Abstract
Background:
There is no current consensus on the management of large hiatal hernias concomitant with performance of a sleeve gastrectomy procedure. Proposed solutions have included performing a modified Nissen fundoplication, performing cruroplasty alone, utilizing the Linx device, performing cruroplasty with reinforcement material, and avoiding the sleeve procedure altogether in favor of a bypass procedure in order to minimize gastroesophageal reflux. Urinary bladder matrix (UBM) represents a biologically derived material for use in hiatal hernia repair reinforcement with the potential to improve durability of repair without incurring the risks of other reinforcement materials.
Methods:
This study reports the results of a retrospective chart review of 32 cases of large hiatal hernia repair utilizing both primary crural repair and UBM reinforcement concomitant with laparoscopic sleeve gastrectomy by a single surgeon. Hernia diameter averaged 6 cm (range 4–9 cm). After an average of 1 year followup, 30 patients were assessed for subjective symptoms of gastroesophageal reflux (GERD) using the Gastroesophageal Reflux Disease-Health Related Quality of Life (GERD-HRQL) score. Twenty patients were evaluated with either upper gastrointestinal (GI) series, endoscopy, or both.
Results:
Each repair was successful and completed laparoscopically concomitant with sleeve gastrectomy. Anterior and posterior cruroplasty was performed using interrupted 0-Ethibond suture using the Endostitch device. The UBM graft exhibited favorable handling characteristics placed as a keyhole geometry sutured to the crura with absorbable suture. A careful chart review was undertaken to assess for complications. There have been no reoperations. After a median of 12 months (range, 4–27 months) of followup, an assessment of recurrences or long-term complications was completed. Median GERD-HRQL score was 6, with a range of 0 to 64 (of possible 75), indicating very low-level reflux symptomatology. Follow-up upper GI radiographs or endoscopy were obtained in 20 cases and show intact repairs.
Conclusion:
In this series of 32 cases, laparoscopic cruroplasty with UBM graft reinforcement has been effective and durable at 12 months of followup. This technique may offer one satisfactory solution for large hiatal hernia repair concomitant with laparoscopic sleeve gastrectomy that may achieve a durable repair with low GERD symptoms.
Keywords: Urinary bladder matrix, Hiatal hernia, Sleeve Gastrectomy, GERD, Upper GI surgery
INTRODUCTION
