Abstract
Dung beetles are increasingly used as a study taxon—both as bioindicators of environmental change, and as a model system for exploring ecosystem functioning. The advantages of this focal taxon approach are many; dung beetles are abundant in a wide range of terrestrial ecosystems, speciose, straightforward to sample, respond to environmental gradients and can be easily manipulated to explore species-functioning relationships. However, there remain large gaps in our understanding of the relationship between dung beetles and the mammals they rely on for dung. Here we review the literature, showing that despite an increase in the study of dung beetles linked to ecosystem functioning and to habitat and land use change, there has been little research into their associations with mammals. We summarize the methods and findings from dung beetle–mammal association studies to date, revealing that although empirical field studies of dung beetles rarely include mammal data, those that do, indicate mammal species presence and composition has a large impact on dung beetle species richness and abundance. We then review the methods used to carry out diet preference and ecosystem functioning studies, finding that despite the assumption that dung beetles are generalist feeders, there are few quantitative studies that directly address this. Together this suggests that conclusions about the effects of habitat change on dung beetles are based on incomplete knowledge. We provide recommendations for future work to identify the importance of considering mammal data for dung beetle distributions, composition and their contributions to ecosystem functioning; a critical step if dung beetles are to be used as a reliable bioindicator taxon.
Keywords: trophic interaction, indicator taxa, scarabaeoidae, conservation, ecosystem functioning, mammals
1. Introduction
Indicator species are often used as a more efficient way to assess ecosystem integrity than sampling a large number of taxa [1]. However, for a focal taxon to be used to assess a community habitat, or the effects of environmental change, robust quantitative data and a detailed understanding of its ecology are needed [2]. Dung beetles are an ideal indicator taxon because of their sensitivity to habitat change [3,4], in combination with broad geographical distributions and ease of collection [5–7]. As such, they have been increasingly used as bioindicators to inform conservation management decisions [8–11]. However, the effect of mammal species compositional change on dung beetles and their associated ecosystem functions has been little explored (but see [12–14]).
Dung beetles primarily feed and breed in dung but are also capable of using carrion, rotting fruit, fungi and decaying plant matter [15,16]. As a result, dung beetles contribute to the ecosystem functions of dung removal [17], seed dispersal [18], nutrient cycling [19,20] and reduction of greenhouse gas emissions [21,22]. In this review, we focus on coprophagic dung beetles (Coleoptera: Scarabaeoidea in the families Geotrupidae, Aphodiinae and Scarabaeinae) that feed primarily on dung, and so are expected to have associations with mammals. Dung beetles can be classified into clearly defined functional groups that can be easily manipulated for ecosystem functioning experiments [23], and as a result are increasingly used for understanding trait-functioning associations [24,25]. However, while studies of dung beetles have provided a large body of information on species' distributions and responses to land use change, they rarely shed light on the biotic interactions between dung beetles and mammals [12–14].
Dung beetles are commonly assumed to display generalist feeding and breeding strategies, with many feeding on more than two dung sources [26,27], or using dung from both native and exotic mammal species [28,29]. Mammalian dung varies in nutrient and fibre content among species [30], but also seasonally based on diet [31]. The quality and quantity of the dung provisioned to larvae have been shown to affect the number, size and development of offspring, and to result in resource allocation trade-offs in adult beetles [32,33]. Dung is used in different ways by adults and larvae [30], and selection of dung for breeding may differ to that used for feeding, although the consequences of this for ecosystem functioning has been little explored (but see [14,34,35]). In addition, variation in the digestive system and gut microbia in dung beetles may play a role in determining feeding preferences [36–38], and while dung volatiles are thought to be key to determining the attractiveness of dung to dung beetles [39,40], how they relate to dung beetle resource use is still not well understood [30,41].
Mammals are also often used as an indicator or flagship taxon [42,43], and there has been extensive research concerning mammal species responses to disturbance (e.g. [44,45]), and species’ associations to habitat types [46]. However, in contrast to dung beetles, mammals are notably harder to survey, requiring more time, effort and at a greater cost [47]. Yet, despite their close ecological association, and despite being two of the best studied vertebrate and invertebrate taxa individually, dung beetles and mammals are rarely studied in combination [48] (but see ‘Avenues for future work’ below for the potential of new molecular methods).
We conducted a systematic review of studies documenting the associations between dung beetles and mammals to address the following questions: (i) what have been the research trends in the study of dung beetle ecology? (ii) what methodological approaches are used to study dung beetle–mammal associations, and dung beetle–dung associations? and (iii) is diet preference and association with mammals accounted for in studies of dung beetle ecosystem functioning? We then highlight the knowledge gaps and give recommendations for how dung beetle–mammal interactions can be incorporated into future work on dung beetle ecology.
2. Material and methods
We carried out a review of the literature to identify trends in the study of dung beetle ecology using Web of Science (as of 18 December 2018). A 1990 start year was chosen to represent a shift in focus towards human land use change and ecosystem functioning in ecology [49]. All searches used the keyword topic (‘dung beetle’ OR scarabaeinae OR geotrupidae OR aphodiinae). We identified literature considering dung beetle–mammal associations using the search term (mammal* OR preference* OR diet*). Additional papers were identified by following publications cited in these articles. The papers were then reviewed to identify the approach to classifying the dung beetle–mammal associations and interactions (box 1). We distinguished between studies that tested interactions directly through dung beetle dietary preference experiments using different dung types (box 1a,b), and those that used mammal and dung beetle co-occurrence data to indirectly infer associations between the two (box 1c,d). We then systematically reviewed the approach taken to classifying the dung beetle–mammal associations in the dung beetle–mammal co-occurrence studies. We recorded three aspects of the studies: (i) how the mammal composition was classified; (ii) how the analysis of the association between dung beetles and mammals was carried out; and (iii) the direction of the effect of the mammal communities on the dung beetle population metrics. For diet preference studies, only those that used at least two dung types were included. For these studies, we identified the location, as well as the dung types and dung volumes used, and the experimental design employed in each of the studies.
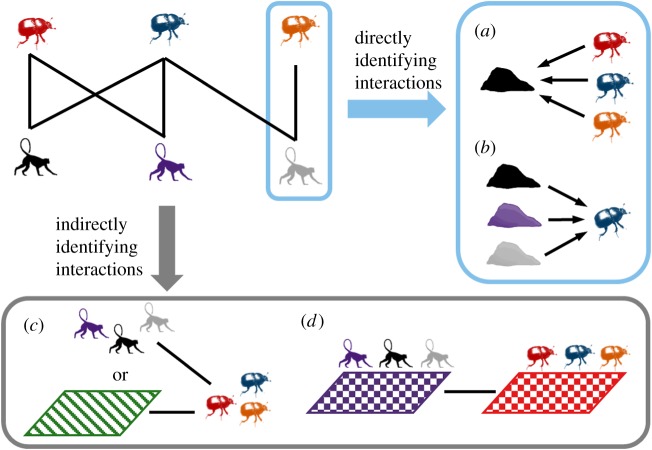 Box 1. The different approaches to studying dung beetle–mammal associations. The dung beetle–mammal interaction network can be estimated directly (a,b) or indirectly (c,d). (a) Directly identifying the dung beetle species composition attracted to a dung type; (b) identifying dung beetle species resource use breadth and dietary preference by identifying attraction to multiple dung types; (c) inferring population level associations between dung beetles and mammals either via pooled mammal composition data or via a proxy for mammal composition such environmental condition; and (d) inferring species-level interactions between dung beetles and mammals via associations between species that co-occur.
