Summary
Background
The recombinant vesicular stomatitis virus (rVSV) vaccine expressing the Zaire Ebola virus (ZEBOV) glycoprotein is efficacious in the weeks following single-dose injection, but duration of immunity is unknown. We aimed to assess antibody persistence at 1 and 2 years in volunteers who received single-dose rVSV-ZEBOV in three previous trials.
Methods
In this observational cohort study, we prospectively followed-up participants from the African and European phase 1 rVSV-ZEBOV trials, who were vaccinated once in 2014–15 with 300 000 (low dose) or 10–50 million (high dose) plaque-forming units (pfu) of rVSV-ZEBOV vaccine to assess ZEBOV glycoprotein (IgG) antibody persistence. The primary outcome was ZEBOV glycoprotein-specific IgG geometric mean concentrations (GMCs) measured yearly by ELISA compared with 1 month (ie, 28 days) after immunisation. We report GMCs up to 2 years (Geneva, Switzerland, including neutralising antibodies up to 6 months) and 1 year (Lambaréné, Gabon; Kilifi, Kenya) after vaccination and factors associated with higher antibody persistence beyond 6 months, according to multivariable analyses. Trials and the observational study were registered at ClinicalTrials.gov (Geneva: NCT02287480 and NCT02933931; Kilifi: NCT02296983) and the Pan-African Clinical Trials Registry (Lambaréné PACTR201411000919191).
Findings
Of 217 vaccinees from the original studies (102 from the Geneva study, 75 from the Lambaréné study, and 40 from the Kilifi study), 197 returned and provided samples at 1 year (95 from the Geneva study, 63 from the Lambaréné, and 39 from the Kilifi study) and 90 at 2 years (all from the Geneva study). In the Geneva group, 44 (100%) of 44 participants who had been given a high dose (ie, 10–50 million pfu) of vaccine and who were seropositive at day 28 remained seropositive at 2 years, whereas 33 (89%) of 37 who had been given the low dose (ie, 300 000 pfu) remained seropositive for 2 years (p=0·042). In participants who had received a high dose, ZEBOV glycoprotein IgG GMCs decreased significantly between their peak (at 1–3 months) and month 6 after vaccination in Geneva (p<0·0001) and Lambaréné (p=0·0298) but not in Kilifi (p=0·5833) and subsequently remained stable at all sites apart from Geneva, where GMC in those given a high dose of vaccine increased significantly between 6 months and 1 year (p=0·0264). Antibody persistence was similar at 1 year and at 6 months in those who had received a low dose of vaccine, with lower titres among participants from the Geneva study at 2 years than at 1 year after vaccination (GMC ratio 0·61, 95% CI 0·49–0·77; p<0·0001). In multivariable analyses, predictors of increased IgG GMCs beyond 6 months included high-dose versus low-dose vaccination (Geneva p=0·0133; Lambaréné p=0·008) and vaccine-related arthritis (p=0·0176), but not sex, age, or baseline seropositivity (all p>0·05). Neutralising antibodies seem to be less durable, with seropositivity dropping from 64–71% at 28 days to 27–31% at 6 months in participants from the Geneva study.
Interpretation
Antibody responses to single-dose rVSV-ZEBOV vaccination are sustained across dose ranges and settings, a key criterion in countries where booster vaccinations would be impractical.
Funding
The Wellcome Trust and Innovative Medicines Initiative 2 Joint Undertaking.
Introduction
The live-attenuated recombinant vesicular stomatitis virus (rVSV) vaccine expressing the glycoprotein of Zaire Ebola virus (ZEBOV) has been granted Breakthrough Therapy Designation status by the US Food and Drug Administration and Priority Medicine (PRIME) status by the European Medicines Agency after it was found to be highly immunogenic for 12 months1–3 and efficacious up to 12 weeks following single-dose injection.4 The long-term durability of immune responses to this single injection and protection over longer periods in areas that are endemic for Ebola virus disease are yet undefined. The durability of the vaccine response becomes increasingly important as more outbreaks occur5 and more information is collected on the persistence of Ebola virus itself within human hosts—eg, replication-competent Ebola viruses have been isolated from patient samples collected up to 9 months after initial Ebola virus disease,6 and viral RNA has been detected up to 2 years after initial infection.7 Indeed, the Wellcome Trust–Center for Infectious Disease Research and Policy Ebola Vaccine Team B initiative8 recommends that any vaccine for the immunisation of disease contacts (ie, anyone who has had any contact with a person who had the disease) should induce protection that lasts at least 2 years. This goal is challenging and has not yet been reported for rVSV-ZEBOV vaccine (also known as rVSVΔG-EBOV-GP). For example, antibody titres to ZEBOV glycoprotein had dropped in all participants at 6 months after a single dose of the recombinant, non-replicating chimpanzee adenovirus 3 (rChAd3)-ZEBOV vaccine.9
Despite the effectiveness of the rVSV-ZEBOV vaccine in the field,4 trends of vaccine-induced protection against Ebola virus disease remain undefined. The WHO-sponsored Guinea ring vaccination trial4 documenting field efficacy did not harvest blood for immunogenicity analyses, and the mechanisms conferring protection in non-human primate models have not been confirmed to be the same in man.10 Although the identification of immune mediators is only at an early phase, passive antibody transfer protects naive non-human primates against lethal Ebola virus,11 and such antibodies are required for protection.12 In human beings, rVSV-ZEBOV-induced antibodies that are likely to contribute to protection include neutralising and non-neutralising antibodies to the ZEBOV glycoprotein.13–15 Thus the presumed durability of rVSV-ZEBOV-induced protection might be best estimated by antibody persistence in people who have been given the vaccine.
Beginning in November, 2014, the VSV-Ebola Consortium (VEBCON)1 did a large (115 participants) phase 1/2 randomised, placebo-controlled trial in Geneva, Switzerland,1,2,16 with parallel dose-escalation trials in Hamburg, Germany (30 participants), Lambaréné, Gabon (75 participants), and Kilifi, Kenya (40 participants).1 Early immunogenicity results of these investigator-initiated trials have been reported.1,2 The Lambaréné trial’s follow-up phase was extended, and participants who received the vaccine from the Geneva trial were invited to participate in a prospective observational study to establish antibody persistence. Here we present persistence data at 1 year for participants who were vaccinated in the Lambaréné, Kilifi, and Geneva studies and at 2 years for the Geneva study. We further explore factors associated with sustained or waning antibody concentrations.
Methods
Study designs and participants
The phase 1 trials that recruited healthy volunteers from the community in Geneva, Switzerland, Lambaréné, Gabon, Kilifi, Kenya, and Hamburg, Germany, launched in 2014–15 and have been described extensively elsewhere (for inclusion and exclusion criteria see ClinicalTrials.gov [NCT02287480 and NCT02296983] and Pan African Trials Registry [PACTR201411000919191]).1,2 We invited participants who had received the vaccine in the Geneva, Lambaréné, and Kilifi studies, to participate in this observational cohort study; the Hamburg study had already terminated at 6 months after vaccination and so participants were not recruited from this trial. Although the Geneva and Kilifi phase 1 trials terminated after 12 months of follow-up, the follow-up phase of the Lambaréné trial was extended to 4 years after intial vaccination, and volunteers from the Geneva study were invited to participate in a 4-year prospective observational study (NCT02933931). We report here antibody concentrations at 1 and 2 years after immunisation, from members of the follow-up population when available, compared with early (28 days after rVSV-ZEBOV vaccination) results to define persistence or waning.
