Abstract
The demographic factors of sex, age, and race/ethnicity are well recognized as relevant to pain sensitivity and clinical pain expression. Of these, sex differences have been the most frequently studied, and most of the literature describes greater pain sensitivity for women. The other two factors have been less frequently evaluated, and current literature is not definitive. Taking advantage of the large OPPERA study cohort, we evaluated the association of sex, age, and self-reported race with 34 measures of pressure, mechanical, and thermal pain sensitivity encompassing both threshold and suprathreshold perception. Women were significantly more pain sensitive than men for 29/34 measures. Age effects were small, and only significant for 7/34 measures, however the age range was limited (18–44 y.o.). Race/ethnicity differences varied across groups and pain assessment type. Non-Hispanic whites (NHW) were less pain sensitive than African-Americans (for 21/34 measures), Hispanics (19/34), and Asians (6/34). No pain threshold measure showed significant racial differences, while several suprathreshold pain measures did. This suggests that racial differences are not related to tissue characteristics or inherent nociceptor sensitivity. Rather, the differences observed for suprathreshold pain ratings or tolerance are more likely related to differences in central nociceptive processing, including modulation imposed by cognitive, psychological, and/or affective factors.
Keywords: quantitative sensory testing, sex differences, racial differences, ethnic differences, pain sensitivity, heat pain, pressure pain
Introduction
Multiple factors are recognized to influence an individual’s pain sensitivity; among these are the major demographic variables of sex, age, and race/ethnicity. A large literature addresses the issues of sex and gender differences in pain sensitivity, clinical pain expression, and mechanisms underlying such differences5,11,20. The majority of these studies report greater pain sensitivity for women vs. men, but several studies fail to find significant sex differences. The conditions leading to the expression of sex differences in pain sensitivity are not clear and do not appear to be distinctly related to types of stimulation or the perceptual metrics. However, sample size issues have been proposed as a reason for at least some of the studies reporting no significant sex differences11.
Considerably less work has been done evaluating age and racial/ethnic factors as related to pain sensitivity. Regarding age differences, a recent meta-analytic review reported that pain thresholds increased with age across several stimulus modalities14. In contrast, findings for measures of pain tolerance revealed no age differences in response to thermal and electrical stimuli, while pressure pain tolerance decreased with age. Racial/ethnic group differences in pain threshold and tolerance have also been the topic of two recent meta-analyses 13,21. The studies revealed that, on average, African Americans reported lower pain thresholds and tolerances across multiple stimulus modalities, with small to moderate effects sizes for threshold and moderate to large effects sizes for tolerance. Notably, most studies of racial/ethnic group differences have compared African Americans and non-Hispanic whites, but infrequently include other racial/ethnic groups in sufficient numbers to permit comparisons.
This paper investigates the demographic predictors of pain sensitivity among a large cohort of volunteers, recruited for the OPPERA study (Orofacial Pain: Prospective Evaluation and Risk Assessment). Specifically, sex, age (years), and race (Non-Hispanic White [NHW], African American [AA], Hispanic, Asian) are used as predictors for 34 quantitative pain sensitivity measures. The sensory domains evaluated include heat pain, cutaneous mechanical (pricking) pain, and muscle/joint pressure pain.
Methods
Study Design and Participant Recruitment
Four sites recruited 3431 study participants: 1) The University of North Carolina at Chapel Hill, NC, 2) The University of Maryland, Baltimore, MD, 3) The University at Buffalo, NY, and 4) The University of Florida at Gainesville, FL. Inclusion criteria permitted either sex, any racial or ethnic group, and ages 18–44. Recruitment targeted individuals who were either 1) of general good health or 2) identified as having temporomandibular disorder (TMD). Demographic statistics are provided in Tables 1 and 2. Detailed descriptions of the study design and recruitment protocols are provided elsewhere25.
Table 1:
Participant numbers by sex and race/ethnicity
| NHW | AA | Asian | Hispanic | Other | Total (%) | |
|---|---|---|---|---|---|---|
| Male | 752 | 432 | 74 | 144 | 21 | 1423 (41.5) |
| Female | 1077 | 616 | 112 | 183 | 20 | 2008 (58.5) |
| Total (%) | 1829 (53.3) | 1048 (30.5) | 186 (5.4) | 327 (9.5) | 41 (1.2) |
Table 2:
Ages for Different Participant Groups
| Male | Female | |||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| NHW | 25.0 | 0.2 | 26.1 | 0.2 |
| AA | 32.3 | 0.4 | 30.2 | 0.3 |
| Asian | 23.0 | 0.6 | 24.2 | 0.5 |
| Hispanic | 25.1 | 0.4 | 24.3 | 0.4 |
The OPPERA study was approved by IRBs at all four sites and at the data coordinating center, Battelle Memorial Institute. All participants provided informed consent for all procedures.
Demographic Data and Psychophysical Protocols
Demographic data were collected on individuals at baseline, including sex (male/female), age (in years), and race/ethnicity. The demographic questionnaire provided the following self-identifying racial/ethnic categories: White, Black/African American, Hispanic, Asian, Native Hawaiian/Pacific Islander, American Indian/Alaskan Native, and Other. (For simplicity, these will be referred to as racial categories throughout the paper.) Due to the low sample size for the latter three racial categories (Table 1), they were not included in any analyses.
Quantitative sensory testing (QST) was conducted in three sensory domains: 1) pressure pain, 2) mechanical cutaneous (pricking) pain, and 3) heat pain. Protocols are outlined below; more detailed information can be found elsewhere10. Pressure pain thresholds (PPT) were measured using a pressure algometer (Somedic; Horby, Sweden). Five facial and upper body sites were tested bilaterally: 1) overlying the temporalis muscle, 2) overlying the masseter muscle, 3) overlying the temporomandibular joint (TMJ), 4) overlying the upper trapezius muscle, and 5) overlying a proximal portion of the flexor carpi ulnaris muscle. The examiner manually applied the algometer to these sites using 1cm2 tip at 30 kPa/s increase in pressure until the participant indicated first feeling a pain sensation by pressing a button. If no pain indication was given by 600 kPa, 600 kPa was used as the threshold value. This procedure was repeated until two values were recorded within 20 kPa or until five trials were conducted. The mean of the two closest values was used as the PPT outcome variable for each body site.
Mechanical cutaneous pain (MCP) was assessed using weighted probes matching those used by the German Neuropathic Pain Network24. Probes exerted forces between 8 and 512 mN and stimuli were applied to the dorsal surface of the middle phalanx of digits two to four. MCP threshold was calculated as the geometric mean of five series of ascending and descending intensities, using a classical Method of Limits protocol. However, if a participant gave two consecutive “no” responses to the most intense stimulus (512mN), the process was stopped and a threshold value of 512 was recorded. After threshold was determined, single stimulus MCP ratings were determined using suprathreshold stimuli. Participants were instructed to provide a numerical rating of pain intensity when asked, using “0” for no pain and “100” for the most intense pain imaginable. Participants reported pain intensity on this 0–100 scale after a single stimulus was applied for 0.6 – 1.0 seconds. Additionally, participants were asked to provide pain intensity ratings once immediately after a series of ten stimuli was applied at a rate of 1Hz (to calculate temporal summation), and then once again at 15 and 30 seconds after this series (as measures of after-sensation). This testing series was conducted four times each with two different stimulus intensities: 256 and 512 mN. Temporal summation (TS) was calculated as the difference between the series of ten stimuli and the single stimulus ratings. If a participant reported a rating of 100, the procedure was stopped. Participants were informed that they could stop the test at any time by request.
