Summary
Complex host-microbiota interactions contribute to systemic autoimmunity outside the gut. On a molecular level, posttranslational modification of, and cross-reactivity with, autoantigens represent mechanisms of how the microbiota mediate autoimmunity. On a cellular level, translocation of live gut bacteria across a dysfunctional gut barrier allows for direct interactions with immune and tissue cells, instigating autoimmunity systemically.
Keywords: Cross-reactivity, orthologs, citrullination, gut barrier, commensal translocation, pathobionts
Introduction:
Systemic autoimmune disorders are characterized by tissue damage in anatomically diverse locations. Inflammation is mediated by innate cells, migrating autoreactive lymphocytes and circulating pathogenic autoantibodies. How these complex processes are triggered and sustained remains incompletely understood but host-microbiota interactions in the context of a genetically susceptible host are increasingly implicated (1). The adaptive immune response to gut commensal bacteria plays an integral role in maintaining homeostasis (2). Environmental factors, genetic polymorphisms and gut microbial diversity shape adaptive immune system responses (3). Furthermore, the neonatal period is critical for the development of lymphoid structures, maturation of T and B cells, and acquisition of immune tolerance to gut commensals (4, 5). Alteration in microbial communities during this critical time can result in immune dysregulation and subsequent immune-mediated diseases such as allergies or autoimmunity.
Various T cell subsets both maintain protective immunity against pathogens and prevent inflammatory immune responses against self and microbiota-derived antigens. Instructed by the environment and innate antigen-presenting cells, CD4+ T helper (Th) cells differentiate into functionally diverse subsets including Th1, Th2, Th17, and regulatory T cells (Tregs). The balance of these cells, in particular that of Th17/Treg, influences the transition from homeostasis to disease and is profoundly affected by the gut microbiota(1, 3). For example, a single commensal microbe, Candidatus Savagella or segmental filamentous bacteria (SFB) colonizing the small intestine, is sufficient to induce mucosal Th17 cells (6). Others exert their effect on T helper cell subsets in concert, such as Treg-inducing Clostridia consortia (7, 8).
Another T cell subset, follicular helper T (Tfh) cells, are specialized CD4+ T cells that enable B cell proliferation and differentiation in germinal centers and are critical for pathogenic autoantibody production in the autoimmune-prone host (9). Tfh biology is also determined through interactions with gut microbiota via innate MyD88 signaling pathways (10). Importantly, B cell development occurs in the intestinal mucosa where microbial colonization influences the immunoglobulin repertoire (11, 12). Both T cell-dependent (TD) and -independent (TI) IgA are induced by the microbiota. In addition, different gut commensals elicit both TD and TI IgA that coat the healthy microbiota (13) and some even co-opt IgA for mucosal colonization (14). Interestingly, excessive IgA coating within the human gut microbiome of patients with inflammatory bowel disease identified pathobionts that confer increased susceptibility to colitis in gnotobiotic models (15). It is unclear whether coating by other isotypes marks pathobionts that elicit systemic immune responses as seen in non-gut rheumatic disease. However, human IgA deficiency, frequently associated with autoimmune conditions, interestingly leads to a perturbed microbial community composition and compensatory IgM coating (16)**.
Systemic IgG responses towards commensals occur in the absence of a functional innate immune system (17). Furthermore, functional gut barrier disturbance during gastrointestinal infection induces inflammatory anti-commensal T cells that acquire a memory phenotype consistent with pathogen-specific T cells (18). Various environmental insults may therefore trigger anti-commensal responses by the adaptive immune system. In individuals with a susceptible MHC haplotype, these responses could theoretically convert into cross-reactive autoimmune responses as recently shown experimentally and discussed further below (19)*. We next go into cross-reactivity and other mechanisms by which the microbiome influences autoimmunity, focusing on rheumatoid arthritis and systemic lupus erythematosus as paradigms.
Gut pathobionts in rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease affecting a variety of organs if left untreated. Inflammation of the articular and extra-articular structures of the body results in synovitis, disability and significant comorbidity (20). Genetics, particularly specific HLA polymorphisms, predispose people to RA via presentation of pathogenic epitopes of posttranslationally modified antigens to autoreactive lymphocytes. Activation of the adaptive immune system by these modified proteins is a result of host genetics interacting with environmental factors such as smoking, periodontitis and other microbial stimuli (21). In particular, mucosal inflammation has been linked with development of RA with implications for oral, gut, and lung microbiota as potential triggers (22, 23). The gut as an inciting origin of RA has long been hypothesized but difficult to study experimentally in humans except through gross surgical interventions undergone for unrelated indications (24).