Box 1. The different approaches to studying dung beetle–mammal associations. The dung beetle–mammal interaction network can be estimated directly (a,b) or indirectly (c,d). (a) Directly identifying the dung beetle species composition attracted to a dung type; (b) identifying dung beetle species resource use breadth and dietary preference by identifying attraction to multiple dung types; (c) inferring population level associations between dung beetles and mammals either via pooled mammal composition data or via a proxy for mammal composition such environmental condition; and (d) inferring species-level interactions between dung beetles and mammals via associations between species that co-occur.
To compare the number of dung beetle–mammal association studies with the number of other studies to provide a minimum indicator of the amount of ecological survey work documenting the study of dung beetles, two additional searches were undertaken. This used the keyword search topic: (habitat* OR environment*) for dung beetle–habitat associations, and: (ecosystem* service* OR ecosystem* function*) for ecosystem function studies. We excluded studies exploring the effects of pesticide and insecticide application on ecosystem functions as this was beyond the scope of this review. This literature search was not exhaustive, as papers that did not refer specifically to these search terms were not further identified. Studies concerning dung beetle ecosystem functioning were assessed for the extent to which they considered feeding variation among dung beetle species. For each study, we recorded location, the number of dung types used, dung volumes and ecosystem function(s) measured.
3. Results
(a). Trends in dung beetle ecological research
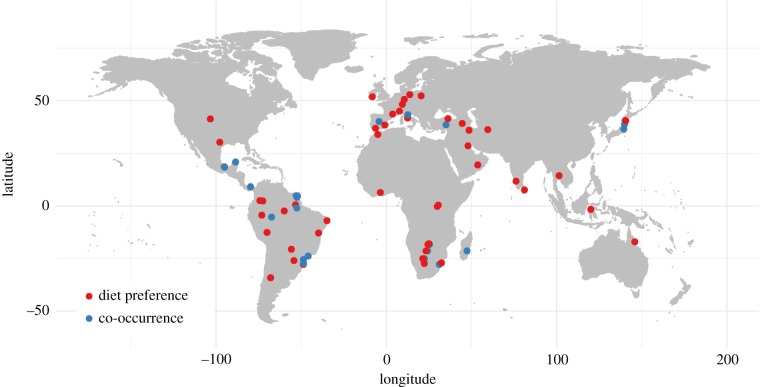
The literature review yielded a total of 359 papers from all three searches. Studies of dung beetle–mammal associations accounted for 65 papers, 68 papers focused on dung beetle–ecosystem functioning research and 226 studies consisted of empirical field studies of dung beetle–habitat associations (see the electronic supplementary material, appendix A). Forty-four of the dung beetle–mammal association studies addressed direct interactions and dung beetle dietary preference, and 21 considered the indirect effect of mammal presence on dung beetle populations or community composition. These two categories were explored separately. There has been an increase in publications on ecosystem functions and habitat associations using dung beetles since 1990 (figure 1). By contrast, studies of dung beetle–mammal associations, both diet preference and species co-occurrence studies, have remained low throughout this period, with studies largely focused in Europe and South America, and with relatively few studies in Asia, Australasia, Africa and North America (figure 2).
Figure 1.
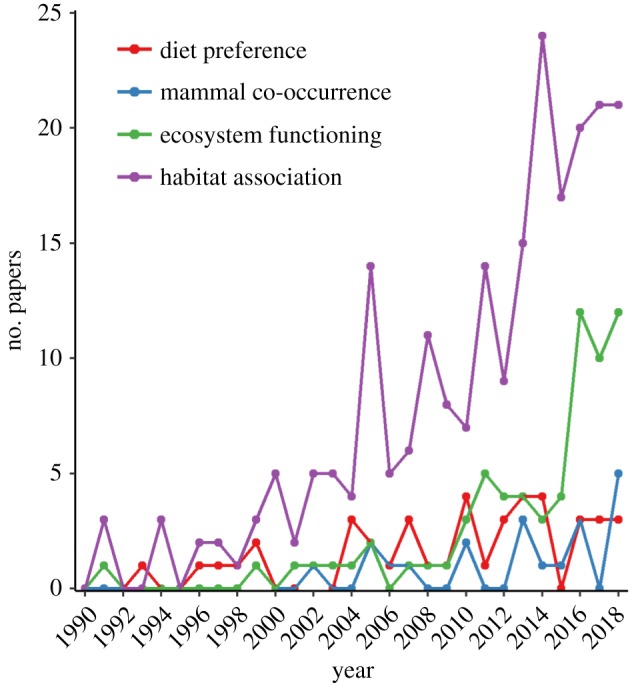
Journal articles published on dung beetle ecology from 1990 to 2018, details of papers given in the electronic supplementary material, appendix A.
Figure 2.
Study locations for papers addressing dung beetle–mammal association studies, separated into mammal co-occurrence and diet preference studies (field-based—see below) (1990–2018). Where the study conducted sampling at disparate locations, multiple points are included.
(b). Dung beetle–mammal association studies: diet preference and direct interactions
Of the 44 diet preference studies, 89% were field experiments and 11% laboratory experiments (box 1a,b). The most commonly used study design for field surveys included only two dung types and distance between traps varied from 1 to 100 m (mode = 50 m) (electronic supplementary material, table S2, figure S1). Experimental methods also varied, with studies either using pitfall traps, directly collecting dung beetles from dung, or using burial intercept traps (see [35]). Cattle dung was the most commonly used dung type. All but one laboratory study [40] assessed diet preference for just one dung beetle species. Across the 68 papers that studied ecosystem functioning, the majority used only one dung type, again with cattle dung the most frequently used bait (electronic supplementary material, figure S1, table S3). Most studies considered only one ecosystem function, dung removal, but other studies also addressed seed removal and dispersal, nutrient cycling and plant growth. The dung volume used in ecosystem functioning studies varied greatly and larger volumes were used in comparison to dietary preference studies (electronic supplementary material, figure S1).
(c). Dung beetle–mammal association studies: co-occurrence data and indirect interactions
There were 21 studies that assessed dung beetle–mammal associations through their co-occurrence (box 1c,d) (electronic supplementary material, table S1). Two studies used co-occurrence data to estimate species-level interactions between dung beetles and mammals [13,50] (box 1d). The other studies assessed population-level associations using a range of approaches to identify mammal composition, including proxies, individual focal mammal species, grazing intensity or estimates of mammal composition based on species richness and raw abundance (box 1c). Thirty-eight per cent of the studies included information on small mammals in their estimates of mammal species composition. Three studies estimated dung beetle occurrence using a range of different dung types [14,51,52]. In each of the studies, we identified the effect a decline in mammal abundance or richness had on the dung beetle population. All studies showed at least one positive association between dung beetle species richness and abundance and the mammal metric used, indicating that a reduction in the mammal community had a negative effect on the dung beetle community. Dung beetle community composition was significantly different between areas of varying mammal composition for all but two studies.