Heat pain (HP) sensitivity was assessed using a commercially available thermal stimulator (Pathway; MEDOC; Ramat Yishai, Israel). Stimuli were applied to the ventral forearm. HP threshold was determined by placing the thermode in contact with the skin at 32°C and the temperature increased by 0.5°C per second until the participant pressed a button to indicate the first perception of pain. This final temperature was recorded as HP threshold. HP tolerance was conducted in the same manner, but the participant indicated when s/he could no longer tolerate the pain sensation. A temperature of 52°C was set as the upper limit for both these procedures. One study site used a different starting temperature (38°C rather than 32°C) for the threshold and tolerance protocols. These data were evaluated and there was no distributional difference among study sites for tolerance measurements; however, there was a noticeable distributional difference between this site and the other three in regards to threshold. Thus, for all statistical analyses of threshold, the HP threshold data collected at the higher starting temperature (809 participants) were omitted from analysis.
Following HP threshold and tolerance measures, ratings of suprathreshold heat stimuli and after-sensation were collected using the same verbal 0–100 rating scale. For this protocol, the thermode was placed on the skin at a temperature of 38°C, then ten pulses were given at 2.5 second intervals with a ramp rate of 20°C per second. The participant was instructed to report his/her peak pain intensity, and was cued to do so by the experimenter when the temperature reached its peak temperature, just before it began to return to the starting temperature. This test was conducted three times, using peak temperatures of 46°C, 48°C, and 50°C. If a participant reported a rating of 100 or requested to stop the stimulus series, the procedure was stopped. Four HP measures were derived for each temperature series: 1) the rating of the first stimulus of the series, 2) the sum of all 10 ratings in a series (area under the pain rating curve), 3) the highest pain rating minus the first stimulus pain rating, and 4) the slope of the first three pain ratings. The last two derived measures were considered indices of TS of pain.
Additionally, after-sensation ratings were collected at 15 and 30 sec after each series of ten heat pulses.
Statistical Methods
Missing data in the pain sensitivity variables were imputed using an expectation maximization (EM) method as described previously10. Inverse probability weighting23 was used to adjust for over-representation of TMD cases in the cohort due to the casecontrol study design. The prevalence of TMD in the general population was assumed to be 5%, which is a conservative estimate of population prevalence. In this case-control study design, TMD cases are overrepresented. Thus, we performed weighted regression where TMD-free controls received a weight of 1 and chronic TMD cases received a weight of (0.05N0)/(0.95N1), where N0 denotes the number of controls in the cohort and N1 represents the number of cases. Under this weighting scheme, the total weight of TMD cases in the regression models is 5%, which would be the proportion of cases we would expect to observe had we sampled from the general population rather than using a case-control study design.
The association between the pain sensitivity variables and the demographic variables was evaluated using a series of regression models. The demographic variables were treated either as categorical (sex, race) or continuous (age). The outcome in each model was the pain sensitivity variable of interest. A separate model was performed for each variable of interest. The covariates in each model included age and dummy variables for study site, sex, and race (NHW, AA, Asian, Hispanic, or other). A second set of models was also calculated that included all possible interactions between age, sex, and race. The variance of the resulting regression coefficients was estimated using generalized estimating equations to compute the sandwich estimator of the variance17.
For each regression model, the coefficients for each demographic variable of interest were computed, and the null hypothesis that a given regression coefficient is equal to 0 (corresponding to the null hypothesis of no association between a demographic variable and a pain sensitivity variable) was evaluated using Wald tests. In order to compare the relative strengths of the associations between a given demographic variable and the different measures of pain sensitivity, the adjusted effect size of each association was calculated by dividing each regression coefficient by its standard deviation. Confidence intervals for these adjusted effect sizes were computed using bootstrapping4. Statistical analysis was performed using SAS version 9.3 and R version 3.0.1.
Results
Sex Differences in Pain Sensitivity
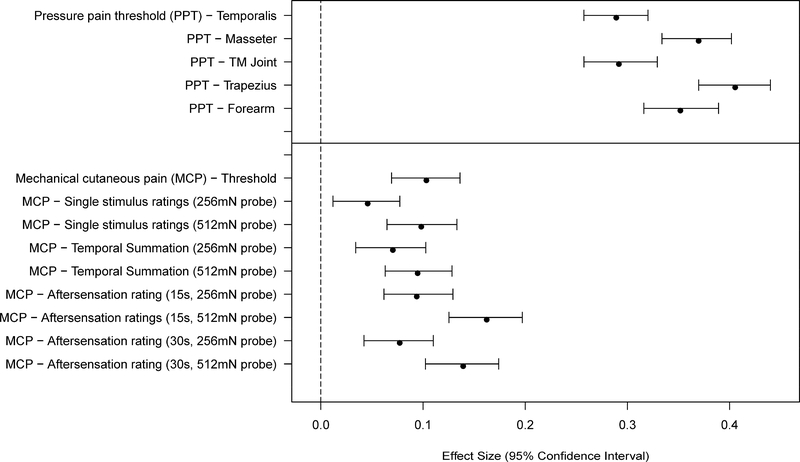
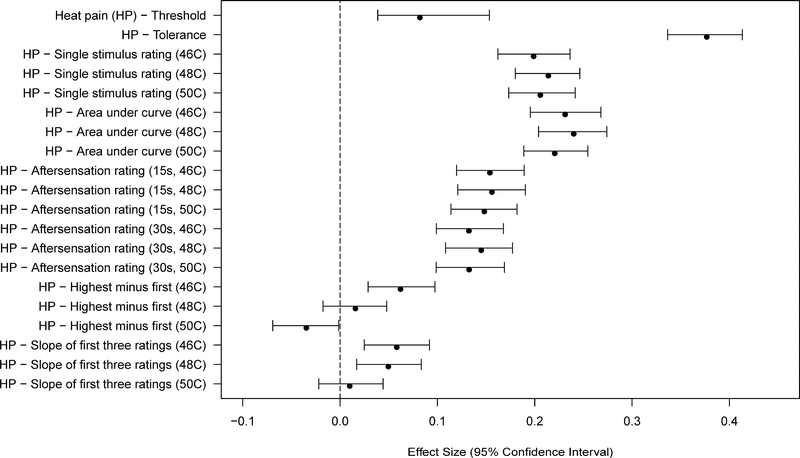
Women showed greater sensitivity than men for 29 of 34 measures of pain sensitivity (Figures 1–2 and Tables 3–4). This included all of the PPT measures, all of the mechanical cutaneous pain measures, and most of the HP measures. Several measures of HP TS did not meet the Bonferroni-adjusted threshold for significance. The largest sex differences were observed in measures of pressure pain threshold and HP tolerance. Most measures of HP sensitivity showed large sex differences, while measures of HP aftersensations and mechanical pain sensitivity showed smaller (although still robust) differences.
Figure 1.
Effect sizes (and 95% confidence intervals) for sex differences are shown for each of the mechanical pain sensitivity measures. Values are shown for each measure such that positive values denote a greater pain sensitivity (lower threshold or higher ratings) for women vs. men.
Figure 2.
Effect sizes (and 95% confidence intervals) for sex differences are shown for each of the heat pain sensitivity measures. Values are shown for each measure such that positive values denote a greater pain sensitivity (lower threshold or higher ratings) for women vs. men.
