Abstract
Objective—
AMP-activated protein kinase (AMPK), an energy and redox sensor, is activated in response to various cellular stresses, including hypoxia, nutrient deprivation, oxidative stress, and fluid shear stress at the site of vessel blockade. The activation of AMPK is involved in angiogenesis. However, it is unknown whether AMPK can influence arteriogenesis. Here, we demonstrate the contribution of macrophage AMPK to arteriogenesis and collateral remodeling and their underlying mechanisms in well-characterized in vivo and in vitro models.
Approach and Results—
AMPKα1, AMPKα2 knockout and wild-type littermates underwent femoral artery ligation. Collateral arteriogenesis was monitored in wild-type, global AMPKα1 knockout, or macrophage-specific AMPKα1 knockout mice, with or without hindlimb ligation. Compared with wild-type mice with ligation, global AMPKα1 knockout mice displayed significant reduction in blood flow recovery and impaired remodeling of collateral arterioles. Similar impairments were observed in macrophage-specific AMPK α1 knockout mice after hindlimb ligation. Mechanistically, we found that AMPKα1 promotes the production of growth factors, such as transforming growth factor β, by directly phosphorylating the inhibitor of nuclear factor κB kinase alpha, resulting in an nuclear factor κB–dependent production of growth factors
Conclusions—
Our findings suggest a novel role for macrophage AMPKα1 in arteriogenesis and collateral remodeling and indicate that AMPKα1 activation might be beneficial for recovery from occlusive vascular disorders.
Keywords: AMP-activated protein kinases; macrophages; mice, knockout; NF-kappa B; transforming growth factor beta
Arteriogenesis is a process of developing collateral circulation through the remodeling and growth of pre-existing collateral arteries after elevated shear stress induced by occlusion.1–3 Arteriogenesis takes place both during embryogenesis and in adult tissues. In the latter case, arteriogenesis, which usually occurs at sites of occlusion or physical disruption of pre-existing arterial conduits such as coronary artery occlusion or femoral artery ligation, plays a vital role in recovery from ischemic insults.4,5 Understanding the biological factors that affect arteriogenesis will aid in the development of new treatments for patients with arterial stenosis and occlusions.
There are 2 commonly considered mechanisms for arteriogenesis: expansion of pre-existing collaterals and de novo arteriogenesis.2,6,7 Inflammation caused by mechanical hemo-dynamic forces, such as shear stress and circumferential wall tension, is considered a pivotal trigger and driver for arteriogenesis.4,8–11 Previous studies have shown that monocytes accumulated in the surrounding tissues of collateral vessels alter arterial occlusion.7,11–13 These macrophages are potent sources of cytokines and growth factors, which are required for natural adaptive arteriogenesis. In spite of a mounting number of putative arteriogenic factors, the exact mechanisms that regulate collateral remodeling are poorly characterized. Furthermore, the processes responsible for arteriogenesis, and its associated molecular signals are poorly understood.
AMP-activated protein kinase (AMPK) is a serine/threo-nine kinase composed of α, β, and γ subunits.14,15 The α sub-unit containing the α1 and α2 isoform is the catalytic subunit, whereas the β and γ are regulatory subunits that maintain the stability of the heterotrimer complex. As an energy sensor, AMPK is activated by various cellular stresses, such as hypoxia, nutrient deprivation, and oxidative stress.16–18 Once activated, AMPK phosphorylates and regulates several downstream kinases that reduce energy demand and increase energy supply to maintain whole-body energy homeostasis.19 In addition, AMPK also regulates many other cellular processes, including cell polarity, cell growth, and proliferation.20–22
Emerging studies have demonstrated that AMPK is activated in response to shear or ischemic stress.23,24 AMPK signaling is required for angiogenesis in vivo and in vitro.25–28 However, there is no information on the consequences of AMPK deletion in arteriogenesis. In this study, we sought to examine the role of monocytes/macrophage AMPK in adult arteriogenesis. We report here that AMPKα1 deletion results in impaired collateral formation, blood flow recovery, and foot movement, as well as necrosis after ligation of the femoral artery.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
AMPKα1 Regulates Plantar Perfusion Recovery After Hindlimb Ischemia
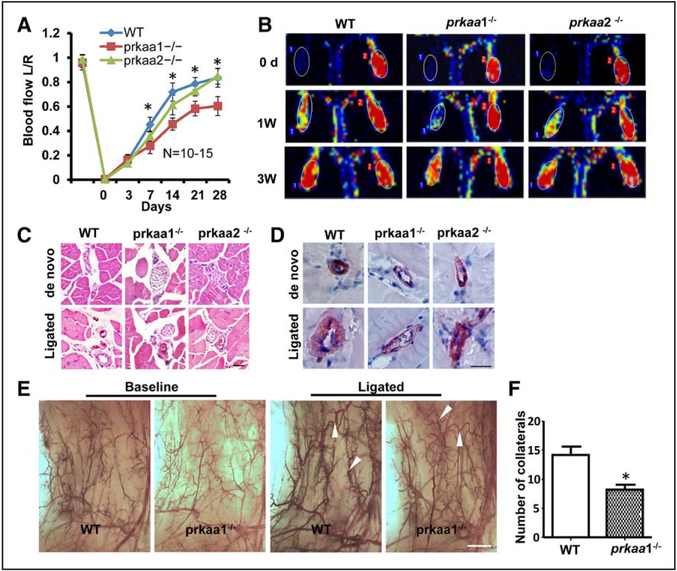
To investigate the role of AMPKα in arteriogenesis, AMPKα1−/−, AMPKα2−/− mice, and wild-type (WT) litter-mates were subjected to hindlimb ischemia brought about by femoral artery ligation, which reduces perfusion to the lower limb, resulting in ischemia in the calf (crural muscle). Blood perfusion in the hind paws (plantar perfusion) was quantified and normalized to sham control to compare various time points (Figure 1A and 1B). The quantitation of perfusion recovery revealed that AMPKα1−/− mice had a greater drop in perfusion than did AMPKα2−/− mice, from 1 to 4 weeks after surgery, P value <0.05 (Figure 1A). However, there was no difference in perfusion recovery among the 3 groups of mice from 0 to 3 days (Figure 1A), implying that AMPKα1 deletion did not affect pre-existing collaterals, and that the impaired recovery in AMPKα1-deleted mice occurred only after 3 days.
Figure 1.