4. Discussion
There has been a rapid increase in the study of coprophagous dung beetles over the last 29 years, yet despite their reliance on mammal dung [48], the understanding of dung beetle–mammal associations is still limited. Increasing data on dung beetle associations with the environment and their functional contributions to ecosystems has not been mirrored by an advancement of the mechanistic understanding of how dung beetles use dung for feeding and breeding, the cascading consequences of defaunation for dung beetle populations, and the ecosystem functions they provide. This lack of research is apparent across both tropical and temperate regions.
(a). Dung beetle–mammal associations
We found that studies on the effects of mammals on dung beetle community composition, species richness and abundance have largely been neglected, despite calls to increase the inclusion of mammal data in dung beetle studies [48]. Although the dung beetle–mammal co-occurrence studies are revealing about the importance of individual mammal species [52,53] and the effects of grazing intensity on dung beetle populations [54,55], the majority were based on proxies or qualitative estimates of mammal composition (box 1c,d). Several studies identified the importance of large bodied mammals for dung beetle species composition [52,56], but only a third of studies accounted for small bodied mammals in their assessment of faunal composition. Overall, studies reported consistent trends towards co-declines in dung beetles and mammals [12,57,58]. This suggests that changes in mammal species composition, such as those occurring as a result of habitat disturbance, are likely to have significant impacts on dung beetle communities, and associated ecosystem functioning (see [48] for review).
(b). Dung beetle diet preference and identifying direct interactions
It is commonly assumed that dung beetles show broad dietary widths [26,59], and a recent meta-analysis suggests generalist feeding preferences in dung beetles across latitudinal gradients [27]. However, the range of experimental methods used in the majority of the studies reviewed here does not provide conclusive evidence to support this. Many studies compared the attractiveness of just two dung types [60–62], or analysed dung beetle species composition attracted to single dung types (box 1a), rather than individual dung beetle species feeding breadth (box 1b) [63–66]. Several studies show that dung beetle species can vary in their attraction to the dung of different mammal trophic groups [67,68], and particular mammal species [69–71]. Equally experimental choice trials in the laboratory have shown variation in dung beetle species resource use [37,72]. Although more time consuming, feeding and breeding choice experiments in the field are key to enabling mechanistic questions surrounding attractiveness of dung to dung beetles to be addressed, such as identifying the association between dung type used for brood provisioning and adult body size [31,73].
In the past 20 years, the study of biodiversity–ecosystem functioning relationships has grown markedly and dung beetles are increasingly used as a focal taxon for such studies [74]. In this review, 60% of ecosystem functioning studies used only one dung type, and over 50% of the time this was domesticated animal dung. As a consequence, variation in the choice between feeding and breeding—two functionally very different interactions—is still unknown. Although livestock dung is important for dung beetle populations globally, especially in agro-ecosystems, it can provide a limited snapshot of the extent of ecosystem functions provided by dung beetles, especially those not performed by domestic animals, such as seed dispersal [75,76], and their important role in non-pasture ecosystems, such as tropical forests [17,77].
(c). Regional variation
Studies exploring dung beetle–mammal associations were concentrated in the Neotropics and Europe, with few studies carried out elsewhere. Variation among biogeographical regions in mammal fauna is likely to have impacted the evolution of dung beetle–mammal associations [78]. In the Neotropics and temperate regions, such as Europe and North America, mammalian biomass is low owing to high rates of mammal extinction in the Pleistocene [72]. This may have resulted in dung beetles switching to non-mammalian dung food sources such as fruit, fungi, carrion, or plant detritus, or developing greater plasticity in their diets [72,79–81]. By contrast, the mammal fauna in Africa is dominated by large herbivorous species and there appears to be a higher number of beetles specialized in coprophagy, with fruit and carrion feeding recorded less frequently [15,79,82]. In addition, regional differences in environmental conditions, such as the dominance of savannahs versus humid forests effect the abundance of dung beetles and attraction to different food sources [83].
(d). Avenues for future work
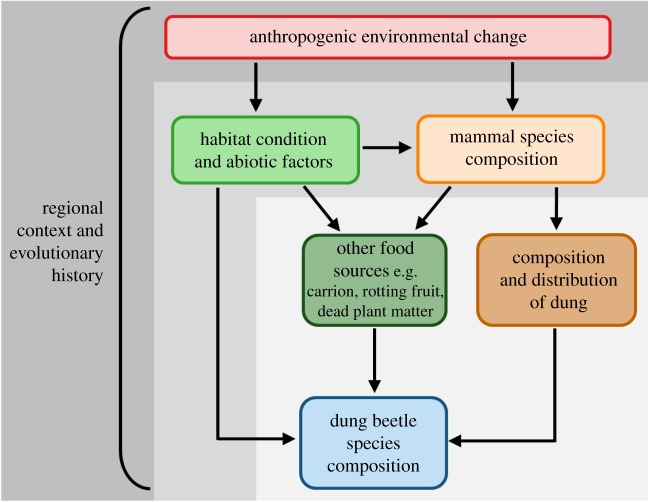
Here we identify areas to improve the understanding of dung beetle–mammal associations within the context of environmental change and advancing the understanding of ecosystem functions provided by dung beetles (figure 3).
Figure 3.
Conceptual diagram of the processes by which environmental change can affect coprophagous dung beetle composition and ecosystem functioning, with avenues for future work represented. Lightest grey: (i) identifying direct interactions between dung beetles and their resources (mammal dung and others); medium grey: (ii) decoupling the effects of biotic factors (availability of resources) from abiotic factors and habitat change on dung beetle populations; dark grey: (iii) identifying dietary switching and plasticity in dung beetles in response to defaunation, within the evolutionary context of different regions.
(i). Identifying dung beetle resource use
Standardized survey methods. Our review highlights the lack of consistent and comparable methods in the experimental design of both diet preference and ecosystem functioning studies, making a synthesis of results challenging. The composition of dung beetles captured in traps is affected by dung volume [84] and trap spacing [85,86], and trapping methods to detect the difference between the choice of dung for feeding and breeding have only recently been developed [34,35]. In addition, the efficacy of different trapping methods and how dung attractiveness to dung beetle species varies across different habitats [87,88] and between life cycle stages [14] is not well understood. Future work is needed to identify standardized experimental designs that strive to minimize the effects of these possible variables. Moreover, the prevalence of studies using only one or two dung types prevents the direct interactions between mammals and dung beetles from being described. We call for standardization of survey methods, and incorporation of surveying with realistic dung sizes of multiple native species. This will enable more accurate predictions of how environmental change and, specifically defaunation will affect these interactions, and the consequences for ecosystem functions and services.
The importance of natural history, taxonomy and museum collections. Establishing species-level interactions is crucial to answering questions about whole interaction networks and the impacts of environmental change at larger scales [89,90]. However, this relies on an understanding of the taxonomy and natural history of the species involved; knowledge which is currently lacking for many dung beetle species, particularly in the tropics. Recently, there has been a renewed interest in the importance of natural history, and concern over the decline of taxonomy, with calls for increased funding and research for these areas [90–92]. As well as increased observational and natural history studies in the field, using the large amounts of data held in museum collections is crucial [89], and unpublished sources, species notes and observations provide a key contribution to our understanding of dung beetle ecology [15].