Table 3:
Model Estimates and Significance for Sex and Age Covariates for Pressure Pain Threshold and Mechanical Cutaneous Pain Outcomes
| ---Sex--- | ---Age--- | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Standard Deviation | Wald t-Statistic | p-value1 | Estimate2 | Standard Deviation | Wald t-Statistic | p-value1 | |
| Pressure Pain Threshold | ||||||||
| Temporalis | −48.30 | 167.07 | −16.901 | <.0001 | 4.75 | 115.81 | 2.399 | 0.0165 |
| Masseter | −55.79 | 150.92 | −21.613 | <.0001 | 5.16 | 106.12 | 2.841 | 0.0045 |
| TMJ | −38.48 | 131.95 | −17.047 | <.0001 | 8.72 | 93.01 | 5.481 | <.0001 |
| Trapezius | −111.04 | 273.94 | −23.699 | <.0001 | 12.09 | 195.55 | 3.614 | 0.0003 |
| Epicondyle | −91.60 | 260.38 | −20.566 | <.0001 | 8.14 | 188.93 | 2.520 | 0.0117 |
| Mechanical Cutaneous Pain | ||||||||
| Threshold | −35.74 | 346.27 | −6.035 | <.0001 | 10.91 | 247.72 | 2.575 | 0.0100 |
| Single Stimulus Rating - 256mN Probe | 1.14 | 24.83 | 2.678 | 0.0074 | −1.43 | 16.58 | −5.040 | <.0001 |
| Single Stimulus Rating - 512mN Probe | 3.76 | 38.30 | 5.743 | <.0001 | −1.83 | 27.18 | −3.945 | <.0001 |
| Temporal Summation - 256mN Probe | 1.67 | 23.71 | 4.116 | <.0001 | −0.75 | 16.82 | −2.594 | 0.0095 |
| Temporal Summation - 512mN Probe | 3.08 | 32.50 | 5.532 | <.0001 | −0.93 | 23.76 | −2.277 | 0.0228 |
| Aftersensation Rating (15s) - 256mN Probe | 1.37 | 14.57 | 5.487 | <.0001 | −0.53 | 10.23 | −3.032 | 0.0024 |
| Aftersensation Rating (15s) - 512mN Probe | 4.58 | 28.22 | 9.487 | <.0001 | −0.86 | 20.84 | −2.417 | 0.0157 |
| Aftersensation Rating (30s) - 256mN Probe | 0.73 | 9.49 | 4.510 | <.0001 | −0.23 | 6.71 | −1.986 | 0.0470 |
| Aftersensation Rating (30s) - 512mN Probe | 2.89 | 20.75 | 8.144 | <.0001 | −0.45 | 14.66 | −1.778 | 0.0754 |
All estimates are compared to a referent White non-Hispanic male, controlling for site and TMD status
Significance evaluated at Bonferroni corrected level of 0.001
Estimate value for age effects represents difference per decade
Table 4:
Model Estimates and Significance for Sex and Age Covariates for Heat Pain Outcomes
| ---Sex--- | ---Age (Decades)--- | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Standard Deviation | Wald t-Statistic | p-value1 | Estimate | Standard Deviation | Wald t-Statistic | p-value1 | |
| Heat Pain | ||||||||
| Threshold | −1.31 | 16.04 | −3.907 | <.0001 | 0.42 | 11.87 | 1.694 | 0.0902 |
| Tolerance | −1.66 | 4.407 | −22.023 | <.0001 | −0.00 | 2.95 | −0.044 | 0.9652 |
| Single Stimulus Rating - 46C | 11.40 | 57.33 | 11.626 | <.0001 | −0.96 | 43.57 | −1.295 | 0.1955 |
| Single Stimulus Rating - 48C | 12.81 | 59.90 | 12.503 | <.0001 | −1.67 | 44.38 | −2.195 | 0.0281 |
| Single Stimulus Rating - 50C | 12.78 | 62.13 | 12.024 | <.0001 | −0.85 | 45.25 | −1.096 | 0.2731 |
| Area Under Curve - 46C | 123.61 | 534.50 | 13.520 | <.0001 | −11.65 | 397.39 | −1.714 | 0.0866 |
| Area Under Curve - 48C | 126.40 | 526.49 | 14.036 | <.0001 | −9.01 | 382.32 | −1.378 | 0.1683 |
| Area Under Curve - 50C | 110.71 | 502.20 | 12.888 | <.0001 | −5.37 | 360.03 | −0.872 | 0.3830 |
| Aftersensation Rating (15s) - 46C | 4.48 | 29.13 | 9.001 | <.0001 | −0.79 | 21.87 | −2.117 | 0.0343 |
| Aftersensation Rating (15s) - 48C | 5.59 | 35.82 | 9.117 | <.0001 | −1.77 | 27.06 | −3.819 | 0.0001 |
| Aftersensation Rating (15s) - 50C | 5.76 | 38.89 | 8.660 | <.0001 | −2.03 | 28.78 | −4.124 | <.0001 |
| Aftersensation Rating (30s) - 46C | 2.78 | 21.03 | 7.740 | <.0001 | −0.50 | 16.00 | −1.812 | 0.0699 |
| Aftersensation Rating (30s) - 48C | 4.08 | 28.13 | 8.473 | <.0001 | −1.02 | 21.68 | −2.751 | 0.0059 |
| Aftersensation Rating (30s) - 50C | 3.95 | 29.80 | 7.753 | <.0001 | −1.41 | 22.16 | −3.707 | 0.0002 |
| Highest Rating Minus First - 46C | 2.66 | 42.91 | 3.626 | 0.0003 | −0.70 | 33.50 | −1.215 | 0.2245 |
| Highest Rating Minus First - 48C | 0.72 | 45.68 | 0.923 | 0.3558 | 0.60 | 36.13 | 0.965 | 0.3343 |
| Highest Rating Minus First - 50C | −1.55 | 44.60 | −2.028 | 0.0426 | 0.13 | 33.40 | 0.228 | 0.8196 |
| Slope of First Three Ratings - 46C | 0.90 | 15.36 | 3.408 | 0.0007 | −0.20 | 11.76 | −0.998 | 0.3182 |
| Slope of First Three Ratings - 48C | 0.82 | 16.53 | 2.900 | 0.0037 | 0.46 | 13.39 | 1.990 | 0.0466 |
| Slope of First Three Ratings - 50C | 0.17 | 16.93 | 0.580 | 0.5617 | 0.28 | 13.57 | 1.203 | 0.2290 |
All estimates are compared to a referent White non-Hispanic male, controlling for site and TMD status
Significance evaluated at Bonferroni corrected level of 0.0015
Estimate value for age effects represents difference per decade
Age Effects in Pain Sensitivity
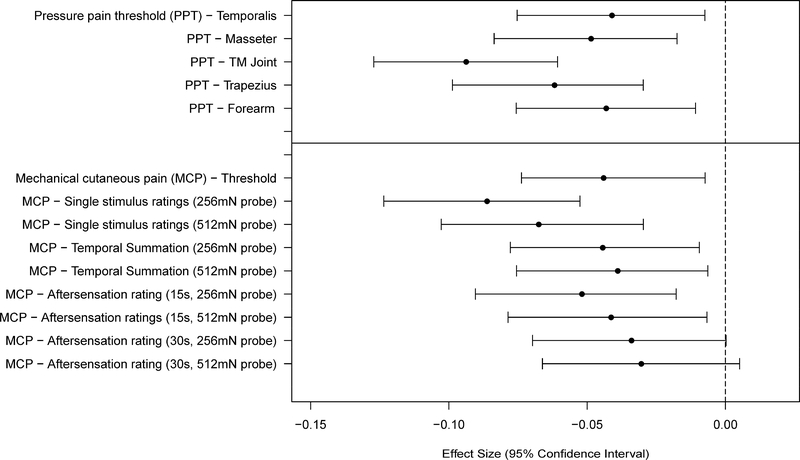
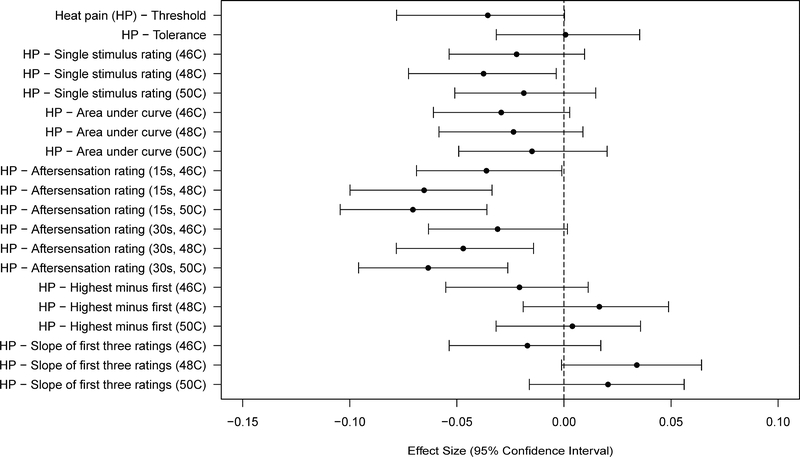
In general pain sensitivity tended to decrease with age, although only seven of 34 variables showed an association that was significant at the Bonferroni-adjusted threshold (Figures 3–4 and Tables 3–4). Specifically, the TMJ and trapezius PPT’s, the MCP single stimulus ratings, and HP aftersensations following more intense stimuli met the Bonferroni-adjusted threshold for significance. Most other measures of pressure pain and mechanical cutaneous pain sensitivity, while not showing statistically significant effects at the Bonferroni-adjusted threshold, showed the same trend of a decrease in pain sensitivity with increasing age. Only a very weak trend in the same direction was observed for age and the measures of HP sensitivity, other than the HP aftersensation measures noted above.