Animal models of RA have demonstrated a mechanistic link between the gut microbiota and RA-like disease. Specific bacterial strains may differ between mice and humans but the immunologic processes induced by murine gut bacteria summarized above help better understand how host-microbiota interactions could occur in human RA. The K/BxN model of autoimmune arthritis is highly dependent on the gut microbiota (25). As stated above, the differentiation of mucosal Th17 cells is promoted by colonization with SFB in the ileum of non-autoimmune-prone animals (6). Remarkably, colonization with SFB alone was sufficient to promote RA-like disease in germ-free K/BxN mice (25) but the mechanisms involved Tfh as opposed to Th17 differentiation (26, 27). SFB colonization drove Tfh differentiation in the Peyer`s patches of the animals and migration of Tfh with dual TCRs to systemic sites, enhancing autoantibody production and polyarthritis. These studies exemplify how a gut commensal can redirect physiologic mucosal adaptive immune responses to systemic autoimmune responses in the right genetic context. Similarly, Treg-inducing Clostridia may not be tolerogenic in some hosts that carry genetic polymorphisms affecting Treg function (28).
The human gut microbiome from RA patients has also been explored by several groups. Not surprisingly, RA patients have microbiomes distinct from healthy controls if studied either in the USA or China (29). 16s rDNA and shotgun sequencing of stool samples from North American patients with new-onset rheumatoid arthritis showed an enrichment of Prevotella copri that was colitogenic in the gnotobiotic setting (30). Fecal metagenomic studies from Chinese RA patients did not show an increased abundance of this species during the first year of disease (29), suggesting geographic variability among other factors contributing to heterogeneity of microbiome studies. Importantly, monocolonization with P. copri in a germ-free arthritis model induced synovitis and peripheral Th17 cells as shown by a Japanese group that studied also a small cohort of early RA patients with P. copri enriched in a subset (31)*. Finally, subgroups of RA patients were shown to mount systemic IgA responses towards antigens from P. copri that correlate with anti-citrullinated peptide antibodies (32). Taken together, these studies support that P. copri is a plausible gut pathobiont in a subset of early RA patients that warrants further investigation.
Oral pathobionts in rheumatoid arthritis
Besides the fecal microbiome, oral and lung microbiota have also been profiled in RA based on the mucosal origin hypothesis (22, 23). A large metagenomic study from China showed that dental microbial community signatures before and after therapy with methotrexate could be predictive of response to therapy (29). Periodontal disease has long been linked to prevalence and severity of RA. Oral commensal bacteria invading the periodontium and alveolar bone are thus intriguing candidates for driving similar processes at the synovial membrane of joints. Indeed, antibodies against Porphyromonas gingivalis, a well-known periodontal pathogen, have been associated with high-risk, autoantibody-positive individuals at increased risk of developing RA (33). The long-standing hypothesis that P. gingivalis could contribute to RA pathogenesis via citrullination has recently been refuted (34, 35)**, but other mechanisms such as those detailed above could be involved besides complement subversion and TLR activation (36, 37). A murine study, for instance, demonstrated that P. gingivalis promotes experimental arthritis via innate TLR2-dependent interleukin-1 signals skewing Th17 differentiation (38). Another oral pathobiont, however, was mechanistically linked to hypercitrullination of autoantigens in human RA (34) (39)**. This elaborate study showed that Aggregatibacter actinomycetemconitams (A. actino.) produces a pore-forming toxin that activates citrullinating enzymes in neutrophils (34)**. The HLA-DRB1 shared epitope alleles associated with anti-citrullinated antibodies only in RA patients with signs of exposure to the toxin. The high prevalence of A. actino. exposure in early RA patients was recently confirmed in a Dutch cohort (39, 40). Additional epidemiologic and mechanistic studies in vivo are needed but this new paradigm how systemic autoimmunity against citrullinated antigen can arise is promising.
