Abstract
Mate choice is an important cause of sexual selection; it can drive the evolution of extravagant ornaments and displays, and promote speciation through the reproductive isolation generated by rapid divergence of sexual traits. Understanding mate choice requires knowledge of the traits involved in generating mate-choice decisions, and how those traits may interact with each other. It has been proposed that mate-choice decisions are the outcome of two components that vary independently: the preference function (the ranking of the attractiveness of prospective mates) and choosiness (the effort invested in mate assessment). Here we test this hypothesis by examining individual variation in female preference functions and choosiness in green treefrogs (Hyla cinerea). We show that measures describing preference functions and choosiness are not correlated. We also show that both components are influenced differently by variation in female body size, and that preference function shape (closed and preferring intermediate values or open-ended and preferring extremes) has a strong influence on this relationship: function traits are positively correlated with body size only for individuals with closed functions, while choosiness is positively correlated with body size for individuals with open functions, but negatively for those with closed functions.
Keywords: preference function, choosiness, sexual selection, mate choice, treefrog
1. Introduction
Mate choice is an important cause of variation in individual fitness, and a strong cause of natural and sexual selection on communication systems and reproductive traits [1–4]. Because of its relevance for selection on signallers and receivers, mate choice has important consequences for speciation and the evolution of extreme traits [5–9]. The consequences of mate choice for these evolutionary processes hinge on the genetic and cognitive architecture of the components involved in the generation of mate-choice decisions [10–14].
Over 20 years ago, Jennions & Petrie [15] discussed the causes and consequences of variation in mate choice in terms of the underlying architecture of mate preferences. They identified two components: mate preference functions (the ranking of prospective mates) and choosiness (the effort invested in mate assessment). To avoid confusing the term ‘mate preference’ with the term ‘preference function’, we suggest referring to the broad topic for analysis as the mate-choice decision. We also suggest viewing choosiness as the effort expended to or willingness to invest in acquiring the preferred mate type, rather than solely mate assessment effort, to distinguish it from mate-searching strategies [11,16–18]. With this modified framework, the hypothesis proposed by Jennions & Petrie [15] can be stated as follows: variation in mate choice decisions arises from the interaction of preference functions and choosiness.
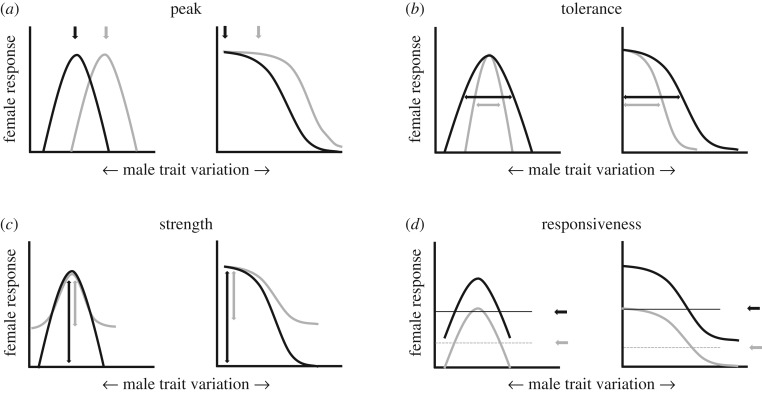
The first component, the preference function, has long been recognized for its usefulness in studying sexual selection, and characteristics of preference functions have been used to generate hypotheses about selection on male traits [3,19–23]. Initially, studies focused on the overall shape of the preference function—open-ended and favouring extreme male trait values, or closed and favouring intermediate male trait values—as well as on the relationship between the peak (the most preferred trait value) and the male trait distribution. Note that function shape (open/closed) does not equate a particular form of selection (directional/stabilizing). It is necessary to relate the shape of the function and the peak of the preference to the male trait distribution to formulate hypotheses about the form of selection; for example, a closed function whose peak is off the mean male trait value will result in directional selection, and an open function that plateaus at a point that includes the majority of males will not. Besides this qualitative characterization of preference functions, recent refinements allow quantitative analysis in terms of preference function traits [22,24–27]. In addition to the preference function trait of ‘peak’, which indicates the most preferred trait value; ‘tolerance’ describes acceptance of trait values that deviate from her peak; ‘strength’ shows how much attractiveness declines as trait values deviate from the peak; and ‘responsiveness’ indicates the mean response levels across all trait values (figure 1; see also [27] for a detailed discussion on the philosophy and analysis of preference functions).
Figure 1.
Examples of potential individual preference functions, illustrating variation in function shape and preference function traits. Preference function shape can be closed, and favour intermediate male trait values (left panels) or open-ended and favouring extreme male trait values (right panels). Preference function traits obtained for our study follow [27]. They were: (a) peak = the most preferred trait value, measured as the trait value that elicits the strongest response; (b) tolerance = a measure of willingness to accept trait values that deviate from the peak, measured as the width of the preference function at 70% elevation relative to the height of the peak; (c) strength = the variation in response across trait values, calculated as [s.d.(response values)/mean(response values)]2; and (d) responsiveness = the average response across all trait values, measured as the overall elevation of the preference function. Note that overall preference shape and preference function traits may in principle vary independently, such that variation in one function trait does not predetermine variation in the other traits (black versus grey curves), and a given function trait may vary to a similar extent within closed and open preferences (arrows). For example, (a) peak may vary to a similar extent within closed and open preferences (depending in the case of open preferences of whether and where they plateau), or (b) tolerance may vary in functions that have the same peak, responsiveness and strength.
The second component, choosiness, can also affect the strength and direction of selection on male traits because it determines how closely a female's actual mate choice matches her preference function. For example, two females could have the same preference function, but vary in their choosiness: the choosier female invests more effort in mate selection, and thus is more likely to obtain her most preferred male. By contrast, a female that is not very choosy probably mates with a male whose traits do not strongly reflect her preference function. Variation in this second component appears to have received less attention in the sexual selection literature than preference functions (but see [28–31]).
Jennions & Petrie [15] proposed that preference functions and choosiness are distinct traits that can evolve independently. Whether this is the case or not is a key question in the theory of sexual selection and speciation [14,32,33]. Theoretical studies vary in whether they view preference functions and choosiness as independent or related in various ways (see discussion in [14]). Some researchers measure willingness to acquire preferred types separately from preference functions and use various terms for such measures, including choosiness [10,28–31,34,35], while others use the term choosiness to refer to an intrinsic aspect of preference functions [4,36–40]. Whether variation in preference functions is independent or related to variation in the willingness to secure preferred types is an empirical question, regardless of how one refers to those components. The answer is of fundamental importance, however: either the mate types that are most attractive evolve independently of how much effort is exerted in obtaining them, or they do not. This, in turn, determines whether preferences and choosiness should be conceptualized as corresponding to different loci, or as corresponding to just one (cf. [14]).