Figure 3.
Effect sizes (and 95% confidence intervals) for age effects are shown for each of the mechanical pain sensitivity measures. Values are shown for each measure such that negative values denote a decreased pain sensitivity (higher threshold or lower ratings).
Figure 4.
Effect sizes (and 95% confidence intervals) for age effects are shown for each of the heat pain sensitivity measures. Values are shown for each measure such that negative values denote a decreased pain sensitivity (higher threshold or lower ratings).
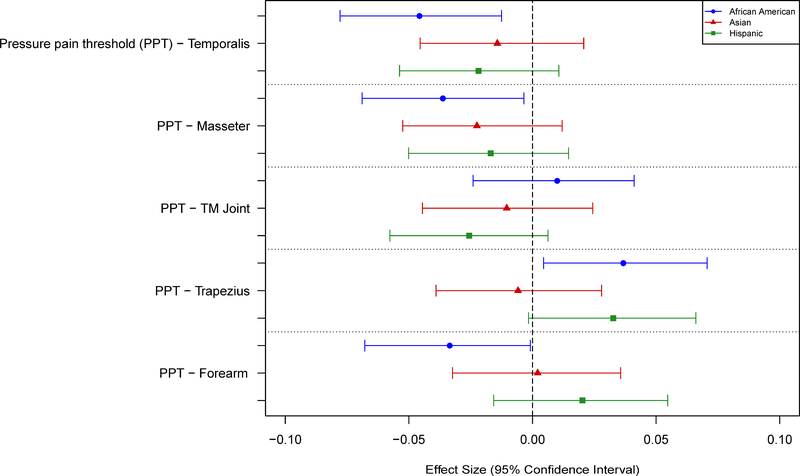
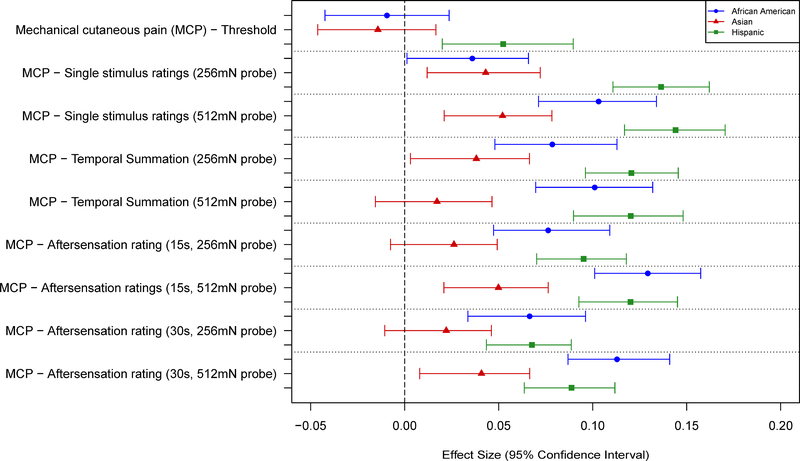
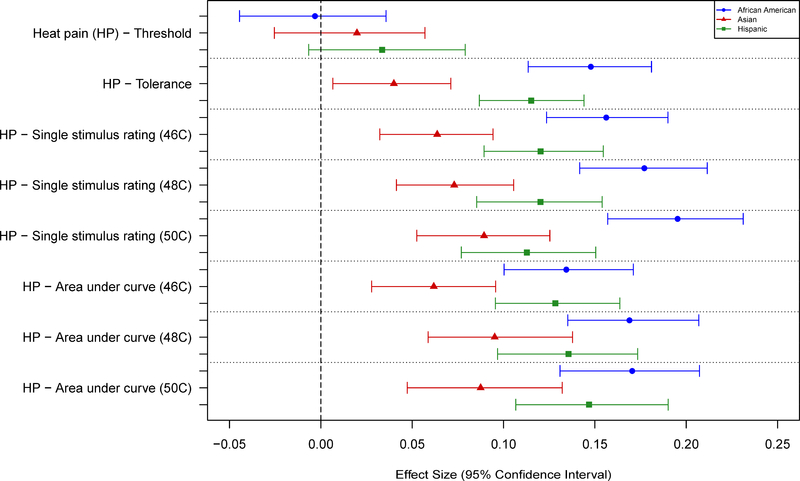
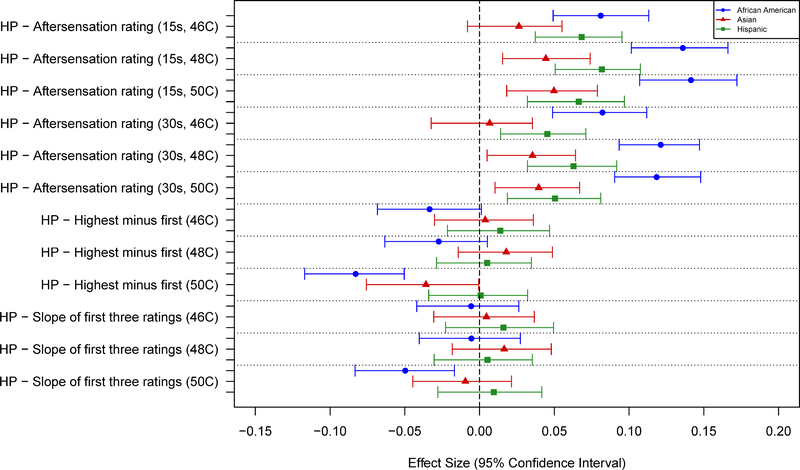
Race Differences in Pain Sensitivity
No significant associations were observed between race and any of the pressure pain thresholds (Tables 5 and 7; Fig. 5). AAs and Hispanics showed greater sensitivity than NHWs for nearly all measures of mechanical cutaneous pain, except for threshold. Asians showed slightly greater sensitivity to mechanical pain compared to NHWs, although the differences were small and generally not statistically significant (Tables 5 and 7; Fig. 6). AAs, Asians, and Hispanics all showed greater HP sensitivity than NHWs, with AAs and Hispanics showing the largest differences. These results were consistent across most measures of HP sensitivity and HP aftersensations (Tables 6 and 8; Figs. 7–8). However, no racial differences were observed with respect to measures of HP threshold, and significant differences in HP TS were observed for AAs, but only with 50°C stimuli (Table 6). Unexpectedly, that difference was in the direction of AAs showing less TS than NHWs.