In the Geneva study, all adults who both participated in the phase 1 trial and received a single dose of either 300 000, 10 million, or 50 million plaque-forming units (pfu) of rVSV-ZEBOV vaccine according to protocol were eligible for follow-up in this observational study. Since the randomised Geneva phase 1 trial had revealed no differences in reactogenicity, viraemia, or early immunogenicity after vaccination with 10 million or 50 million pfu of vaccine,1,2 volunteers from the study receiving these doses were again grouped as high-dose participants, whereas those who had received 300 000 pfu of vaccine were grouped as low-dose participants.
The follow-up population consisted of volunteers from the three sites who adhered to the studies’ protocols (eg, did not undergo further rVSV-ZEBOV vaccination) and who had no suspected or documented clinical exposure to Ebola virus throughout the study period (ie, from vaccination to end of follow-up). A detailed description of the Geneva, Lambaréné, and Kilifi cohorts is provided in the appendix.
All phase 1 trials received ethics approval from WHO’s Ethics Committee and from their local and regional ethics committees (for the Geneva study: the Geneva Cantonal Ethics Commission; for the Lambaréné study: the Institutional Ethics Committee of the Centre de Recherche Médicales de Lambaréné; and for the Kilifi study: the National Ethics Committee of Gabon, the Institutional Ethics Committee of the Universitätsklinikum Tübingen, and the Kilifi Ethics Committee). The follow-up observational study in Geneva received additional ethics approval from the Geneva Cantonal Ethics Commission (approval no. 2016-00918).
All Geneva volunteers provided written informed consent before enrolment in this follow-up observational study. The Kilifi and Lambaréné volunteers had given written informed consent to be included in their respective original trials.
Procedures
Serum samples from all studies were frozen at −20°C before transfer to the Non-Clinical Development laboratory at the US Army Medical Research Institute for Infectious Diseases (USAMRIID), Fort Detrick, MD, USA. ZEBOV glycoprotein-specific antibodies were quantified with the Filovirus Animal Non-Clinical Group (FANG)-approved ELISA by use of the homologous Zaire-Kikwit strain glycoprotein, following USAMRIID’s standard operating procedure (SOP AP-03–35; USAMRIID ELISA).1 To improve interassay comparisons, the relative amounts oxOV glycoprotein-specific antibodies previously reported as endpoint titres1,2 were recalculated as arbitrary ELISA units per mL (EU/mL) compared with a reference standard. To convert from EU/mL to IU/mL, EU/mL is divided by 27 135·90. The mean optical density for cutoff values of negative samples was 0·218 EU/mL (SD 0·0321). By solving x when y=0·218 for each of the four parameter logistic regression curves, using seven Human Reference Standards, we found that the lower limit of quantification was 48·7 EU/mL (SD 5·07). Values were log10-transformed and reported as geometric mean concentrations (GMCs) in arbitrary EU/mL with 95% CIs, as indicated. Samples were taken at 0, 28, 84, 168, 365, and 730 days in the Geneva group, 0, 28, 56, 84, 180, and 365 days in the Lambaréné group, and 0, 30, 60, 90, 180, and 365 days in the Kilifi group. For the purposes of this analysis, we refer to measurements taken at 168 and 180 days as 6-month measurements. Neutralising antibodies were assessed in serum samples that were harvested from volunteers from the Geneva study at baseline (before vaccination), day 28, and month 6 (appendix).
Outcomes
Unless protection only requires very low antibody concentrations, persistence of seropositivity is an unlikely correlate of protection. However, assuming IgG measured by ELISA was a marker of immunity, maintenance of the GMCs seen at day 28, at which point a high dose of rVSV-ZEBOV vaccine (20 million pfu) was effective in a ring vaccination setting,4 could be indicative of protection. The main outcome in this observational study was ZEBOV glycoprotein-specific GMC measured by ELISA at yearly timepoints, compared with GMCs measured at 28 days. Other endpoints were seropositivity at 1 and 2 years; seropositivity persistence at 1 and 2 years compared with day 28; the geometric mean fold increase between two timepoints (eg, 6 months and 1 year); GMCs of neutralising antibody titres in the Geneva group at baseline and on days 28 and 168; the association between 1-year GMCs of ZEBOV glycoprotein-specific antibodies and baseline characteristics (eg, sex, age, and baseline seropositivity); and the correlation between vaccine-related arthritis, GMC status, and dose of vaccine. Seropositivity was experimentally defined by adding two SDs to the mean of negative samples, defining a seropositivity threshold of 58·84 EU/mL or higher. In the absence of established correlates of protection, antibody persistence was defined by the maintenance of seropositivity or the ratio of anti-ZEBOV IgG GMCs at a given follow-up timepoint compared with day 28, or both. Seroconversion occurred when a previously negative sample reached a concentration equal to or greater than 58·84 EU/mL. For neutralising antibodies, a titre of 1:8 or higher was experimentally defined as seropositive (appendix).
Statistical analysis
The sample size of the three follow-up cohorts was not calculated but predetermined by the number of participants who fulfilled the defined eligibility criteria and provided serum samples at a given timepoint.
We calculated GMCs of ZEBOV glycoprotein-specific IgG antibodies and 95% CIs for all volunteers with available data using a log10 transformation. Given the USAMRIID ELISA’s limit of seropositivity of 58·84 EU/mL, we arbitrarily assigned titres below this value a lower value of 29·42 EU/mL (half the limit of seropositivity) for statistical analyses. We report seropositivity with Clopper-Pearson’s 95% CIs. We did comparisons of GMCs and seropositivity between independent groups using t test and Fisher’s exact test. We did comparisons of GMCs and seropositivity between two timepoints using t test for paired data and McNemar’s test. We assessed geometric mean fold increases between two timepoints. We investigated associations between GMCs at 1 year after vaccination and baseline demographic factors (sex, age) by comparing GMCs between subgroups or by assessing Spearman’s correlation coefficients. We applied a linear regression model to test whether the magnitude of the association between vaccine-related arthritis and GMC 2 years after vaccination was the same in volunteers from the Geneva study who were given a low dose of vaccine and those who were given a high dose. We applied linear regression models with mixed effects to investigate ZEBOV-IgG antibody persistence, accounting for repeated measures of ELISA titre and adjusting for sex, age, baseline seropositivity, and vaccine-related arthritis. A detailed description of statistical methods we used can be found in the appendix. All analyses were done by the R Development Core Team, 2008 (R Foundation for Statistical Computing, Vienna, Austria). The trials and the observational study were registered at ClinicalTrials.gov (Geneva NCT02287480 and NCT02933931; Kilifi NCT02296983) and the Pan-African Clinical Trials Registry (Lambaréné PACTR201411000919191).