Here we test the hypothesis that preference functions and choosiness are independent traits [15]. To our knowledge, the relationship between preference functions and choosiness as conceptualized by Jennions & Petrie [15] has not been assessed empirically. This hypothesis makes two predictions: (i) variation in preference functions (i.e. preference shape, preference peak and so on) should not be correlated with variation in choosiness; and (ii) preference functions and choosiness should respond differently to different causes of variation. The rationale for the second prediction comes from recent research that has identified several factors that generate individual variation in mate-choice decisions. These can be grouped into internal factors such as age or condition of the choosing individual [11,41,42], and external factors such as experience with potential mates [24,25]. If preference functions and choosiness are independent traits, they should be influenced differently by the above factors, and this influence could either take the form of functions being influenced by one variable and choosiness being influenced by another; or functions and choosiness being influence by the same variable but in different ways (i.e. the sign or slope of the correlation may be different).
To test these predictions we took advantage of the well-studied acoustic communication system of green treefrogs, Hyla cinerea (Anura: Hylidae). Green treefrogs are a common anuran species that has been the focus of intense research on neurophysiological, behavioural and evolutionary aspects of mate choice and sexual selection [35,43–46]. During the breeding season males gather at ponds to form large choruses, where males produce advertisement calls for several hours each night. When female green treefrogs are ready to mate, they approach a calling male they deem attractive. In our focal population, females prefer lower-frequency calls [30]. Call frequency is negatively correlated with male body size [45], but whether females use call frequency as an indicator for good genes (for longevity, good foraging ability or efficient metabolism) or this preference is simply arbitrary [4,13] is currently unknown.
We used field playback experiments to describe individual variation in preference functions and choosiness for call frequency, and examined how body size and/or social experience affected variation in these traits. We focused on body size and social experience because they are frequent causes of variation in preference functions and/or choosiness [24–26,47,48], and they show substantial variation in green treefrogs. Body size differences of mate searching females amount to up to 60% (34–59 mm, n = 590; G.H. 1999–2015, unpublished data). And the social environment females experience when they reach the pond is highly variable due to the high turnover rate of males participating in the chorus (individual males generally only call for a few nights over a season that can last weeks or month [45]). Thus, for green treefrogs, the architecture proposed by Jennions & Petrie [15] predicts that: (i) preference function traits may covary with each other but will vary independently of choosiness; and that (ii) preference function traits and choosiness will relate in different ways to body size and social experience.
2. Methods and materials
(a). Study species and sampling
Our focal population of H. cinerea inhabits the western part of the species's range, at the East Texas Conservation Center, in Jasper, TX. We performed all trials during May–July of 2013. During the breeding season (April–August), males congregate at ponds and swampy areas where they produce advertisement calls to attract females. The calls are short, ranging from 100–200 ms in duration, with a repetition rate of 80 calls min−1. Calls contain two spectral bands, in the low-frequency range (0.68–1.2 kHz), and the high-frequency range (2.3–3.7 kHz) (G.H. 1999–2015, unpublished data). Females show preferences for a number of call traits (i.e. duration, relative amplitude and repetition rate), but the strongest preference is for call frequency [43–46]. There is geographical variation in preference functions and in choosiness for call frequency [35]. There is also evidence of socially mediated plasticity in choosiness [30].
We collected females in the late evening at the beginning of chorus formation from amplexed pairs to ensure sexual receptivity. We released all frogs at the site of capture after the conclusion of the night's trials.
(b). Testing preference functions and choosiness
We used synthetic playback stimuli varying in their spectral features (see below) and fixed at the mean values of our focal population for duration (160 ms), rise time (25 ms) and fall time (50 ms). Females readily respond to these synthetic calls via phonotaxis. We used a custom-written DOS program (courtesy of J. J. Schwartz; available upon request) to generate the playbacks.
We tested females in an outdoor arena set up at our study site, but away from active choruses and at a time when males generally had ceased calling (between 01.00 and 05.00 h). Females remain responsive to playback stimuli for several hours after peak calling has ceased, and only stop responding upon sunrise (when they would naturally move from the pond to their diurnal retreats in the surrounding vegetation). The arena consisted of a plywood floor (2 × 1 m) surrounded by a wood frame 50 cm in height, screened with a visually opaque but acoustically transparent black cloth. Just outside of the cloth, two loudspeakers (JBL Control 1X) were placed opposite one another along the central long axis of the arena, so that females could hear the broadcast stimuli, but not see the speakers. Stimuli were broadcast from a PC laptop using Audacity software (Audacity Team, 2013). Stimulus amplitudes were verified using a Lutron SL-4001 sound-level meter (fast RMS with ‘C’ weighting) prior to each test.
During each playback trial, females were placed in an acoustically transparent wire cage, 10 cm wide by 5 cm tall, in the centre of the arena, the lid of which was removed remotely after five rounds of alternating stimuli. Females were able to climb out of the cage and free to move about the arena for up to 5 min, and a choice was scored once the female reached a 10 cm ‘choice area’ in front of a speaker. Females were rested 5–15 min between consecutive trials.
To examine the relationship between preference functions and choosiness, we obtained data on both for each female. We switched each night the order of playback trials conducted to describe preference functions or choosiness, to guard against sequence/time biases. We detected no such biases: neither preference function traits nor choosiness differed between females whether either was obtained first or second that night (t-tests: p ≥ 0.08).
(i). Preference functions
Preference functions are representations of how attractiveness varies with ornament trait values (i.e. [19,22]). Because mate preferences are expressed in terms of the ornaments encountered by an individual, they are function-valued traits best represented as curves [23,26,27,49,50]. Variation in these functions can be analysed in terms of their overall shape: open preference functions favour extreme trait values, and closed preference functions favour intermediate trait values and discriminate against extremes. Within these shape categories, the curves can be further characterized by preference function traits. These include peak (a female's most preferred trait value), tolerance (the range of trait values for which attractiveness remains relatively high), strength (the overall variation in female response across trait values) and responsiveness (a female's average response across all trait values (figure 1) [24,25,27]. Note that preference shape and the different preference function traits may in principle vary independently (e.g. peak may vary to a similar extent within closed and open preferences; figure 1a). We therefore tested for differences in preference function traits according to the overall shape of the preferences.
To describe female mate preference functions for call frequency we conducted a series of six two-choice trials, using synthetic calls generated as described above. Trials presented a ‘standard’ call with a dominant frequency of 900 Hz (grand species average) against alternatives that were either lower (600, 700, 800 Hz) or higher (1000, 1100, 1200 Hz) in dominant frequency. The frequency range covered by these trials (600–1200 Hz) slightly exceeds the natural range of the species (679–1172 Hz, n = 549 males from 13 sites across the range; G.H. 1999–2015, unpublished data), as is recommended to capture the full shape of the preference function within biological relevance [26,27]. We randomized stimulus sequences for each female and periodically switched the speaker presenting the standard call (every seven trials) so that each female was tested with both speaker configurations.