Cross-reactive commensals in systemic lupus erythematosus
Systemic lupus erythematosus is a prototypical systemic autoimmune disease of unknown etiology with a highly variable prevalence based on geography, suggesting environmental factors in addition to genetics contribute to its pathogenesis (41). SLE presents with a variety of clinical subphenotypes, which are partly related to activation of different innate and adaptive immune pathways (42). The plasmacytoid dendritic cell (pDC)/interferon (IFN) axis as well as neutrophil-mediated inflammation are two major innate immune pathways in SLE (43). Adaptive immune targets are diverse due to the fact that autoantibodies in SLE target various nuclear and cell membrane structures released during apoptosis. Autoantibodies against the Ro60 antigen are considered the earliest antibodies detected, often years before the first occurrence of symptoms, followed by epitope spreading to other autoantigens (44). Environmental or mucosal triggers within the microbiome are thus plausible in subjects with the HLA predisposition to cross-react with microbial antigens similar in sequence or structure to the Ro60 autoantigen.
Cross-reactive triggers within the murine microbiome have also been identified in other non-gut autoimmune disease such as models of uveitis or autoimmune diabetes (45, 46). Interestingly, the cross-reactive target in the autoimmune diabetes model was recently shown to be protective against colitis when targeted by CD8+ T cells (47). This phenomenon might explain why several non-mucosal autoimmune diseases spare the gut as in SLE where overt gut inflammation is exceedingly rare. Peptide cross-reactivity with Ro60 epitopes targeted in SLE patients was shown with a murine T cell hybridoma (48). Consistent with this finding, we identified cross-reactive responses between human gut, oral, and skin microbiota from SLE patients and human Ro60 (19)*. This study was based on the peculiar fact that commensal orthologs of Ro60 exist in certain bacteria. Since Ro60 ortholog-expressing commensal bacteria chronically colonize the skin and mucosal sites of genetically-predisposed hosts, cross-reactive responses are likely to sustain autoimmunity in vivo. Indeed, we demonstrated that monocolonization of germ-free mice with one Ro60 commensal bacterium was able to induce and sustain anti-human Ro60 IgG autoantibodies in the systemic circulation (19)*. Interestingly, one bacterial Ro60 ortholog was expressed in a skin commensal, Propionibacterium propionicum, which was detected in the skin lesions of patients with subacute cutaneous lupus erythematosus (SCLE). Because anti-Ro60 antibodies are well known to contribute to SCLE immunopathology, it is tempting to speculate that this skin commensal drives SCLE lesions in colonized patients. Although sunlight is a trigger for SCLE flares, UV light would be expected to uniformly lead to skin eruptions. Instead, SCLE lesions are either psoriasiform or annular, both of which cannot be explained by UV light alone. We therefore hypothesize that environmental triggers such as UV light together with outgrowth of P. propionicum contribute to the phenotype of SCLE lesions, which needs to be tested experimentally in vitro and vivo using gnotobiotic models. Importantly, the commensal Ro60 ortholog-directed responses may also promote systemic autoimmune responses. Sera from anti-Ro60+ lupus patients immunoprecipitated commensal Ro60 ribonucleoproteins and CD4+ memory T cell clones cross-reacted with Ro60 orthologs. Unpublished data from our group also suggests that Ro60 knockout mice, crossed with the lupus-prone TLR7.1 transgenic mice, produce significant levels of serum anti-human Ro60 IgG (49). The production of anti-Ro60 antibodies in the absence of murine Ro60 further supports the hypothesis that microbial Ro60 contributes to anti-Ro60 autoimmunity. This paradigm should be advanced further by generating microbial mutants of Ro60 and by extending peripheral blood and microbiome studies to pre-SLE cohorts.
As more evidence arises on cross-reactivity, further efforts should be made to investigate the remaining ortholog candidates. Corynebacterium amycolatum, for example, can colonize the lacrimal duct and could therefore be involved in the pathogenesis of dry eye symptoms in anti-Ro60+ Sjögren’s syndrome patients. In addition to cross-reactivity, naturally polyreactive antibodies recognizing the microbiota also contribute to autoantibodies with germline-encoded gene segments (50). Lastly, cross-specific antibodies recognizing only a limited set of microbes were recently defined and could theoretically also target certain host antigens in genetically susceptible individuals (51).