Role of the funding source
The funders of the phase 1 trials (Wellcome Trust Foundation) and the follow-up observational study (Innovative Medicines Initiative) had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Results
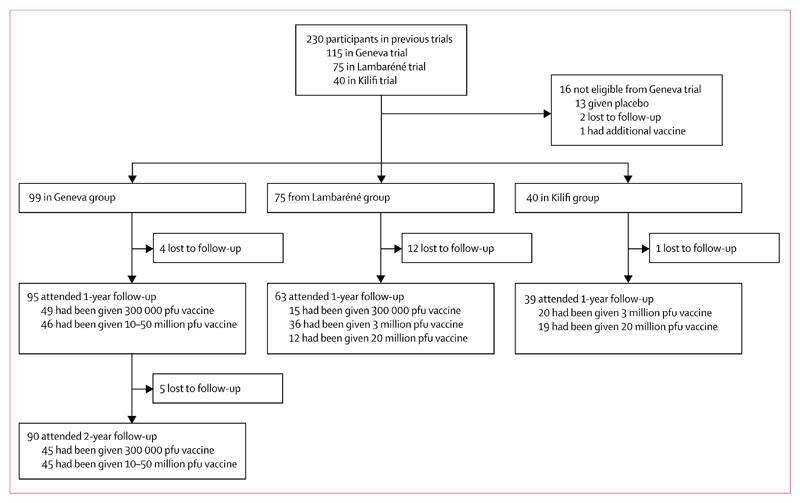
Between vaccination during the period November, 2014, to January, 2015, and 1-year follow-up for the Lambaréné and Kilifi groups in the period November, 2015, to January, 2016, or 2-year follow-up for the Geneva group in the period November, 2016, to January, 2017, the 230 participants in the previous phase 1/2 trials on rVSV-ZEBOV vaccination in Geneva (115 participants), Lambaréné (75 participants), and Kilifi (40 participants) were assessed, and 214 were eligible to participate in the observational study (99 from the Geneva study [102 had been vaccinated; however, three were not eligible for invitation to this observational study; one had had another vaccination, and two were lost to follow-up], 75 from the Lambaréné study, and 40 from the Kilifi study). 197 attended the 1-year follow-up, 95 (96%) from the Geneva study, 63 (84%) from the Lambaréné study, and 39 (98%) from the Kilifi study, and, for the Geneva study population, 90 (91%) attended the 2-year follow-up (figure 1). All demographic, clinical, and immunological characteristics of volunteers attending the 1-year after vaccination visit are in table 1.
Figure 1. Trial profile.
pfu=plaque-forming units.
Table 1. Demographic, clinical, and immunological characteristics at follow-up enrolment (12 months after immunisation).
| All | Geneva group | Lambaréné group | Kilifi group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low dose; 300 000 pfu |
High dose; 10 million pfu |
High dose; 50 million pfu |
Low dose; 300 000 pfu |
Intermediate dose; 3 million pfu |
High dose; 20 million pfu |
Intermediate dose; 3 million pfu |
High dose; 20 million pfu |
||
| Group size at vaccination | 217 | 51 | 35 | 16 | 20 | 39 | 16 | 20 | 20 |
| Group size at 12 months | 197 | 49 | 32 | 14 | 15 | 36 | 12 | 20 | 19 |
| Age, years | 35 (11) | 40 (11) | 42 (12) | 43 (14) | 29 (8) | 28 (8) | 25 (5) | 34 (7) | 32 (7) |
| Sex | |||||||||
| Female | 63 (32%) | 26 (53%) | 14 (44%) | 4 (29%) | 5 (31%) | 3 (9%) | 1 (6%) | 6 (30%) | 4 (21%) |
| Male | 134 (68%) | 23 (47%) | 18 (56%) | 10 (71%) | 10 (67%) | 33 (92%) | 11 (92%) | 14 (70%) | 15 (79%) |
| Ethnic origin | |||||||||
| Black | 100 (51%) | 0 | 1 (3%) | 0 | 16 (100%) | 35 (100%) | 12 (100%) | 17 (85%) | 19 (100%) |
| White | 92 (47%) | 49 (100%) | 26 (81%) | 14 (100%) | 0 | 0 | 0 | 3 (15%) | 0 |
| Other | 5 (3%) | 0 | 5 (16%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Clinical characteristics | |||||||||
| Vaccine-related arthritis | 24 (12%) | 12 (24%) | 8 (25%) | 3 (21%) | 0 | 0 | 0 | 1 (5%) | 0 |
| Immunological characteristics | |||||||||
| Seropositivity before vaccination* | 18 (9%) | 1 (2%) | 1 (3%) | 1 (7%) | 2 (13%) | 7 (20%) | 5 (42%) | 1 (5%) | 0 |
| Seropositivity at day 28 | 184 (94%)† | 39 (80%) | 32 (100%) | 13 (100%)† | 13 (87%) | 36 (100%) | 12 (100%) | 20 (100%) | 19 (100%) |
Data are n, n (%), or mean (SD). Percentages have been calculated with the group size at 12 months as the denominator. pfu=plaque-forming units.
Seropositivity was experimentally defined by a Zaire Ebola virus glycoprotein-specific IgG antibody titre ≥58·84 EU/mL.
One sample for day 28 seropositivity was missing.
For the Geneva study population, at the 1-year visit, 49 (52%) of 95 attendees had been given the low dose of vaccine (ie, 300 000 pfu), and 46 (48%) had been given a high dose of vaccine (10–50 million pfu). Most participants (92 [97%]) had been seronegative before vaccination (table 1); at the 2-year visit, 45 volunteers who had been given a low dose of vaccine and 45 volunteers who had been given a high dose of vaccine returned; baseline seronegativity remained 97%. Among the 75 Lambaréné phase 1 participants, 63 returned for the 1-year follow-up: 15 (24%) had been given 300 000 pfu of vaccine, 36 (57%) had been given 3 million pfu, and 12 (19%) had been given 20 million pfu. Baseline seropositivity in this group 1 year after vaccination was much higher (14 [22%] of 63) in this Ebola virus disease-endemic area than in the Geneva or Kilifi groups. In the Kilifi phase 1 trial population, 39 (98%) of 40 attended the 1-year follow-up; only one (3%) of 39 attendees had been seropositive at baseline (table 1).
In the Geneva group, 45 (100%) of 45 volunteers who were given the high-dose vaccine and completed 1-year follow-up (and had day-28 data) were seropositive on day 28, compared with 39 (80%) of 49 given the low-dose vaccine (p=0·001). In the Lambaréné group, all (48 [100%] volunteers who were given 3 million pfu or more of vaccine were seropositive on day 28 compared with 13 (87%) of 15 who were given the lower dose of 300 000 pfu of vaccine (p=0·079). In the Kilifi group, all participants had been given 3 million or 20 million pfu of vaccine and they were all seropositive by day 28. Thus, vaccine dose influenced early (ie, day 28) antibody responses, as described previously by us1,2 and others.3
In the absence of established correlates of protection, seropositivity persistence was taken as a first, low-stringency marker for the persistence of vaccine-induced responses. Seropositivity at 1 year at all sites and 2 years after vaccination in the Geneva group did not differ significantly among dose groups (appendix). In volunteers who had been given 3 million pfu or more of vaccine, seropositivity persisted (45 [100%] of 45 volunteers in the Geneva group at 2 years, 48 [100%] of 48 in the Lambaréné group at 1 year, and 39 [100%] of 39 tested [one volunteer was not tested] in the Kilifi group at 1 year). Delayed seropositivity responses were occasionally seen in volunteers who had been given 300 000 pfu of vaccine, with 39 (80%) of 49 volunteers in the Geneva study seropositive at 1 year and 41 (91%) of 45 seropositive at 2 years; and 17 (85%) of 20 volunteers in the Lambaréné group seropositive at day 28 and 15 (100%) of 15 seropositive at 1 year (p=0·244; appendix). Thus, 1 year and 2 years after vaccination seropositivity remained high and dose dependency was lost.
In the Geneva group, 44 (100%) of 44 participants who had been given a high dose (ie, 10–50 million pfu) of vaccine and who were seropositive at day 28 remained seropositive at 2 years, whereas 33 (89%) of 37 who had been given the low dose (ie, 300 000 pfu) of vaccine and who were seropositive at day 28 remained seropositive for 2 years (p=0·042). Further details of the similarly high proportions of participants with seropositivity persistence in the Lambaréné and Kilifi groups are shown in the appendix.