Using new molecular methods to understand direct interactions. Advances in DNA barcoding mean it is now possible to identify vertebrate genetic material from invertebrates that feed on them [93,94]. DNA in dung beetle gut contents has been used to successfully identify the mammal species dung they fed on in pilot studies [95–97]. Such studies have the potential to be an important addition to mammal survey data, particularly in detecting rare or cryptic species. The attractiveness of these new molecular methods for studying dung beetle mammal interactions is that they have the potential to sidestep the issues affecting ecological sampling (e.g. the size and dung type of the bait used in the trap) and allow direct identification of species interactions under natural conditions.
(ii). Identifying drivers of dung beetle responses to environmental change
Incorporating mammals into dung beetle studies. To understand the responses of dung beetles to disturbance, it is necessary to consider both biotic and abiotic conditions. There has been much focus recently on the declines of insects owing to habitat loss [98] but the importance of abiotic and biotic factors in driving this response is unknown for most species. Thus, decoupling these drivers is crucial to inform conservation planning for insect populations globally. In this context, the lack of studies including mammals as a biotic factor in analyses of dung beetle responses to environmental change neglects an important factor influencing their distribution and responses. However, disentangling the effects of mammal species presence, abundance and richness on dung beetle species composition from abiotic factors is still a challenge which can only be addressed by accounting for both taxa in population level assessments. Often expensive field equipment employed in the field over long time periods is required to gather the high-resolution mammal data required for this kind of analysis (but see above for molecular advances). This work can be furthered to understand how the reintroduction of mammal species can affect dung beetle recovery in restoration projects [8,52].
Identifying important functional traits. Dung beetle responses to environmental change can also be considered in terms of shared functional traits among species that both shape their use of the resource (effect traits) and how they respond to their environment and changes in it (response traits) [25]. Although several dung beetle functional traits are known to be important in influencing how species respond to environmental change [77], the traits relating to resource use and dietary preference (such as olfaction [41,99], flight capacity [100], digestion [101] and searching behaviour [102]) are not well understood.
Modelling interactions from co-occurrence data. Identifying ecological interactions from community patterns in species occurrence from spatial data is commonplace [103], where non-random species occurrence patterns are used to infer interspecific interactions [104,105]. Advances in the use of species distribution modelling have recently been applied to dung beetle–mammal co-occurrence data to reveal interactions between the two trophic levels, and also identify the effect of ecological processes such as dispersal, in addition to abiotic factors, on dung beetle species distributions [106–108].
(iii). Identifying dietary switching and plasticity in dung beetles
Dietary switching and plasticity in resource use. The relative importance of alternative food sources such as rotting fruit, fungi and carrion, and plant detritus impact the extent of interactions between dung beetles and mammals and should be included in dung beetle feeding studies. Research addressing dietary plasticity has mostly been conducted in the Neotropics (see [15] for review), and the importance of these resources as alternatives in dung beetle diets in other regions is largely unknown. Pleistocene extinctions of the large mega-fauna in the Neotropics and Europe [72,109], and historical mammal species introductions have resulted in dung use switching in native adult dung beetle species in these regions, either to alternative dung sources [110,111] or to frugivory [112]. In addition, flexibility in resource choice for breeding, and the impact that this has on larval development and survival is not well understood [15,41]. A quantitative network approach may be a valuable way to assess resource use and flexibility in dung beetles, especially under changing environmental conditions [14].
Regional trends in research and evolutionary context. The current abiotic and biotic conditions in combination with historical context affects the ability of dung beetles to adapt to new environmental conditions and the introduction of exotic mammal dung [13,77,83,113]. However, the extent to which each of these factors contributes to the ability of dung beetles to adapt to changing conditions and the extent to which resource switching and diet plasticity exist is still vastly understudied [79]. Few studies fully address the range and availability of food sources available in a study area, and in particular, the dung of small mammals is rarely included. More studies are needed outside of the Neotropics to test whether biogeographical differences in diet flexibility are a result of differences in resource availability and adaptation in dung beetles among regions, or simply a sampling effect from the limited research conducted to date [15,72].
(e). Synthesis
Although there has been an increase in dung beetle research related to their response to disturbance and their importance for ecosystem functioning, further work is now needed to put this into context with regard to their resource use, interactions with mammals and evolutionary history. The use of dung beetles as bioindicators is currently constrained by a lack of understanding of the associations between dung beetles and mammals; a necessary pre-requisite if data are to be scaled up to give a broader understanding of the extent of functioning provided by dung beetles within whole ecosystems, and their response to environmental change.
Supplementary Material
Acknowledgements
We are grateful to Nico Blüthgen, Owen Lewis and three anonymous reviewers for constructive comments on the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
E.M.S. conceived the study, E.H.R. carried out data collection and analysis, and the manuscript was written jointly by E.H.R. and E.M.S.
Competing interests
We declare we have no competing interests.
