56–68. ( 10.1016/j.exger.2015.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the results are available as part of the electronic supplementary material.