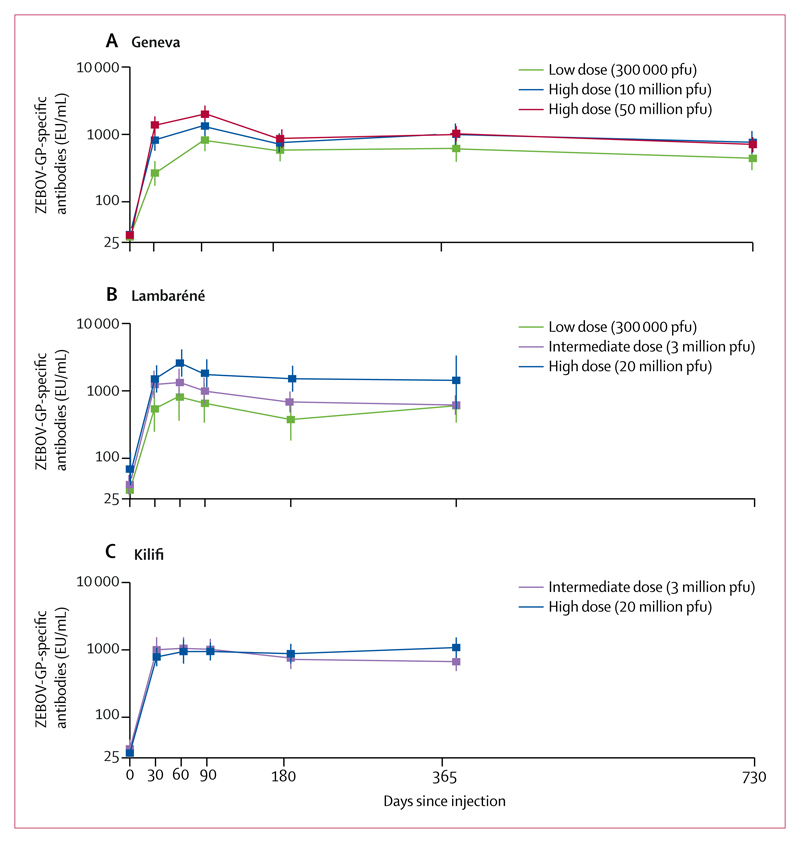
Figure 2 shows and table 2 lists GMCs of rVSV-ZEBOV glycoprotein-specific antibodies for each dose group at each site over time, and GMC ratios across each timepoint in each group are shown in the appendix. GMCs peaked between 1 and 3 months after vaccination in all dose groups, although this finding was less pronounced in the Kilifi group. Baseline seropositivity, frequent in Lambaréné, did not influence follow-up GMCs compared with seronegative volunteers receiving the same dose, at this site or in Geneva or Kilifi (table 3). In all sites and across all doses, GMC peaks were followed by an initial decline until month 6 (table 3). GMCs then plateaued between 6 and 12 months across doses and settings, and up to 2 years in participants who had been given a high dose of vaccine in the Geneva group (table 3). In the Geneva group, lower 2-year titres than 1-year titres were seen in participants who were given the low dose of vaccine (GMC ratio 0·61, 95% CI 0·49–0·77; p<0·0001).
Figure 2. GMCs of ZEBOV-GP-specific antibodies in Geneva (A), Lambaréné (B), and Kilifi (C).
See appendix for GMC ratios and descriptive statistics. Error bars show 95% CI. EU=ELISA arbitrary units.
GMCs=geometric mean concentrations. pfu=plaque-forming units. ZEBOV-GP=Zaire Ebola virus glycoprotein.
Table 2. GMCs of ZEBOV glycoprotein-specific antibodies across doses and studies at different timepoints.
| Day 0 | Day 28 | Day 56 | Day 84 | Month 6 | Year 1 | Year 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | GMC, EU/mL | n | GMC, EU/mL | n | GMC, EU/mL | n | GMC, EU/mL | n | GMC, EU/mL | n | GMC, EU/mL | n | GMC, EU/mL | |
| Geneva group | ||||||||||||||
| 300 000 pfu | 51 | 30·0 (28·9–31·1; p=0·19*) |
51 | 267·4 (175·3–407·9; p<0·001*) |
ND | ND | 50 | 824·9 (576·7–1179·9; p=0·006*) |
51 | 583·9 (407·5–836·5; p=0·19*) |
49 | 618·2 (412·0–927·6; p=0·04*) |
45 | 440·8 (297·8–652·5; p=0·03*) |
| 10 million pfu | 35 | 32·6 (28·2–37·7) |
34 | 821·2 (579·7–1163·5) |
ND | ND | 33 | 1332·1 (955·9–1856·5) |
34 | 747·6 (539·1–1036·9) |
32 | 1005·7 (697·4–1450·4) |
31 | 761·3 (516·2–1122·8) |
| 50 million pfu | 16 | 31·9 (28·4–36·0; p=0·86†) |
14 | 1383·0 (996·0–1920·4; p=0·09†) |
ND | ND | 16 | 1993·7 (1547·9–2567·8; p=0·10†) |
15 | 865·7 (630·6–1188·6; p=0·06†) |
14 | 1037·9 (804·2–1339·6; p=0·90†) |
14 | 707·8 (546·6–916·4; p=0·78†) |
| 10 or 50 million pfu |
51 | 32·4 (28·2–37) |
48 | 956·1 (680·1–1344·0) |
ND | ND | 49 | 1591·6 (1118·6–2064·3) |
49 | 782·6 (521·7–1069·6) |
46 | 1015·4 (732·7–1407·1) |
45 | 744·3 (528·2–1048·6) |
| Lambaréné group | ||||||||||||||
| 300 000 pfu | 19 | 33·7 (27·6–41·1; p=0·02‡) |
20 | 540·2 (254·4–1146·7; p=0·04‡) |
17 | 809·9 (355·3–1846·1; p=0·02‡) |
17 | 654·0 (333·1–1283·9; p=0·02‡) |
16 | 375·2 (189·9–741·2; p<0·001‡) |
15 | 602·3 (357·9–1013·7; p=0·06‡) |
ND | ND |
| 3 million pfu | 39 | 40·6 (29·2–56·5) |
39 | 1245·0 (778·4–1991·2) |
37 | 1330·7 (829·7–2134·1) |
35 | 994·0 (629·0–1571·0) |
37 | 684·6 (479·6–977·2) |
36 | 616·1 (441·3–860·1) |
ND | ND |
| 20 million pfu | 16 | 69·1 (39·7–120·2) |
16 | 1503·0 (943·6–2394·0) |
13 | 2589·5 (1625·2–4126·0) |
14 | 1825·7 (1133·6–2940·2) |
15 | 1514·4 (972·1–2359·3) |
12 | 1433·3 (571·8–3592·7) |
ND | ND |
| Kilifi group | ||||||||||||||
| 3 million pfu | 20 | 34·0 (25·1–46·2; p=0·33§) |
20 | 1005·2 (655·2–1542·1; p=0·34§) |
20 | 1054·9 (721·6–1542·3; p=0·68§) |
20 | 1018·9 (711·0–1460·2; p=0·75§) |
20 | 756·9 (520·1–1101·5; p=0·54§) |
20 | 667·9 (484·7–920·2; p=0·04§) |
ND | ND |
| 20 million pfu | 18 | 29·4 (29·4–29·4) |
20 | 785·3 (571·8–1078·7) |
20 | 944·6 (625·8–1425·7) |
20 | 946·8 (695·5–1288·9) |
20 | 877·9 (625·7–1231·9) |
19 | 1083·1 (766·8–1529·8) |
ND | ND |
Data are GMC (95% CI; p value) or GMC (95% CI); n is number of samples available at timepoint. GMC=geometric mean concentration. EU=ELISA arbitrary units. ZEBOV=Zaire Ebola virus. pfu=plaque-forming units. ND=not done.