Funding
Financial support came from an NERC studentship to E.H.R. (grant no. NE/L02612/1) and E.M.S. was funded under UK Natural Environment Research Council grant (no. NE/K016407/1).
References
- 1.Carignan V, Villard M-A. 2002. Selecting indicator species to monitor ecological integrity: a review. Environ. Monit. Assess. 78, 45–61. ( 10.1023/A:1016136723584) [DOI] [PubMed] [Google Scholar]
- 2.Dufrêne M, Legendre P. 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr. 67, 345–366. ( 10.1890/0012-9615(1997)067) [DOI] [Google Scholar]
- 3.Davis AJ, Holloway JD, Huijbregts H, Krikken J, Kirk-Spriggs AH, Sutton SL. 2001. Dung beetles as indicators of change in the forests of northern Borneo. J. Appl. Ecol. 38, 593–616. ( 10.1046/j.1365-2664.2001.00619.x) [DOI] [Google Scholar]
- 4.Gollan JR, de Bruyn LL, Reid N, Wilkie L. 2013. Monitoring the ecosystem service provided by dung beetles offers benefits over commonly used biodiversity metrics and a traditional trapping method. J . Nat. Conserv. 21, 183–188. ( 10.1016/j.jnc.2012.12.004) [DOI] [Google Scholar]
- 5.Nichols ES, Gardner TA. 2011. Dung beetles as a candidate study taxon in applied biodiversity conservation research. In Ecology and evolution of dung beetles (eds LW Simmons, TJ Ridsdill-Smith), pp. 267–293. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 6.Oliveira VHF, Barlow J, Gardner T, Louzada J. 2017. Are we selecting appropriate metrics to assess human impacts on biodiversity? Basic Appl. Ecol. 21, 85–93. ( 10.1016/j.baae.2017.03.002) [DOI] [Google Scholar]
- 7.Didham RKR, Hammond PMP, Lawton JH, Eggleton P, Stork NE. 1998. Beetle species responses to tropical forest fragmentation. Ecol. Monogr. 68, 295–323. ( 10.1890/0012-9615(1998)068) [DOI] [Google Scholar]
- 8.Audino LD, Louzada J, Comita L. 2014. Dung beetles as indicators of tropical forest restoration success: is it possible to recover species and functional diversity? Biol. Conserv. 169, 248–257. ( 10.1016/j.biocon.2013.11.023) [DOI] [Google Scholar]
- 9.Gollan JR, Reid CAMM, Barnes PB, Wilkie L. 2011. The ratio of exotic-to-native dung beetles can indicate habitat quality in riparian restoration. Insect Conserv. Divers. 4, 123–131. ( 10.1111/j.1752-4598.2010.00115.x) [DOI] [Google Scholar]
- 10.Filgueiras BKCC, Tabarelli M, Leal IR, Vaz-de-Mello FZ, Iannuzzi L. 2015. Dung beetle persistence in human-modified landscapes: combining indicator species with anthropogenic land use and fragmentation-related effects. Ecol. Indic. 55, 65–73. ( 10.1016/j.ecolind.2015.02.032) [DOI] [Google Scholar]
- 11.Davis A, Scholtz C. 2004. Scarabaeine dung beetles as indicators of biodiversity, habitat transformation and pest control chemicals in agro-ecosystems: review article. S. Afr. J. Sci. 100, 415–424. [Google Scholar]
- 12.Culot L, Bovy E, Zagury Vaz-de-Mello F, Guevara R, Galetti M. 2013. Selective defaunation affects dung beetle communities in continuous Atlantic rainforest. Biol. Conserv. 163, 79–89. ( 10.1016/j.biocon.2013.04.004) [DOI] [Google Scholar]
- 13.Nichols E, Peres CA, Hawes JE, Naeem S. 2016. Multitrophic diversity effects of network degradation. Ecol. Evol. 6, 4936–4946. ( 10.1002/ece3.2253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raine EH, Mikich SB, Lewis OT, Riordan P, Vaz-de-Mello FZ, Slade EM. 2018. Extinctions of interactions: quantifying a dung beetle–mammal network. Ecosphere 9, e02491 ( 10.1002/ecs2.2491) [DOI] [Google Scholar]
- 15.Halffter G, Halffter V. 2009. Why and where coprophagous beetles (Coleoptera: Scarabaeinae) eat seeds, fruits or vegetable detritus. Boletín Soc. Entomol. Aragon. 45, 1–22. [Google Scholar]
- 16.Hill CJC, Hill J. 1996. Habitat specificity and food preferences of an assemblage of tropical Australian dung beetles. J. Trop. Ecol. 12, 449–460. ( 10.1017/S026646740000969X) [DOI] [Google Scholar]
- 17.Slade EM, Mann DJ, Lewis OT. 2011. Biodiversity and ecosystem function of tropical forest dung beetles under contrasting logging regimes. Biol. Conserv. 144, 166–174. ( 10.1016/j.biocon.2010.08.011) [DOI] [Google Scholar]
- 18.Lugon AP, Boutefeu M, Bovy E, Vaz-de-Mello FZ, Huynen M, Galetti M, Culot L. 2017. Persistence of the effect of frugivore identity on post-dispersal seed fate: consequences for the assessment of functional redundancy. Biotropica 49, 293–302. ( 10.1111/btp.12418) [DOI] [Google Scholar]
- 19.Nervo B, et al. 2017. Ecological functions provided by dung beetles are interlinked across space and time: evidence from 15 N isotope tracing. Ecology 98, 433–446. ( 10.1002/ecy.1653) [DOI] [PubMed] [Google Scholar]
- 20.Menéndez R, Webb P, Orwin KH. 2016. Complementarity of dung beetle species with different functional behaviours influence dung–soil carbon cycling. Soil Biol. Biochem. 92, 142–148. ( 10.1016/j.soilbio.2015.10.004) [DOI] [Google Scholar]
- 21.Piccini I, Arnieri F, Caprio E, Nervo B, Pelissetti S, Palestrini C, Roslin T, Rolando A. 2017. Greenhouse gas emissions from dung pats vary with dung beetle species and with assemblage composition. PLoS ONE 12, e0178077 ( 10.1371/journal.pone.0178077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slade EM, Kirwan L, Bell T, Philipson CD, Lewis OT, Roslin T. 2017. The importance of species identity and interactions for multifunctionality depends on how ecosystem functions are valued. Ecology 98, 2626–2639. ( 10.1002/ecy.1954) [DOI] [PubMed] [Google Scholar]
- 23.Stokstad E. 2004. Loss of dung beetles puts ecosystems in deep doo-doo. Science 305, 1230a ( 10.1126/science.305.5688.1230a) [DOI] [PubMed] [Google Scholar]
- 24.Slade EM, Mann DJ, Villanueva JF, Lewis OT. 2007. Experimental evidence for the effects of dung beetle functional group richness and composition on ecosystem function in a tropical forest. J. Anim. Ecol. 76, 1094–1104. ( 10.1111/j.1365-2656.2007.01296.x) [DOI] [PubMed] [Google Scholar]
- 25.Larsen TH, Lopera A, Forsyth A. 2008. Understanding trait-dependent community disassembly: dung beetles, density functions, and forest fragmentation. Conserv. Biol. 22, 1288–1298. ( 10.1111/j.1523-1739.2008.00969.x) [DOI] [PubMed] [Google Scholar]
- 26.Hanski I, Cambefort Y. 1991. Dung beetle ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 27.Frank K, Krell FT, Slade EM, Raine EH, Chiew LY, Schmitt T, Vairappan CS, Walter P, Blüthgen N. 2018. Global dung webs: high trophic generalism of dung beetles along the latitudinal diversity gradient. Ecol. Lett. 21, 1229–1236. ( 10.1111/ele.13095) [DOI] [PubMed] [Google Scholar]