Laparoscopic sleeve gastrectomy provides durable weight-loss results that are comparable to Roux-en-Y gastric bypass at 5 years, but with lower overall complications.1,2 However, published series report both de novo and worsening of existing GERD symptoms following the sleeve procedure, and hiatal hernia contributes to this problem.3 As sleeve gastrectomy has risen in popularity, bariatric surgeons must grapple with the best approach when a large hiatal hernia is present so as to minimize postoperative GERD, maximize weight loss, and minimize complications. No current consensus exists on the management of large hiatal hernias concomitant with performance of a sleeve gastrectomy procedure. Unique challenges include the risk of esophagitis, GERD symptoms after sleeve gastrectomy, and the durability of repairs of large hiatal hernias. While some authors have advised against the sleeve procedure when a large hiatal hernia is present, others have proposed solutions such as performing a modified Nissen fundoplication,4 performing cruroplasty alone,5 utilizing the Linx device,6 and performing cruroplasty with reinforcement material.7 Synthetic and biologically derived mesh reinforcement result in lower rates of hiatal hernia recurrence than native tissue repair alone in reports of stand-alone hiatal hernia repair.8–12 Concerns have been raised in the literature with respect to the complications of synthetic mesh repairs.13,14 Biologically derived graft materials, proposed as an alternative to minimize mesh-related complications of erosion and infection, are increasingly utilized for hiatal hernia repairs.10–12 Urinary bladder matrix (UBM) consists of the epithelial basement membrane and lamina propria of the porcine urinary bladder. After decellularization, it retains biochemical diversity, an architecture that is similar to the normal tissue, and robust mechanical behavior.15 UBM has shown promise in animal studies for surgical repair of soft tissue with connective tissue remodeling in anatomic settings as diverse as esophageal, urinary bladder, pelvic floor, body wall repair.15,16 In the clinical setting, UBM has shown efficacy for management of a variety of complex wounds, and in the setting of soft-tissue reinforcement in general surgery, where the efficacy has been observed without reports of complications associated with synthetic mesh.17–19 While UBM undergoes a resorption process, site-appropriate tissue is deposited and remodels to support the local physiologic loads as the UBM implant is resorbed.20 A previously published series demonstrated effective resolution of reflux symptoms as well as radiographic durability of hiatal hernia with UBM repair over 3 years.21
METHODS
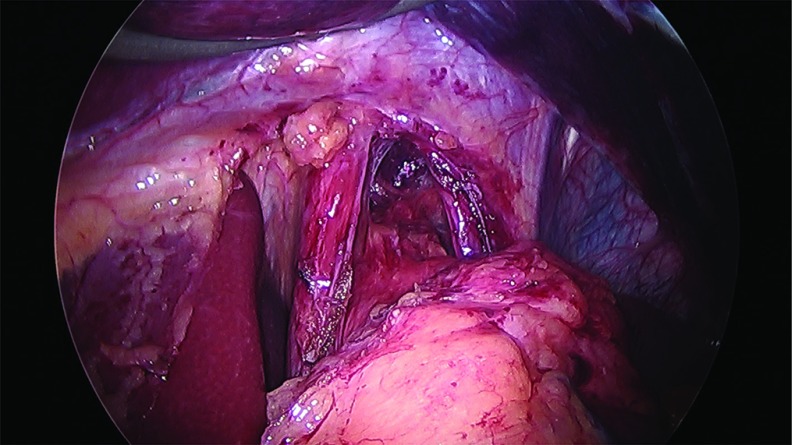
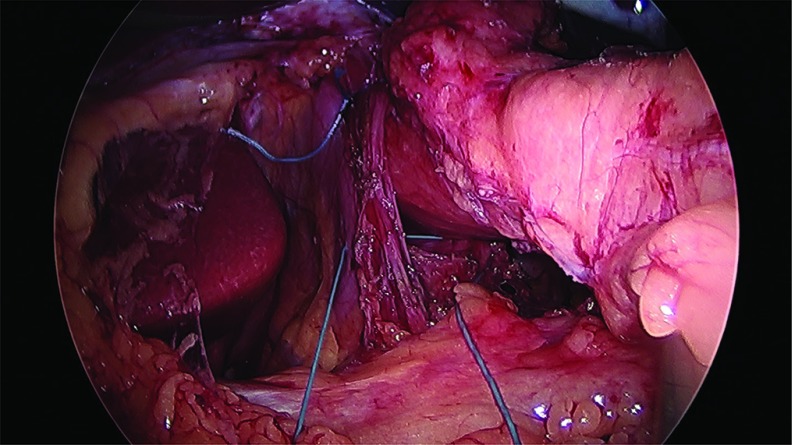
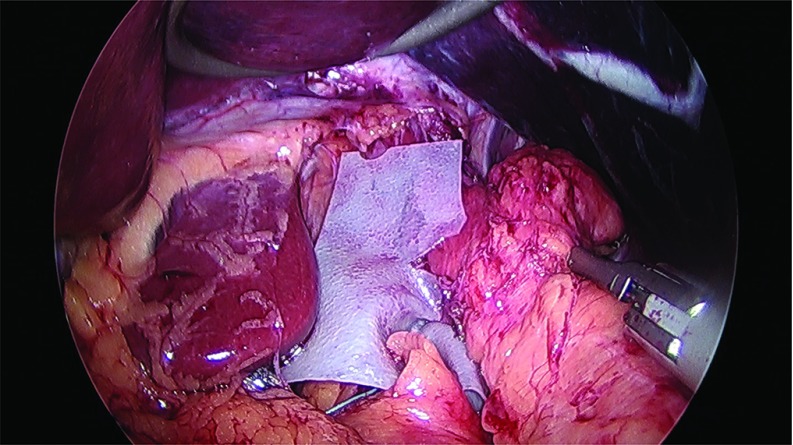
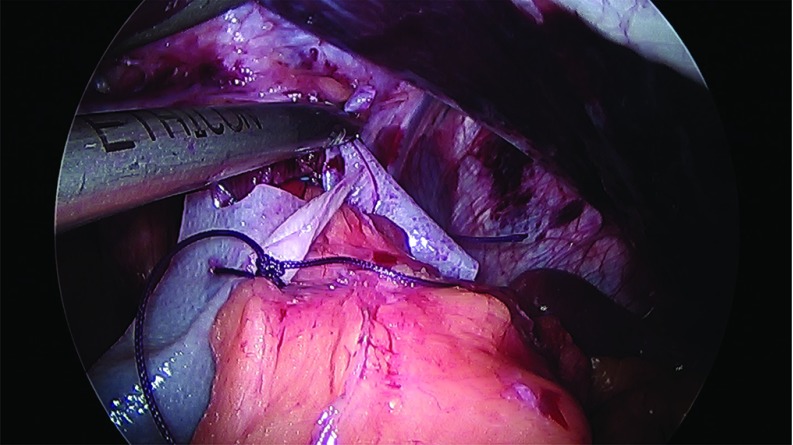