Gut commensal translocation in systemic lupus erythematosus
In addition to T and B cell cross-reactivity on a molecular level, Th17 and Tfh cells also contribute to the pathogenesis of SLE at a cellular level (42). Recent work from our laboratory shows that an unusual gram-positive gut commensal, Enterococcus gallinarum, translocates across the gut barrier and is capable of inducing Th17 and Tfh cells as well as innate immune pathways such as the pDC/IFN axis (52)*. Monocolonization of E. gallinarum alone in germ-free non-autoimmune-prone mice was sufficient to induce peripheral Th17 and to break tolerance, generating anti-dsDNA and anti-RNA autoantibodies (52)*. Importantly, E. gallinarum was found to cross the gut barrier and colonize extraintestinal organs such as the mesenteric lymph nodes, liver and spleen, contributing also to organ-specific autoimmunity (i.e., autoimmune hepatitis-like inflammation). E. gallinarum DNA and anti-E. gallinarum adaptive immune responses were detectable in blood and liver tissues from both patients with either autoimmune hepatitis or SLE, and human hepatocyte co-cultures with E. gallinarum products elicited similar pathways as in mice in vivo (52)*. Translocation of E. gallinarum, the autoimmune pathways, and the autoimmune phenotypes were prevented in mice treated with an oral antibiotic targeting gram-positive bacteria or, more specifically, with an intramuscular vaccine targeting E. gallinarum but not the phylogenetically related E. faecalis or unrelated gut bacteria (52)*.
In addition to E. gallinarum, we explore other microbiota, most notably Lactobacilli, which also translocated to human livers along with Enterococcus spp. (52)*. Lactobacilli are highly immunomodulatory bacteria with a wide range of host effects including promotion of tolerogenic and proinflammatory pathways. We recently identified a Lactobacillus reuteri strain, typically viewed as a probiotic, that was enriched in the fecal and ileal microbiome of lupus-prone TLR7.1 transgenic mice (53)* This lupus model is predominantly driven by the pDC/IFN pathway. L. reuteri exacerbated this pathway in the transgenic model and an inducible lupus model triggered by exogenous TLR7 activation via imiquimod. Importantly, even in the germ-free setting, L. reuteri monocolonization alone was able to promote the pDC and type I IFN pathway induced by imiquimod. Interestingly, L. reuteri could be recovered from mesenteric lymph nodes, liver and spleen of these animals and translocation was mechanistically linked to TLR7 using TLR7 KO mice, but the autoimmunity-promoting effects of L. reuteri developed before detectable translocation, suggesting metabolites secreted by L. reuteri may contribute to lupus-like manifestations (53)*. Furthermore, fecal Lactobacillus spp. were enriched in a subset of SLE patients with concomitant decrease of Clostridiales, as seen in the TLR7-driven murine models (53)*. In mice, Clostridiales and other short-chain fatty acid (SCFA)-producing gut commensals could be enriched by a starch diet that in turn suppressed L. reuteri via SCFAs in vitro and vivo. Consistent with suppressed L. reuteri growth, starch feeding prevented lupus-like disease and all pDC and type I IFN signatures induced by TLR7 signals (53)*
In summary, an otherwise innocuous gut commensal, promoted as a probiotic for healthy subjects, can become a pathobiont in genetically or environmentally prone hosts suffering from enhanced TLR7 pathway-related signals. This study cautions against the unrestricted use of probiotics and provides a dietary intervention that may be beneficial for autoimmune-prone subjects with a type I IFN signature, a hypothesis that could be tested in a human pilot study. SLE patient subsets carrying the type I IFN signature should be explored more specifically for a dysbiosis of Lactobacillus spp. versus Clostridiales. Clearly, the SLE microbiome will not be uniformly altered given the subphenotypes that are already well defined both clinically and genetically.
Conclusion:
We summarized recent studies that identified mechanistic links between the mucosal (gut, oral, gingival) or skin microbiota and autoimmune pathways in RA and SLE, respectively. Older studies and numerous association studies without deeper mechanistic insights were not part of this review but contribute equally to the burgeoning literature on the microbiome in systemic autoimmune diseases. Several pathobionts have been identified in RA and SLE (Fig. 1) that warrant further mechanistic work as well as investigation of their prevalence in geographically distinct regions. The microbiome is, however, a paradigm for personalized medicine, so even if all technical and study-related biases are avoided, biologic differences in patient microbiomes will likely persist. Personalized approaches, such as longitudinal studies in individual patients as well as microbiota-targeted interventions in pre-screened patient subsets might be solutions to this hurdle.