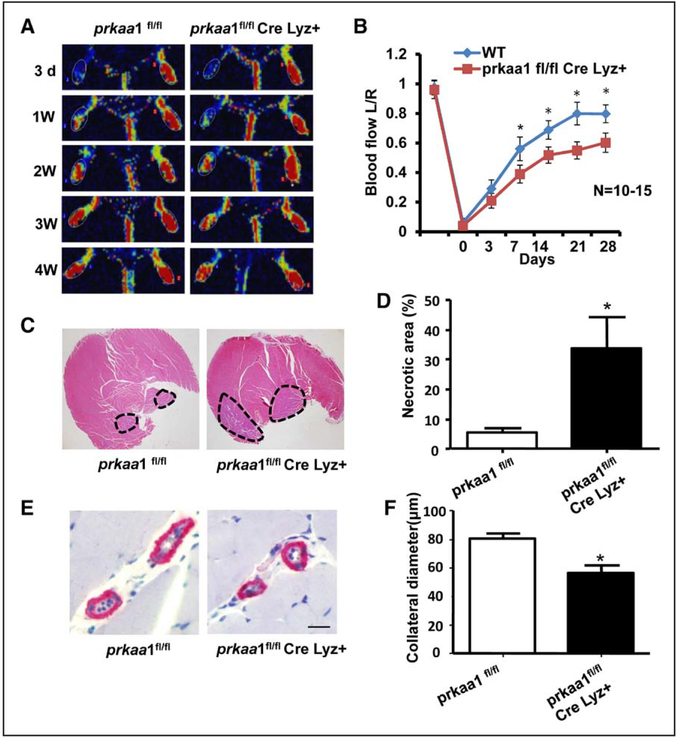
Deletion of AMPKα1 affects blood flow and collateral remodeling after ligation of the femoral artery (FAL). Blood flow, collateral remodeling were assessed in wild-type (WT) control and knockout (KO) mice. A, Quantitative Laser Doppler analyses showing the ratio of left plantar perfusion:right plantar perfusion after occlusion of the femoral artery. AMP-activated protein kinase (AMPK) α1, but not AMPKα2 deletion, mice displayed delayed restoration of perfusion compared with WT control mice. B, Representative images obtained using Laser Doppler Imaging, showing the efficiency of the surgery distal to the occlusion side and the recovery of perfusion at the indicated time points. C, Representative hematoxylin and eosin staining of adductor muscle sections from ligated and nonligated limbs in KO and WT mice (scale bar, 50 μm). D, Staining for smooth muscle α-actin to analyze collateral lumen wall (scale bar, 50 μm). E, Whole-mount visualization of hindlimb arteries through pigment particle perfusion (scale bar, 1 mm). F, Transparent hindlimb muscle of the ligated side of the mice at 7 d post surgery showed a significantly lower number of enlarged collateral arteries in AMPKα1−/− mice than in WT mice (*P<0.05 vs WT, n=7).
AMPKα1 Deletion Impairs Adult Arteriogenesis and Angiogenesis
Next, we measured the diameter of collaterals in adductor muscle by histomorphometry. There was no difference in the baseline (de novo) diameter of collaterals between the 3 genotypes (Figure 1C; Figure IA in the online-only Data Supplement). However, the diameters of AMPKα1−/− collaterals (46.25±2.09 μm) were markedly smaller than those of WT collaterals (78.67±3.82 μm) at 1 week post ligation (Figure 1C; Figure IA in the online-only Data Supplement), indicating that impaired perfusion recovery in the absence of AMPKα1 was likely ascribed to poor arteriogenesis.
During arteriogenesis, collateral vessels undergo extensive remodeling involving thickening of the tunica media, which is composed of α-smooth muscle actin-positive smooth muscle cells (SMCs), and extension of vessel diameter. Numbers and total areas of α-smooth muscle actin-positive collateral vessels were comparable in both genotypes at baseline, but lower in AMPKα1−/− adductors after ischemia, compared with WT (Figure 1D; Figure IB and IC in the online-only Data Supplement). Based on the protocol, significant growth and the characteristic changes of collateral vessels occur at day 7 after artery occlusion. Therefore, we observe arteriogenesis at this time point. Whole-mount visualization of hindlimb arteries by pigment particle perfusion showed that the number of collaterals that grew to large caliber conduction vessels with a corkscrew pattern was significantly reduced (8.2±0.86) in the AMPKα1−/− hindlimb 7 days after femoral artery ligation, compared with WT mice (14.2±1.43; Figure 1E and 1F). Compared with baseline (de novo) collateral vessel, femoral artery ligation induces expansion of pre-existing collateral vessels in both genotype mice (Figure 1C and 1E). The collateral remodeling involves the expansion of pre-existing collaterals and de novo arteriogenesis (Figure 1C and 1F). These data show that the collateral vessels of AMPKα−/− mice at baseline were similar to those of WT mice, but the capacity for arteriogenesis (expansion of pre-existing collaterals and de novo arteriogenesis) in AMPKα1−/− mice is decreased after femoral artery ligation.
In addition, we also observed capillary growth (angiogenesis) in ischemic calf tissues by endothelial immunofluorescent staining of the cross sections with anti-CD31 antibody (Figure IIA in the online-only Data Supplement). Consistent with our earlier study,26 we found that capillary density was lower in calf tissues from AMPKα1−/− mice than in those from AMPKα2−/− or WT mice at day 7 after femoral artery ligation (Figure IIB in the online-only Data Supplement). As expected, AMPKα1 deletion significantly reduced levels of vascular endothelial growth factor (VEGF) and transforming growth factor (TGF)-β (Figure IIC and IID in the online-only Data Supplement). To determine the contributions of endothelial progenitor cells, we detected endothelial progenitor cell numbers by flow cytometry in circulating blood of mice with hindlimb ischemia. Global deletion of AMPK α1 did not alter the number of endothelial progenitor cells (Figure IIE in the online-only Data Supplement). Taken together, these results suggest that macrophage AMPKα1 is important in arteriogenesis.
Because of impaired collateral vessel remodeling and angiogenesis, muscles from ligated limbs in AMPKα1−/− mice exhibited more extensive areas of necrosis than those in control mice at day 7 after surgery (Figure IIIA and IIIB in the online-only Data Supplement). By using a scoring system based on active foot movements, the functional outcomes of impaired revascularization in AMPKα1−/− mice were further observed. Foot movements in the ligated limb were markedly reduced, P<0.05 at days 7, 14, 21, and 28 in AMPKα1−/− mice compared with control mice (Figure IIIC in the online-only Data Supplement), indicating that AMPK activity is required for proper vascular recovery after femoral artery ligation.
