Abstract
Enabled by the discovery of novel cinchona alkaloid-derived chiral phase-transfer catalysts, highly chemo-, regio-, diastereo- and enantioselective umpolung additions of trifluoromethyl imines to α, β-unsaturated N-acylpyrroles were realized. With a catalyst loading ranging from 0.2 to 5.0 mol%, this new catalytic asymmetric transformation provides facile and high-yield access to highly enantiomerically enriched chiral trifluoromethylated γ-amino acids and γ-lactams.
Keywords: Amino acids, Asymmetric catalysis, C-C forming, Phase-transfer catalyst, Umpolung
Graphical Abstract
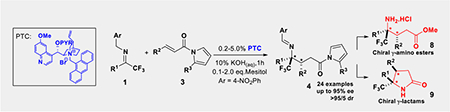
Enabled by the discovery of novel cinchona alkaloid-derived chiral phase-transfer catalysts, highly chemo-, regio-, diastereo- and enantioselective umpolung additions of trifluoromethyl imines to α, β-unsaturated N-acylpyrroles were realized. This new catalytic asymmetric transformation provides facile and high-yield access to highly enantiomerically enriched chiral trifluoromethylated γ-amino acids and γ-lactams.
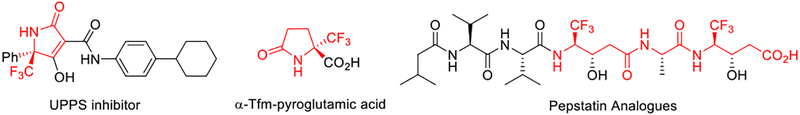
Chiral γ-amino acids as a structural motif are presented in a wide variety of natural products and synthetic compounds of biological interests and pharmaceutical importance.[1–2] Accordingly, the development of new catalytic methodology for the efficient asymmetric synthesis of γ-amino acid derivatives continues to attract great attention.[3] Recently, γ-trifluoromethylated γ-amino acid motifs began to appear in pharmaceutically significant compounds (Figure 1).[4] On the other hand, a general enantioselective access toward these chiral building blocks is not yet available. Herein, we wish to report the discovery and development of new catalysts to enable an unprecedented asymmetric umpolung addition of trifluoromethyl imines to α, β-unsaturated N-acylpyrroles, thereby establishing a general synthetic strategy for the asymmetric synthesis of trifluoromethylated γ-amino acid derivatives.
Figure 1.
Biologically active and medicinally important trifluoromethylated γ-amino acids.
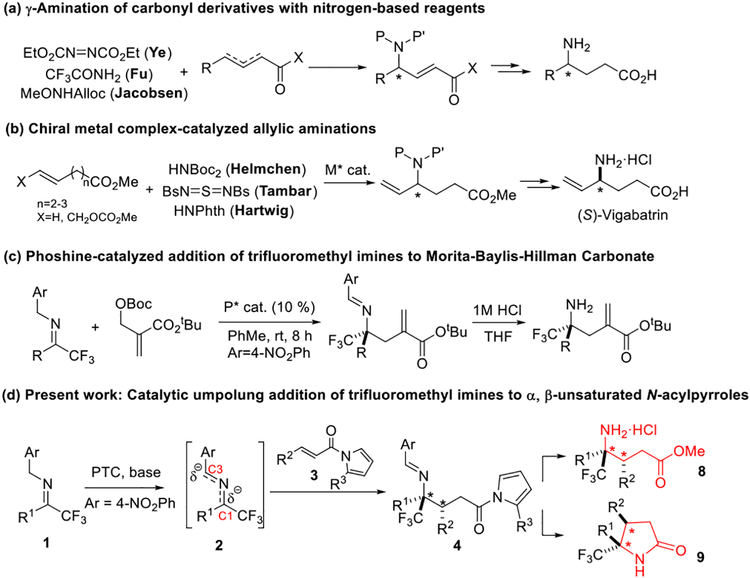
While numerous approaches towards α/β-substituted γ-amino acids have been successfully established,[5–7] the catalytic asymmetric generation of γ-substituted γ-amino acids from achiral starting materials remains underdeveloped. One approach focuses on the development of enantioselective C-N bond forming γ-aminations of carbonyl derivatives with nitrogen-based electrophilic or nucleophilic reagents. Ye and coworkers[8] reported the use of a cinchona alkaloid-derived chiral nucleophilic catalyst to mediate a highly enantioselective γ-amination of α, β-unsaturated acyl chlorides with azodicarboxylates, after which the products were transformed into enantiomerically enriched γ-substituted γ-amino acids (Scheme 1a). By exploring phosphine nucleophilic catalysis, Fu and coworkers[9] developed a chiral phosphine catalyst to realize an efficient enantioselective γ-amination of allenoates with 2,2,2-trifluoroacetamide to generate optically active α, β-unsaturated γ-amino esters (Scheme 1a). Jacobsen and coworkers[10] designed a chiral phosphinothiourea bifunctional catalyst for the asymmetric