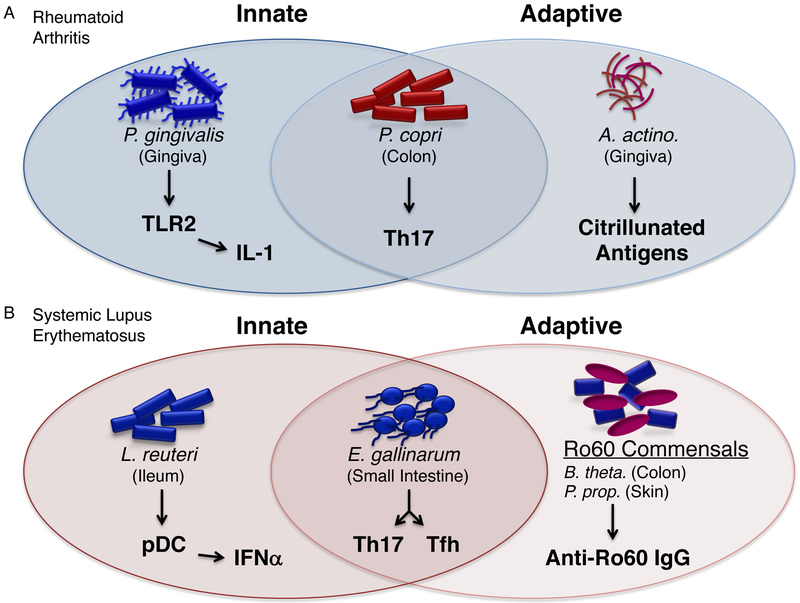
Figure.
Examples of microbiota in RA and SLE that propagate systemic autoimmunity through innate and adaptive immune pathways.
A. Examples of pathobionts linked to rheumatoid arthritis (RA). In the oral cavity, P. gingivalis and Aggregatibacter actinomycetemconitams (A. actino.) contribute to the pathogenesis of RA. P. gingivalis skews autoimmune responses through innate signaling via TLR2 leading to inflammatory cytokines(38). Complement activation by P. gingivalis contributes also to innate inflammation (not shown)(36,37). Posttranslational modification of autoantigens in RA is mediated by a toxin, leukotoxin A, from A. actino. This toxin induces citrullinated peptides in neutrophils, which can be recognized as neo-epitopes by the adaptive immune system(39). A. actino. also mediates neutrophil-specific cytolysis leading to neutrophil extracellular traps or NETosis. This process contributes to innate inflammation, not shown here(39). In the colon, P. copri promotes Th17 differentiation, engaging both innate and adaptive signaling pathways(30, 31).
B. Examples of pathobionts linked to systemic lupus erythematosus (34) and subacute cutaneous lupus erythematosus (SCLE). In the ileum, L. reuteri promotes pDC and type I IFN innate pathways in genetically prone hosts leading to SLE-related manifestations (53). E. gallinarum colonizes the jejunum and ileum but drives autoimmunity by translocating to extraintestinal organs(52). E. gallinarum induces thereby autoreactive Th17 and Tfh responses and generation of autoantibodies. Monocolonization with E. gallinarum further induces pDCs, not shown here. Commensal organisms such as B. thetaiotaomicron (B. theta.), P. propionicum, or Corynebacterium amycolatum (not shown), express orthologs of the human Ro60 autoantigen targeted by SLE and Sjogren`s syndrome patients(19). These Ro60 ortholog-expressing commensals reside on the skin and mucosal sites, where they may stimulate and sustain autoreactive T and B cells in genetically predisposed hosts, contributing to the pathogenesis of both SLE and SCLE.
In summary, posttranslational modification and cross-reactivity are two molecular mechanisms of how the microbiota and its antigens or toxins can promote systemic autoimmunity. Other processes such as bystander activation and epitope spreading are also likely to contribute to disease (1). Cellular mechanisms include translocation of bacteria to target organs and T helper cell skewing. Furthermore, apoptosis induced in infected host cells may lead to autoreactivity as shown for pathogens (54), and is also plausible for translocating gut bacteria. In general, recent studies highlight gut commensal translocation to internal organs as an important step in autoimmune host-microbiota responses. These studies put the gut barrier at the center of non-gut autoimmune diseases - barriers that are frequently dysfunctional in humans exposed to medications (e.g., NSAIDs, proton pump inhibitors), mucosal pathogens, or other barrier-altering factors.