AMPKα1 Deletion Reduces Accumulation of Macrophages in Ischemic Hindlimb
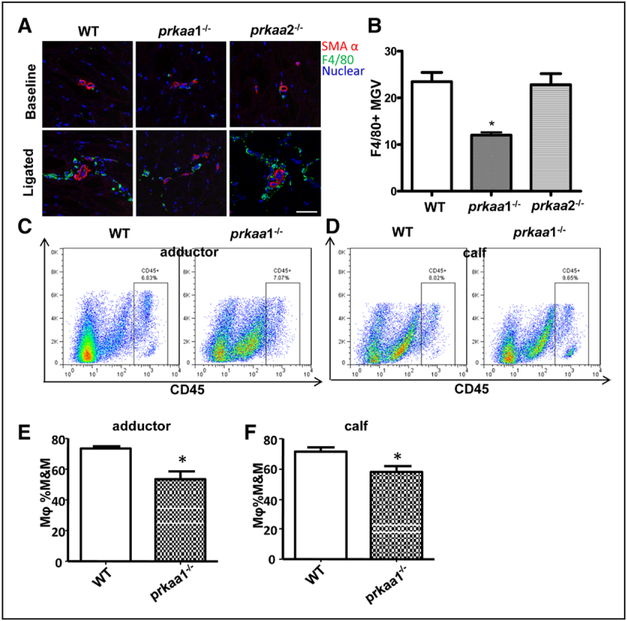
Because inflammatory cells, particularly macrophages, are mainly responsible for collateral vessel remodeling, macrophage accumulation in the adjacent tissues of collateral vessels reach peak at 3 to 4 day after ligation; therefore, we first conducted immunostaining to analyze a macrophage marker (F4/80) in adductor muscle sections from AMPKα1−/−, AMPKα2−/− and WT mice 3 days after femoral artery ligation. There was significant accumulation of macrophage in perivessel of adductor tissues in all 3 genotype mice after hindlimb ischemia (Figure 2A and 2B). However, the numbers of accumulated macrophage in AMPKα1−/− mice were significantly lower than those in WT and AMPKα2−/− mice. To further test whether AMPKα1−/− influences macrophage and other inflammatory cells involved in ligation of the femoral artery (FAL)–triggered arteriogenesis, we analyzed the accumulation of various leukocyte subsets in the ischemic hindlimb. Flow cytometry analysis showed that neither the number of CD45+ leukocytes in tissues (Figure 2C and 2D; Figure IVA and IVB in the online-only Data Supplement) nor the ratios of monocytes and macrophages in CD45+ leukocytes (Figure IVC and IVD in the online-only Data Supplement) were different between WT and AMPKα1 deletion mice 3 days after surgery. In contrast, the percentage of macrophages was more significantly reduced in adductor and calf tissues from AMPKα1−/− mice than in their counterparts from control mice (Figure 2E and 2F), and the ratios of monocytes to macrophages were significantly higher in adductor and calf tissues from AMPKα1−/− mice than ratios from control tissues (Figure IVE and IVF in the online-only Data Supplement). Altogether, these results suggest that AMPKα1 deletion impairs monocyte to macrophage terminal differentiation in ischemic tissues.
Figure 2.
Deletion of AMPKα1 decreases the accumulation of macrophages in the muscle from ischemic hind limbs in vivo. A, Representative immunostaining with an antibody against F4/80, a macrophage marker, of adductor muscle sections from wild-type (WT), AMPKα1−/− and AMPKα2−/− mice 3 d after femoral artery ligation (scale bar, 50 μm). B, Leica Application Suite Advanced Fluorencence Lite (LAS AF Lite) quantification of F4/80+ signal mean gray value (MGV) in 1259.57 μm2 region of interest in adductor sections from mice 3 d after ligation of the femoral artery. *P<0.05 versus WT, n=5. C and D, Representative flow cytometric contour plots for CD45+ cells in adductor (C) and calf tissue (D). E and F, Quantification of the ratio of macrophages in blood-derived mononuclear cells by flow cytometry in adductor muscle (E) and calf muscle (F) from WT and AMPKα1−/− mice 3 d after femoral artery ligation. *P<0.05 vs WT, n=7. M&M indicates monocyte and macrophage.
AMPKα1 Deletion Inhibits the Expression of Growth Factors in Macrophages
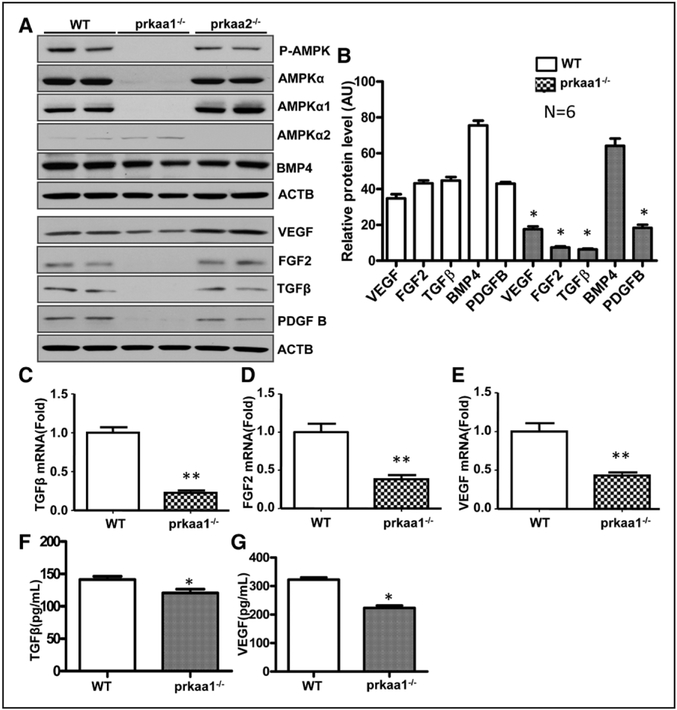
To further explore whether AMPKα1 deletion influences macrophage functions, macrophages were sorted from ischemic hindlimb tissue by flow cytometry, and their ability to generate growth factors was determined through real-time polymerase chain reaction. In addition, Western blot was utilized to determine protein levels in bone marrow–derived macrophages (BMDMs). As depicted in Figure 3A through 3E, both mRNA and protein levels of TGFβ, fibroblast growth factor (FGF)-2, VEGF, and platelet-derived growth factor subunit B were significantly reduced in AMPKα1−/− macrophages, but not in AMPKα1−/− macrophages. In addition, we measured the capability of macrophage to secrete soluble factors in WT and AMPKα1−/− mice and found that there was a lower level of VEGF and TGFβ in AMPKα1−/− BMDM supernatant than in WT BMDM supernatant (Figure 3F and 3G). We also detected levels of VEGF and TGFβ in circulation of mice with FAL. As shown in Figure IIC and IID in the online-only Data Supplement, levels of VEGF and TGFβ in circulation were lower in AMPKα1−/− than in WT mice 3 days after FAL. Overall, these results suggest that AMPKα1 deletion inhibits the generation or secretion of growth factors in macrophages.
Figure 3.
AMP-activated protein kinase (AMPK)-α1 deletion affects growth factor expression in macrophages. A, Western blot determination of transforming growth factor (TGF)-β1, fibroblast growth factor (FGF) 2, vascular endothelial growth factor (VEGF), bone morphogenetic protein 4 (BMP4), and platelet-derived growth factor subunit B (PDGF-B) protein levels in bone marrow–derived macrophages from wild-type (WT) and AMPKα1−/− mice. B, Quantification of data in A. *P<0.05 vs WT, n=4. C–E, Real-time polymerase chain reaction analyses of the mRNA levels of TGFβ1 (C), FGF2 (D), and VEGF (E) in sorted macrophages from adductor muscle in WT and AMPKα1−/− mice 3 d after femoral artery ligation. *P<0.05 vs WT, n=4. F, ELISA determination of TGFβ level in BMDM supernatant. *P<0.05 vs WT, n=5. G, ELISA determination of VEGF level in BMDM supernatant. *P<0.05 vs WT, n=5.