Thirty-two cases of large hiatal hernia repair with UBM graft reinforcement and concomitant laparoscopic sleeve gastrectomy were performed between 2012 and 2017. Under an approved Institutional Review Board (IRB) protocol, each chart was retrospectively reviewed, and patients were assessed for symptoms of GERD. Thirty female and 2 male patients, with an average age of 51 years and an average body mass index (BMI) of 42 kg/m2, underwent surgery. Twenty-eight patients reported GERD symptoms preoperatively and 25 patients were known to have a hiatal hernia on preoperative upper GI radiographs. Patient information is presented in Table 1. The sleeve gastrectomy was performed with the use of a 40 French sizing calibration tube, with a previously published technique,22 with full exposure of the left and right crura, and reduction of any herniated stomach and fat pad. Laparoscopic repair was performed in all 32 cases utilizing crural repair followed by UBM device placement. In each case, 2–3 cm of distal esophagus was freed below the crura (Figure 1). The crural defect averaged 6 cm in diameter, and posterior and anterior cruroplasty was performed using 0-Ethibond sutures (Figure 2), The crural reinforcement was performed with a keyhole-shaped UBM graft ranging in size from 5 × 5 cm to 10 × 15 cm, (Gentrix® Surgical Matrix Thin or Gentrix Surgical Matrix, ACell®, Inc., Columbia, Maryland, USA) secured circumferentially with absorbable sutures to the crura (Figures 3 and 4).
Table 1.
Patient and Procedure Information
| N | 32 |
| Mean age | 51 (25–73) |
| Female/male | 30/2 |
| Mean BMI | 42 (33–54) |
| Mean LOS (days) | 1.5 |
| Mean OR time (minutes) | 54 minutes |
| Complication | 1 gastric sleeve stenosis |
| Follow-up (months) | 12 (4–27) |
| Recurrence | 0 |
| 1-year GERD-HRQL score (mean) | 6 (0–64 out of possible 75) |
BMI, Body Mass Index; GERD, Gastroesophageal reflux disease; HRQL, Health-related quality of life; LOS, Length of stay; OR, Operating room.
Figure 1.
Hiatal hernia defect exposed and 2–3 cm intra-abdominal esophagus freed.
Figure 2.
Crural closure sutures anterior and posterior.
Figure 3.
Placement of UBM graft and securing to crura.
Figure 4.
Securing UBM graft circumferentially.
After an average of 12 months, 30 patients completed GERD-HRQL reflux score survey23 responses via telephone or in-person interview.24 Follow-up upper GI radiographs or endoscopy, or both, were obtained in 20 cases and show intact repairs. Sixteen patients were studied with radiographic upper GI series examinations (Figures 5 and 6). Six patients were evaluated with surveillance esophagogastroduodenoscopy by the primary surgeon or have records from esophagogastroduodenoscopy performed at outside facilities.
Figure 5.