Table 5:
Model Estimates and Significance for Self-Identified Race (NHW vs. African American or Asian) for Pressure Pain Threshold and Mechanical Cutaneous Pain Outcomes
| ---African American--- | ---Asian--- | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Standard Deviation | Wald t-Statistic | p-value1 | Estimate | Standard Deviation | Wald t-Statistic | p-value1 | |
| Pressure Pain Threshold | ||||||||
| Temporalis | 10.05 | 219.50 | 2.676 | 0.0075 | 4.73 | 332.63 | 0.832 | 0.4056 |
| Masseter | 7.17 | 197.43 | 2.122 | 0.0338 | 7.03 | 312.74 | 1.315 | 0.1887 |
| TMJ | −1.64 | 164.64 | −0.583 | 0.5602 | 2.89 | 277.62 | 0.609 | 0.5422 |
| Trapezius | −12.86 | 350.15 | −2.148 | 0.0317 | 3.33 | 570.43 | 0.342 | 0.7327 |
| Epicondyle | 11.38 | 339.41 | 1.960 | 0.0500 | −1.16 | 557.92 | −0.121 | 0.9033 |
| Mechanical Cutaneous Pain | ||||||||
| Threshold | 4.13 | 439.06 | 0.549 | 0.5827 | 11.18 | 785.62 | 0.832 | 0.4055 |
| Single Stimulus Rating - 256mN Probe | 1.19 | 33.12 | 2.104 | 0.0354 | 2.62 | 60.85 | 2.520 | 0.0117 |
| Single Stimulus Rating - 512mN Probe | 5.48 | 53.15 | 6.030 | <.0001 | 4.92 | 94.54 | 3.044 | 0.0023 |
| Temporal Summation - 256mN Probe | 2.58 | 32.88 | 4.591 | <.0001 | 2.27 | 59.63 | 2.229 | 0.0258 |
| Temporal Summation - 512mN Probe | 4.51 | 44.64 | 5.909 | <.0001 | 1.29 | 75.00 | 1.006 | 0.3146 |
| Aftersensation Rating (15s) - 256mN Probe | 1.56 | 20.43 | 4.460 | <.0001 | 1.19 | 45.46 | 1.534 | 0.1251 |
| Aftersensation Rating (15s) - 512mN Probe | 5.42 | 41.91 | 7.559 | <.0001 | 3.89 | 77.92 | 2.916 | 0.0035 |
| Aftersensation Rating (30s) - 256mN Probe | 0.89 | 13.45 | 3.885 | 0.0001 | 0.68 | 30.95 | 1.294 | 0.1956 |
| Aftersensation Rating (30s) - 512mN Probe | 3.61 | 32.00 | 6.601 | <.0001 | 2.40 | 58.76 | 2.388 | 0.0169 |
All estimates are compared to a referent White non-Hispanic male, controlling for site and TMD status
Significance evaluated at Bonferroni corrected level of 0.0015
Table 7:
Model Estimates and Significance for Self-Identified Race (NHW vs. Hispanic or Other) for Pressure Pain Threshold and Mechanical Cutaneous Pain Outcomes
| ---Hispanic--- | ---Other--- | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Standard Deviation | Wald t-Statistic | p-value1 | Estimate | Standard Deviation | Wald t-Statistic | p-value1 | |
| Pressure Pain Threshold | ||||||||
| Temporalis | 6.47 | 296.22 | 1.276 | 0.2019 | 3.48 | 628.98 | 0.324 | 0.7461 |
| Masseter | 4.33 | 256.10 | 0.988 | 0.3229 | 4.35 | 581.74 | 0.437 | 0.6619 |
| TMJ | 5.83 | 227.52 | 1.497 | 0.1343 | 2.48 | 459.50 | 0.315 | 0.7528 |
| Trapezius | −15.09 | 462.08 | −1.910 | 0.0562 | 11.21 | 1184.93 | 0.553 | 0.5803 |
| Epicondyle | −9.34 | 462.16 | −1.182 | 0.2374 | 18.35 | 1192.83 | 0.900 | 0.3684 |
| Mechanical Cutaneous Pain | ||||||||
| Threshold | −29.60 | 564.55 | −3.065 | 0.0022 | 15.21 | 1458.37 | 0.610 | 0.5421 |
| Single Stimulus Rating - 256mN Probe | 8.79 | 64.44 | 7.975 | <.0001 | 0.73 | 76.51 | 0.554 | 0.5794 |
| Single Stimulus Rating - 512mN Probe | 12.08 | 83.87 | 8.423 | <.0001 | 3.39 | 159.31 | 1.243 | 0.2138 |
| Temporal Summation - 256mN Probe | 6.18 | 51.28 | 7.050 | <.0001 | 3.94 | 154.12 | 1.496 | 0.1347 |
| Temporal Summation - 512mN Probe | 7.44 | 61.90 | 7.031 | <.0001 | 4.20 | 191.62 | 1.281 | 0.2003 |
| Aftersensation Rating (15s) - 256mN Probe | 3.60 | 37.78 | 5.564 | <.0001 | −0.02 | 28.22 | −0.051 | 0.9593 |
| Aftersensation Rating (15s) - 512mN Probe | 7.47 | 62.12 | 7.025 | <.0001 | 6.30 | 189.94 | 1.939 | 0.0525 |
| Aftersensation Rating (30s) - 256mN Probe | 1.66 | 24.46 | 3.956 | <.0001 | 0.22 | 18.97 | 0.681 | 0.4957 |
| Aftersensation Rating (30s) - 512mN Probe | 3.85 | 43.36 | 5.192 | <.0001 | 4.69 | 153.74 | 1.785 | 0.0742 |
All estimates are compared to a referent White non-Hispanic male, controlling for site and TMD status
Significance evaluated at Bonferroni corrected level of 0.0015
Figure 5.
Effect sizes (and 95% confidence intervals) for race/ethnicity differences are shown for the pressure pain threshold measures. Values are shown for each measure such that positive values denote a greater pain sensitivity (lower threshold) for a given race/ethnicity vs non-Hispanic whites.
Figure 6.
Effect sizes (and 95% confidence intervals) for race/ethnicity differences are shown for the mechanical cutaneous pain measures. Values are shown for each measure such that positive values denote a greater pain sensitivity (lower threshold or higher ratings) for a given race/ethnicity vs non-Hispanic whites.