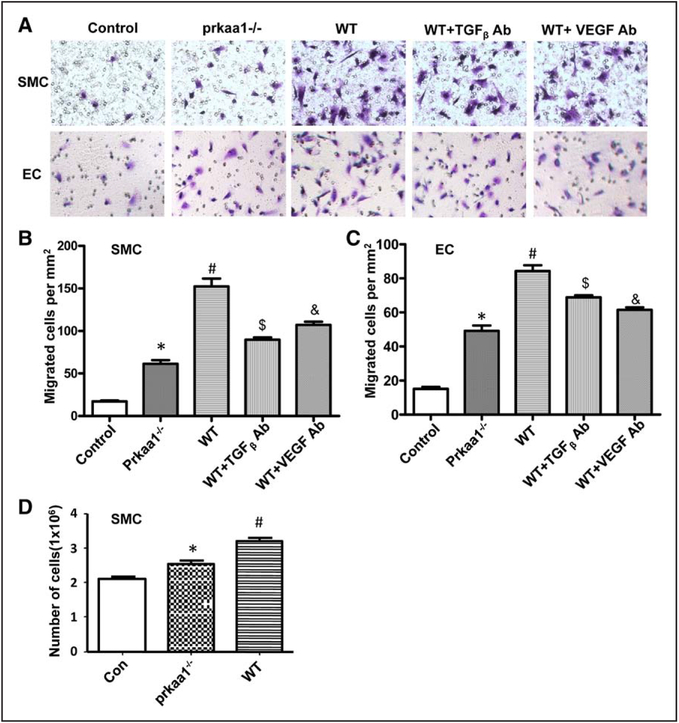
Next, we assessed the effects of macrophages isolated from WT and AMPKα1−/− mice on the migration and proliferation of endothelial cells and vascular smooth muscle cells, the 2 main cellular components of arteries. Soluble factors released by both AMPKα1−/− and WT BMDMs increased the migration and proliferation of vascular smooth muscle cells s (Figure 4A and 4B). However, the ability of AMPKα1−/− macrophage–conditioned medium to enhance the migration and proliferation of vascular smooth muscle cells was significantly lower than that of WT macrophage–conditioned medium (Figure 4A, 4B, and 4D). Consistent with this, endothelial cells exposed to conditioned medium from AMPKα1−/− macrophages showed less migration than those exposed to WT macrophage–conditioned medium (Figure 4C). To test a relative contribution of soluble factors secreted by macrophages to SMC and endothelial cell functions, neutralizing antibodies (anti-VEGF and anti-TGFβ) were used to block corresponding factors in WT macrophage supernatant. As expected, the neutralizing antibodies diminished the effect of WT macrophage supernatant on SMC and endothelial cell (Figure 4A through 4C), indicating that macrophage impacts the behavior of SMC and endothelial cell through secretion of growth factors. Collectively, these data show that the ability of macrophages from AMPKα1−/− mice to promote the recruitment and growth of the 2 main cellular components of arteries is reduced compared with that of WT macrophages, and the reduction is attributed to AMPKα1−/− macrophage–secreting less growth factors.
Figure 4.
Macrophage-secreted growth factor sustaining smooth muscle cell (SMC) and endothelial cell (EC) migration. A, Micrographs of crystal-violet–stained SMC and EC migration triggered by macrophage-conditioned medium, from 3 independent experiments. B and C, Quantification of migration of crystal-violet–stained SMCs (B) and ECs (C) toward macrophage-conditioned medium. *P<0.05 vs WT, $P<0.05 vs WT, &P<0.05 vs WT, #P<0.05 vs control group, from 3 independent experiments. D, Quantification of SMC growth in response to macrophage-conditioned medium. *P<0.05 vs WT, from 3 independent experiments. TGF indicates transforming growth factor; and VEGF, vascular endothelial growth factor.
Impairment of Arteriogenesis Is Because of AMPKα1 Deficiency in Macrophages
To more precisely assess the intrinsic role of AMPK in macrophage function, and to demonstrate whether the impairment of arteriogenesis in AMPKα1−/− mice is macrophage dependent, myeloid specific, AMPKα1-deletion mice (called macrophage-specific knockout mice) were generated by breeding Lysozyme Cre and AMPKα1 flox/flox mice and were subjected to femoral artery ligation as was done in the global AMPK knockout mice. Blood flow perfusion was monitored after ligation at different times. As shown in Figure 5A and 5B, hindlimb perfusion recovery was attenuated in AMPKα1flox/flox; Lys Cre mice, compared with littermate controls. In addition, compared with control mice at day 7 after ligation, collateral modeling in AMPKα1flox/flox; Lys Cre mice was markedly decreased (Figure 5E and 5F) in parallel with an increase in calf muscle necrosis (Figure 5C and 5D). Taken together, these results suggest that AMPKα1 deletion impairs arteriogenesis by regulating the amount and function of monocytes and macrophages in ischemic tissues.
Figure 5.
Impairment of arteriogenesis is caused by AMP-activated protein kinase (AMPK)-α1 deficiency in macrophages. A, Representative images recorded by Laser Doppler showing the blood flow recovery process in macrophage-specific AMPKα1-deletion mice and control mice after femoral artery ligation. B, Quantitative analyses showing the ratio of left plantar:right plantar perfusion after surgery for the occlusion of the femoral artery. Macrophage-specific AMPKα1 deletion mice exhibited deferred recovery in perfusion compared with AMPKα1 flox mice. *P<0.05 vs control group, n=10 to 15. C, Hematoxylin and eosin–stained sections of calf muscle from ligated limbs from control and macrophage-specific AMPKα1-deletion mice. The dashed outlines denote injured and necrotic area within the muscle. D, The percentage of injured and necrotic area in each section was determined for the ligated limb. *P<0.05 vs control group, n=5. E, Histochemical staining of collaterals for smooth muscle α-actin (scale bar, 50 μm). F, Quantification of collateral diameter in adductor muscles from mice 7 d after surgery. *P<0.05 vs control group, n=10.
Decreased Expression of Growth Factors in Macrophages Under Hypoxia Conditions In Vivo and In Vitro
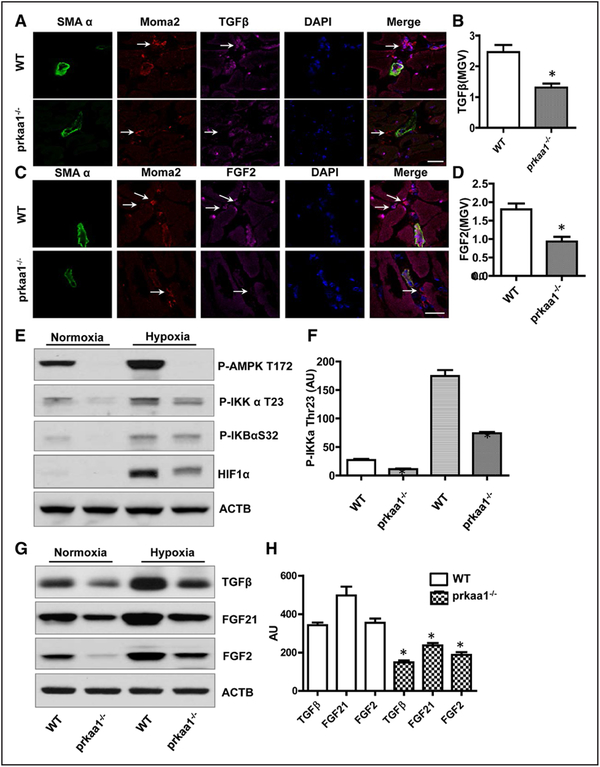
To further demonstrate that AMPKα1 deletion influences growth factor generation in macrophages in vivo, we performed immunofluorescence staining of TGFβ or FGF2 and macrophage markers in ischemic adductor tissues to observe vascular growth factors in macrophages in situ. As shown in Figure 6A through 6D, TGFβ and FGF2 levels were reduced in macrophages from AMPKα1−/− mice when compared with those in WT mice. We also analyzed VEGF, TGFβ, FGF2, and bone morphogenetic protein 4 (BMP4) levels in ischemic adductor tissue by Western blot. As depicted in Figure VA and VB in the online-only Data Supplement, proangiogenic factors were reduced in ischemic settings in AMPKα1−/− mice in comparison with WT mice. In contrast, the level of BMP4, a TGFβ signal pathway, was comparable between the 2-genotype mice.