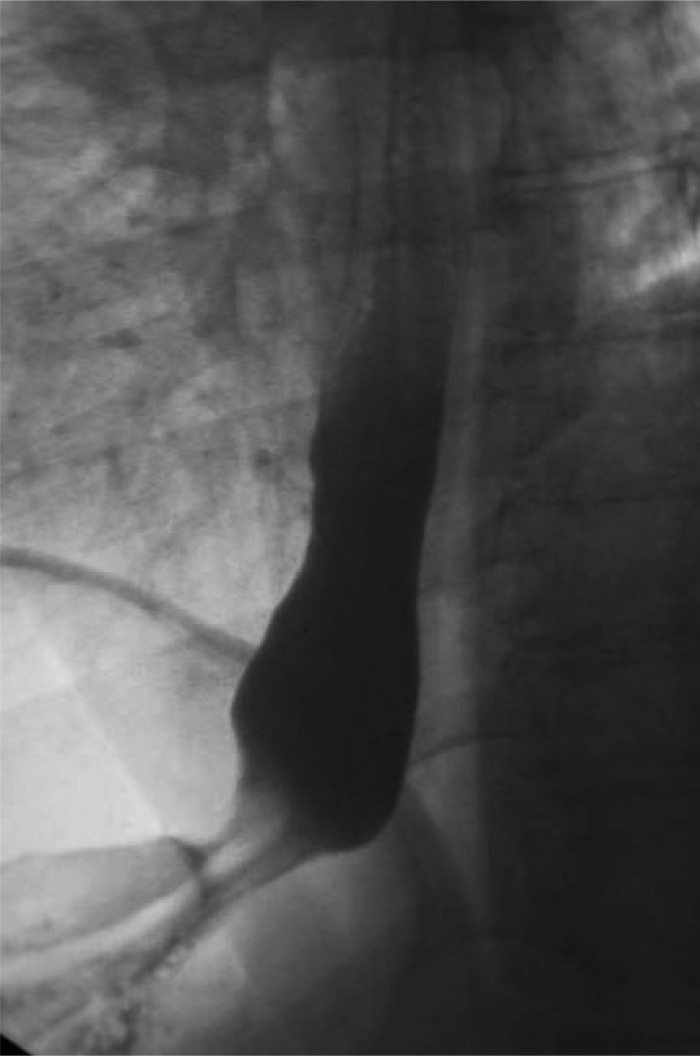
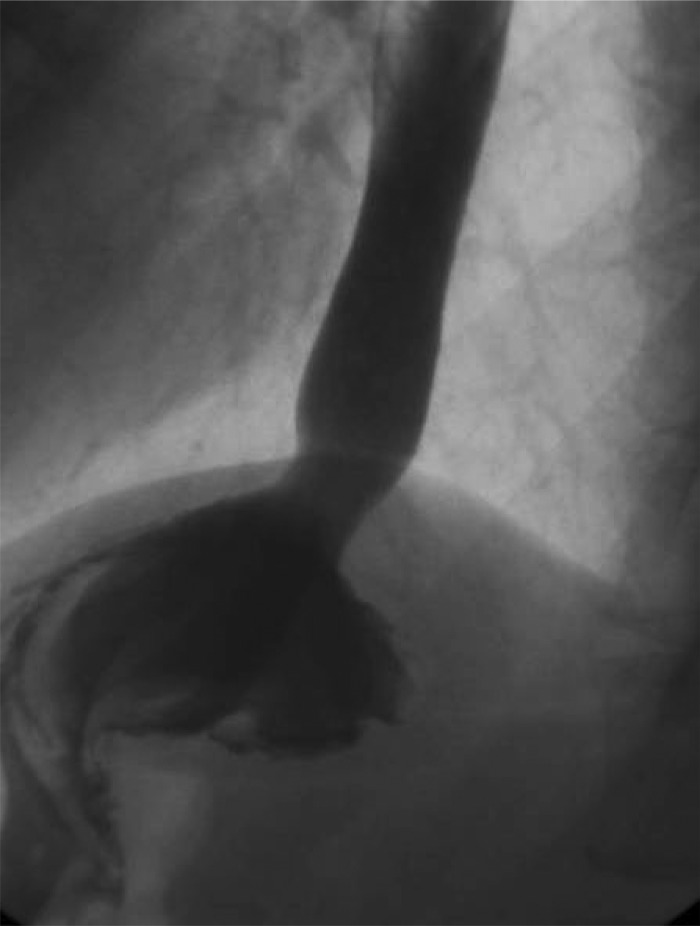
Preoperative esophogram showing presence of hiatal hernia.
Figure 6.
Postoperative Upper GI series 13 months showing intact repair of hiatal hernia with UBM reinforcement and concomitant sleeve gastrectomy.