Table 6:
Model Estimates and Significance for Self-Identified Race (NHW vs. African American or Asian) for Heat Pain Outcomes
| ---African American--- | ---Asian--- | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Standard Deviation | Wald t-Statistic | p-value1 | Estimate | Standard Deviation | Wald t-Statistic | p-value1 | |
| Heat Pain | ||||||||
| Threshold | 0.07 | 20.71 | 0.153 | 0.8782 | −0.41 | 20.72 | −0.942 | 0.3461 |
| Tolerance | −0.81 | 5.47 | −8.639 | <.0001 | −0.38 | 9.53 | −2.337 | 0.0194 |
| Single Stimulus Rating - 46C | 12.26 | 78.47 | 9.131 | <.0001 | 8.23 | 129.20 | 3.724 | 0.0002 |
| Single Stimulus Rating - 48C | 14.21 | 80.30 | 10.349 | <.0001 | 9.96 | 136.35 | 4.268 | <.0001 |
| Single Stimulus Rating - 50C | 15.65 | 80.14 | 11.415 | <.0001 | 12.45 | 139.35 | 5.223 | <.0001 |
| Area Under Curve - 46C | 95.24 | 708.93 | 7.854 | <.0001 | 75.11 | 1215.19 | 3.614 | 0.0003 |
| Area Under Curve - 48C | 114.21 | 676.25 | 9.873 | <.0001 | 107.97 | 1134.57 | 5.563 | <.0001 |
| Area Under Curve - 50C | 104.53 | 613.43 | 9.962 | <.0001 | 95.49 | 1091.84 | 5.113 | <.0001 |
| Aftersensation Rating (15s) - 46C | 3.41 | 42.14 | 4.737 | <.0001 | 1.85 | 70.22 | 1.540 | 0.1236 |
| Aftersensation Rating (15s) - 48C | 6.93 | 50.99 | 7.948 | <.0001 | 3.84 | 86.54 | 2.592 | 0.0095 |
| Aftersensation Rating (15s) - 50C | 7.74 | 54.71 | 8.268 | <.0001 | 4.79 | 96.18 | 2.909 | 0.0036 |
| Aftersensation Rating (30s) - 46C | 2.61 | 31.81 | 4.802 | <.0001 | 0.31 | 46.59 | 0.395 | 0.6927 |
| Aftersensation Rating (30s) - 48C | 5.04 | 41.56 | 7.084 | <.0001 | 2.28 | 64.30 | 2.070 | 0.0384 |
| Aftersensation Rating (30s) - 50C | 5.10 | 43.08 | 6.925 | <.0001 | 3.02 | 76.26 | 2.314 | 0.0207 |
| Highest Rating Minus First - 46C | −1.89 | 56.62 | −1.954 | 0.0507 | 0.35 | 93.50 | 0.221 | 0.8254 |
| Highest Rating Minus First - 48C | −1.64 | 59.81 | −1.600 | 0.1096 | 1.92 | 107.48 | 1.045 | 0.2960 |
| Highest Rating Minus First - 50C | −4.84 | 58.42 | −4.845 | <.0001 | −3.33 | 92.78 | −2.097 | 0.0360 |
| Slope of First Three Ratings - 46C | −0.12 | 21.06 | −0.330 | 0.7416 | 0.16 | 33.59 | 0.271 | 0.7868 |
| Slope of First Three Ratings - 48C | −0.13 | 22.97 | −0.321 | 0.7479 | 0.63 | 37.94 | 0.964 | 0.3352 |
| Slope of First Three Ratings - 50C | −1.15 | 23.07 | −2.906 | 0.0037 | −0.37 | 39.20 | −0.555 | 0.5786 |
All estimates are compared to a referent White non-Hispanic male, controlling for site and TMD status
Significance evaluated at Bonferroni corrected level of 0.0015
Table 8:
Model Estimates and Significance for Self-Identified Race (NHW vs. Hispanic or Other) for Heat Pain Outcomes
| ---Hispanic--- | ---Other--- | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Standard Deviation | Wald t-Statistic | p-value1 | Estimate | Standard Deviation | Wald t-Statistic | p-value1 | |
| Heat Pain | ||||||||
| Threshold | −0.67 | 19.99 | −1.599 | 0.1097 | −0.58 | 26.07 | −1.052 | 0.2928 |
| Tolerance | −0.95 | 8.23 | −6.733 | <.0001 | −0.65 | 22.57 | −1.690 | 0.0910 |
| Single Stimulus Rating - 46C | 12.27 | 101.98 | 7.031 | <.0001 | 8.65 | 278.18 | 1.818 | 0.0691 |
| Single Stimulus Rating - 48C | 12.74 | 105.98 | 7.029 | <.0001 | 10.59 | 295.48 | 2.095 | 0.0361 |
| Single Stimulus Rating - 50C | 12.11 | 107.40 | 6.592 | <.0001 | 10.33 | 290.08 | 2.082 | 0.0373 |
| Area Under Curve - 46C | 117.88 | 918.41 | 7.504 | <.0001 | 55.85 | 2531.39 | 1.290 | 0.1971 |
| Area Under Curve - 48C | 118.99 | 877.59 | 7.927 | <.0001 | 67.82 | 2440.65 | 1.625 | 0.1043 |
| Area Under Curve - 50C | 115.16 | 784.44 | 8.582 | <.0001 | 71.59 | 2131.18 | 1.964 | 0.0495 |
| Aftersensation Rating (15s) - 46C | 3.81 | 55.75 | 3.991 | <.0001 | 4.77 | 186.33 | 1.498 | 0.1342 |
| Aftersensation Rating (15s) - 48C | 5.41 | 66.04 | 4.787 | <.0001 | 5.52 | 204.56 | 1.579 | 0.1144 |
| Aftersensation Rating (15s) - 50C | 4.46 | 67.23 | 3.880 | 0.0001 | 4.00 | 201.32 | 1.163 | 0.2449 |
| Aftersensation Rating (30s) - 46C | 1.82 | 40.15 | 2.652 | 0.0080 | 4.18 | 165.66 | 1.477 | 0.1398 |
| Aftersensation Rating (30s) - 48C | 3.22 | 51.27 | 3.674 | 0.0002 | 5.00 | 181.56 | 1.611 | 0.1071 |
| Aftersensation Rating (30s) - 50C | 2.57 | 50.95 | 2.949 | 0.0032 | 3.85 | 168.16 | 1.338 | 0.1810 |
| Highest Rating Minus First - 46C | 1.01 | 72.77 | 0.814 | 0.4156 | −2.47 | 191.23 | −0.756 | 0.4499 |
| Highest Rating Minus First - 48C | 0.41 | 79.41 | 0.299 | 0.7646 | −4.04 | 200.46 | −1.179 | 0.2383 |
| Highest Rating Minus First - 50C | 0.06 | 81.50 | 0.043 | 0.9657 | −3.42 | 222.36 | −0.895 | 0.3709 |
| Slope of First Three Ratings - 46C | 0.39 | 24.55 | 0.931 | 0.3517 | 0.19 | 80.96 | 0.138 | 0.8902 |
| Slope of First Three Ratings - 48C | 0.13 | 25.10 | 0.310 | 0.7567 | −0.60 | 79.92 | −0.440 | 0.6602 |
| Slope of First Three Ratings - 50C | 0.28 | 29.36 | 0.555 | 0.5791 | −1.10 | 91.95 | −0.702 | 0.4825 |
All estimates are compared to a referent White non-Hispanic male, controlling for site and TMD status
Significance evaluated at Bonferroni corrected level of 0.0015
Figure 7.
Effect sizes (and 95% confidence intervals) for race/ethnicity differences are shown for several heat pain measures. Values are shown for each measure such that positive values denote a greater pain sensitivity (lower threshold or higher ratings) for a given race/ethnicity vs non-Hispanic whites.