γ-amination of allenyl esters with N-methoxy carbamates (Scheme 1a). Chiral metal complex-catalyzed asymmetric allylic aminations of γ, δ or δ, ε-unsaturated esters constitute another enantioselective C-N bond forming approach for the generation of γ-amino acid derivatives. Helmchen and coworkers[11] reported an iridium-catalyzed highly enantioselective allylic amination of allylic carbonates with diacylamides (Scheme 1b). By employing the imido-sulfur compounds as the nitrogen source, Tambar and coworkers[12] established a palladium-catalyzed highly enantioselective allylic functionalization of methyl hexenoate via an ene reaction/[2,3]-rearrangement (Scheme 1b). More recently, Hartwig and coworkers[13] disclosed a one-pot sequence of palladium catalyzed allylic C-H bond oxidation followed by the enantioselective iridium-catalyzed allylic amination of methyl hexenoate with phthalimide to form optically active γ-vinyl γ-amino acids (Scheme 1b).
Scheme 1.
Catalytic asymmetric approaches to γ-substituted γ-amino acid derivatives
The catalytic activation of imines as 2-azaallylanions for asymmetric C-C bond forming reactions has recently emerged as a new strategy for the design and development of efficient asymmetric access to chiral amines.[14–15] These latest advances in asymmetric catalysis pointed to the development of nucleophilic addition of imines to α, β-unsaturated carboxylic acid derivatives as a conceptually distinct C-C bond forming strategy for the direct generation of optically active γ-substituted γ-amino acids. With this strategy direct enantioselective synthesis of chiral γ-amino acids not yet accessible by existing enantioselective C-N bond forming methods, such as γ, γ-disubstituted and β, γ-disubstituted γ-amino acids, is possible. Zhang and coworkers[16] reported the development of a phosphine nucleophilic catalyst for the activation of a Morita-Baylis-Hillman carbonate for the nucleophilic addition of trifluoromethyl imines to generate enantiomerically enriched α-methylene γ-trifluoromethylated γ-amino acids (Scheme 1c).
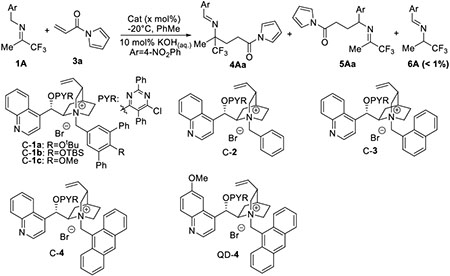
Our group recently discovered that cinchonium salts bearing an N-terphenyl substituent such as C-1a could efficiently activate imines for asymmetric nucleophilic additions to enals and enones.[17] Thus, we commenced our studies with an investigation of C-1a as a catalyst for the model reaction of methyl trifluoromethyl imine 1A and α, β-unsaturated N-acylpyrrole 3a. However, this attempt to extend the application of catalyst C-1a proved to be unsuccessful. Specifically, the model reaction with 1.0 mol% of C-1a was found to proceed with virtually no enantioselectivity and poor regioselectivity, affording the desirable adduct 4Aa in only 5% ee and the undesirable regioisomer 5Aa as a significant side product, although no isomerization product 6A (< 1%) was detected (Table 1, entry 1). We next examined two additional N-terphenyl catalysts C-1b and C-1c, and found that the latter, with a sterically less bulky electron-donating substituent, afforded a significantly improved regioselectivity albeit little enhancement in enantioselectivity (4Aa/5Aa = 89/11, Table 1, entry 3 vs 1).
Table 1.