- 28.Whipple SD, Hoback WW. 2012. A comparison of dung beetle (Coleoptera: Scarabaeidae) attraction to native and exotic mammal dung. Environ. Entomol. 41, 238–244. ( 10.1603/EN11285) [DOI] [PubMed] [Google Scholar]
- 29.Stavert JR, Gaskett AC, Scott DJ, Beggs JR. 2014. Dung beetles in an avian-dominated island ecosystem: feeding and trophic ecology. Oecologia 176, 259–271. ( 10.1007/s00442-014-3001-z) [DOI] [PubMed] [Google Scholar]
- 30.Holter P, Scholtz CH. 2007. What do dung beetles eat? Ecol. Entomol. 32, 690–697. ( 10.1111/j.1365-2311.2007.00915.x) [DOI] [Google Scholar]
- 31.Edwards P. 1991. Seasonal variation in the dung of African grazing mammals and its consequences for coprophagous insects. Funct. Ecol. 5, 617–628. ( 10.2307/2389480) [DOI] [Google Scholar]
- 32.Moczek A. 1998. Horn polyphenism in the beetle Onthophagus taurus: larval diet quality and plasticity in parental investment determine adult body size and male horn morphology. Behav. Ecol. 9, 636–641. ( 10.1093/beheco/9.6.636) [DOI] [Google Scholar]
- 33.Schwab DB, Casasa S, Moczek AP. 2017. Evidence of developmental niche construction in dung beetles: effects on growth, scaling and reproductive success. Ecol. Lett. 20, 1353–1363. ( 10.1111/ele.12830) [DOI] [PubMed] [Google Scholar]
- 34.Goh TG, Hashim R. 2018. A comparison of the dung beetle (Coleoptera: Scarabaeidae: Scarabaeinae) collecting performance of pitfall traps and burrowing interception traps. Coleopt. Bull. 72, 195–202. ( 10.1649/0010-065X-72.1.195) [DOI] [Google Scholar]
- 35.Arellano L. 2016. A novel method for measuring dung removal by tunneler dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) in pastures. Coleopt. Bull. 70, 185–188. ( 10.1649/072.070.0129) [DOI] [Google Scholar]
- 36.Estes AM, Hearn DJ, Snell-Rood EC, Feindler M, Feeser K, Abebe T, Dunning Hotopp JC, Moczek AP. 2013. Brood ball-mediated transmission of microbiome members in the dung beetle, Onthophagus taurus (Coleoptera: Scarabaeidae). PLoS ONE 8, e79061 ( 10.1371/journal.pone.0079061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shukla SP, Sanders JG, Byrne MJ, Pierce NE. 2016. Gut microbiota of dung beetles correspond to dietary specializations of adults and larvae. Mol. Ecol. 25, 6092–6106. ( 10.1111/mec.13901) [DOI] [PubMed] [Google Scholar]
- 38.Franzini PZN, Ramond J-B, Scholtz CH, Sole CL, Ronca S, Cowan DA. 2016. The gut microbiomes of two Pachysoma Macleay desert dung beetle species (Coleoptera: Scarabaeidae: Scarabaeinae) feeding on different diets. PLoS ONE 11, e0161118 ( 10.1371/journal.pone.0161118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frank K, Brückner A, Hilpert A, Heethoff M, Blüthgen N. 2017. Nutrient quality of vertebrate dung as a diet for dung beetles. Sci. Rep. 7, 1–12. ( 10.1038/s41598-017-12265-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dormont L, Rapior S, McKey D, Lumaret J. 2007. Influence of dung volatiles on the process of resource selection by coprophagous beetles. Chemoecology 17, 23–30. ( 10.1007/s00049-006-0355-7) [DOI] [Google Scholar]
- 41.Holter P. 2016. Herbivore dung as food for dung beetles: elementary coprology for entomologists. Ecol. Entomol. 41, 367–377. ( 10.1111/een.12316) [DOI] [Google Scholar]
- 42.Morrison JC, Sechrest W, Dinerstein E, Wilcove DS, Lamoreux JF. 2007. Persistence of large mammal faunas as indicators of global human impacts. J. Mammal. 88, 1363–1380. ( 10.1644/06-MAMM-A-124R2.1) [DOI] [Google Scholar]
- 43.Walpole MJ, Leader-Williams N. 2002. Tourism and flagship species in conservation. Biodivers. Conserv. 11, 543–547. ( 10.1023/A:1014864708777) [DOI] [Google Scholar]
- 44.Canale GR, Peres CA, Guidorizzi CE, Gatto CAF, Kierulff MCM. 2012. Pervasive defaunation of forest remnants in a tropical biodiversity hotspot. PLoS ONE 7, e41671 ( 10.1371/journal.pone.0041671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Effiom EO, Nunez-Iturri G, Smith HG, Ottosson U, Olsson O. 2013. Bushmeat hunting changes regeneration of African rainforests. Proc. R. Soc. B 280, 20130246 ( 10.1098/rspb.2013.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yackulic CB, Sanderson EW, Uriarte M. 2011. Anthropogenic and environmental drivers of modern range loss in large mammals. Proc. Natl Acad. Sci. USA 108, 4024–4029. ( 10.1073/pnas.1015097108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardner TA, et al. 2008. The cost-effectiveness of biodiversity surveys in tropical forests. Ecol. Lett. 11, 139–150. ( 10.1111/j.1461-0248.2007.01133.x) [DOI] [PubMed] [Google Scholar]
- 48.Nichols E, Gardner TA, Peres CA, Spector S. 2009. Co-declining mammals and dung beetles: an impending ecological cascade. Oikos 118, 481–487. ( 10.1111/j.1600-0706.2009.17268.x) [DOI] [Google Scholar]
- 49.McDonnell MJ, Pickett STA. 1990. Ecosystem structure and function along urban-rural gradients: an unexploited opportunity for ecology. Ecology 71, 1232–1237. ( 10.2307/1938259) [DOI] [Google Scholar]
- 50.Raine EH, Gray CL, Mann DJ, Slade EM. 2018. Tropical dung beetle morphological traits predict functional traits and show intraspecific differences across land uses. Ecol. Evol. 8, 8686–8696. ( 10.1002/ece3.4218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tshikae BP, Davis ALV, Scholtz CH. 2013. Does an aridity and trophic resource gradient drive patterns of dung beetle food selection across the Botswana Kalahari? Ecol. Entomol. 38, 83–95. ( 10.1111/j.1365-2311.2012.01409.x) [DOI] [Google Scholar]
- 52.Enari H, Koike S, Enari HS, Seki Y, Okuda K, Kodera Y. 2018. Early-stage ecological influences of population recovery of large mammals on dung beetle assemblages in heavy snow areas. Acta Oecol. 92, 7–15. ( 10.1016/j.actao.2018.07.007) [DOI] [Google Scholar]
- 53.Kanda N, Yokota T, Shibata E, Sato H. 2005. Diversity of dung-beetle community in declining Japanese subalpine forest caused by an increasing sika deer population. Ecol. Res. 20, 135–141. ( 10.1007/s11284-004-0033-6) [DOI] [Google Scholar]
- 54.Tonelli M, Verdú JR, Zunino ME. 2017. Effects of grazing intensity and the use of veterinary medical products on dung beetle biodiversity in the sub-mountainous landscape of Central Italy. PeerJ 5, e2780 ( 10.7717/peerj.2780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvarado F, Escobar F, Williams DR, Arroyo-Rodríguez V, Escobar-Hernández F. 2017. The role of livestock intensification and landscape structure in maintaining tropical biodiversity. J. Appl. Ecol. 55, 185–194. ( 10.1111/1365-2664.12957) [DOI] [Google Scholar]
- 56.Bogoni JA, et al. 2016. Contributions of the mammal community, habitat structure, and spatial distance to dung beetle community structure. Biodivers. Conserv. 25, 1661–1675. ( 10.1007/s10531-016-1147-1) [DOI] [Google Scholar]