RESULTS
Each repair with concomitant sleeve gastrectomy was successful. The average BMI prior to surgery was 42 kg/m2, and the BMI after 12 months averaged 32 kg/m2. The handling properties of the UBM material were favorable, including ease of insertion via a 12-mm trocar, maneuvering into position, and suturing to the crura. There were no major complications, no leaks, and no mortality. With an average followup of 12 months, there has been no evidence of erosion, infection, fistulization, seroma, abscess, stenosis, or late stricture. A median GERD-HRQL score of 6 was observed in 30 patients 1 year after surgical repair of the hiatal hernia and concomitant sleeve procedure (range 0 to 64 of possible 75). All patients but one reported little to no reflux, with scores ranging from 0 to 29 (Table 2). One patient has experienced dysphagia and reflux related to a stenosis of the mid stomach related to the sleeve gastrectomy, managed with gastric balloon dilation. No patients have required endoscopic or surgical intervention related to the hiatal hernia repair. Upper GI series radiographs in 16 patients at 1–2 years after repairs showed intact repair compared to preoperative upper GI series images, and no evidence of recurrence (Figures 5 and 6). Esophagogastroduodenoscopy in a subset of 6 patients demonstrated no recurrent hiatal hernias and no other complications.
Table 2.
Patient-Reported Symptoms of Reflux, GERD-HRQL Scores
| Score Range | Number of Participants |
|---|---|
| 0–9 | 24 |
| 10–19 | 4 |
| 20–29 | 1 |
| 30–39 | 0 |
| 40–49 | 0 |
| 50–59 | 0 |
| 60–69 | 1 |
| 70–75 | 0 |
GERD-HRQL, Gastroesophageal Reflux Disease-Health Related Quality of Life.
DISCUSSION
In the past, more authors favored avoidance of sleeve gastrectomy when GERD and a large hiatal hernia was present, but recent trends indicate a greater comfort among surgeons in performing hiatal hernia repair concomitant with sleeve gastrectomy.6,19,25 If GERD and the other potential complications of hiatal hernia may be durably resolved using UBM graft reinforcement concomitant with sleeve, then surgeons and patients who favor the sleeve may be more comfortable choosing this approach. While we do not have clear data on rates of recurrence following hiatal hernia repair at the time of sleeve gastrectomy, we do know that recurrence of hiatal hernia after stand-alone repairs is not uncommon. Short-term recurrence rates of 16.7% and 3.7% for suture repair and mesh repair, respectively, were reported in a review of cases of hiatal hernia repair reported by Antoniou et al.9 There is no consensus on a single best method for hiatal hernia repair.8–14 The cases presented in this series represent successful treatment of hiatal hernias concomitant with sleeve gastrectomy, using biologically derived graft reinforcement UBM material, with satisfactorily durable repairs to 12 months on average with little symptomatic reflux.
Stand-alone repair of hiatal hernias with mesh derived from small intestinal submucosa has been shown to reduce early recurrence rates but not at 5 years when compared to native tissue repair alone.10,26 Reoperations and repairs of recurrent hiatal hernias are challenging cases with higher potential for complications.24
Mesh erosion or infection remain late complications in hiatal hernia repair with synthetic mesh, including polytetrafluoroethylene (PTFE).11 Synthetic mesh repair has been found to offer superior rates of durability when compared to primary sutured repair, but has not been measured against biologically derived graft repairs. In a randomized, controlled prospective trial comparing use of mesh to primary repair for hiatal hernia, use of mesh significantly reduced the rate of recurrent hernias over native tissue repair alone.8 Widespread use of UBM in hiatal, abdominal wall and rectopexy reinforcement have resulted in no reports of erosion or fistulization over the past 10 years.17–19,21 The UBM grafts handle favorably in the laparoscopic surgical environment and prove easier to suture than some materials. At a cost of $650 to $1600 per graft, the UBM grafts cost more than synthetic grafts and less than a Linx device which costs approximately $5000 at our institution,25 and many comparable biological grafts, which range from $1400 to $3000 at our facility.19
A rationale for utilizing biologically derived grafts is the potentially improved durability over cruroplasty alone with a lower rate of erosion or infection compared to synthetic.10–12 UBM has been shown to facilitate a remodeling process with less scar tissue formation and restoration of more physiologic, site-appropriate tissue.15,16,18 While UBM undergoes a degradation process, the UBM implant provides mechanical support in the immediate postoperative period, and then is replaced by host connective tissue that has mechanical characteristics and histology similar to normal healthy tissue, as reported in both animal and human studies.15,16,27 In a hiatal hernia animal model, UBM implants showed an improvement in strength of repair compared to a nonreinforced control between 2 to 3 months after surgery.28 Two recent retrospective series of hiatal hernia repairs reinforced with UBM showed rare short-term complications.17,19 One study of 62 patients, including 5 patients with concomitant sleeve gastrectomy, patients reported only one reoperation at 24 months for symptomatic recurrence.19 Based on these published early experiences, and the detailed study of biological mechanism in conferring strength in hernia repairs, UBM material is considered potentially advantageous in the reinforcement of the repair of large hiatal hernias.