Comparison of GMCs between participants who were given a low dose (300 000 pfu) and those who were given a high dose (10 million or 50 million pfu) of vaccine.
Comparison of GMCs between participants who were given 10 million and 50 million pfu of vaccine in the Geneva study.
Comparison of GMCs between participants who were given 300 000 and 20 million pfu of vaccine in the Lambaréné study.
Comparison of GMCs between participants who were given 3 million and 20 million pfu of vacccine in the Kilifi study.
Table 3. Multivariable linear regression model with mixed effects assessing determinants of GMC ratios by dose of vaccine and study group.
| Low dose* | Intermediate dose† | High dose‡ | ||||
|---|---|---|---|---|---|---|
| Ratio of GMC (95% CI) | p value | Ratio of GMC (95% CI) | p value | Ratio of GMC (95% CI) | p value | |
| Geneva group | ||||||
| Days since vaccination | ||||||
| 84 | 1·44 (1·15–1·79) | 0·0016 | NA | ·· | 1·81 (1·51–2·17) | <0·0001 |
| 168 | 1 (ref) | ·· | NA | ·· | 1 (ref) | ·· |
| 365 | 1·05 (0·84–1·31) | 0·65 | NA | ·· | 1·23 (1·03–1·48) | 0·026 |
| 730 | 0·65 (0·51–0·81) | 0·0003 | NA | ·· | 0·91 (0·76–1·09) | 0·32 |
| Sex | ||||||
| Male | 1 (ref) | ·· | ·· | ·· | 1 (ref) | ·· |
| Female | 1·53 (0·80–2·91) | 0·20 | NA | ·· | 1·46 (0·91–2·35) | 0·12 |
| Baseline seropositivity | ||||||
| No | 1 (ref) | ·· | ·· | ·· | 1 (ref) | ·· |
| Yes | 2·18 (0·22–21·80) | 0·51 | NA | ·· | 2·18 (0·82–5·79) | 0·12 |
| Age, per 10 years | 1·30 (0·96–1·76) | 0·096 | NA | ·· | 1·12 (0·92–1·36) | 0·26 |
| Vaccine-related arthritis | ||||||
| No | 1 (ref) | ·· | ·· | 1 (ref) | ||
| Yes | 1·98 (0·90–4·38) | 0·096 | NA | ·· | 1·39 (0·79–2·43) | 0·26 |
| Lambaréné group | ||||||
| Days since vaccination | ||||||
| 56 | 1·81 (1·25–2·62) | 0·0031 | 1·79 (1·51–2·12) | <0·0001 | 1·70 (1·07–2·69) | 0·030 |
| 84 | 1·62 (1·12–2·33) | 0·013 | 1·37 (1·15–1·63) | 0·0007 | 1·16 (0·74–1·81) | 0·52 |
| 168 | 1 (ref) | ·· | 1 (ref) | ·· | 1 (ref) | ·· |
| 365 | 1·16 (0·78–1·73) | 0·45 | 0·89 (0·75–1·05) | 0·18 | 1·20 (0·73–1·95) | 0·48 |
| Sex | ||||||
| Male | 1 (ref) | ·· | 1 (ref) | ·· | 1 (ref) | ·· |
| Female | 3·16 (0·84–11·81) | 0·11 | 1·06 (0·47–2·41) | 0·89 | 0·19 (0·02–2·26) | 0·20 |
| Baseline seropositivity | ||||||
| No | 1 (ref) | ·· | 1 (ref) | ·· | 1 (ref) | ·· |
| Yes | 3·08 (0·49–19·27) | 0·25 | 1·42 (0·75–2·70) | 0·29 | 1·19 (0·45–3·17) | 0·73 |
| Age, per 10 years | 0·58 (0·25–1·35) | 0·22 | 0·99 (0·69–1·42) | 0·95 | 0·86 (0·35–2·13) | 0·75 |
| Kilifi group | ||||||
| Days since vaccination | ||||||
| 60 | NA | ·· | 1·39 (1·07–1·81) | 0·014 | 1·08 (0·83–1·40) | 0·58 |
| 90 | NA | ·· | 1·35 (1·04–1·75) | 0·027 | 1·08 (0·83–1·40) | 0·57 |
| 180 | NA | ·· | 1 (ref) | ·· | 1 (ref) | ·· |
| 365 | NA | ·· | 0·88 (0·68–1·15) | 0·35 | 1·14 (0·88–1·49) | 0·32 |
| Sex | ||||||
| Male | NA | ·· | 1 (ref) | ·· | 1 (ref) | ·· |
| Female | NA | ·· | 0·68 (0·35–1·32) | 0·27 | 1·49 (0·69–3·21) | 0·32 |
| Baseline seropositivity | ||||||
| No | NA | ·· | 1 (ref) | ·· | NA | ·· |
| Yes | NA | ·· | 1·17 (0·28–4·80) | 0·83 | NA | ·· |
| Age, per 10 years | NA | ·· | 0·89 (0·59–1·35) | 0·60 | 1·04 (0·67–1·60) | 0·88 |
Log10 ELISA values were modelled and regression coefficients were back-transformed to express results as ratio of GMC. The model includes a random intercept (for variability between participants), excludes placebo recipients and day 0 and 28 titres, and is adjusted for sex, age, and baseline seropositivity. Unless specified otherwise (days since vaccination), this models GMC at 1 year after immunisation. GMC=geometric mean concentration. NA=not applicable. pfu=plaque-forming units.
300 000 pfu.
3 million pfu.
10 million or 50 million pfu in Geneva group and 20 million pfu in Lambaréné and Kilifi groups.
Because comparing GMCs could mask antibody disappearance in a subset of participants, individual values are given at each timepoint in the appendix; this analysis shows that the lower GMCs at day 28 in people who were given the low dose of vaccine (300 000 pfu) are due to a slow response in some volunteers to this low dose, whereas higher doses (≥3 million pfu) induce a more prompt and stronger response. Furthermore, complete antibody loss was rare after 6 months, and was only observed in four (9%) of 45 participants given the low dose.
For the Geneva group, neutralising antibody geometric mean titres assessed at baseline, day 28, and at month 6 with Ebola virus are shown in the appendix. The proportion of participants with seropositivity increased from zero at baseline to 71% (ten of 14 participants who received 50 million pfu) on day 28. By month 6, titres had fallen to low levels in all dose groups, with seropositivity dropping to 27–31%.
Univariable analyses detected higher GMCs of ZEBOV glycoprotein-specific antibodies at 1 year in female participants than in male participants who received a high dose in the Geneva group (appendix), although in multivariable models significance was not reached (table 3). Similarly, although univariable analyses detected an association between increasing age and increased GMCs in participants who received the low dose in the Geneva group (appendix), a multivariable regression model with mixed effects and adjusted for sex, age, and seropositivity at baseline indicates that GMC ratios were influenced by whether the participant was given a high dose versus a low dose of vaccine in Geneva (1·66, 95% CI 1·12–2·46; p=0·0133) and Lambaréné (2·56, 1·30–5·03; p=0·008; appendix). GMC ratios were not influenced by sex, age, or baseline seropositivity (table 3).