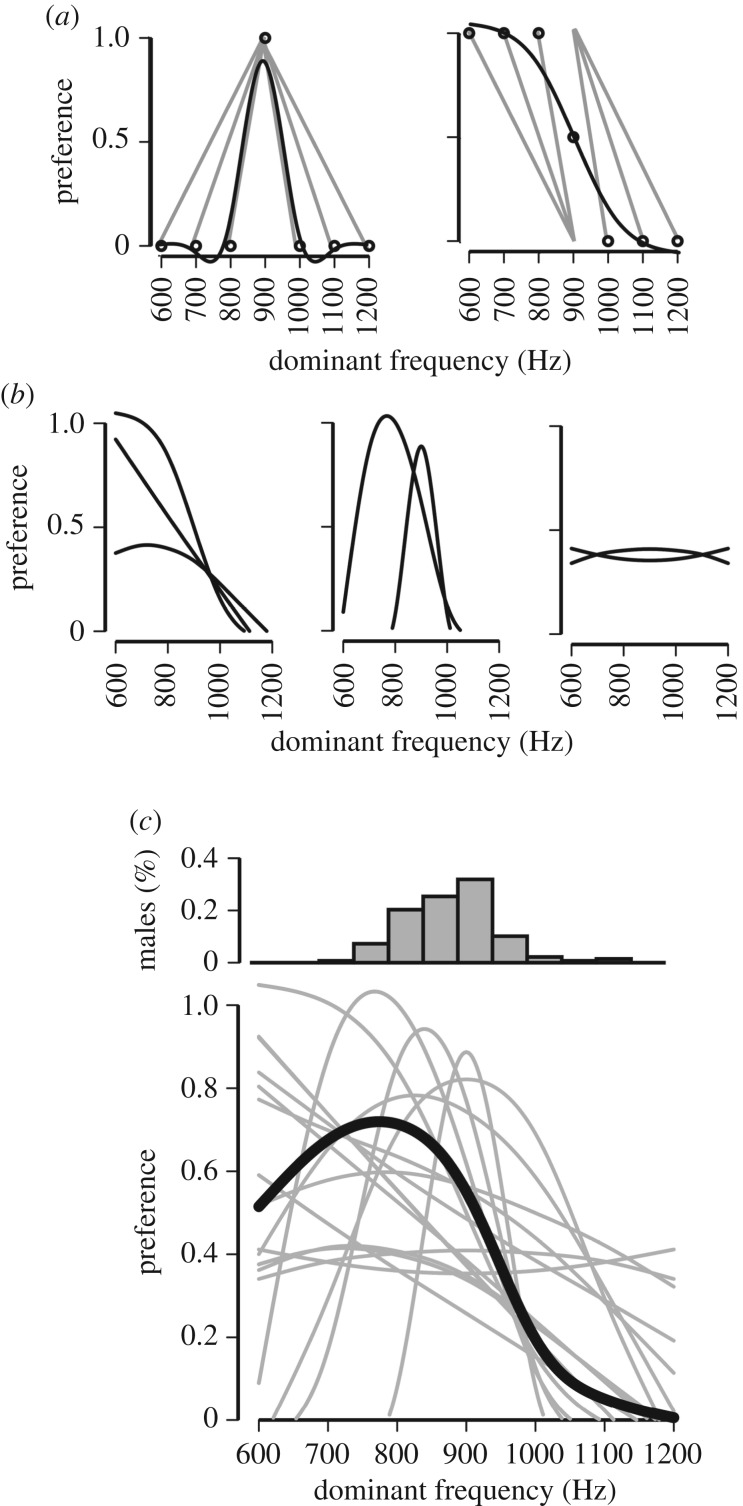
To obtain stimulus-specific response values from our two-choice data structure where females chose between two alternatives, we scored the trials as follows: The chosen alternative was awarded a score of ‘1’, the rejected alternative a score of ‘0’. Because each female was presented with each of the alternative stimuli only once, but presented with the standard stimulus in all of her trials, we calculated the final score of the standard stimulus as [(sum of standard scores across all trials)/6]. Scores for each of the six alternatives could thus be either 0 or 1, and scores for the standard could range from 0 to 1 (figure 2a).
Figure 2.
Variation in preference functions for the low-frequency peak of H. cinerea male advertisement call. (a) Preference functions were obtained by conducting two-choice trials (grey lines indicate stimulus exemplars tested in each trial), which were converted into preference scores (symbols), onto which non-parametric splines were fitted (black line). Shown are an example of a closed (left) and an open (right) preference function. (b) Functions could be grouped into three general shapes: open (53% of females; left), closed (42% of females; centre) or flat (5% of females; right). (c) Comparison between the distribution of the dominant frequency peak of male advertisement calls in the population (n = 138; bin size is 50 Hz) with the preference functions of individual females (grey lines), and for comparison, the composite function for the population (black line).
We then used a function-valued approach to describe mate preference functions on the basis of these scores. We used the program PFunc [27] to fit non-parametric regressions for each individual tested (figure 2a). This method makes no assumption about the shape of the functions, other than that they should have some level of smoothness (e.g. it does not pre-specify a linear or quadratic shape, but allows each function to be determined by the responses of the individual females). We then used the program to calculate the above preference function traits for each individual female. This method has been used extensively to measure individual, genetic and plasticity-related variation in preference functions [26,51,52]. Finally, we obtained a population function and population function traits.
(ii). Choosiness
Our assay of choosiness is based on the natural behaviour of green treefrog females: if a female has a choice between an attractive male that is farther away and a less attractive male that is nearer, she will often choose the closer, less attractive male. We manipulated apparent distance to the playback stimuli by changing their relative amplitude—as per the inverse square law of sound attenuation, amplitude decreases by 6 dB for each doubling of distance to the sound source [53]. This offers a simple but effective assay of the distance a female is willing to go in securing the mate she prefers [30,31,34,35].
We assessed choosiness with two-choice playback trials presenting an unattractive call (1100 Hz) played at constant amplitude against an attractive call (800 Hz) that is attenuated. For each trial, the unattractive alternative was broadcast at a constant amplitude of 85 dB SPL. To start a trial, we presented the attractive alternative at 73 dB SPL, and then adjusted its amplitude up or down in steps of 6 dB and then 3 dB until we identified the lowest amplitude at which she still approached the quieter attractive stimulus rather than the louder unattractive stimulus. Arriving at a choosiness measure for each female thus required 3–4 trials. The lowest amplitude required to arrive at these choosiness scores (61 dB SPL) is well within the auditory threshold (derived from single auditory fibre recordings) of H. cinerea (30 dB SPL [54]) and the phonotaxis threshold of a related species of similar size (43 dB SPL; Hyla versicolor [55]). As with the preference function trials, we switched speaker locations periodically.
(c). Causes of variation
One of the predictions of the hypothesis that preference functions and choosiness are independent traits is that they will be influenced differently by variables such as social experience and body size.