Figure 6.
The expression of growth factor decreased in AMPKα1−/− macrophages under hypoxic condition in vitro and in vivo. A, Immunofluorescence staining showing transforming growth factor (TGF)-β levels in monocytes/macrophages in ischemic adductor muscle (scale bar, 50 μm). B, LAS AF Lit quantification of TGFβ mean gray value (MGV) in monocytes/macrophages in adductor sections from mice 3 d after ligation of the femoral artery (FAL). *P<0.05 vs wild-type (WT), n=5. C, Immunostaining was used to examine FGF2 levels in macrophages in ischemic adductor muscle (scale bar, 50 μm). D, Quantification of FGF2 MGV in monocytes/macrophages in adductor sections from mice 3 d after FAL. *P<0.05 vs WT, n=5. E, Western blot was utilized to analyze phospho-AMP-activated protein kinase (AMPK) Thr172, phospho-inhibitor of nuclear factor kappa B (NF-κB) kinase (IKK)-α Thr23, and phospho-IκB α Ser32 levels in macrophages under hypoxic conditions for 3 h. The blot is representative of 3 blots from 3 independent experiments (n=3). F, Quantitation of phospho-IKKα Thr23 level in macrophages under normoxia and hypoxia conditions, *P<0.05 vs WT, n=3. G, Under hypoxic conditions, macrophage growth factor levels were detected by Western blot. The blot is representative of 5 blots from 5 individual mice (n=5). H, Quantitation of TGFβ, FGF21, and FGF2 level in macrophages under hypoxic conditions, *P<0.05 vs WT, n=5. ACTB indicates actin beta; and HIF, hypoxia-inducible factor.
To mimic ischemia and hypoxia condition in vivo, BMDMs were exposed to 1% oxygen for 3 hours. In agreement with in vivo findings, Western blot showed that hypoxia activated both AMPK and nuclear factor κB (NF-κB) pathways (Figure 6E) and increased TGF-β, FGF2, and FGF21 levels (Figure 6G). However, under hypoxia condition, increase of TGF-β, FGF2, and FGF21 in AMPKα1−/− macrophages was much less when compared with increases in WT macrophages (Figure 6G and 6H), suggesting that AMPKα1 deletion mitigates expression of vascular growth factors in macrophage response to hypoxia. In addition, hypoxia-inducible factor (HIF)-1α levels was significantly reduced (P<0.05) in macrophages from AMPKα1−/− mice under both normoxia and hypoxia conditions (Figure 6E), indicating that AMPK may be involved in HIF1α stability in macrophages.
NF-κB Regulates Growth Factor Expression in Macrophages
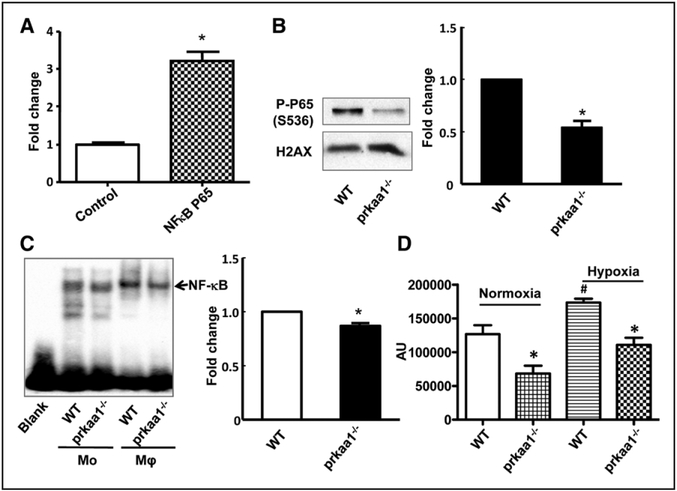
We reasoned that the mechanism by which AMPKα1 deletion impairs growth factor expression in macrophages might involve NF-κB because this signaling molecule is not only a central regulator of the stress response but also a transcription factor for many genes. To test whether NF-κB regulates TGFβ1 and FGF2 gene expression, human embryonic kidney 293T (HEK293T) cells were cotransfected with plasmids containing a TGFβ1 promoter reporter and NF-κB p65. The luciferase activity assay showed that NF-κB p65 significantly increased TGFβ1 promoter activity (Figure 7A), indicating that NF-κB may function as a transcription factor for TGFβ1.
Figure 7.
Reduced nuclear factor kappa B (NF-κB) activity in AMPKα1−/− macrophages. A, Luciferase assay measuring NFκB P65 activation of transforming growth factor (TGF)-β promoter: the data are from 3 independent experiments. B, Phospho-P65 levels were detected by Western blot in nuclear fractions isolated from bone marrow–derived macrophages (BMDMs), and histone H2AX was used as the loading control for the nuclear fraction. The blot is a representative of 3 blots from 3 independent experiments (n=3). C, The nuclear fraction was isolated from BMDMs and mononuclear cells, and NF-κB activity was detected by electrophoretic mobility shift assay (n=3). D, BMDMs were subjected to hypoxia (1% O2) for 1 h, and NF-κB activity was determined using the NF-κB activity kit (*P<0.05 vs WT, #P<0.05 vs normoxia), data from 3 independent experiments (n=3).
AMPKα1 Deletion Leads to Reduction of NF-κB Transcription Factor Activity in Macrophages
To test whether AMPKα1 deletion leads to a reduction of NF-κB activity in macrophages, the nuclear fraction was isolated from BMDMs and subjected to Western blot, to determine the level of phosphorylated NF-κB P65 (on residue serine 536). A distinct reduction in phospho-NF-κB P65 serine 536 levels was observed in AMPKα1−/− BMDMs, compared with WT (Figure 7B). Subsequently, we utilized an electro-phoretic mobility shift assay to assess the DNA-binding ability of NF-κB in macrophages. As shown in Figure 7C, the DNA-binding affinity of NF-κB was modestly reduced in BMDMs from AMPKα1−/− mice when compared with those from WT mice. To explore NF-κB transcription factor activity in AMPKα1−/− macrophages under ischemic conditions in vivo, we analyzed NF-κB activity in macrophages under hypoxic conditions in vitro to mimic ischemic condition in vivo. We found that NF-κB activity is noticeably lower in AMPKα1−/− macrophages than in WT macrophages under hypoxic conditions (Figure 7D). Taken together, these results suggest that AMPKα1 deletion impairs NF-κB activation in the macrophage response to hypoxic stress.