Development of phase transfer catalysts.[a]
 | ||||
|---|---|---|---|---|
| entry | cat. (x mol%) | time (h); conv.(%)[b] | 4Aa/5Aa[b] | ee of 4Aa (%)[c] |
| 1 | C-1a (1.0) | 1; 100 | 73/27 | −5 |
| 2 | C-1b (1.0) | 1; 100 | 80/20 | 5 |
| 3 | C-1c (1.0) | 1; 100 | 89/11 | 9 |
| 4 | C-2 (1.0) | 1; 100 | >99/1 | 2 |
| 5 | C-3 (1.0) | 1; 100 | >99/1 | 0 |
| 6 | C-4 (1.0) | 1; 100 | >99/1 | 85 |
| 7 | QD-4 (1.0) | 1; 100 | >99/1 | 89 |
| 8[d] | QD-4 (1.0) | 1; 100 | >99/1 | 90 |
| 9[d,e] | QD-4 (1.0) | 1/4; 100 | >99/1 | 93 |
| 10[d,e] | QD-4 (0.2) | 10; 98 | >99/1 | 91 |
Unless noted, reactions were performed with 1A (0.025 mmol), 3a (0.05 mmol), aqueous KOH (0.22 μL, 50 wt%, 10 mol%) in PhMe (0.25 mL) with catalyst (x mol%).
Determined by 19F NMR analysis.
Determined by HPLC analysis.
PhMe/CHCl3 (4/1) was used as solvent.
10 mol% mesitol was added.
These initial results, though disappointing from the view point of developing an efficient catalyst, led us to formulate the working hypothesis that a further decrease of the steric bulk of the N-substituent might provide a catalyst to afford further improved regioselectivity. To test this working hypothesis, catalyst C-2 bearing N-benzyl as a substituent was applied to our model reaction. To our delight, the C-2 mediated reaction proceeded with essentially perfect regioselectivity although in virtually no enantioselectivity (Table 1, entry 4). Although we were not able to address the issue of enantioselectivity at this point, this result directed us to a new focus in catalyst development; namely focusing on extending the spatial reach of the pi-moiety in the N-substituent without drastically increasing its steric bulk.
Following this consideration, we prepared and examined catalysts C-3 and C-4, respectively. While the reaction with C-3 proceeded in essentially the same fashion as that mediated by C-2 (Table 1, entry 5), a dramatic leap in enantioselectivity was observed from the reaction mediated by catalyst C-4 (Table 1, entry 6). Importantly, C-4 afforded this improved enantioselectivity without compromising the perfect regioselectivity. We next tested QD-4, the quinidium analogue of C-4, and found it to afford even higher enantioselectivity (Table 1, entry 7). Subsequent optimization studies identified the mixed solvent of toluene/chloroform (4/1) to be the optimal solvent (Table 1, entry 8). Lygo and Maruoka previously demonstrated that the additions of phenol derivatives could significantly improve the reaction conversion and yield of phase transfer catalyst-mediated C-C bond forming reactions.[18] We also found the addition of 10 mol% mesitol resulted in a significantly accelerated reaction rate and slightly improved stereoselectivity, allowing a complete reaction to occur with perfect regioselectivity and high enantioselectivity in only 15 minutes with 1.0 mol% QD-4 (Table 1, entry 9). Notably, the reaction also went to completion in 10 hours with only 0.2 mol% catalyst without deterioration in either regioselectivity or enantioselectivity (Table 1, entry 10).
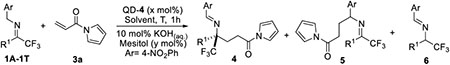
Under the optimized conditions, we investigated the substrate scope of this new reaction. As summarized in Table 2, the trifluoromethyl imines bearing simple and functionalized linear aliphatic alkyl groups (1A-1D) reacted with 3a in not only high enantioselectivity but also perfect chemo- and regioselectivity to furnish the desired products 4Aa-4Da in 93–96% yields and 91–93% ee (Table 2, entries 1–4). Similarly, excellent enantioselectivities and high yields were obtained from reactions with α, β and γ-branched alkyl groups (entries 5–8). Such highly efficient catalytic control could also be extended to the reaction with an alkenyl substituted trifluoromethyl imine 1I (entry 9). Trifluoromethyl aldimine 1J was shown to be a more active substrate, allowing a complete reaction in one hour with only 0.5 mol% QD-4. However, this reaction occurred in a decreased enantioselectivity (entry 10).