(i). Social experience treatments
We manipulated the social experience of females using playbacks simulating variation in chorus composition. Females were randomly assigned to one of four experience treatments: (i) attractive stimulus only (800 Hz playback); (ii) unattractive stimulus only (1100 Hz playback); (iii) a 1 : 1 mixture of the unattractive and attractive stimuli; or (iv) a silent treatment (no playback). The body size of females entering each experience treatment did not differ significantly (F3,57 = 1.46, p = 0.24).
We created the treatment playbacks with Audacity by pasting the synthetic stimuli into longer sound files that we played to females. The temporal pattern of the calls in the sound file mimicked natural male chorusing behaviour, with a mean inter-call interval of 400 ms that ranged from the equivalent of one male in isolation to several interacting males [56].
We broadcast the playbacks from a MP3 player (RCA TH2002RDR) through an iHome rechargeable mini speaker (iHM60) adjusted to a sound pressure level of 73 dB, which is representative of natural chorus noise [57]. These were placed next to mesh screen cages (Exo Terra Explorarium; 45 × 60 cm) containing 2–5 females. The playback apparatus and cages were placed into quiet areas of the study site (i.e. away from active frog choruses). Females entered the experience treatments immediately after capture (21.00–22.00 h), and remained exposed to the playbacks for 3 h. Immediately after the conclusion of their playback treatment females were tested for their preference functions and their choosiness.
(ii). Variation in body size
We measured body size as snout–vent length (SVL), which is measured from the tip of the nose to the end of the vent. We took advantage of the natural size variation of reproductively active females in our study population (range: 34.5–56.8 mm, n = 63) to test whether the relationship between body size and preference functions was different from the relationship between body size and choosiness.
(d). Statistical analysis
We started the analysis by examining the extent of individual variation in preference function shape, preference function traits (peak, tolerance, strength, responsiveness), and choosiness. This analysis revealed substantial variation in function shape, which prompted us to explore variation separately for open and closed functions (preference function traits differed according to function shape; see below).
Then we tested the hypothesis that preference functions and choosiness are independent traits. First, we looked at trait correlations. If preference functions and choosiness are independent, the function traits (peak, tolerance, strength, responsiveness) may be correlated with each other, but they should not be correlated with choosiness. We ran this analysis separately for open and closed preferences because preference function traits differed according to the shape of the preferences (see below). Then we conducted a principal component analysis including the four preference functions traits (peak, tolerance, strength, responsiveness) and choosiness. Here, independence of trait types would be supported if preference function traits and choosiness loaded differently onto the principal components. This was indeed the case (see results).
Second, we examined how social experience and body size relate to preference function traits and choosiness. Here the prediction is that if they are indeed independent, then any changes in preference function traits associated with variation in body size or social experience should be uncorrelated with any changes in choosiness. We tested this using standard least square regressions that had PC1 (loading with the function traits of peak, tolerance, strength, responsiveness) or PC2 (loading primarily with choosiness) as the dependent variable. The models had the following explanatory terms: preference function shape, female body size and social experience treatment. Initially, we included all two-way and three-way interactions. The three-way interactions were never significant (p ≥ 0.24), and we removed them to increase statistical power.
All statistical analyses were performed using JMP Pro v. 11.1.0 (SAS Institute Inc., 2015).
3. Results
(a). Variation in preference functions and choosiness
Hyla cinerea females varied in the shape of their preference functions. Out of 62 individuals for which we completed the full battery of trials, 53% had open functions, 42% had closed functions and 5% had flat functions (figure 2b). We removed the three females with flat preference functions from further analysis because those females respond similarly to every tested call trait value and thus did not allow us to extract meaningful preference function traits. Body size differed only slightly between females with open and closed preferences (mean ± s.d. SVL = 47.8 ± 5.1 versus 45.6 ± 4.2 mm, respectively), and this 4% difference was barely detectable (t-test: t = 1.74, d.f. = 55.6, p = 0.09). Function shape was also not influenced by social experience (, p = 0.98). Nota bene: removal of the females with flat preferences did not bias our subsequent analysis: females with flat or curved (either open or closed) functions did not differ significantly in body size (t-test: t = −1.70, d.f. = 2.18, p = 0.22) or choosiness (t = −2.14, d.f. = 2.27, p = 0.15), and flat functions were not more common after certain experience treatments (, p = 0.30).
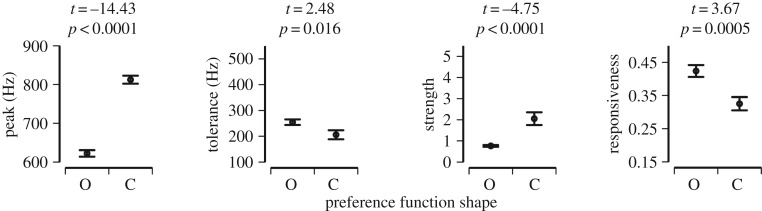
Individual females varied widely in their preference function characteristics (both shape as well as function traits; figures 2–5). All preference function traits (peak, tolerance, strength and responsiveness) differed significantly between functions of open and closed shape (figure 3).
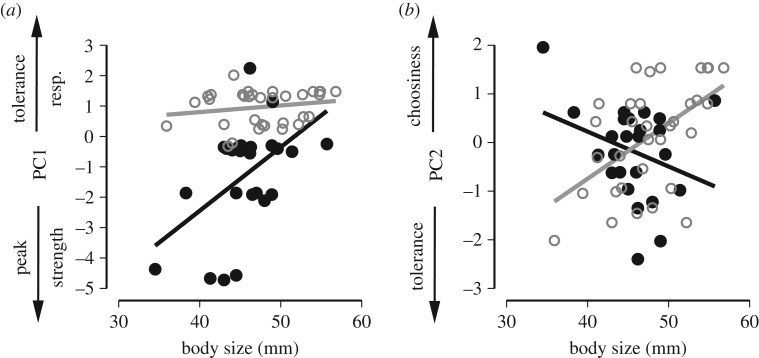
Figure 5.
Influence of body size and function shape on female mating preferences. (a) Body size influenced preference functions traits (summarized as PC1) in females with closed preference functions, but not open ones. (b) Body size also influenced choosiness (summarized as PC2); large females with open preference functions and small females with closed preference functions are more choosy.
Figure 3.
Differences in preference functions traits between open and closed preference functions. y-axis indicates range of individual variation in preference trait values. Shown are mean ± s.e., and the results of t-tests comparing function shapes.
Individual females also varied in choosiness, and the range of choosiness scores across all females spanned the gamut from 0 to 24 dB attenuation difference (mean ± s.d.: 13.3 ± 6.9 dB). This range of choosiness is remarkable, especially when translated from dB difference to the distance the choosy females are willing to walk to reach their preferred mate. Using the inverse square law of sound attenuation, a very choosy female with a 24 dB score would walk 16 m further than a non-choosy female with a 0 dB score (only willing to walk to the more attractive call if it is equidistant to the unattractive one).