AMPK Directly Phosphorylates Inhibitor of NF-κB Kinase α Threonine 23
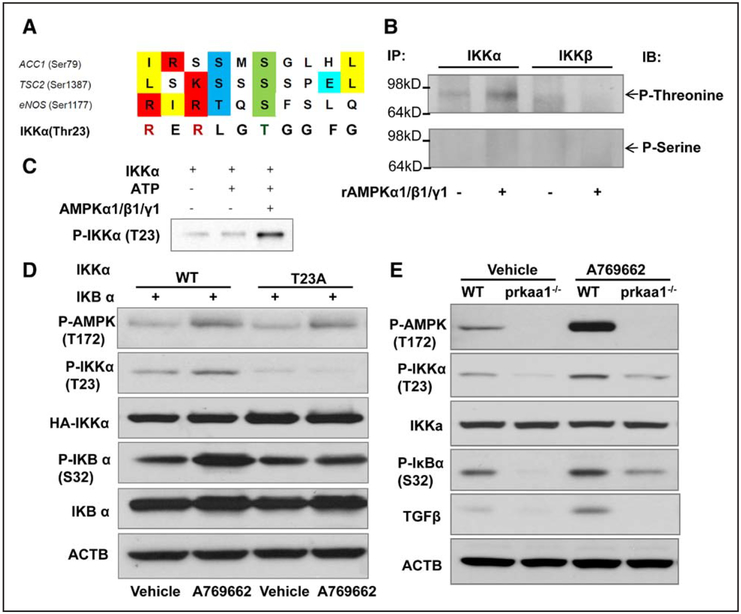
We next tested how AMPKα1 regulates NF-κB activation in macrophages, and whether phosphorylation of inhibitor kappa B kinase alpha (IKKα) at threonine 23 affects NF-κB activity and function. AMPK is a Thr/Ser protein kinase that can phosphorylate various kinds of substrates, including acetyl-CoA carboxylase, endothelial nitric oxide synthase, Unc-51 like autophagy activating kinase 1, tuberous sclerosis complex 2, and mammalian target of rapamycin to regulate all aspects of cellular function, including autophagy, cell polarity, cell growth, and cell proliferation. Based on the consensus site of the substrate recognized by AMPK20 (Figure 8A), we speculated that AMPK might directly phosphorylate IKK. To test this, we first measured total threonine/serine phosphorylation of IKK in BMDMs. As depicted in Figure 8B, AMPKα1 may increase IKKα threonine phosphorylation, but not that of serine or IKKβ. Next, we determined whether AMPKα1 directly phosphorylates IKKα, and which threonine residues in the IKKα protein are phosphorylated, by measuring AMPK kinase activity in vitro. As shown in Figure 8C, recombinant AMPKα1/β1/γ1 may significantly increase the phosphorylation of recombinant IKKα protein at threonine 23, suggesting that AMPK may directly phosphorylate IKKα at Thr23.
Figure 8.
AMP-activated protein kinase (AMPK)-α1 directly phosphorylates inhibitor of nuclear factor kappa B kinase (IKKα) threonine 23. A, The IKKα amino acid sequence contains a conserved motif recognized by AMPK kinase. B, IKKα or IKKβ immunoprecipitated from bone marrow–derived macrophage (BMDM) lysates with anti-IKKα or IKKβ antibody was incubated as a substrate with recombinant AMPKα1/β1/γ1 complex for 15 min at 37°C in kinase buffer. The substrate was subjected to Western blot to determine phosphorylated threonine levels using the antiphospho-threonine/serine antibody as a probe. Top, Phospho-IKKα/β threonine levels, and the bottom shows phospho-IKKα/β IKKβ serine levels. The blot is representative of 3 blots from 3 independent experiments (n=3). C, Recombinant IKKα as substrate was incubated with recombinant AMPKα1/β1/γ1 complex for 15 min at 37°C in kinase buffer. Anti-IKKα Thr23 was used as a probe, and Western blot was performed to determine the phospho-IKKα Thr23 levels. The blot is representative of 3 blots from 3 independent experiments (n=3). D, HEK293T cells were cotransfected with a plasmid encoding IκB α, and a plasmid encoding IKKα wild-type (WT) or the IKKα T23A mutant for 24 h and then treated with the AMPK activator A769662 for 3 h. Western blot was utilized to measure the phospho-IKKα Thr23 level in the treated cells. The blot is representative of 3 blots from 3 independent experiments (n=3). E, BMDMs were treated with A769662 for 3 h, cell lysates were subjected to Western blot to determine the levels of phospho-IKKα Thr23, phospho-IκB α Ser 32, and TGFβ. ACTB indicates actin beta.
To further confirm Thr23 of IKKα as the phosphorylation target of AMPKα1, we generated a site-directed mutant construct of IKKα (threonine to alanine, T23A). HEK293 cells were transfected with IKKα or the IKKα T23A mutant. After the transfection, they were treated with A769662, a selective activation for AMPK. As expected, A769662 promoted AMPK phosphorylation at Thr172 in cells transfected with IKKα or the IKKα T23A mutant (Figure 8D). IKKα phosphorylation at Thr23 was markedly increased in cells transfected with WT IKKα, but not in those transfected IKKα mutants (Figure 8D), confirming that threonine 23 is the target of AMPKα threo-nine phosphorylation.
To further test whether AMPKα1 directly phosphorylates IKKα threonine 23 in cells in vivo, we measured the levels of IKKα Thr 23 phosphorylation in WT and AMPKα1−/− peritoneal macrophages and BMDMs. We observed significantly decreased levels of Thr 23 phosphorylation in AMPKα1−/− compared with WT macrophages (Figure 6E and 8E) under both normoxic and hypoxic conditions.
Discussion
In the present study, we have demonstrated that AMPKα1 plays an essential role in the arteriogenesis of collateral remodeling. We found that the AMPKα1 knockout mouse displayed a manifest decrease in the restoration of blood flow after femoral artery ligation. Histological evaluation revealed defects in arteriogenesis as a result of impaired collateral remodeling. An AMPK α1 flox/flox; Lyz-Cre mouse, in which AMPK α1 was deleted in macrophages, exhibited similar phenotypes in terms of blood flow recovery and remodeling of collateral arterioles, suggesting macrophage AMPK as a regulator for arteriogenesis. We further found less accumulation of AMPKα1−/− macrophages in the arteriogenesis setting and lower levels of growth factor production in AMPKα1−/− macrophages. In addition, we demonstrated that AMPK directly phosphorylates IKKα and subsequently activates NF-κB, which functions as a transcription factor for TGFβ.
The most important finding in this study was that macrophage AMPK plays an essential role in arteriogenesis through its regulation of growth factor generation. We did observe a decrease in macrophage accumulation and macrophage growth factor generation in AMPKα1−/− mice and further generated macrophage-specific AMPKα1−/− mice to demonstrate that the impairment of arteriogenesis can be attributed to the loss of macrophage AMPK. The data from both global knockout and AMPK α1 flox/flox; Lyz-Cre mouse models suggest that macrophage AMPK is important in growth factor generation and arteriogenesis.