Table 2.
Substrate scope of trifluoromethyl imines with α, β-unsaturated N-acylpyrrole 3a.[a]
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | R1 | cat. (x mol%) | mesitol (y mol%) | T (°C) | 4/5; 4/6[b] | Yield of 4 (%)[c] | ee of 4 (%)[d] |
| 1 | Me; 1A | 1.0 | 10 | −20 | >99/1; >99/1 | 96 (4Aa) | 93 |
| 2 | Et: 1B | 2.0 | 10 | −20 | >99/1; >99/1 | 95 (4Ba) | 92 |
| 3 | n-Bu; 1C | 2.0 | 10 | −20 | >99/1; >99/1 | 94 (4Ca) | 92 |
| 4 | CH2=CH(CH2)3; 1D | 2.0 | 10 | −20 | >99/1; >99/1 | 93 (4Da) | 91 |
| 5 | Cy; 1E | 5.0 | 10 | −20 | >99/1; >99/1 | 90 (4Ea) | 92 |
| 6 | CyCH2; 1F | 5.0 | 10 | −20 | >99/1; >99/1 | 90 (4Fa) | 94 |
| 7 | Bn; 1G | 2.0 | 10 | −20 | >99/1; >99/1 | 93 (4Ga) | 92 |
| 8 | PhCH2CH2; 1H | 2.0 | 10 | −20 | >99/1; >99/1 | 94 (4Ha) | 90 |
| 9 | PhCH=CH2; 1I | 2.0 | 200 | −50 | >99/1; >99/1 | 93 (41a) | 95 |
| 10 | H; 1J | 0.5 | 10 | −20 | >99/1; >99/1 | 90 (4Ja) | 76 |
| 11 | Ph; 1K | 3.0 | 100 | −20 | 99/1; 96/4 | 80 (4Ka) | 93 |
| 12 | p-FC6H4; 1L | 5.0 | 200 | −30 | 99/1; 96/4 | 77 (4La) | 93 |
| 13 | p-CIC6H4; 1M | 5.0 | 200 | −40 | 99/1; 97/3 | 75 (4Ma) | 93 |
| 14 | p-BrC6H4; 1N | 5.0 | 200 | −40 | 99/1; 97/3 | 70 (4Na) | 93 |
| 15 | p-CF3C6H4; 1O | 5.0 | 200 | −40 | 98/2; 95/5 | 78 (40a) | 91 |
| 16 | p-MeOC6H4; 1P | 5.0 | 100 | −20 | 98/2; 94/6 | 79 (4Pa) | 92 |
| 17 | p-MeC6H4; 1Q | 5.0 | 100 | −20 | 98/2; 97/3 | 77 (4Qa) | 94 |
| 18 | m-MeOC6H4; 1R | 5.0 | 100 | −20 | 98/2; 97/3 | 78 (4Ra) | 91 |
| 19 | m-MeC6H4; 1S | 5.0 | 100 | −20 | 98/2; 96/4 | 70 (4Sa) | 92 |
| 20 | 2-Naphthyl; 1T | 5.0 | 200 | −30 | 98/2; 96/4 | 78 (4Ta) | 93 |
Unless noted, reactions were performed with 1 (0.2 mmol), 3a (0.4 mmol), mesitol (y mol%) and aqueous KOH (2.2 μL, 50 wt%, 10 mol%) in PhMe/CHCl3 (4/1, totally 2.0 mL) with catalyst QD-4 (x mol%) at indicated temperature. For entries 9, 11–20, PhMe (2.0 mL) was used as the solvent.
Determined by 19F NMR analysis.
Isolated yield of product.
Determined by HPLC analysis.
A variety of aryl substituted trifluoromethyl imines were also examined. These imines, in comparison to aliphatic trifluoromethyl imines, appeared to be more challenging substrates. In particular, the reactions with imines bearing electron-deficient aryl groups proceeded in compromised chemoselectivity and regioselectivity. Importantly, these side reactions could be efficiently suppressed in the presence of an increased amount of mesitol (1.0–2.0 equiv.). Consequently, reactions with trifluoromethyl imines with para-substituted phenyls of varying electronic properties proceeded in useful yield and high enantioselectivity (Table 2, 1L-1Q, entries 12–17). Moving the substituent from the para- to the meta-position appeared to have little influence on the reaction outcome (entries 18–19). The presence of a more extended aromatic group such as naphthyl was readily tolerated by the catalyst (entry 20).