- 57.Andresen E, Laurance SGW. 2007. Possible indirect effects of mammal hunting on dung beetle assemblages in Panama. Biotropica 39, 141–146. ( 10.1111/j.1744-7429.2006.00239.x) [DOI] [Google Scholar]
- 58.Escobar F, Lobo JM, Halffter G. 2006. Assessing the origin of Neotropical mountain dung beetle assemblages (Scarabaeidae: Scarabaeinae): the comparative influence of vertical and horizontal colonization. J. Biogeogr. 33, 1793–1803. ( 10.1111/j.1365-2699.2006.01528.x) [DOI] [Google Scholar]
- 59.Simmons L, Ridsdill-Smith T. 2011. Ecology and evolution of dung beetles. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 60.Galante E, Cartagena MC. 1999. Comparison of Mediterranean dung beetles (Coleoptera: Scarabaeoidea) in cattle and rabbit dung. Environ. Entomol. 28, 420–424. ( 10.1093/ee/28.3.420) [DOI] [Google Scholar]
- 61.Puker A, Correa CMA, Korasaki V, Ferreira KR, Oliveira NG. 2013. Dung beetles (Coleoptera: Scarabaeidae) attracted to dung of the largest herbivorous rodent on earth: a comparison with human feces. Environ. Entomol. 42, 1218–1225. ( 10.1603/EN13100) [DOI] [PubMed] [Google Scholar]
- 62.Hidayat P, Manuwoto S, Noerdjito WA, Tscharntke T, Schulze CH. 2010. Diversity and body size of dung beetles attracted to different dung types along a tropical land-use gradient in Sulawesi, Indonesia. J. Trop. Ecol. 26, 53–65. ( 10.1017/S0266467409990423) [DOI] [Google Scholar]
- 63.Plewińska B. 2007. The effect of food odour on food preference, activity and density of dung beetle Geotrupes stercorosus (Scriba, 1791) in a mixed coniferous forest. Polish J. Ecol. 55, 495–509. [Google Scholar]
- 64.Carpaneto GM, Mazziotta A, Ieradi M. 2010. Use of habitat resources by scarab dung beetles in an African savanna. Environ. Entomol. 39, 1756–1764. ( 10.1603/EN09249) [DOI] [PubMed] [Google Scholar]
- 65.Martín-Piera F, Lobo J. 1996. A comparative discussion of trophic preferences in dung beetle communities. Misc. Zool. 19, 13–31. [Google Scholar]
- 66.Tshikae BP, Davis AL, Scholtz CH. 2013. Species richness: energy relationships and dung beetle diversity across an aridity and trophic resource gradient. Acta Oecol. 49, 71–82. ( 10.1016/j.actao.2013.02.011) [DOI] [Google Scholar]
- 67.Filgueiras BKC, Iannuzzi L, Leal IR. 2011. Habitat fragmentation alters the structure of dung beetle communities in the Atlantic Forest. Biol. Conserv. 144, 362–369. ( 10.1016/j.biocon.2010.09.013) [DOI] [Google Scholar]
- 68.Bogoni JA, Hernández MIM. 2014. Attractiveness of native mammal's feces of different trophic guilds to dung beetles (Coleoptera: Scarabaeinae). J. Insect Sci. 14, 1–7. ( 10.1093/jisesa/ieu161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Enari H, Koike S, Sakamaki H. 2013. Influences of different large mammalian fauna on dung beetle diversity in beech forests. J. Insect Sci. 13, 1–13. ( 10.1673/031.013.5401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos-Heredia C, Andresen E, Zárate DA. 2010. Secondary seed dispersal by dung beetles in a Colombian rain forest: effects of dung type and defecation pattern on seed fate. J. Trop. Ecol. 26, 355–364. ( 10.1017/S0266467410000192) [DOI] [Google Scholar]
- 71.Noriega JA. 2012. Dung beetles (Coleoptera: Scarabaeinae) attracted to Lagothrix lagotricha (Humboldt) and Alouatta seniculus (Linnaeus) (Primates: Atelidae) dung in a Colombian Amazon Forest. Psyche A J. Entomol. 2012, 1–6. ( 10.1155/2012/437589) [DOI] [Google Scholar]
- 72.Galetti M, et al. 2017. Ecological and evolutionary legacy of megafauna extinctions. Biol. Rev. 93, 845–862. ( 10.1111/brv.12374) [DOI] [PubMed] [Google Scholar]
- 73.Arellano L, Castillo-Guevara C, Huerta C, Germán-García A, Lara C. 2015. Effect of using different types of animal dung for feeding and nesting by the dung beetle Onthophagus lecontei (Coleoptera: Scarabaeinae). Can. J. Zool. 93, 337–343. ( 10.1139/cjz-2014-0226) [DOI] [Google Scholar]
- 74.Noriega JA, et al. 2018. Research trends in ecosystem services provided by insects. Basic Appl. Ecol. 26, 8–23. ( 10.1016/j.baae.2017.09.006) [DOI] [Google Scholar]
- 75.Santos-Heredia C, Andresen E, Stevenson P. 2011. Secondary seed dispersal by dung beetles in an Amazonian forest fragment of Colombia: influence of dung type and edge effect. Integr. Zool. 6, 399–408. ( 10.1111/j.1749-4877.2011.00261.x) [DOI] [PubMed] [Google Scholar]
- 76.Culot L, Huynen M-C, Heymann EW. 2015. Partitioning the relative contribution of one-phase and two-phase seed dispersal when evaluating seed dispersal effectiveness. Methods Ecol. Evol. 6, 178–186. ( 10.1111/2041-210X.12317) [DOI] [Google Scholar]
- 77.Nichols E, et al. 2013. Trait-dependent response of dung beetle populations to tropical forest conversion at local and regional scales. Ecology 94, 180–189. ( 10.1890/12-0251.1) [DOI] [PubMed] [Google Scholar]
- 78.Viljanen H, Escobar F, Hanski I. 2010. Low local but high beta diversity of tropical forest dung beetles in Madagascar. Glob. Ecol. Biogeogr. 19, 886–894. ( 10.1111/j.1466-8238.2010.00552.x) [DOI] [Google Scholar]
- 79.Falqueto SAS, Vaz-de-Mello FZ, Schoereder JJH. 2005. Are fungivorous Scarabaeidae less specialist? Ecol. Austral. 15, 17–22. [Google Scholar]
- 80.Larsen TH, Lopera A, Forsyth A. 2006. Extreme trophic and habitat specialization by Peruvian dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Coleopt. Bull. 60, 315–324. ( 10.1649/0010-065X(2006)60[315:ETAHSB]2.0.CO;2) [DOI] [Google Scholar]
- 81.Salomão RP, Iannuzzi L. 2015. Dung beetle (Coleoptera, Scarabaeidae) assemblage of a highly fragmented landscape of Atlantic forest: from small to the largest fragments of northeastern Brazilian region. Rev. Bras. Entomol. 59, 126–131. ( 10.1016/j.rbe.2015.03.008) [DOI] [Google Scholar]
- 82.Ururahy-Rodrigues A, Rafael JA, Wanderley RF, Marques H, Pujol-Luz JR. 2008. Coprophanaeus lancifer (Linnaeus, 1767) (Coleoptera, Scarabaeidae) activity moves a man-size pig carcass: relevant data for forensic taphonomy. Forensic Sci. Int. 182, 1–5. ( 10.1016/j.forsciint.2008.09.009) [DOI] [PubMed] [Google Scholar]
- 83.Favila ME. 2012. Historical, biogeographical and ecological factors explain the success of some native dung beetles after the introduction of cattle in Mexico. Pastos 42, 161–181. [Google Scholar]
- 84.Peck SB, Howden HF. 1984. Response of a dung beetle guild to different sizes of dung bait in a Panamanian rainforest. Biotropica 16, 235 ( 10.2307/2388057) [DOI] [Google Scholar]