Another important finding is how AMPK regulates the production of growth factors. We revealed that AMPK regulates NF-κB activation through the phosphorylation of threo-nine 23 on IKKα (IκB kinase), which in turn phosphorylates IκBα in macrophages under either hypoxic or normoxic conditions. Next, we validated that NF-κB as a transcription factor can bind to the TGFβ promoter to enhance its expression (Figure 7). On deletion of AMPKα1 in macrophages, the ability of macrophages to generate growth factors was markedly reduced. This link between AMPK, NF-κB, and growth factor levels, to our knowledge, has not yet been reported. Indeed, monocytes/macrophages have long been thought to be crucial to arteriogenesis, in part, because of their production of VEGF and FGF2.29 Apart from VEGF and FGF2, we found that there were significantly decreased levels of TGFβ, FGF21, and platelet-derived growth factor subunit B in AMPKα1-deficient macrophages compared with WT macrophages, not only under hypoxic but also under normoxic conditions.
Although studies have reported that TGFβ expression is increased in growing collaterals and that local application of exogenous TGFβ might foster arteriogenesis,30–32 the generation and regulation of TGFβ is elusive. Our findings show that macrophages express high levels of TGFβ and that AMPK affects its expression via the regulation of NF-κB. However, emerging studies indicate that AMPK signaling can suppress the inflammatory responses induced by the NF-κB system.33,34 NF-κB sub-units are not direct phosphorylation substrates of AMPK; rather, the inhibition of NF-κB signaling is mediated through its downstream targets, eg, SIRT1 (NAD+-dependent histone deacetylase 1), PGC-1α (peroxisome proliferator-activated receptor-γ coactivator-1α), p53, and FoxO1,3 in different cell types.35
Previous studies36–38 mainly focused on endothelial NF-κB and found that NF-κB was a key regulator of adult and developmental arteriogenesis and collateral formation. Shear stress7 and inflammation8,13 have long been considered key drivers for arteriogenesis in adult tissues. During arterial occlusion, increased shear stress causes NF-κB activation in the endothelium. This activation of the endothelial NF-κB cascade can augment the expression of adhesion molecules to recruit blood-derived monocytes/macrophages. Although endothelial NF-κB is squarely at the center of both developmental and adult arteriogenesis,36,39 we focused on the mechanism underlying the activation of recruited monocytes/macrophages during adult arteriogenesis. We found that AMPKα1 deletion results in decreased NF-κB activity, which subsequently leads to a lack of macrophage-secreting growth factors, thereby affecting the interaction between macrophages and SMCs/endothelial cells. In this regard, macrophage NF-κB also plays an important role in arteriogenesis. However, AMPK might play additional roles in other cell types (such as endothelial cells) in response to fluid shear stress, hypoxia, and ischemic condition during arteriogenesis and angiogenosis. Both in vivo and in vitro studies40–42 indicate that AMPK might be activated by hypoxia, ischemia, and fluid shear stress in endothelial cells, with the subsequent interplay between AMPK, SIRT1, and endothelial nitric oxide synthase performing an atheroprotective function. Findings in this study demonstrate that hypoxia activates macrophage AMPK and sequentially increases generation of growth factors that are beneficial for arteriogenesis and angiogenesis (Figure 6). Moreover, we found that small-molecule AMPK activator, A769662, enhances NF-κB signal, and increases TGFβ level in macrophage (Figure 8E), indicating that activation of macrophage AMPK is helpful for neovascularization. However, the clinical application of macrophage-specific AMPKα1-selective pharmacological activator is unclear because this molecule may promote proinflammatory and atherogenic signaling mechanisms in other organs.
Consistent with recent studies, which have suggested that HIF levels are controlled by transcription factor NF-κB and that NF-κB can stabilize HIF1α levels both under hypoxic and normoxic conditions,43–45 our findings demonstrated that the HIF1α levels in AMPK−/− macrophages are significantly lower than those in WT macrophages under both normoxia and hypoxia, indicating that AMPK might regulate VEGF levels indirectly through the NF-κB -HIF1α pathway.
In summary, we found that macrophage AMPKα1 plays an important role in the regulation of adult arteriogenesis and the formation of collateral circulation via-NF-κB signaling and through the control of monocyte-macrophage production of TGF-β, platelet-derived growth factor subunit B, FGF2, and VEGF.
Supplementary Material
Highlights.
Occlusive vascular diseases remain the most important causes of morbidity and death in developed countries and cause >50% of mortalities before the age of 75 years.
Therapies that promote the compensatory growth of blood vessels could prove superior to palliative surgical intervention in managing occlusive vascular diseases.
However, therapies that improve collateral growth are not available because the underlying mechanisms that control the complex process of arteriolar remodeling remain undefined.
To define suitable targets for pharmacological therapies, a description of key molecules orchestrating the activity of macrophages, vascular smooth muscle cells, and endothelial cells is necessary.
Our study suggests that AMPK signaling plays a key role in collateral remodeling and arteriogenesis.
Genetic ablation of AMPKα1 in mice impairs collateral growth by inhibiting both monocyte to macrophage terminal differentiation and the secretion of arteriogenic factors by macrophages in the area surrounding an arterial occlusion.
From a clinical point of view, enhancing the activation of AMPK favors collateral remodeling and arteriogenesis and thus may be an alternative therapeutic strategy for ischemic disorders.
Acknowledgments
We acknowledge Dr Lijun Xia for his helpful suggestion, which proved critical for this study.
Sources of Funding
This study was supported by National Institutes of Health (NIH) grants (HL079584, HL080499, HL089920, HL110488, and AG047776) from the NIH. This work was, in part, supported by the Georgia Research Alliance. Dr Zou is a Georgia Research Alliance Eminent Scholar in Molecular Medicine.
Nonstandard Abbreviations and Acronyms
- AMPK
AMP-activated protein kinase
- BMDM
bone marrow–derived macrophage
- FGF2
fibroblast growth factor 2
- HIF1α
hypoxia-inducible factor 1 alpha
- IKK
inhibitor of nuclear factor kappa B (NF-κB) kinase
- KO
knockout
- NF-κB
nuclear factor kappa B
- SMCs
smooth muscle cells
- TGFβ
transforming growth factor beta
- VEGF
vascular endothelial growth factor
- WT
wild-type
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.116.307743/-/DC1.
Disclosures
None.