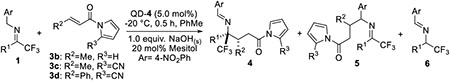
We next focused our attention to expand the scope of the electrophiles to β-substituted α, β-unsaturated N-acylpyrroles 3a-d. The β-methyl-α, β-unsaturated N-acylpyrrole 3b turned out to be completely inactive in an attempted reaction with imine 1A in the presence of QD-4 (Table 3, entry 1). By incorporating an electron-withdrawing cyano group on N-acylpyrrole moiety, the β-alkyl and β-aryl α, β-unsaturated N-acylpyrroles such as 3c and 3d became suitably active electrophiles. As summarized in Table 3, the additions of trifluoromethyl imines 1A, 1B and 1J to 3c-d proceeded in useful chemo-, regio-, diastereo-, and enantioselectivity (entries 2–5). To our knowledge, these results represent the first examples of highly diastereoselective and enantioselective formations of β, γ-disubstituted γ-amino acid derivatives from achiral precursors. Interestingly, we found that TBAB could also catalyze a racemic reaction of trifluoromethyl imine 1A and 3c, producing 4Ac in high diastereoselectivity (> 95/5) but moderate regioselectivity (4Ac/5Ac = 70/30).[19]
Table 3.
Substrate scope of trifluoromethyl imines with β-substituted α, β-unsaturated N-acylpyrroles.[a]
 | ||||||
|---|---|---|---|---|---|---|
| entry | R1 | 3 | 4/5; 4/6[b] | d.r[b] | Yield of 4[c] | ee of 4 (%)[d] |
| 1 | Me; 1A | 3b | --; -- | -- | -- | -- |
| 2 | Me; 1A | 3c | 85/15; >99/1 | >95/5 | 72 (4Ac) | 87 |
| 3 | Et; 1B | 3c | 76/24; >99/1 | >95/5 | 55(4Bc) | 81 |
| 4[e] | H; 1J | 3c | 95/5; 85/15 | >95/5 | 73 (4JC) | 85 |
| 5[f] | H; 1J | 3d | 95/5; 80/20 | >95/5 | 52 (4Jd) | 80 |
Unless noted, reactions were performed with 1 (0.2 mmol), 3 (0.4 mmol), mesitol (20 mol%) and anhydrous NaOH solid powder (1.0 equiv.) in PhMe (2.0 mL) with catalyst QD-4 (5.0 mol%) at −20 °C.
Determined by 19F NMR analysis.
Isolated yield of product.
Determined by HPLC analysis.
The absolute configuration of 4Jc was determined to be (S, S) by X-ray crystallographic analysis.[20]
PhMe/Ch2Ch2 (4/1, totally 2.0 mL) was used as solvent.
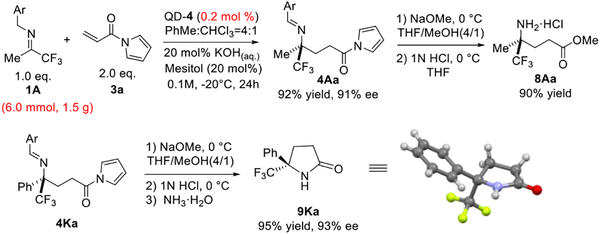
We carried out a gram-scale reaction of 1A with 3a (Scheme 2). With only 0.2 mol% of QD-4 the reaction went to completion in 24 hours to afford the desired product 4Aa in 92% yield and 91% ee. In addition, the straightforward and high-yield respective conversions of 4Aa and 4Ka to γ-amino ester 8Aa and γ-lactam 9Ka illustrated the utility of 4 as versatile chiral building blocks. The absolute configuration of 9Ka was further established by the X-ray crystallographic analysis.[20]
Scheme 2.