The wide range of variation in individual peak preferences (600–900 Hz; figures 2 and 3) meant that the attractive stimulus used in the choosiness trials (800 Hz, derived from population-based trials) did not correspond to every female's preferred value. We used the formula ABS(800-peak) to calculate the difference of each female's function peak to the 800 Hz stimulus, and then correlated those values with their measures of choosiness. This analysis revealed no significant correlation (n = 58, r = 0.15, p = 0.26), and the positive slope (i.e. choosiness increased with larger 800-peak differences) was opposite the pattern expected had preference function peak affected our choosiness measure.
(b). Correlation between preference functions and choosiness
Preference function traits (peak, tolerance, strength and responsiveness) were highly correlated with each other, but were almost never correlated with choosiness (table 1). Preference function shape played an important role for determining correlations between different preference function traits, as well as between function traits and choosiness. Only two out of six trait correlations (tolerance–strength, and peak–responsiveness) were similar in sign and magnitude between closed and open preference functions, while four trait correlations were different in sign and/or magnitude (table 1). Further, only in open preference functions was a preference function trait (strength) significantly correlated with choosiness—but here our criterion for analysis discounts this difference as potentially spurious [58], and in any case the correlation was not strong (table 1).
Table 1.
Correlations between preference function traits and choosiness. The strongest correlations are those between the four preference function traits: peak, tolerance, strength and responsiveness. Correlations calculated separately for closed and open preference functions; significant correlations are shown in italics.
| tolerance | strength | responsiveness | choosiness | |
|---|---|---|---|---|
| closed | ||||
| peak | −0.44 | 0.76 | −0.77 | −0.04 |
| tolerance | −0.76 | 0.86 | −0.17 | |
| strength | −0.94 | 0.01 | ||
| responsiveness | −0.10 | |||
| open | ||||
| peak | 0.83 | −0.68 | −0.76 | −0.07 |
| tolerance | −0.70 | −0.33 | 0.05 | |
| strength | 0.51 | 0.38 | ||
| responsiveness | 0.28 | |||
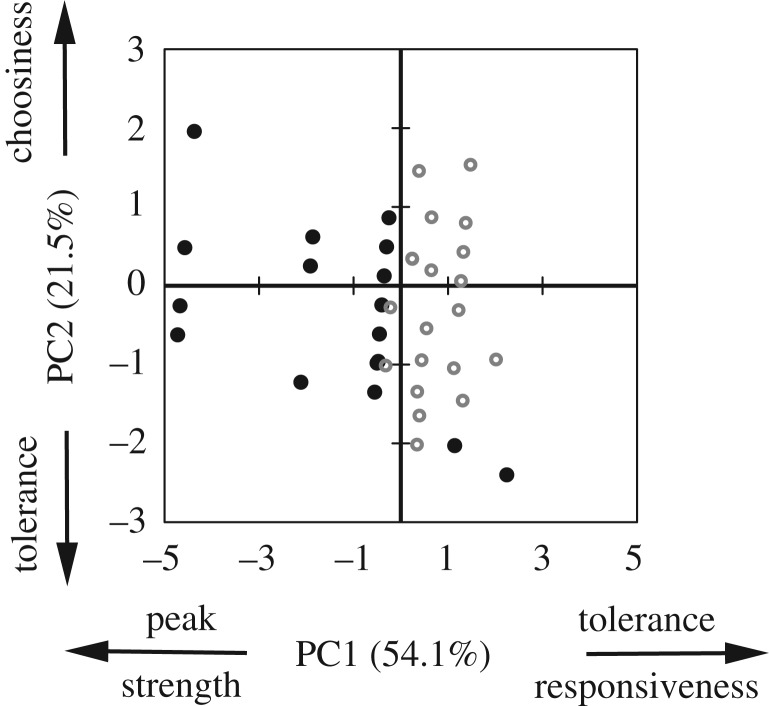
This result was corroborated by the principal component analysis. The PCA returned two principal components with eigenvalues larger than 1. PC1 had an eigenvalue of 2.70, and loaded with the four preference function traits (peak, tolerance, strength, responsiveness). Note that factor loadings differ in sign, such that a decrease in strength is related to an increase in tolerance and responsiveness (table 2, figure 4). PC2 had an eigenvalue of 1.08, and loaded mainly with choosiness, and also with tolerance; note that factor loadings differ in sign, such that an increase in choosiness is related to a decrease in tolerance (table 2, figure 4). Together the two first PCs accounted for 75.6% of the variation.
Table 2.
Factor loading on the first two principal components, which together account for 75.6% of variation in mate choice traits. Factor loading values >0.4 are shown in italics.
| factor | PC1 | PC2 |
|---|---|---|
| peak | −0.489 | −0.235 |
| strength | −0.554 | 0.214 |
| tolerance | 0.412 | −0.433 |
| responsiveness | 0.520 | 0.171 |
| choosiness | 0.113 | 0.826 |
Figure 4.
Principal components of female mating preferences. PC1 loaded mainly with the four preference function traits (peak, strength, tolerance, responsiveness; table 2). PC2 loaded mainly with choosiness, and also one function trait (tolerance) (table 2).
(c). Relationship of body size and social experience with preference functions (PC1) and choosiness (PC2)
The variables we assessed related differently to preference function traits (PC1) and choosiness (PC2) (table 3, figure 5). Overall, social experience never had an effect. Body size did affect preference functions and choosiness, yet in different ways.
Table 3.
Sources of variation on PC1 (preference function traits) and PC2 (mainly choosiness). Significant or marginally significant factors are shown in italics.
| factor | d.f. | F | p |
|---|---|---|---|
| PC1 | |||
| treatment | 3,45 | 1.52 | 0.22 |
| female size | 1,45 | 4.94 | 0.03 |
| function shape | 1,45 | 41.15 | <0.0001 |
| treatment × female size | 3,45 | 0.25 | 0.87 |
| treatment × function shape | 3,45 | 1.35 | 0.27 |
| female size × function shape | 1,45 | 3.08 | 0.058 |
| PC2 | |||
| treatment | 3,45 | 1.50 | 0.23 |
| female size | 1,45 | 1.40 | 0.24 |
| function shape | 1,45 | 1.15 | 0.29 |
| treatment × female size | 3,45 | 0.34 | 0.79 |
| treatment × function shape | 3,45 | 0.78 | 0.51 |
| female size × function shape | 1,45 | 4.42 | 0.04 |
Preference function traits (PC1) differed between individuals with open and closed preference functions, and were related to body size. Body size mainly had an influence on individual with closed preference functions (see significant size × shape interaction term in table 3 and figure 5a). Larger females with closed functions preferred lower-frequency calls, but (due to low strength and high tolerance and responsiveness) have a high likelihood of accepting trait values other than their preferred value. Small females with closed functions, on the other hand, prefer higher-frequency calls, and due to high strength and low tolerance and responsiveness are not expected to accept trait values that deviate much from their peak.