Weaknesses of the study include the relatively short duration of followup and the lack of complete radiographic and endoscopic evaluation of the cohort who may have enjoyed favorable results from the cruroplasty alone. While low GERD-HRQL scores 12 months after surgery are favorable, this cohort does not have preoperative scores for comparison, and hiatal hernia recurrences, as well as GERD, may be asymptomatic and thus under-reported. A further weakness is that follow-up endoscopy to assess asymptomatic reflux esophagitis and metaplasia has not taken place for most of the patients.
CONCLUSIONS
In this series of 32 cases, laparoscopic cruroplasty with a UBM graft resulted in successful repair, no recurrences at 12 months, and little reflux symptomatology among patients undergoing concomitant sleeve gastrectomy. This technique may offer one satisfactory solution for repair of large hiatal hernias concomitant with laparoscopic sleeve gastrectomy. After an average of 12 months of followup, there were no recurrences, strictures, abscesses, or erosions. Future investigation and long-term followup will determine which techniques and implants offer the most cost-effective hernia repair reinforcement that reduces the risk of erosion, stenosis, GERD, and recurrence while providing the most durable repair among sleeve gastrectomy patients.
Contributor Information
Kent C. Sasse, K Sasse Surgical Associates, Reno, Nevada, USA.; Reno School of Medicine, University of Nevada, Reno, Nevada, USA.
Jonathan Gevorkian, K Sasse Surgical Associates, Reno, Nevada, USA.; Reno School of Medicine, University of Nevada, Reno, Nevada, USA.
Rachel Lambin, K Sasse Surgical Associates, Reno, Nevada, USA.; Reno School of Medicine, University of Nevada, Reno, Nevada, USA.
Rami Afshar, K Sasse Surgical Associates, Reno, Nevada, USA..
Amy Gardner, K Sasse Surgical Associates, Reno, Nevada, USA..
Aradhana Mehta, Reno School of Medicine, University of Nevada, Reno, Nevada, USA..
John-Henry Lambin, K Sasse Surgical Associates, Reno, Nevada, USA.; Reno School of Medicine, University of Nevada, Reno, Nevada, USA.
Austin Shinagawa, Reno School of Medicine, University of Nevada, Reno, Nevada, USA..
References:
- 1. Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: The SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barr AC, Frelich MJ, Bosler ME, Goldblatt MI, Gould JC. GERD and acid reduction medication use following gastric bypass and sleeve gastrectomy. Surg Endosc. 2017;31(1):410–415. [DOI] [PubMed] [Google Scholar]
- 4. Nocca D, Skalli EM, Boulay E, Nedelcu M, Michel Fabre J, Loureiro M. Nissen Sleeve (N-Sleeve) operation: Preliminary results of a pilot study. Surg Obes Relat Dis. 2016;12(10):1832–1837. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen H, Steele K, McIver B, Lidor A, Schweitzer M. Laparoscopic sleeve gastrectomy as treatment for patients with morbid obesity with concurrent paraesophageal hernia. Presented at the 2009 Scientific Session of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), April 22–25, 2009, Phoenix, AZ, USA. [Google Scholar]
- 6. Hawasli A, Tarakji M, Tarboush M. Laparoscopic management of severe reflux after sleeve gastrectomy using the LINX® system: Technique and one year follow up case report. Int J Surg Case Rep. 2017;30:148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruscio S, Abdelgawad M, Badiali D, et al. Simple versus reinforced cruroplasty in patients submitted to concomitant laparoscopic sleeve gastrectomy: Prospective evaluation in a bariatric center of excellence. Surg Endosc. 2016;30(6):2374–2381. [DOI] [PubMed] [Google Scholar]
- 8. Frantzides CT, Madan AK, Carlson MA, Stavropoulos GP. A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg. 2002;137(6):649–652. [DOI] [PubMed] [Google Scholar]
- 9. Antoniou SA, Müller-Stich BP, Antoniou GA, et al. Laparoscopic augmentation of the diaphragmatic hiatus with biologic mesh versus suture repair: A systematic review and meta-analysis. Langenbecks Arch Surg. 2015;400(5):577–583. [DOI] [PubMed] [Google Scholar]