Gram-scale reactions and synthetic applications
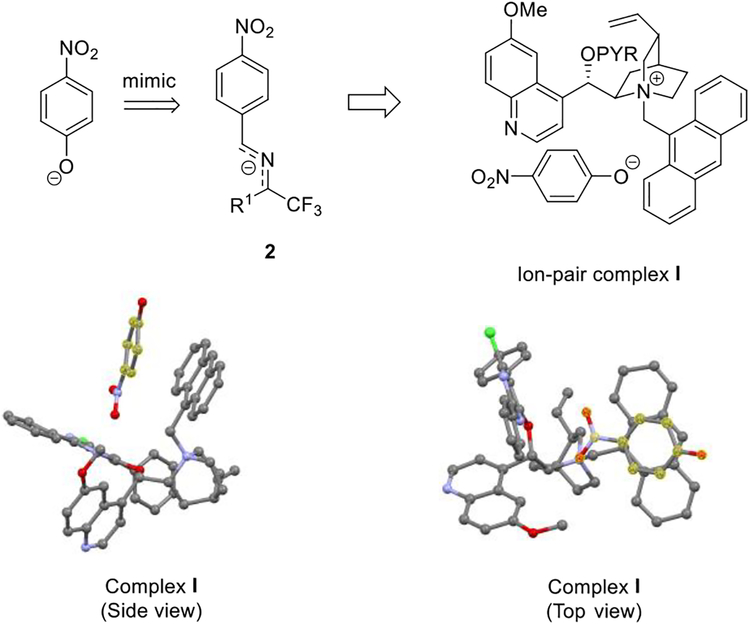
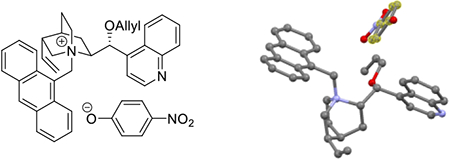
To provide experimental data to support our hypothesis that the quinidium unit of QD-4 interacted with 2-azaallylanion 2 via a pi-pi interaction between the anthracene and the 4-nitrophenyl groups, we prepared ion-pair complex I by replacing the bromide anion in QD-4 with 4-nitrophenoxide (Figure 2). The 4-nitrophenoxide was selected to mimic the unstable 2-azaallyanion 2 in that both contain a 4-nitrophenyl group in conjugation with an anionic moiety. We were able to determine the structure of ion-pair complex I by X-ray crystallographic analysis.[21] Significantly, a face-to-face pi-stacking interaction between the anthracene ring and the 4-nitrophenoxide was observed. Our structural studies were inspired by the X-ray crystallographic structural determination of an N-(9-methylanthracene) cinchonidiumphenoxide complex. Interestingly, no pi-stacking interaction was observed in that complex.[22] These experimental results suggested that a pi-pi interaction between the anthracene ring in QD-4 and 2-azaallyanion 2 is feasible, which was a key hypothesis in our catalyst development studies.
Figure 2.
Single crystal X-ray structure of ion-pair complex I. The phenyl ring of 4-nitrophenoxide is highlighted. The protons and solvent molecules have been hided for clarity (C: grey, O: red, N: blue, Cl: green).
In conclusion, we have developed a new chiral phase-transfer catalyst to realize an unprecedented asymmetric umpolung reaction of trifluoromethyl imines with α, β-unsaturated N-acylpyrroles. This catalyst addressed the issues of inadequate regioselectivity and poor enantioselectivity encountered by existing catalysts. By complementing existing methods toward optically active γ-amino acids derivatives and γ-lactams, this new asymmetric imine umpolung reaction should be valuable for asymmetric synthesis of chiral amino compounds.
Supplementary Material
Acknowledgements
We are grateful for the financial support from the National Institute of General Medical Science (NIH, GM-61591) and the Keck Foundation. We are grateful to Dr. Mark Bezpalko, Dr. Diane Dickie and Prof. Bruce Foxman for X-ray crystallographic characterizations of structures.
Footnotes
Publisher's Disclaimer: This manuscript has been accepted after peer review and appears as an Accepted Article online prior to editing, proofing, and formal publication of the final Version of Record (VoR). This work is currently citable by using the Digital Object Identifier (DOI) given below. The VoR will be published online in Early View as soon as possible and may be different to this Accepted Article as a result of editing. Readers should obtain the VoR from the journal website shown below when it is published to ensure accuracy of information. The authors are responsible for the content of this Accepted Article.
Supporting information for this article is given via a link at the end of the document.