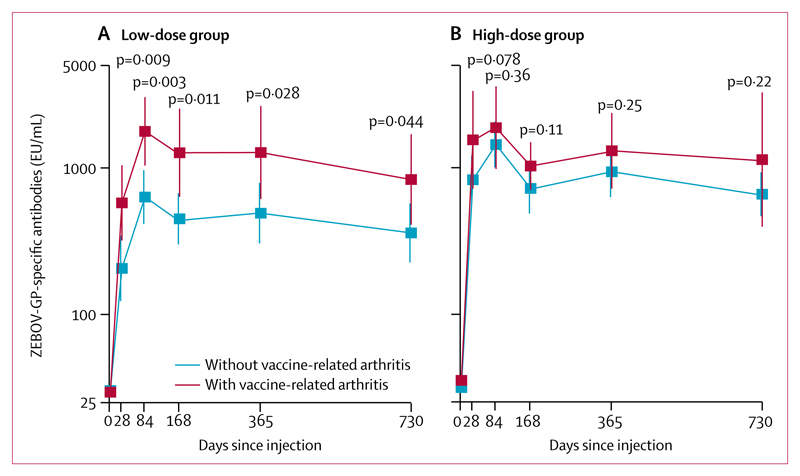
In the Geneva group, 13 (25%) of 51 people who were given the low dose of vaccine and 11 (22%) of 51 who were given the high dose of vaccine reported vaccine-related arthritis at a median of 10 days (IQR 9–14) after immunisation.1,2 In the 6 months following vaccination, two participants with early vaccine-related arthritis had suspected, self-limited recurrences of arthritis.1 Since then, no episodes were reported up until March 22, 2018. However, the occurrence of vaccine-related arthritis after vaccination was associated with increased ZEBOV glycoprotein-specific IgG GMCs throughout the 2-year follow-up period (figure 3). The association was only significant in the low-dose group; anti-ZEBOV GMCs of participants with vaccine-related arthritis were significantly higher than those without vaccine-related arthritis at every timepoint (p<0·05 for all), and by 2 years after vaccination, the anti-ZEBOV GMCs of participants with vaccine-related arthritis were higher than those of participants who received a high dose who did not have vaccine-related arthritis (1270·1 EU/mL, 95% CI 612·4–2634·2 vs 659·5 EU/mL, 467·3–931·0; p=0·5189; figure 3; appendix). Volunteers who had been given the high dose of vaccine who had vaccine-related arthritis also achieved higher GMCs than those who did not get vaccine-related arthritis, but the difference did not reach significance (appendix). A multivariable linear regression model to investigate interactions between vaccine dose and vaccine-related arthritis did not reveal an influence of the dose on the GMC ratios and 2-year titres of those with and those without vaccine-induced arthritis (pinteraction=0·596; appendix). The multivariable regression model with mixed effects confirmed the association of arthritis with increased antibody persistence when adjusting for sex, age, and baseline seropositivity (table 3). The occurrence of vaccine-related arthritis did have a significant effect on GMC when participants who received low doses and high doses of vaccine were combined in the Geneva group (GMC ratio 1·76, 95% CI 1·11–2·78; p=0·0176; appendix).
Figure 3. GMCs of ZEBOV glycoprotein-specific antibodies in the Geneva group with and without vaccine-related arthritis by dose group.
(A) Low-dose group—ie, 300 000 pfu of vaccine. (B) High-dose group—ie, 10 million or 50 million pfu of vaccine. p values are for the comparison of dose groups at each timepoint (see appendix for a listing of values and descriptive statistics). Error bars show 95% CI. GMC=geometric mean concentration. EU=ELISA arbitrary units. pfu=plaque-forming units. ZEBOV-GP=Zaire Ebola virus glycoprotein.
Discussion
The humoral response induced by a single injection of the replication-competent rVSV-ZEBOV vaccine persisted for at least 1–2 years across three populations in two different continents and with different doses, with kinetics enabling the prediction of long-term antibody persistence in most people who were given the vaccine. The data from the Geneva group show that 100% of early responders given 10 million pfu or more of vaccine remained seropositive at 2 years after vaccination, with similar patterns seen in two African countries (Lambaréné, Gabon, and Kilifi, Kenya). Even at the lowest dose of 300 000 pfu, 89% of participants with seroconversion remained seropositive 2 years after vaccination. Thus, a single injection of rVZV-ZEBOV induced sustained antibody responses in almost all participants who were given the vaccine.
The kinetics of ZEBOV glycoprotein-specific IgG antibodies were typical of other live-attenuated vaccines. After an early peak at 2–3 months after the single-dose vaccination, during which ZEBOV glycoprotein-specific antibodies were most likely produced by short-lived plasmablasts,17 antibody concentrations followed an initial decline, with no significant subsequent decreases in GMCs between 28 days and up to 1 or 2 years after vaccination, or 6 months and up to 1 or 2 years after vaccination. In the absence of exposure to filoviruses after immunisation (in the Geneva or Kilifi groups) that could have boosted vaccine responses, this result probably reflects an effective switch from short-lived to long-lived plasma cells following rVSV-ZEBOV immunisation17 and provides an immunological basis for long-lasting protection, should the protection be mediated by vaccine antibodies. Multivariable analyses indicating a decline in 2-year GMCs in participants who were given the low dose of vaccine (but not those who were given the high dose) in the Geneva group warrant further follow-up.
Protective humoral responses to natural viral infections can be extremely sustained, even lifelong. Circulating, antigen-specific antibodies have been detected in patients with no interim exposure for as long as 65 years for measles infection and 75 years for yellow fever infection.18,19 Some live-attenuated vaccines appear to induce similar lifelong humoral immunity—eg, antigen-specific IgG concentrations have been consistent for several decades after single-dose injection with the smallpox20 and yellow fever21 vaccines in patients without pathogen exposure. The results from this observational study allow some degree of cautious optimism regarding the long-term persistence of antibody responses and of protection should vaccine antibodies confer protection. The heavily glycosylated glycoprotein of Ebola virus behaves as a rather weak immunogen, at least when presented in some vaccine formulations, as shown by the rapid disappearance of glycoprotein-specific antibodies following a single dose of the glycoprotein delivered by the non-replicating (single-cycle) rChAd3 vector vaccine.9 Therefore, we presume that the sustainability of humoral responses to rVSV-ZEBOV vaccine essentially results from the strong influence of rVSV on early immune responses.22
This finding is of substantial interest since, similarly, rVSV might have the capacity to induce sustained responses to glycoproteins from other emerging viruses, such as the Nipah or Lassa viruses.23 This sustained persistence of antibody titres is consistent with that observed by Heppner and colleagues3 in US volunteers in a phase 1b study of rVSV-ZEBOV vaccine at 1 year (last timepoint assessed) after vaccination, but contrasts with a study by Khurana and colleagues13 in which rapid antibody decline was reported in recipients of two doses of rVSV-ZEBOV vaccine. Our understanding is that the surface plasmon resistance assay used by Khurana and colleagues13 in their study of human antibodies after VSV-Ebola vaccination essentially detected high levels of IgM antibodies, whereas the FANG ELISA assay (used in our study and that of Heppner and colleagues3) specifically measures IgG antibodies. The rapid decline of IgM versus the longer persistence of IgG antibodies could explain this discrepancy and be consistent with the rapid waning of some neutralising antibodies found in our study (appendix).