By contrast, choosiness (PC 2) did not vary on average between individuals with closed and open preferences (table 3). However, because of the inverse relationship between function shape and body size (significant size × shape interaction term in table 3 and figure 5b), larger females with open preferences are choosier, as are smaller females with closed preferences.
4. Discussion
Our results support the hypothesis that mate preference functions and choosiness are independent traits [15]. In our population of H. cinerea, preference function traits and choosiness were predominantly uncorrelated, and variation in preference function traits and choosiness, though both associated with body size, were affected by body size in different ways. This finding has several implications for our understanding of sexual selection via mate choice and its consequences for speciation. First, the absence of a phenotypic correlation between the preference function traits and choosiness suggests that the genetic correlation too may be absent or weak [59]. If so, preference functions and choosiness may evolve independently, each being tweaked or optimized by natural and sexual selection, potentially without major trade-offs. Additionally, it also supports the intuition that preferences and choosiness should be modelled as being influenced by different loci (cf. [14]), and that choosiness should not be automatically equated with aspects of the preference function such as what we term tolerance, strength and responsiveness (see discussion in [27]). More studies in other species and groups are necessary to determine how widespread our findings may be.
A second interesting point is that variation in preference functions and choosiness, whether between individuals, populations or species, may interact in ways that bring about various evolutionary consequences. For instance, choosiness may determine the strength of selection due to mate choice, while the tolerance of the preference function may determine the amount of variation permitted around the peak of the preference (figure 1). If so, choosiness may determine the speed at which equilibrium is attained, and preference tolerance may in turn determine the variation sustained at equilibrium. The interplay between preference functions and choosiness may generate considerable variation in the resulting patterns of assortative mating and signal-preference linkage disequilibrium, as well as in the consequences for the maintenance of genetic variation and the promotion of divergence. A related question: which is a stronger determinant of the strength of sexual selection due to mate choice—choosiness or the preference function traits that describe its curvature around the peak?
Also of particular interest is our finding of considerable variation in all the components of mate choice: overall preference shape, the different preference function traits, and choosiness. The population-level preference function had a closed shape with a peak beyond the population mean for the call trait (figure 2c). This would suggest moderately strong directional selection on male call frequency. However, the population contained near equal numbers of females with open and closed preferences. Their relative contributions to selection on signals will vary according to their choosiness, as per the above rationale. However, their contributions will also vary in an additional sense: the peak of open preferences was further away from the male mean than the peak of closed preferences (figure 2c). Consequently, females with open preferences favour more extreme male signals than females with closed preferences—although their greater tolerance, lower strength and higher responsiveness suggest they would exert overall weaker selection. These patterns of variation suggest how female preferences may actually be involved in the maintenance of variation in signals. While relatively understudied, there is evidence that such within-population variation may be common, at least in terms of individual differences in peak preference, preference tolerance, and whether females have a preference or not [15,60–62].
Both preference functions and choosiness varied continuously, suggesting inputs from many loci. This seems to be common in nature, at least for preference functions [26], as well as the ability of preference functions and choosiness to be modified by variables that span the gamut from the age, condition, or reproductive stage of the choosing individual to the social environment they experienced during certain stages of their life [24–26,30,31,41,42,47,63–65]. We find that if a given factor changes the preference function, it will probably not change choosiness, or it will change it in a different way. For example, body size affected both function traits and choosiness, but for function traits it was the closed functions that were mostly affected, while for choosiness both closed and open functions were affected, but in opposite directions. Moreover, although body size affected both function traits and choosiness of females with closed functions, the effect was with opposite signs. One outcome of this independent influence is a higher likelihood that the plastic response by each of these components may also be shaped independently by selection.
In conclusion, we find that preference functions and choosiness are distinct traits that may interact in various ways to generate mate choice decisions. A full understanding of how mate choice contributes to sexual selection and speciation will require the joint study of variation in both of these components to establish whether this pattern is widespread in nature. It will also require assessing how these components interact with other determinants of mate choice decisions, such as the mate sampling rules that are followed by individuals varying in preferences and choosiness [11,15,20,66].
Acknowledgements
We thank K. Kosnicki and C. Lange with their invaluable assistance with data collection and G. Caulkins for access to the East Texas Conservation Center. We would also like to thank J. Kilmer for assistance with technical support, and S. Bertram and an anonymous reviewer for helpful and encouraging comments on the manuscript.
Ethics
Experimental procedures were approved by the Animal Care and Use Committee of the University of Wisconsin–Milwaukee (IACUC 07-08#38).
Data accessibility
Data are archived in the Dryad Digital Repository: https://doi.org/10.5061/dryad.5r6t1mh [67].
Authors' contributions
D.P.N. designed research, performed research, analysed data and wrote paper. R.L.R. analysed data and wrote paper. G.H. designed research, analysed data and wrote paper.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the University of Wisconsin–Milwaukee Research Growth Initiative grant no. 101X104.