References
- [1].For selected examples of biologically interesting natural products and analogues, see:Luesch H, Moore RE, Paul VJ, Mooberry SL, Corbett TH, J. Nat. Prod 2001, 64, 907–910;Maderna A, Doroski M, Subramanyam C, Porte A, Leverett CA, Vetelino BC, Chen Z, Risley H, Parris K, Pandit J, Varghese AH, Shanker S, Song C, Sukuru SCK, Farley KA, Wagenaar MM, Shapiro MJ, Musto S, Lam M-H, Loganzo F, O’Donnell CJ, J. Med. Chem 2014, 57, 10527–10543;Colombo R, Wang Z, Han J, Balachandran R, Daghestani HN, Camarco DP, Vogt A, Day BW, Mendel D, Wipf P, J. Org. Chem 2016, 81, 10302–10320;Khalil MW, Sasse F, Lünsdorf H, Elnakady YA, Reichenbach H, Chembiochem 2006, 7, 678–683;Palomo C, Oiarbide M, García JM, González A, Pazos R, Odriozola JM, Bañuelos P, Tello M, Linden A, J. Org. Chem 2004, 69, 4126–4134.
- [2].For selected examples of medical agents, see:Gulder TAM, Moore BS, Angew. Chem. Int. Ed 2010, 49, 9346–9367; Angew. Chem. 2010, 122, 9534–9556;Froestl W, Adv. Pharmacol 2010, 58, 19–62;Silverman RB, Angew. Chem. Int. Ed 2008, 47, 3500–3504; Angew. Chem. 2008, 120, 3552–3556;Al-Majed A, Profiles of Drug Substances, Excipients and Related Methodology 2010, 35, 309–345.
- [3].For selected reviews on the asymmetric synthesis of γ-amino acid/lactam derivatives see:Ordóñez M, Cativiela C, Tetrahedron: Asymmetry 2007, 18, 3–99;Ordóñez M, Cativiela C, Romero-Estudillo I, Tetrahedron: Asymmetry 2016, 27, 999–1055;Caruano J, Muccioli GG, Robiette R, Org. Biomol. Chem 2016, 14, 10134–10156.
- [4].a) Pesenti C, Arnone A, Bellosta S, Bravo P, Canavesi M, Corradi E, Frigerio M, Meille SV, Monetti M, Panzeri W, Viani F, Venturini R, Zanda M, Tetrahedron 2001, 57, 6511–6522; [Google Scholar]; b) Chaume G, Van Severen M-C, Ricard L, Brigaud T, J. Fluorine Chem 2008, 129, 1104–1109; [Google Scholar]; c) Berger AA, Völler J-S, Budisa N, Koksch B, Acc. Chem. Res 2017, 50, 2093–2103. [DOI] [PubMed] [Google Scholar]
- [5].a) Li H, Wang Y, Tang L, Deng L, J. Am. Chem. Soc 2004, 126, 9906–9907; [DOI] [PubMed] [Google Scholar]; b) Li H, Wang Y, Tang L, Wu F, Liu X, Guo C, Foxman BM, Deng L, Angew. Chem. Int. Ed 2005, 44, 105–108; Angew. Chem. 2005, 117, 107–110; [DOI] [PubMed] [Google Scholar]; c) Okino T, Hoashi Y, Furukawa T, Xu X, Takemoto Y, J. Am. Chem. Soc 2005, 127, 119–125. [DOI] [PubMed] [Google Scholar]
- [6].a) Barnes DM, Ji J, Fickes MG, Fitzgerald MA, King SA, Morton HE, Plagge FA, Preskill M, Wagaw SH, Wittenberger SJ, Zhang J, J. Am. Chem. Soc 2002, 124, 13097–13105; [DOI] [PubMed] [Google Scholar]; b) Mitsunuma H, Matsunaga S, Chem. Commun 2011, 47, 469–471. [DOI] [PubMed] [Google Scholar]
- [7].a) Mase N, Watanabe K, Yoda H, Takabe K, Tanaka F, Barbas CF, J. Am. Chem. Soc 2006, 128, 4966–4967; [DOI] [PubMed] [Google Scholar]; b) Chi Y, Guo L, Kopf NA, Gellman SH, J. Am. Chem. Soc 2008, 130, 5608–5609; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wiesner M, Revell JD, Tonazzi S, Wennemers H, J. Am. Chem. Soc 2008, 130, 5610–5611. [DOI] [PubMed] [Google Scholar]