The influence of rVSV-ZEBOV vaccine is dose dependent. Vaccine doses below 3 million pfu, which induce lower cytokine responses,22 led to weaker early (ie, 28 day) antibody responses, suggesting weaker induction of short-term plasma cells than might be achieved with a higher dose of vaccine.1–3 But this weaker response could just have been a delay in response; by 1 year after vaccination, GMCs in the participants in the Lambaréné and Geneva groups who were given low doses of vaccine (300 000 pfu) resembled those of participants immunised with doses that were ten and 100 times higher, and by 2 years (for the Geneva group), GMCs in those given the low dose of vaccine were 1·6-times higher than on day 28. Thus, although high doses of vaccine might contribute to early protection, lower (less reactogenic1,2,22) doses than these could be attractive should preventive campaigns be considered necessary. We postulate that the induction of delayed but ultimately high humoral responses by low doses of rVSV-ZEBOV vaccine result from a facilitated escape of the vaccine load from lower early antiviral responses,22 enabling longer antigen persistence and thus potentially more durable immune responses than those achieved with a higher dose of vaccine.
The same mechanism could be true in participants with vaccine-related arthritis. We previously reported the onset of vaccine-related arthritis in the second week after immunisation in a substantial proportion (24%) of the Geneva group,1,2,16 an observation that was less frequent in the Lambaréné and Kilifi groups. Reasons for the discrepancy have been explored elsewhere.1,2 The phase 1 rVSV-ZEBOV trial3 in the USA confirmed the occurrence of vaccine-related arthritis after immunisation, at an incidence of 5%, with no association with race or ethnic origin. Along with the similarities of vaccine plasma signatures observed in the Geneva and Lambaréné groups,22 this similarity between studies suggests that volunteer perception, ascertainment, and reporting methods could have a role in the reported variable incidence of vaccine-related arthritis. In a subsequent study,22 we showed that participants who were given high doses of vaccine (ie, ≥10 million pfu) had significantly weaker cytokine and chemokine responses than those given low doses of vaccine (ie, 300 000 pfu), suggesting weaker initial viral control among the high-dose population than the low-dose population. We now show that vaccine-related arthritis is associated with significantly higher GMCs, especially in participants given low doses of vaccine, than among those who did not get vaccine-related arthritis (figure 3). Participants who were given a low dose of vaccine versus those given a high dose, and those given a high dose of vaccine with vaccine-related arthritis versus those without vaccine-related arthritis, have weaker cytokine and chemokine responses than their respective counterparts.22 Therefore, vaccine escape from early innate responses, and thus extended viral persistence, could contribute to the onset of rVSV-ZEBOV-related arthritis and result in increased antibody responses; therefore, the so-called benefit of extended antigen presentation contributes less in people given a high dose of vaccine than in those given a low dose.
Participants in the Lambaréné group had a higher proportion of baseline seropositivity than those in the other groups, although none of the participants had had known contacts in Gabon’s 2002 outbreak of Ebola virus disease. Repeated antigenic stimulation or aborted infection has been postulated to occur through the handling or eating of fruitbats (a common source of bushmeat) or fruits contaminated by bat saliva, urine, or faeces containing infectious virus, inactivated virus, or viral antigens.24 Therefore, baseline antibodies could reflect cross-reactive antibodies to other filoviruses, since baseline seropositivity did not influence anti-ZEBOV glycoprotein antibody persistence.
We observed a pattern toward higher ZEBOV glycoprotein-specific IgG titres at 1 year after vaccination in female participants than male participants in Geneva. Women are known to have more vigorous innate and adaptive responses,25 and stronger responses have occasionally been documented in female vaccinees.26 The finding was not, however, confirmed by multivariable analyses.
By contrast with the ZEBOV glycoprotein-binding IgG con-centrations, neutralising antibody titres of the Geneva group were significantly lower at month 6 than at day 28 for all dose groups. This finding differs from the persistence reported at 6 months after vaccination by another group using a similar assay to assess the Lambaréné and Kilifi cohorts.1,3 A similar reduction of titres had been observed in the Geneva group, Lambaréné group, and Kilifi group volunteers using a pseudovirion neutralisation assay,2 but not in US volunteers.3 This variability highlights the poor correlation among neutralisation assays27 and the need for collaborative efforts to validate and standardise assays before conclusions can be reached on the persistence of neutralising antibodies and their putative role in protection against disease.
Our study has limitations. Not all people who had been vaccinated in the previous studies were available for later-phase sampling, but the missing samples are few. The immune correlates of protection against Ebola virus disease have not been defined. The role of vaccine-induced T cells in protection against Ebola virus is still unknown and their assessment was not part of this study protocol. IgM vaccine antibodies were shown to contribute to in-vitro virus neutralisation, but over time IgM responses are replaced by IgG responses.13 Neutralising antibodies have been assessed only up to 6 months after immunisation, pending the availability of validated sensitive assays. Their role in protection is still unclear. Participants who were seropositive at baseline were not excluded from the analysis, but baseline seropositivity did not affect antibody persistence. If ZEBOV glycoprotein-specific IgG mediates protection, the protective concentration remains unknown. We addressed this limitation by using low-stringency (seropositivity) and high-stringency (yearly GMC ratio compared with that at day 28, at which protection had been observed) markers. Although a protective threshold would likely rank somewhere in between, the use of both markers gave the same conclusion—ie, that the humoral response is durable in the 2 years following so-called one-shot rVSV-ZEBOV vaccination. Given the logistical challenges inherent in vaccine campaigns in Ebola-endemic regions, the importance of single-injection vaccination goes beyond mere convenience.
Supplementary Material
Research in context.
Evidence before this study
Since the durability of recombinant vesicular stomatitis virus (rVSV)-Zaire Ebola virus (ZEBOV)-induced protection is probably best estimated by antibody persistence in people who have been vaccinated, we sought to identify information on this durability after single-dose vaccination. We did this by searching the MEDLINE and ClinicalTrials.gov databases, with no language restrictions, for studies that were published before Sept 1, 2017, using keywords including “Ebola”, “vaccine”, “VSV-Ebola”, “rVSV-ZEBOV”, “rVSV-EBOV”, “VSV-EBOV”, “durability”, and “long-term immunity”. These terms were then combined with the additional terms “antibody persistence”, “long-term protection”, “durable immune response”, and “antibody response over time” and the search was broadened to include the Google search engine. We identified one clinical trial reporting antibody concentrations at 1 year after vaccination with rVSV-ZEBOV but found no information on antibody persistence in human beings beyond this interval. Antibody persistence in this study, which tested a wide range of rVSV-ZEBOV doses in a US population, was remarkably robust, with 1-year geometric mean concentrations (GMCs) of anti-ZEBOV IgG antibodies in the higher dose groups found to not be significantly lower than GMCs at 28 days after vaccination.
Added value of this study
This study provides new data on the durability of the humoral immune response up to 2 years after single-dose injection with rVSV-ZEBOV vaccine in a population of primarily European ancestry, and additional data on durability at 1 year after vaccination in two African populations.
Implications of all the available evidence
The Wellcome Trust-Center for Infectious Disease Research and Policy Ebola Vaccine Team B initiative recommends that any vaccine used for immunisation of contacts of patients with Ebola virus disease should induce protection lasting at least 2 years. These results suggest that a single injection of rVSV-ZEBOV vaccine is likely to fulfill this recommendation, and larger, observational studies after vaccination are needed to confirm this hypothesis.
Acknowledgments
We thank all volunteers for their generous participation in the phase 1 trials and follow-up studies. We also thank the Clinical Research Centre, University Hospital and Faculty of Medicine, Geneva, Switzerland and Julie-Anne Dayer, Laurent Kaiser, Sheri Dubey, Beth-Ann Coller, Jakub Simon, and Rituparna Das for their support.