References
- 1.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: Murray. [Google Scholar]
- 2.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Andersson M, Simmons LW. 2006. Sexual selection and mate choice. Trends Ecol. Evol. 21, 296–302. ( 10.1016/j.tree.2006.03.015) [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal GG. 2017. Mate choice: the evolution of sexual decision making from microbes to humans. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.West-Eberhard MJ. 1983. Sexual selection, social competition, and speciation. Q Rev. Biol. 58, 155–183. ( 10.1086/413215) [DOI] [Google Scholar]
- 6.Coyne JA, Orr HA. 2004. Speciation. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 7.Rodríguez RL, Boughman JW, Gray DA, Hebets EA, Höbel G, Symes LB. 2013. Diversification under sexual selection: the relative roles of mate preference strength and the degree of divergence in mate preferences. Ecol. Lett. 16, 964–974. ( 10.1111/ele.12142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West-Eberhard MJ. 2014. Darwin's forgotten idea: the social essence of sexual selection. Neurosci. Biobehav. Rev. 46(Pt 4), 501–508. ( 10.1016/j.neubiorev.2014.06.015) [DOI] [PubMed] [Google Scholar]
- 9.Prum RO. 2017. The evolution of beauty: how Darwin's forgotten theory of mate choice shapes the animal world. New York, NY: Anchor. [Google Scholar]
- 10.Chenoweth SF, Blows MW. 2006. Dissecting the complex genetic basis of mate choice. Nat. Rev. Genet. 7, 681 ( 10.1038/nrg1924) [DOI] [PubMed] [Google Scholar]
- 11.Cotton S, Small J, Pomiankowski A. 2006. Sexual selection and condition-dependent mate preferences. Curr. Biol. 16, 755–765. ( 10.1016/j.cub.2006.08.022) [DOI] [PubMed] [Google Scholar]
- 12.Mendelson TC, Fitzpatrick CL, Hauber ME, Pence CH, Rodríguez RL, Safran RJ, Stern CA, Stevens JR. 2016. Cognitive phenotypes and the evolution of animal decisions. Trends Ecol. Evol. 31, 850–859. ( 10.1016/j.tree.2016.08.008) [DOI] [PubMed] [Google Scholar]
- 13.Ryan M. 2018. A taste for the beautiful: the evolution of attraction. Princeton, NJ: Princeton University Press. [Google Scholar]
- 14.Kopp M, et al. 2018. Mechanisms of assortative mating in speciation with gene flow: connecting theory and empirical research. Am. Nat. 19, 1–20. ( 10.1086/694889) [DOI] [PubMed] [Google Scholar]
- 15.Jennions MD, Petrie M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. 72, 283–327. ( 10.1017/S0006323196005014) [DOI] [PubMed] [Google Scholar]
- 16.Janetos AC. 1980. Strategies of female mate choice: a theoretical analysis. Behav. Ecol. Sociobiol. 7, 107–112. ( 10.1007/BF00299515) [DOI] [Google Scholar]
- 17.Parker GA. 1983. Mate quality and mating decisions. In Mate choice (ed. PPG Bateson), pp. 141–166 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 18.Dombrovsky Y, Perrin N. 1994. On adaptive search and optimal stopping in sequential mate choice. Am. Nat. 144, 355–361. ( 10.1086/285680) [DOI] [Google Scholar]
- 19.Ritchie MG. 1996. The shape of female mating preferences. Proc. Natl Acad. Sci. USA 93, 14 628–14 631. ( 10.1073/pnas.93.25.14628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner RH. 1998. Hidden leks: sexual selection and the clustering of avian territories. Ornithol. Monogr. 49, 123–145. ( 10.2307/40166721) [DOI] [Google Scholar]
- 21.Shaw KL, Herlihy DP. 2000. Acoustic preference functions and song variability in the Hawaiian cricket Laupala cerasina. Proc. R. Soc. Lond. B 267, 577–584. ( 10.1098/rspb.2000.1040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez RL, Ramaswamy K, Cocroft RB. 2006. Evidence that female preferences have shaped male signal evolution in a clade of specialized plant-feeding insects. Proc. R. Soc. B 273, 2585–2593. ( 10.1098/rspb.2006.3635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez RL, Rebar D, Fowler-Finn KD. 2013. The evolution and evolutionary consequences of social plasticity in mate preferences. Anim. Behav. 85, 1041–1047. ( 10.1016/j.anbehav.2013.01.006) [DOI] [Google Scholar]
- 24.Fowler-Finn KD, Rodríguez RL. 2012. Experience mediated plasticity in mate preferences: mating assurance in a variable environment. Evolution 66, 459–468. ( 10.1111/j.1558-5646.2011.01446.x) [DOI] [PubMed] [Google Scholar]
- 25.Fowler-Finn KD, Rodríguez RL. 2012. The evolution of experience-mediated plasticity in mate preferences. J. Evol. Biol. 25, 1855–1863. ( 10.1111/j.1420-9101.2012.02573.x) [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez RL, Hallett AC, Kilmer J, Fowler-Finn KD. 2013. Curves as traits: genetic and environmental variation in mate preference functions. J. Evol. Biol. 26, 434–442. ( 10.1111/jeb.12061) [DOI] [PubMed] [Google Scholar]
- 27.Kilmer JT, Fowler-Finn KD, Gray DA, Höbel G, Rebar D, Reichert MS, Rodríguez RL. 2017. Describing mate preference functions and other function-valued traits. J. Evol. Biol. 30, 1658–1673. ( 10.1111/jeb.13122) [DOI] [PubMed] [Google Scholar]
- 28.Lindström K, Lehtonen TK. 2013. Mate sampling and choosiness in the sand goby. Proc. R. Soc. B 280, 20130983 ( 10.1098/rspb.2013.0983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Judge KA, Ting JJ, Gwynne DT. 2014. Condition dependence of female choosiness in a field cricket. J. Evol. Biol. 27, 2529–2540. ( 10.1111/jeb.12509) [DOI] [PubMed] [Google Scholar]
- 30.Neelon DP, Höbel G. 2017. Social plasticity in choosiness in green tree frogs, Hyla cinerea. Behav. Ecol. 28, 154–1546. ( 10.1093/beheco/arx103) [DOI] [Google Scholar]
- 31.Kuczynski MC, Getty T, Gering E. 2017. Larger females are choosier in the gray treefrog (Hyla versicolor). Behav. Processes 135, 29–35. ( 10.1016/j.beproc.2016.11.019) [DOI] [PubMed] [Google Scholar]
- 32.Mead LS, Arnold SJ. 2004. Quantitative genetic models of sexual selection. Trends Ecol. Evol. 19, 264–271. ( 10.1016/j.tree.2004.03.003) [DOI] [PubMed] [Google Scholar]
- 33.Kuijper B, Pen I, Weissing FJ. 2012. A guide to sexual selection theory. Annu. Rev. Ecol. Evol. Syst. 43, 287–311. ( 10.1146/annurev-ecolsys-110411-160245) [DOI] [Google Scholar]
- 34.Gerhardt HC, Tanner SD, Corrigan CM, Walton HC. 2000. Female preference functions based on call duration in the gray tree frog (Hyla versicolor). Behav. Ecol. 11, 663–669. ( 10.1093/beheco/11.6.663) [DOI] [Google Scholar]
- 35.Höbel G, Gerhardt HC. 2003. Reproductive character displacement in the acoustic communication system of green treefrogs (Hyla cinerea). Evolution 57, 894–904. ( 10.1111/j.0014-3820.2003.tb00300.x) [DOI] [PubMed] [Google Scholar]
- 36.Gray DA. 1999. Intrinsic factors affecting female choice in house crickets: time cost, female age, nutritional condition, body size, and size-relative reproductive investment. J. Insect Behav. 12, 691–700. ( 10.1023/A:1020983821436) [DOI] [Google Scholar]