- 85.Dormont L, Epinat G, Lumaret J-P. 2004. Trophic preferences mediated by olfactory cues in dung beetles colonizing cattle and horse dung. Environ. Entomol. 33, 370–377. ( 10.1603/0046-225X-33.2.370) [DOI] [Google Scholar]
- 86.Dormont L, Jay-Robert P, Bessiere J-M, Rapior S, Lumaret J-P. 2010. Innate olfactory preferences in dung beetles. J. Exp. Biol. 213, 3177–3186. ( 10.1242/jeb.040964) [DOI] [PubMed] [Google Scholar]
- 87.da Silva PG, Hernández MIM. 2015. Spatial patterns of movement of dung beetle species in a tropical forest suggest a new trap spacing for dung beetle biodiversity studies. PLoS ONE 10, e0126112 ( 10.1371/journal.pone.0126112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larsen TH, Forsyth A. 2005. Trap spacing and transect design for dung beetle biodiversity studies. Biotropica 37, 322–325. ( 10.1111/j.1744-7429.2005.00042.x) [DOI] [Google Scholar]
- 89.Schilthuizen M, Vairappan CS, Slade EM, Mann DJ, Miller JA. 2015. Specimens as primary data: museums and ‘open science’. Trends Ecol. Evol. 30, 237–238. ( 10.1016/j.tree.2015.03.002) [DOI] [PubMed] [Google Scholar]
- 90.Cardoso P, Erwin TL, Borges PAV, New TR. 2011. The seven impediments in invertebrate conservation and how to overcome them. Biol. Conserv. 144, 2647–2655. ( 10.1016/j.biocon.2011.07.024) [DOI] [Google Scholar]
- 91.Tewksbury JJ, et al. 2014. Natural history's place in science and society. 64, 300–310. ( 10.1093/biosci/biu032) [DOI] [Google Scholar]
- 92.Packer L, Monckton SK, Onuferko TM, Ferrari RR. 2018. Validating taxonomic identifications in entomological research. Insect Conserv. Divers. 11, 1–12. ( 10.1111/icad.12284) [DOI] [Google Scholar]
- 93.Calvignac-Spencer S, Leendertz FH, Gilbert MTP, Schubert G. 2013. An invertebrate stomach's view on vertebrate ecology: certain invertebrates could be used as ‘vertebrate samplers’ and deliver DNA-based information on many aspects of vertebrate ecology. Bioessays 35, 1004–1013. ( 10.1002/bies.201300060) [DOI] [PubMed] [Google Scholar]
- 94.Ji Y, et al. 2013. Reliable, verifiable and efficient monitoring of biodiversity via metabarcoding. Ecol. Lett. 16, 1245–1257. ( 10.1111/ele.12162) [DOI] [PubMed] [Google Scholar]
- 95.Gómez A, Kolokotronis S. 2017. Genetic identification of mammalian meal source in dung beetle gut contents. Mitochondrial DNA A 28, 612–615. ( 10.3109/24701394.2016.1155120) [DOI] [PubMed] [Google Scholar]
- 96.Gillett CPDT, Johnson AJ, Barr I, Hulcr J. 2016. Metagenomic sequencing of dung beetle intestinal contents directly detects and identifies mammalian fauna. bioRxiv, 074849 ( 10.1101/074849) [DOI] [Google Scholar]
- 97.Kerley GIH, Landman M, Ficetola GF, Boyer F, Bonin A, Rioux D, Taberlet P, Coissac E. 2018. Diet shifts by adult flightless dung beetles Circellium bacchus, revealed using DNA metabarcoding, reflect complex life histories. Oecologia 188, 1–9. ( 10.1007/s00442-018-4203-6) [DOI] [PubMed] [Google Scholar]
- 98.Hallmann CA, et al. 2017. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12, e0185809 ( 10.1371/journal.pone.0185809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frank K, Brückner A, Blüthgen N, Schmitt T. 2018. In search of cues: dung beetle attraction and the significance of volatile composition of dung. Chemoecology 28, 145–152. ( 10.1007/s00049-018-0266-4) [DOI] [Google Scholar]
- 100.Verdú JR, Lobo JM. 2008. Ecophysiology of thermoregulation in endothermic dung beetles: ecological and geographical implications. Insect Ecol. Conserv. 661, 1–28. [Google Scholar]
- 101.Holter P, Scholtz CH. 2013. Elongated hindguts in desert-living dung beetles (Scarabaeidae: Scarabaeinae) feeding on dry dung pellets or plant litter. J. Morphol. 274, 657–662. ( 10.1002/jmor.20123) [DOI] [PubMed] [Google Scholar]
- 102.Jacobs J, Nole I, Palminteri S, Ratcliffe B. 2008. First come, first serve: ‘sit and wait’ behavior in dung beetles at the source of primate dung. Neotrop. Entomol. 37, 641–645. ( 10.1590/S1519-566X2008000600003) [DOI] [PubMed] [Google Scholar]
- 103.Götzenberger L, et al. 2012. Ecological assembly rules in plant communities-approaches, patterns and prospects. Biol. Rev. 87, 111–127. ( 10.1111/j.1469-185X.2011.00187.x) [DOI] [PubMed] [Google Scholar]
- 104.Gotelli NJ, McCabe DJ. 2002. Species co-occurrence : a meta-analysis of J. M. Diamond's assembly rules model. Ecology 83, 2091–2096. ( 10.1890/0012-9658) [DOI] [Google Scholar]
- 105.Ulrich W, Gotelli NJ. 2013. Pattern detection in null model analysis. Oikos 122, 2–18. ( 10.1111/j.1600-0706.2012.20325.x) [DOI] [Google Scholar]
- 106.D'Amen M, Mod HK, Gotelli NJ, Guisan A. 2018. Disentangling biotic interactions, environmental filters, and dispersal limitation as drivers of species co-occurrence. Ecography (Cop.) 41, 1233–1244. ( 10.1111/ecog.03148) [DOI] [Google Scholar]
- 107.Warton DI, Blanchet FG, O'Hara RB, Ovaskainen O, Taskinen S, Walker SC, Hui FKC. 2015. So many variables: joint modeling in community ecology. Trends Ecol. Evol. 30, 766–779. ( 10.1016/j.tree.2015.09.007) [DOI] [PubMed] [Google Scholar]
- 108.Zurell D, Pollock LJ, Thuiller W. 2018. Do joint species distribution models reliably detect interspecific interactions from co- occurrence data in homogenous environments? Ecography (Cop.) 125, 336–342. ( 10.1111/ecog.03315) [DOI] [Google Scholar]
- 109.Schweiger AH, Svenning JC. 2018. Down-sizing of dung beetle assemblages over the last 53 000 years is consistent with a dominant effect of megafauna losses. Oikos 127, 1243–1250. ( 10.1111/oik.04995) [DOI] [Google Scholar]
- 110.Jones AG, Forgie SA, Scott DJ, Beggs JR. 2012. Generalist dung attraction response in a New Zealand dung beetle that evolved with an absence of mammalian herbivores. Ecol. Entomol. 37, 124–133. ( 10.1111/j.1365-2311.2012.01344.x) [DOI] [Google Scholar]
- 111.Kohlmann B, Solís Á, Alvarado GE. 2018. Did Costa Rican dung beetles (Coleoptera: Scarabaeidae) feed on bision dung before the arrival of Spanish cattle? Coleopt. Bull. 72, 295–300. ( 10.1649/0010-065X-72.2.295) [DOI] [Google Scholar]
- 112.Sarges R, Halffter G, Rojas AD. 2012. The importance of frugivory to the survival of the dung beetle Onthophagus rhinolophus Harold (Coleoptera: Scarabaeidae: Scarabaeinae) under changing ecological conditions. Coleopt. Bull. 66, 166–168. ( 10.1649/072.066.0216) [DOI] [Google Scholar]
- 113.Hanski I, Wirta H, Nyman T, Rahagalala P. 2008. Resource shifts in Malagasy dung beetles: contrasting processes revealed by dissimilar spatial genetic patterns. Ecol. Lett. 11, 1208–1215. ( 10.1111/j.1461-0248.2008.01239.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.