Figure 8.
Effect sizes (and 95% confidence intervals) for race/ethnicity differences are shown for heat pain measures related to aftersensation and temporal summation. Values are shown for each measure such that positive values denote a greater pain sensitivity (higher ratings) for a given race/ethnicity vs non-Hispanic whites.
Interactions among Demographic Variables
A series of separate regression analyses were performed to identify any statistically significant interactions among the three major demographic variables examined. Evaluating each QST measure separately, and all possible combinations of demographic variables, we found only two of 306 analyses to be significant at p<0.001. These two were 1) a sex by race interaction for MCP TS with the 512mN probe, and 2) an age by race interaction for HP tolerance. Based on these few effects, showing no apparent pattern, no further analysis of interaction effects were performed.
Discussion
Pain sensitivity measures varied significantly according to sex, age, and self-reported race across multiple pain domains: pressure, mechanical cutaneous, and heat pain. However, not all of the pain sensitivity measures showed the same profile of significant effects, reflecting underlying differences in the neurophysiological basis for the separate pain measures. Sex differences produced the largest effect sizes in this study, with women showing greater pain sensitivity than men in nearly all measures (29/34). Pain sensitivity decreased with age, however this effect was only statistically significant for 7/34 measures, and of very weak magnitude. Racial differences were observed for many of the pain sensitivity measures, largely in the direction of NHW showing the least pain sensitivity and AAs showing the greatest pain sensitivity. However, patterns of racial differences in pain sensitivity varied according to the individual pain measures, and were not found for any threshold measures.
Sex Differences in Pain Sensitivity
Uniformly, women had significantly lower pain thresholds across all three domains, and higher pain ratings for most MCP and HP measures compared to men. Women also showed greater HP TS, although significant differences for HP TS were only observed for the lowest temperature. Despite these statistically significant effects, the magnitude of the effects varied across measures. The sex differences for PPTs and HP tolerance were the largest (ES: 0.3–0.4), followed by those for ratings of individual HP stimuli (0.2–0.3), all of which are likely clinically meaningful. However, the sex differences for other measures, while statistically significant, were of smaller effect sizes (all < 0.20), and could easily have failed to achieve statistical significance with a smaller sample size.
Many studies have evaluated sex differences in human pain sensitivity, and a majority finds women to be more sensitive than men. Nearly all other studies failed to find a significant sex difference, while only rarely does a study report greater pain sensitivity for men 5,11,20. It is not clear what factors determine the expression of sex differences in experimental pain sensitivity, since significant sex differences can be observed (or not) for different stimulus modalities and different types of pain measures. The power of any given study is certainly critical to its ability to identify a significant sex difference. However, the literature reviews on this topic reveal that weaker statistical power, while certainly relevant, does not explain all the failures to observe significant sex differences. Sex differences clearly exist on the basis of both physiological and psychological features, and many factors from both domains can influence pain perception and reporting9. Thus, “sex differences” reflects a constellation of factors that have a role in determining one’s pain sensitivity, the influence of which can vary according to situational variables. Accordingly, it has been shown that the testing environment can influence whether or not significant sex differences in pain sensitivity are found. For instance, some studies have demonstrated a significant effect of experimenter gender upon subjects’ experimental pain sensitivity, particularly with respect to male study participants1,7,15. Yet, the failure to find such effects in other reports2,18,19 supports the idea that multiple factors play a role in determining the expression of sex differences in pain sensitivity, with any one of them having greater of lesser prominence in any particular situation.
Age-Related Effects upon Pain Sensitivity
Despite a consistent trend of reduced pain sensitivity with age across many measures in this study, few variables showed significant age effects. Furthermore, the magnitude of the significant age effects was very small (all effect sizes < 0.1). The variables that did show significant age effects were scattered among the different stimulus modalities and the different types of pain measures, thus not showing any pattern with respect to these factors.
The literature on age-related effects upon experimental pain sensitivity has produced variable results. One systematic review characterized the literature as showing “consistent, although not invariable, age differences” in experimental pain. The most common differences were less sensitivity with age for weakly painful stimuli (higher thresholds and lower ratings for weaker suprathreshold stimuli), but greater sensitivity to stronger stimuli (higher pain ratings for more intense stimuli and reduced tolerance).6 However, nearly every study compares a “younger” and “older” age group, and the cohort of the present study encompasses an age range that did not represent an “older” age group. Thus, this report is the first, to our knowledge, to explore age effects upon multiple measures of pain sensitivity within a more restricted 18–44 year old age range. The modest reduction in pain sensitivity observed within this age range likely reflects the more significant age effects observed in other studies which include an older age group.
Racial Differences in Pain Sensitivity
There were varying patterns of racial differences observed, depending upon the groups compared and the particular pain sensitivity variable evaluated. Contrasts of NHWs and AAs showed the largest number of significant racial differences, including 1) nearly all of the MCP tests, and 2) many of the HP tests, including suprathreshold ratings and aftersensations. These contrasts uniformly indicated greater pain sensitivity for AAs compared to NHW. Unexpectedly, HP TS showed no significant AA-NHW racial difference with lower temperatures, while the significant difference seen at 50°C was in the direction of less TS for AAs. This contrasts with several reports of greater HP TS for AAs13,16. This data set did show significant TS overall, with rating increases averaging 20–25 points on a 0–100 VAS10. One possible factor affecting these 50°C TS results is that the initial ratings for AAs were higher than for NHWs, leaving less room for ratings increases compared to NHWs. Of note, none of the threshold measures indicated a significant racial difference.
These results are generally congruent with previous literature. According to recent meta-analyses, AAs show greater pain sensitivity than NHWs in multiple studies evaluating HP intensity ratings, and tolerance, with small or no differences in HP threshold13,21. The only study evaluating AA-NHW differences in PPTs, reported no statistically significant differences, when tested on two of the same body sites as in the current study22.
Hispanics showed greater pain sensitivity than NHW in all MCP measures (except threshold), and several measures of HP sensitivity. No difference was seen for any threshold or HP TS measure. Furthermore, there were no significant differences recognized between AAs and Hispanics, with the exception of HP aftersensation measures for 50°C stimuli (based on the absence of overlap of 95% CIs; Figure 8). Few comparable studies exist. Similar to our results, one study found significant HP tolerance differences between Hispanic and NHW groups, but no significant HP or pressure pain threshold differences22.
Asians were more similar to NHW than AAs or Hispanics with respect to nearly all pain sensitivity measures. Compared to NHWs, Asians provided significantly higher HP ratings for suprathreshold stimuli, but showed no differences for HP aftersensation or TS measures. There were also no significant differences between Asians and NHWs for PPTs or any MCP measure. While some previous work has compared NHWs with specific Asian groups (i.e., Japanese, Indian, and others reviewed by Rahim-Williams et al.21), these are not sufficiently comparable to our sample of mixed Asian participants to warrant comparison.
The fact that pain thresholds from all types of stimuli failed to show significant racial differences, while other measures did show such differences, suggests that the basis for any racial differences is not related to tissue characteristics or inherent sensitivity of nociceptors. Rather, any differences observed for suprathreshold pain ratings or tolerance are more likely related to differences in central nociceptive processing, including the modulation of such processing imposed by cognitive, psychological, and/or affective differences21.
Despite the many significant racial differences noted above, effect sizes were modest. The largest effect sizes observed - HP ratings and tolerance comparing NHW and AA – were between 0.14–0.20. For these same measures comparing NHW and Hispanics, the effect sizes were between 0.10–0.15. For all the statistically significant mechanical cutaneous pain measures and HP aftersensation measures, effect sizes were under 0.15. Thus, even with the consistency of racial differences observed across multiple pain measures in the current study and parallel results from previous studies, quantitative differences among the races are small.