- 37.McPeek MA, Gavrilets S. 2006. The evolution of female mating preferences: differentiation from species with promiscuous males can promote speciation. Evolution 60, 1967–1980. ( 10.1111/j.0014-3820.2006.tb01835.x) [DOI] [PubMed] [Google Scholar]
- 38.Bailey NW. 2008. Love will tear you apart: different components of female choice exert contrasting selection pressures on male field crickets. Behav. Ecol. 19, 960–966. ( 10.1093/beheco/arn054) [DOI] [Google Scholar]
- 39.Edward DA. 2014. The description of mate choice. Behav. Ecol. 26, 301–310. ( 10.1093/beheco/aru142) [DOI] [Google Scholar]
- 40.Reinhold K, Schielzeth H. 2015. Choosiness, a neglected aspect of preference functions: a review of methods, challenges and statistical approaches. J. Comp. Physiol. A 201, 171–182. ( 10.1007/s00359-014-0963-6) [DOI] [PubMed] [Google Scholar]
- 41.Hunt J, Brooks R, Jennions MD. 2005. Female mate choice as a condition-dependent life-history trait. Am. Nat. 166, 79–92. ( 10.1086/430672) [DOI] [PubMed] [Google Scholar]
- 42.DuVal EH, Kapoor JA. 2015. Causes and consequences of variation in female mate search investment in a lekking bird. Behav. Ecol. 26, 1537–1547. ( 10.1093/beheco/arv110) [DOI] [Google Scholar]
- 43.Gerhardt HC. 1974. The significance of some spectral features in mating call recognition in the green treefrog (Hyla cinerea). J. Exp. Biol. 61, 229–241. [DOI] [PubMed] [Google Scholar]
- 44.Gerhardt HC. 1981. Mating call recognition in the green treefrog (Hyla cinerea): importance of two frequency bands as a function of sound pressure level. J. Comp. Physiol. 144, 9–16. ( 10.1007/BF00612792) [DOI] [Google Scholar]
- 45.Gerhardt HC, Daniel RE, Perrill SA, Schramm S. 1987. Mating behaviour and male mating success in the green treefrog. Anim. Behav. 35, 1490–1503. ( 10.1016/S0003-3472(87)80021-0) [DOI] [Google Scholar]
- 46.Höbel G. 2010. Interaction between signal timing and signal feature preferences: causes and implications for sexual selection. Anim. Behav. 79, 1257–1266. ( 10.1016/j.anbehav.2010.02.026) [DOI] [Google Scholar]
- 47.Hebets EA, Sullivan-Beckers L. 2010. Mate choice and learning. In Encylcopedia of animal behavior (eds MD Breed, J Moore), vol. 2, pp. 389–393. London, UK: Academic Press. [Google Scholar]
- 48.Wong RY, So P, Cummings ME. 2011. How female size and male displays influence mate preference in a swordtail. Anim. Behav. 82, 691–697. ( 10.1016/j.anbehav.2011.06.024) [DOI] [Google Scholar]
- 49.Meyer K, Kirkpatrick M. 2005. Up hill, down dale: quantitative genetics of curvaceous traits. Phil. Trans. R. Soc. B 360, 1443–1455. ( 10.1098/rstb.2005.1681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stinchcombe JR, Kirkpatrick M, Function-valued Traits Working Group. 2012. Genetics and evolution of function-valued traits: understanding environmentally responsive phenotypes. Trends Ecol. Evol. 27, 637–647. ( 10.1016/j.tree.2012.07.002) [DOI] [PubMed] [Google Scholar]
- 51.Reichert MS, Höbel G. 2015. Modality interactions alter the shape of acoustic mate preference functions in gray treefrogs. Evolution 69, 2384–2398. ( 10.1111/evo.12750) [DOI] [PubMed] [Google Scholar]
- 52.Underhil VA, Höbel G. 2017. Variation in nocturnal light levels does not alter mate choice behavior in female eastern gray treefrogs (Hyla versicolor). Behav. Ecol. Sociobiol. 71, 151 ( 10.1007/s00265-017-2386-1) [DOI] [Google Scholar]
- 53.Speaks CE. 1997. Introduction to sound acoustics for the hearing and speech sciences. San Diego, CA: Cengage Learning, [Google Scholar]
- 54.Ehret G, Capranica RR. 1980. Masking patterns and filter characteristics of auditory nerve fibers in the green treefrog (Hyla cinerea). J. Comp. Physiol. 141, 1–12. ( 10.1007/BF00611872) [DOI] [Google Scholar]
- 55.Beckers OM, Schul J. 2004. Phonotaxis in Hyla versicolor (Anura, Hylidae): the effect of absolute call amplitude. J. Comp. Physiol. A 190, 869–876. [DOI] [PubMed] [Google Scholar]
- 56.Höbel G, Gerhardt HC. 2007. Sources of selection on signal timing in a tree frog. Ethology 113, 973–982. ( 10.1111/j.1439-0310.2007.01404.x) [DOI] [Google Scholar]
- 57.Vélez A, Höbel G, Gordon NM, Bee MA. 2012. Dip listening or modulation masking? Call recognition by green treefrogs (Hyla cinerea) in temporally fluctuating noise. J. Comp. Physiol. A 198, 891–904. ( 10.1007/s00359-012-0760-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moran MD. 2003. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100, 403–405. ( 10.1034/j.1600-0706.2003.12010.x) [DOI] [Google Scholar]
- 59.Roff DA. 1997. Evolutionary quantitative genetics. New York, NY: Chapman and Hall. [Google Scholar]
- 60.Wagner WE, Murray AM, Cade WH. 1995. Phenotypic variation in the mating preferences of female field crickets, Gryllus integer. Anim. Behav. 49, 1269–1281. ( 10.1006/anbe.1995.0159) [DOI] [Google Scholar]
- 61.Hedrick A, Weber T. 1998. Variance in female responses to the fine structure of male song in the field cricket, Gryllus integer. Behav. Ecol. 9, 582–591. ( 10.1093/beheco/9.6.582) [DOI] [Google Scholar]
- 62.Murphy CG, Gerhardt HC. 2000. Mating preference functions of individual female barking treefrogs, Hyla gratiosa, for two properties of male advertisement calls. Evolution 54, 660–669. ( 10.1111/j.0014-3820.2000.tb00067.x) [DOI] [PubMed] [Google Scholar]
- 63.Rosenqvist G, Houde A. 1997. Prior exposure to male phenotypes influences mate choice in the guppy, Poecilia reticulata. Behav. Ecol. 8, 194–198. ( 10.1093/beheco/8.2.194) [DOI] [Google Scholar]
- 64.Byers JA, Byers AA, Dunn SJ. 2006. A dry summer diminishes mate search effort by pronghorn females: evidence for a significant cost of mate search. Ethology 112, 74–80. ( 10.1111/j.1439-0310.2006.01127.x) [DOI] [Google Scholar]
- 65.Uetz GW, Norton S. 2007. Preference for male traits in female wolf spiders varies with the choice of available males, female age and reproductive state. Behav. Ecol. 61, 631–641. ( 10.1007/s00265-006-0293-y) [DOI] [Google Scholar]
- 66.Uy JAC, Patricelli GL, Borgia G (2001) Complex mate searching in the satin bowerbird Ptilonorhynchus violaceus. Am. Nat. 158:530–542. ( 10.1086/323118) [DOI] [PubMed] [Google Scholar]
- 67.Neelon DP, Rodríguez RL, Höbel G. 2019. Data from: On the architecture of mate choice decisions: preference functions and choosiness are distinct traits Dryad Digital Repository. ( 10.5061/dryad.5r6t1mh) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Neelon DP, Rodríguez RL, Höbel G. 2019. Data from: On the architecture of mate choice decisions: preference functions and choosiness are distinct traits Dryad Digital Repository. ( 10.5061/dryad.5r6t1mh) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are archived in the Dryad Digital Repository: https://doi.org/10.5061/dryad.5r6t1mh [67].