References
- 1.Carmeliet P Mechanisms of angiogenesis and arteriogenesis. Nat Med.2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 2.Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin (Shanghai). 2008;40:681–692. [PubMed] [Google Scholar]
- 3.Heil M, Eitenmüller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front Physiol. 2012;3:353. doi: 10.3389/fphys.2012.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heil M, Schaper W. Cellular mechanisms of arteriogenesis. Exs. 2005:181–191 [DOI] [PubMed] [Google Scholar]
- 6.Heil M, Schaper W. Insights into pathways of arteriogenesis. Curr Pharm Biotechnol. 2007;8:35–42. [DOI] [PubMed] [Google Scholar]
- 7.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23:1143–1151. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 8.Khmelewski E, Becker A, Meinertz T, Ito WD. Tissue resident cells play a dominant role in arteriogenesis and concomitant macrophage accumulation. Circ Res. 2004;95:E56–E64. doi: 10.1161/01.RES.0000143013.04985.E7. [DOI] [PubMed] [Google Scholar]
- 9.Eitenmüller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, Heil M, Pipp F, Fischer S, Horrevoets AJ, Schmitz-Rixen T, Schaper W. The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res. 2006;99:656–662. doi: 10.1161/01.RES.0000242560.77512.dd. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Rubin J, Tzima E. Role of PECAM-1 in arteriogenesis and specification of preexisting collaterals. Circ Res. 2010;107:1355–1363. doi: 10.1161/CIRCRESAHA.110.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda Y, Costa S, Delamarre E, et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479:122–126. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ Res. 2004;95:449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 13.la Sala A, Pontecorvo L, Agresta A, Rosano G, Stabile E. Regulation of collateral blood vessel development by the innate and adaptive immune system. Trends Mol Med. 2012;18:494–501. doi: 10.1016/j.molmed.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Hardie DG, Carling D. The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. [DOI] [PubMed] [Google Scholar]
- 15.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. [DOI] [PubMed] [Google Scholar]
- 16.Hardie DG. AMPK and autophagy get connected. EMBO J. 2011;30:634–635. doi: 10.1038/emboj.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Takano-Ohmuro H, Mukaida M, Kominami E, Morioka K. Autophagy in embryonic erythroid cells: its role in maturation. Eur J Cell Biol. 2000;79:759–764. doi: 10.1078/0171-9335-00096. [DOI] [PubMed] [Google Scholar]
- 19.Hardie DG. The AMP-activated protein kinase pathway–new players upstream and downstream. J Cell Sci. 2004;117(pt 23):5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 20.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of bcl-2-beclin1 complex by activated ampk enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62(4):1270–1281. doi: 10.2337/db12-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu H, Foretz M, Xie Z, Zhang M, Zhu Z, Xing J, Leclerc J, Gaudry M, Viollet B, Zou MH. PRKAA1/AMPKα1 is required for autophagy-dependent mitochondrial clearance during erythrocyte maturation. Autophagy. 2014;10:1522–1534. doi: 10.4161/auto.29197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland T Jr, Shyy JY. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 24.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 25.Zippel N, Malik RA, Frömel T, Popp R, Bess E, Strilic B, Wettschureck N, Fleming I, Fisslthaler B. Transforming growth factor-β-activated kinase 1 regulates angiogenesis via AMP-activated protein kinase-α1 and redox balance in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:2792–2799. doi: 10.1161/ATVBAHA.113.301848. [DOI] [PubMed] [Google Scholar]
- 26.Xu MJ, Song P, Shirwany N, Liang B, Xing J, Viollet B, Wang X, Zhu Y, Zou MH. Impaired expression of uncoupling protein 2 causes defective postischemic angiogenesis in mice deficient in AMP-activated protein kinase α subunits. Arterioscler Thromb Vasc Biol. 2011;31:1757–1765. doi: 10.1161/ATVBAHA.111.227991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng RJ, Du J, Afolayan AJ, Eis A, Shi Y, Konduri GG. AMP kinase activation improves angiogenesis in pulmonary artery endothelial cells with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2013;304:L29–L42. doi: 10.1152/ajplung.00200.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi D, Young LH. AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab. 2015;26:422–429. doi: 10.1016/j.tem.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simons M Angiogenesis: where do we stand now? Circulation. 2005;111:1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 30.Olieslagers S, Pardali E, Tchaikovski V, ten Dijke P, Waltenberger J. TGF-β1/ALK5-induced monocyte migration involves PI3K and p38 pathways and is not negatively affected by diabetes mellitus. Cardiovasc Res. 2011;91:510–518. doi: 10.1093/cvr/cvr100. [DOI] [PubMed] [Google Scholar]
- 31.van Royen N, Hoefer I, Buschmann I, Heil M, Kostin S, Deindl E, Vogel S, Korff T, Augustin H, Bode C, Piek JJ, Schaper W. Exogenous application of transforming growth factor beta 1 stimulates arteriogenesis in the peripheral circulation. FASEB J. 2002;16:432–434. doi: 10.1096/fj.01-0563fje. [DOI] [PubMed] [Google Scholar]
- 32.van Royen N, Piek JJ, Schaper W, Fulton WF. A critical review of clinical arteriogenesis research. J Am Coll Cardiol. 2009;55:17–25. doi: 10.1016/j.jacc.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 33.Cacicedo JM, Yagihashi N, Keaney JF Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 34.Green CJ, Pedersen M, Pedersen BK, Scheele C. Elevated NF-κB activation is conserved in human myocytes cultured from obese type 2 diabetic patients and attenuated by AMP-activated protein kinase. Diabetes. 2011;60:2810–2819. doi: 10.2337/db11-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on health-span and lifespan. J Mol Med (Berl). 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tirziu D, Jaba IM, Yu P, Larrivée B, Coon BG, Cristofaro B, Zhuang ZW, Lanahan AA, Schwartz MA, Eichmann A, Simons M. Endothelial nuclear factor-κB-dependent regulation of arteriogenesis and branching. Circulation. 2012;126:2589–2600. doi: 10.1161/CIRCULATIONAHA.112.119321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweet DT, Chen Z, Givens CS, Owens AP 3rd, Rojas M, Tzima E. Endothelial Shc regulates arteriogenesis through dual control of arterial specification and inflammation via the notch and nuclear factor-κ-light-chain-enhancer of activated B-cell pathways. Circ Res. 2013;113:32–39. doi: 10.1161/CIRCRESAHA.113.301407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lähteenvuo JE, Lähteenvuo MT, Kivelä A, Rosenlew C, Falkevall A, Klar J, Heikura T, Rissanen TT, Vähäkangas E, Korpisalo P, Enholm B, Carmeliet P, Alitalo K, Eriksson U, Ylä-Herttuala S. Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arterio-genesis via vascular endothelial growth factor receptor-1- and neuropilin receptor-1-dependent mechanisms. Circulation. 2009;119:845–856. doi: 10.1161/CIRCULATIONAHA.108.816454. [DOI] [PubMed] [Google Scholar]
- 39.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixit M, Bess E, Fisslthaler B, Härtel FV, Noll T, Busse R, Fleming I. Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc Res. 2008;77:160–168. doi: 10.1093/cvr/cvm017. [DOI] [PubMed] [Google Scholar]
- 41.Fisslthaler B, Fleming I, Keserü B, Walsh K, Busse R. Fluid shear stress and NO decrease the activity of the hydroxy-methylglutaryl coenzyme A reductase in endothelial cells via the AMP-activated protein kinase and FoxO1. Circ Res. 2007;100:e12–e21. doi: 10.1161/01.RES.0000257747.74358.1c. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci USA. 2010;107:10268–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonello S, Zähringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Görlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 44.van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.