Footnotes
Contributors
AH, STA, JFF, BL, SG, and C-AS collected the data. AH, STA, CC, JFF, EBB, LK, FMN, JB, TPM, BL, SG, MB, OE, JP, DS, PB, PS, PK, and C-AS analysed and interpreted the data. AH, STA, the VEBCON and VSV-EBOLA Consortia, PK, and C-AS made substantial contributions to the conception and design of the study. AH, CC, and C-AS wrote the manuscript. All authors contributed to the revision of the manuscript.
Declaration of interests
TPM is an employee of NewLink Genetics Corporation. All other authors declare no competing interests.
Contributor Information
Angela Huttner, Division of Infectious Diseases, University Hospitals of Geneva, Geneva, Switzerland; Infection Control Programme, University Hospitals of Geneva, Geneva, Switzerland; Centre for Vaccinology, University Hospitals of Geneva, Geneva, Switzerland.
Selidji Todagbe Agnandji, Centre de Recherches Médicales de Lambaréné, Hôpital Albert Schweitzer, Lambaréné, Gabon; Institut für Tropenmedizin and German Centre for Infection Research (DZIF) partner sites, Universitätsklinikum Tübingen, Tübingen, Germany.
Christophe Combescure, Division of Clinical Epidemiology, University Hospitals of Geneva, Geneva, Switzerland.
Francis Maina Ndungu, KEMRI-Wellcome Trust Research Programme, Kilifi, Kenya.
Jessica Brosnahan, Centre de Recherches Médicales de Lambaréné, Hôpital Albert Schweitzer, Lambaréné, Gabon; Institut für Tropenmedizin and German Centre for Infection Research (DZIF) partner sites, Universitätsklinikum Tübingen, Tübingen, Germany.
Thomas P Monath, NewLink Genetics Corporation, Devens, MA, USA.
Miriam Botto, Non-clinical Development, US Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD, USA.
Prof Philip Bejon, KEMRI-Wellcome Trust Research Programme, Kilifi, Kenya.
Peter Silvera, Non-clinical Development, US Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD, USA.
Prof Peter Kremsner, Centre de Recherches Médicales de Lambaréné, Hôpital Albert Schweitzer, Lambaréné, Gabon; Institut für Tropenmedizin and German Centre for Infection Research (DZIF) partner sites, Universitätsklinikum Tübingen, Tübingen, Germany.
Prof Claire-Anne Siegrist, Centre for Vaccinology, University Hospitals of Geneva, Geneva, Switzerland.
References
- 1.Agnandji ST, Huttner A, Zinser ME, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med. 2016;374:1647–60. doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huttner A, Dayer JA, Yerly S, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2015;15:1156–66. doi: 10.1016/S1473-3099(15)00154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heppner DG, Jr, Kemp TL, Martin BK, et al. Safety and immunogenicity of the rVSVΔG-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: a phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infect Dis. 2017;17:854–66. doi: 10.1016/S1473-3099(17)30313-4. [DOI] [PubMed] [Google Scholar]
- 4.Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) Lancet. 2017;389:505–18. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Ebola virus disease Democratic Republic of the Congo: external situation report 28. Brazzaville: World Health Organization; 2017. [accessed Sept 1, 2017]. http://apps.who.int/iris/bitstream/10665/255793/1/EbolaDRC-30062017.pdf?ua=1. [Google Scholar]
- 6.Jacobs M, Rodger A, Bell DJ, et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet. 2016;388:498–503. doi: 10.1016/S0140-6736(16)30386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soka MJ, Choi MJ, Baller A, et al. Prevention of sexual transmission of Ebola in Liberia through a national semen testing and counselling programme for survivors: an analysis of Ebola virus RNA results and behavioural data. Lancet Glob Health. 2016;4:e736–43. doi: 10.1016/S2214-109X(16)30175-9. [DOI] [PubMed] [Google Scholar]
- 8.Osterholm M, Moore K, Ostrowsky J, Kimball-Baker K, Farrar J. The Ebola Vaccine Team B: a model for promoting the rapid development of medical countermeasures for emerging infectious disease threats. Lancet Infect Dis. 2016;16:e1–9. doi: 10.1016/S1473-3099(15)00416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Santis O, Audran R, Pothin E, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis. 2016;16:311–20. doi: 10.1016/S1473-3099(15)00486-7. [DOI] [PubMed] [Google Scholar]
- 10.Mire CE, Geisbert TW, Feldmann H, Marzi A. Ebola virus vaccines—reality or fiction? Expert Rev Vaccines. 2016;15:1421–30. doi: 10.1080/14760584.2016.1178068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dye JM, Herbert AS, Kuehne AI, et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci USA. 2012;109:5034–39. doi: 10.1073/pnas.1200409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marzi A, Engelmann F, Feldmann F, et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci USA. 2013;110:1893–98. doi: 10.1073/pnas.1209591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khurana S, Fuentes S, Coyle EM, Ravichandran S, Davey RT, Jr, Beigel JH. Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nat Med. 2016;22:1439–47. doi: 10.1038/nm.4201. [DOI] [PubMed] [Google Scholar]
- 14.Howell KA, Brannan JM, Bryan C, et al. Cooperativity enables non-neutralizing antibodies to neutralize ebolavirus. Cell Rep. 2017;19:413–24. doi: 10.1016/j.celrep.2017.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Q, Fan C, Li Q, et al. Antibody-dependent-cellular-cytotoxicity-inducing antibodies significantly affect the post-exposure treatment of Ebola virus infection. Sci Rep. 2017;7 doi: 10.1038/srep45552. 45552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dayer JA, Siegrist CA, Huttner A. Volunteer feedback and perceptions after participation in a phase I, first-in-human Ebola vaccine trial: an anonymous survey. PLoS One. 2017;12:e0173148. doi: 10.1371/journal.pone.0173148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegrist CA. Vaccine immunology. In: Plotkin S, editor. Vaccines. 6th edn. London: Elsevier; 2017. pp. 14–32. [Google Scholar]
- 18.Panum LM. Beobachtungen über das maserncontagium. Virchows Arch. 1847;1:492–512. [Google Scholar]
- 19.Sawyer WA. Persistence of yellow fever immunity. J Prev Med. 1931;5:413–28. [Google Scholar]
- 20.Taub DD, Ershler WB, Janowski M, et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med. 2008;121:1058–64. doi: 10.1016/j.amjmed.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotuzzo E, Yactayo S, Córdova E. Efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am J Trop Med Hyg. 2013;89:434–44. doi: 10.4269/ajtmh.13-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huttner A, Combescure C, Grillet S, et al. A dose-dependent plasma signature of the safety and immunogenicity of the rVSV-Ebola vaccine in Europe and Africa. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aaj1701. eaaj1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coalition for Epidemic Preparedness Innovations. Resources: priority diseases. Oslo: Coalition for Epidemic Preparedness Innovations; 2017. [accessed Sept 5, 2017]. http://cepi.net/resources. [Google Scholar]
- 24.Becquart P, Wauquier N, Mahlakõiv T, et al. High prevalence of both humoral and cellular immunity to Zaire ebolavirus among rural populations in Gabon. PLoS One. 2010;5:e9126. doi: 10.1371/journal.pone.0009126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–38. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 26.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26:3551–55. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson DE, Page M, Mattiuzzo G, et al. Comparison of platform technologies for assaying antibody to Ebola virus. Vaccine. 2017;35:1347–52. doi: 10.1016/j.vaccine.2016.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.