- [8].a) Shen L-T, Sun L-H, Ye S, J. Am. Chem. Soc 2011, 133, 15894–15897; [DOI] [PubMed] [Google Scholar]; b) Chen X-Y, Xia F, Cheng J-T, Ye S, Angew. Chem. Int. Ed 2013, 52, 10644–10647; Angew. Chem. 2013, 125, 10838–10841. [DOI] [PubMed] [Google Scholar]
- [9].Lundgren RJ, Wilsily A, Marion N, Ma C, Chung YK, Fu GC, Angew. Chem. Int. Ed 2013, 52, 2525–2528; Angew. Chem. 2013, 125, 2585–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fang Y-Q, Tadross PM, Jacobsen EN, J. Am. Chem. Soc 2014, 136, 17966–17968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gnamm C, Franck G, Miller N, Stork T, Brödner K, Helmchen G, Synthesis 2008, 2008, 3331–3350. [Google Scholar]
- [12].Bao H, Tambar UK, J. Am. Chem. Soc 2012, 134, 18495–18498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sharma A, Hartwig JF, J. Am. Chem. Soc 2013, 135, 17983–17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].For examples of metal-catalyzed asymmetric C-C bond forming coupling reactions of 2-azaallylanions, seeZhu Y, Buchwald SL, J. Am. Chem. Soc 2014, 136, 4500–4503;Liu J, Cao C-G, Sun H-B, Zhang X, Niu D, J. Am. Chem. Soc 2016, 138, 13103–13106;Su Y-L, Li Y-H, Chen Y-G, Han Z-Y, Chem. Commun 2017, 53, 1985–1988.
- [15].For examples of metal-catalyzed racemic C-C bond forming coupling reactions of 2-azaallylanions, seeNiwa T, Yorimitsu H, Oshima K, Org. Lett 2008, 10, 4689–4691;Li M, Berritt S, Walsh PJ, Org. Lett 2014, 16, 4312–4315;Li M, Yucel B, Jiménez J, Rotella M, Fu Y, Walsh PJ, Adv. Synth. Catal 2016, 358, 1910–1915;Li M, Gutierrez O, Berritt S, Pascual-Escudero A, Ye§ilçimen A, Yang X, Adrio J, Huang G, Nakamaru-Ogiso E, Kozlowski MC, Walsh PJ, Nat. Chem 2017, 9, 997–1004.
- [16].Chen P, Yue Z, Zhang J, Lv X, Wang L, Zhang J, Angew. Chem. Int. Ed 2016, 55, 13316–13320; Angew. Chem. 2016, 128, 13510–13514. [DOI] [PubMed] [Google Scholar]
- [17].a) Wu Y, Hu L, Li Z, Deng L, Nature 2015, 523, 445–450; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hu L, Wu Y, Li Z, Deng L, J. Am. Chem. Soc 2016, 138, 15817–15820; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Waser M, Novacek J, Angew. Chem. Int. Ed 2015, 54, 14228–14231; Angew. Chem. 2015, 127, 14434–14437. [DOI] [PubMed] [Google Scholar]
- [18].a) Lygo B, Beynon C, Lumley C, McLeod MC, Wade CE, Tetrahedron Lett 2009, 50, 3363–3365; [Google Scholar]; b) Ooi T, Kameda M, Taniguchi M, Maruoka K, J. Am. Chem. Soc 2004, 126, 9685–9694; [DOI] [PubMed] [Google Scholar]; c) Shirakawa S, Makino H, Yoshidome T, Maruoka K, Tetrahedron 2014, 70, 7128–7132. [Google Scholar]
- [19].For detailed analysis, see Supporting Information.
- [20].CCDC 1581585 (4Jc) and 1581586 (9Ka) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Center.
- [21].CCDC 1586429 (Ion-pair complex I) contains the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Center.
-
[22].Corey EJ, Xu F, Noe MC, J. Am. Chem. Soc
1997, 119, 12414–12415.Corey and coworkers have determined the structure of an ion-pair compex derived from a cinchonidium and 4-nitrophenoxide by X-ray crystallographic analysis.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.