- 10. Oelschlager BK, Pellegrini CA, Hunter J, et al. Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: A multicenter, prospective, randomized trial. Ann Surg. 2006;244(4):481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diaz DF, Roth JS. Laparoscopic paraesophageal hernia repair with acellular dermal matrix cruroplasty. JSLS. 2011;15(3):355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reznichenko AA. Different biologic grafts for diaphragmatic crura reinforcement during laparoscopic repair of large hiatal hernia: A 6-year single surgeon experience. J Med Imp Surg. 2015;1(1):2–6. [Google Scholar]
- 13. Lebenthal A, Waterford SD, Fisichella PM. Treatment and controversies in paraesophageal hernia repair. Front Surg. 2015;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frantzides CT, Carlson MA, Loizides S, et al. Hiatal hernia repair with mesh: A survey of SAGES members. Surg Endosc. 2010;24(5):1017–1024. [DOI] [PubMed] [Google Scholar]
- 15. Gilbert TW, Wognum S, Joyce EM, Freytes DO, Sacks MS, Badylak SF: Collagen fiber architecture and biaxial mechanical behavior of extracellular matrix scaffolds derived from the porcine urinary bladder. Biomaterials. 2008;29(36):4775–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilbert TW, Nieponice A, Spievack AR, Holcomb J, Gilbert S, Badylak SF. Repair of the thoracic wall with an extracellular matrix scaffold in a canine model. J Surg Res. 2007;147(1):61–67. [DOI] [PubMed] [Google Scholar]
- 17. Howell RS, Fazzari M, Petrone P, et al. Paraesophageal Hiatal Hernia Repair With Urinary Bladder Matrix Graft. JSLS. 2018;22(2):e2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sasse KC, Ackerman EM, Brandt JR. Complex wounds treated with MatriStem xenograft material: case series and cost analysis. OA Surg. 2013;1(1):3. [Google Scholar]
- 19. Zografakis J, Johnston G, Haas J, et al. Urinary Bladder Matrix Reinforcement for Laparoscopic Hiatal Hernia Repair. JSLS. 2018;22(2)22:e2017.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Young DA, Jackson N, Ronaghan CA, Brathwaite CE, Gilbert TW. Retrorectus repair of incisional ventral hernia with urinary bladder matrix reinforcement in a long-term porcine model. Regen Med. 2018;13(4):395–408. [DOI] [PubMed] [Google Scholar]
- 21. Sasse KC, Warner DL, Ackerman E, Brandt J. Hiatal hernia repair with novel biological graft reinforcement. JSLS. 2016;20(2).e2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Warner DL, Sasse KC. Technical details of laparoscopic sleeve gastrectomy leading to lowered leak rate: Discussion of 1070 consecutive cases. Minim Invasive Surg. 2017;2017:4367059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. 2007;20(2):130–134. [DOI] [PubMed] [Google Scholar]
- 24. Poulose BK, Gosen C, Marks JM, et al. Inpatient mortality analysis of paraesophageal hernia repair in octogenarians. J Gastrointest Surg. 2008;12(11):1888–1892. [DOI] [PubMed] [Google Scholar]
- 25. Garg H, Vigneshwaran B, Aggarwal S, Ahuja V. Impact of concomitant laparoscopic sleeve gastrectomy and hiatal hernia repair on gastro-oesophageal reflux disease in morbidly obese patients. J Minim Access Surg. 2017;13(2):103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: Long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg. 2011;213(4):461–468. [DOI] [PubMed] [Google Scholar]
- 27. Sasse KC, Lambin JH, Gevorkian J, et al. Long-term clinical, radiological, and histological follow-up after complex ventral incisional hernia repair using urinary bladder matrix graft reinforcement: A retrospective cohort study. Hernia. 2018;22(6):899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Costa A, Naranjo JD, Turner NJ, et al. Mechanical strength vs. degradation of a biologically-derived surgical mesh over time in a rodent full thickness abdominal wall defect. Biomaterials. 2016;108:81–90. [DOI] [PubMed] [Google Scholar]