Limitations
The size of the study cohort and the wide array of pain sensitivity measures are very strong features of this study. However, the limited age range (18–44) in the study sample did not allow for a full evaluation of pain sensitivity across the life span. This prevented any meaningful comparisons with existing literature on age effects upon pain. Another limitation, relevant to sex differences, is the absence of control for menstrual cycle variation. While the literature contains reports of pain sensitivity fluctuations across the menstrual cycle of healthy women, a comparable number of studies report finding no cycle effects11. A recent review of this topic concluded: “… the majority of the more recent, well-controlled studies show that menstrual cycle phase has no effect on the perception of pain in healthy, pain-free women” (Iacovides et al., p. 1398).12
Conclusions
This large-scale study allowed for a powerful analysis of sex, age, and racial/ethnic effects upon a wide range of pain sensitivity measures. Greater pain sensitivity for women was robustly found for nearly all pain measures, however, only some of the effect sizes could be considered clinically significant. Age effects were weak or nonexistent, but interpretations are limited given the restricted age range evaluated. While significant racial/ethnic differences were observed for several pain measures, no differences were found for pain thresholds. With respect to suprathreshold pain, sensitivity generally followed the following pattern: AA>Hispanic>Asian>NHW, although few significant differences were observed between AA and Hispanic, or between Asian and NHW. These demographic differences in experimental pain sensitivity are likely to have relevance to clinical pain expression3, as was recently demonstrated in the context of racial differences in OA knee pain8.
Highlights.
Greater female pain sensitivity is seen across many domains and measures.
Non-Hispanic whites are often less pain sensitive than other racial/ethnic groups.
Racial/ethnic differences are seen with suprathreshold, but not threshold pain.
While many racial/ethnic differences are significant, they are of small effect size.
Only a weak trend of decreased pain sensitivity with age is seen among 18–44 y.o.
Perspective.
The influence of sex, age, and race/ethnicity upon various aspects of pain sensitivity, encompassing threshold and suprathreshold measures and multiple stimulus modalities, allows for a more complete evaluation of the relevance of these demographic factors to acute pain perception.
Acknowledgments
This work was supported by NIH grant U01DE017018. The authors would like to thank the OPPERA research staff at each of four study sites and the data coordination center for their invaluable contributions to this work. In addition, we express our gratitude to the participants who have devoted time and effort in support of this research.
Disclosures
This work was supported by NIH grant U01DE017018. The OPPERA program also acknowledges resources specifically provided for this project by the respective host universities: University at Buffalo, University of Florida, University of Maryland-Baltimore, and University of North Carolina-Chapel Hill. Roger Fillingim and Gary Slade are consultants and equity stock holders, and William Maixner is cofounder and equity stock holder in Algynomics, Inc., a company providing research services in personalized pain medication and diagnostics. Richard Ohrbach, Joel Greenspan, Cara Ostrom, Ronald Dubner, and Eric Bair declare that they have no conflicts of interest.
This work was done at: University of North Carolina at Chapel Hill, NC; University at Buffalo, NY; University of Maryland-Baltimore, MD; University of Florida, FL; and Battelle Memorial Institute, NC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aslaksen PM, Myrbakk IN, Hoifodt RS, Flaten MA: The effect of experimenter gender on autonomic and subjective responses to pain stimuli. Pain 129:260–8, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bush FM, Harkins SW, Harrington WG, Price DD: Analysis of gender effects on pain perception and symptom presentation in temporomandibular pain. Pain 53:73–80, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Edwards RR, Sarlani E, Wesselmann U, Fillingim RB: Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain 114:315–9, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Efron B, Tibshirani R. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1994. [Google Scholar]
- 5.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL III: Sex, Gender, and Pain: A Review of Recent Clinical and Experimental Findings. Journal of Pain 10:447–85, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson SJ, Helme RD: Age-related differences in pain perception and report. Clinics in Geriatric Medicine 17:433–56, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Gijsbers K, Nicholson F: Experimental pain thresholds influenced by sex of experimenter. Percept Mot Skills 101:803–7, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Goodin BR, Bulls HW, Herbert MS, Schmidt J, King CD, Glover TL, Sotolongo A, Sibille KT, CruzAlmeida Y, Staud R, Fessler BJ, Redden DT, Bradley LA, Fillingim RB: Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and older with knee osteoarthritis: ethnic differences. Psychosom Med 76:302–10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ: Studying sex and gender differences in pain and analgesia: A consensus report. Pain 132 Suppl 1:S26–S45, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Mulkey F, Rothwell R, Maixner W: Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J Pain 12:T61–T74, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenspan JD, Traub RJ. Gender differences in pain and its relief In: Wall and Melzack’s Textbook of Pain. 6 ed. (McMahon SB, Koltzenburg M, Tracey I, Turk DC, editors.) Elsevier (Saunders), Philadelphia, 2013. p. 221–31. [Google Scholar]
- 12.Iacovides S, Avidon I, Baker FC: Does pain vary across the menstrual cycle? A review. Eur J Pain 19:1389405, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Yang GS, Greenspan JD, Downton KD, Griffith KA, Renn CL, Johantgen M, Dorsey SG: Racial and ethnic differences in experimental pain sensitivity: Systematic review and meta-analysis. Pain. 2016. September 24 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Lautenbacher S: Experimental Approaches in the Study of Pain in the Elderly. Pain Medicine 13:S44–S50, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Levine FM, De Simone LL: The effects of experimenter gender on pain report in male and female subjects. Pain 44:69–72, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Mechlin B, Heymen S, Edwards CL, Girdler SS: Ethnic differences in cardiovascular-somatosensory interactions and in the central processing of noxious stimuli. Psychophysiol 48:762–73, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Monsees GM, Tamimi RM, Kraft P: Genome-wide association scans for secondary traits using case-control samples. Genet Epidemiol 33:717–28, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers CD, Robinson ME, Riley JL III, Sheffield D: Sex, gender, and blood pressure: Contributions to experimental pain report. Pschosom Med 63:545–50, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Otto MW, Dougher MJ: Sex differences and personality factors in the responsivity to pain. Percept Mot Skills 61:383–90, 1985. [DOI] [PubMed] [Google Scholar]
- 20.Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinière M: A systematic literature review of 10 years of research on sex/gender and experimental pain perception − Part 1: Are there really differences between women and men? Pain 153:602–18, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Rahim-Williams B, Riley JL, Williams AKK, Fillingim RB: A Quantitative Review of Ethnic Group Differences in Experimental Pain Response: Do Biology, Psychology, and Culture Matter? Pain Medicine 13:522–40, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahim-Williams FB, Riley JL III, Herrera D, Campbell CM, Hastie BA, Fillingim RB: Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain 129:177–84, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson DB, Rzehak P, Klenk J, Weiland SK: Analyses of case-control data for additional outcomes. Epidemiology 18:441–5, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD: Quantitative sensory testing: a comprehensive protocol for clinical trials. EJP 10:77–88, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Slade GD, Bair E, By K, Mulkey F, Baraian C, Rothwell R, Reynolds M, Miller V, Gonzalez Y, Gordon S, Ribeiro-Dasilva M, Lim PF, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Dampier D, Knott C, Ohrbach R: Study Methods, Recruitment, Sociodemographic Findings, and Demographic Representativeness in the OPPERA Study. J Pain 12:T12–T26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]