Consistent with this paradigm in SLE, other gut commensals identified in RA microbiome profiling studies have recently been found to alter gut permeability in addition to disease severity in experimental arthritis(55). In addition, synovial fluid 16S rDNA sequencing suggests candidates such as P. copri, discussed above, might also reach target tissues in RA (56). Lastly, some systemic rheumatic diseases, most notably ankylosing spondylitis, are associated with well-documented subclinical gut inflammation and altered permeability (57). Gut commensal translocation to joints or other systemic tissues likely contribute to autoimmune pathology in this setting. Overall, this review summarized molecular and cellular mechanisms of how recently identified pathobionts contribute to systemic autoimmunity. Microbial antigens, toxins, soluble factors or direct interactions with host tissues after translocation from mucosal sites are all mechanisms to be explored further in this fascinating field.
Key points:
Gut and oral pathobionts in RA may promote Th17 systemically and induce hypercitrullination of autoantigens.
A defined set of Ro60 ortholog-expressing gut, oral, and skin commensals trigger cross-reactivtiy with human Ro60 in Ro60+ SLE.
Translocation of live gut commensals across the gut barrier triggers several innate and adaptive autoimmune pathways that are reversible with a commensal vaccine.
TLR7 controls gut barrier integrity and TLR7-mediated IFN and autoimmune pathology can be exacerbated by a diet-sensitive Lactobacillus strain.
Purpose of review
The resident bacterial communities and the host immune system have coevolved for millennia. However, recent changes in modern societies have disrupted this coevolutionary homeostasis and contributed to a rise in immune-mediated conditions. The purpose of this review is to provide an overview of recently elucidated mechanisms of how certain taxa within the bacterial microbiome propagate autoimmunity.
Recent findings
Interactions between the bacterial microbiome with innate and adaptive immune cells propagate autoreactivity, chronic inflammation, and tissue damage in susceptible hosts. These interactions contribute to autoimmune diseases such as rheumatoid arthritis or systemic lupus erythematosus, which are the focus of this review. Recent data suggest that autoimmune manifestations in genetically susceptible individuals can arise through cross-reactivity with commensal orthologs of autoantigens or commensal-mediated posttranslational modification of autoantigens. Physiologic responses to gut, oral or skin commensal bacteria can thus be misdirected towards such autoantigens in susceptible hosts. In addition, recent studies highlight that a breach of the gut barrier and translocation of commensal bacteria to non-gut organs can trigger several autoimmune pathways that can be prevented by commensal vaccination or dietary interventions.
Acknowledgements
We would like to thank all members of the Kriegel lab for fruitful discussions on this topic.
Financial support and sponsorship
Work discussed in this review was supported by grants from the National Institutes of Health (NIH) (K08AI095318, R01AI118855, T32AI07019, T32DK007017–39), the O’Brien Center at Yale (NIH P30DK079310), the Yale Rheumatic Diseases Research Core (NIH P30 AR053495), the Yale Liver Center (NIH P30 DK34989), the Women’s Health Research at Yale, the Arthritis National Research Foundation, the Arthritis Foundation, the Lupus Research Institute, and the Lupus Foundation of America.
Footnotes
Conflicts of interest
M.A.K. holds an international patent on the use of antibiotics and commensal vaccination to treat autoimmunity and receives salary support from Roche. The remaining authors have no conflict of interest.
References and recommended reading:
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Ruff WE, Kriegel MA. Autoimmune host-microbiota interactions at barrier sites and beyond. Trends Mol Med. 2015;21(4):233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson DA, Cardona RA. Specificity of the adaptive immune response to the gut microbiota. Adv Immunol. 2010;107:71–107. [DOI] [PubMed] [Google Scholar]
- 3.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330(6012):1768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Q, Elson CO. Adaptive immune education by gut microbiota antigens. Immunology. 2018;154(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeissig S, Blumberg RS. Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol. 2014;15(4):307–10. [DOI] [PubMed] [Google Scholar]
- 6.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagano Y, Itoh K, Honda K. The induction of Treg cells by gut-indigenous Clostridium. Curr Opin Immunol. 2012;24(4):392–7. [DOI] [PubMed] [Google Scholar]
- 8.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–6. [DOI] [PubMed] [Google Scholar]
- 9.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8(6):337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubinak JL, Petersen C, Stephens WZ, Soto R, Bake E, O’Connell RM, et al. MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe. 2015;17(2):153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013;501(7465):112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Chaudhary N, Yang N, Granato A, Turner JA, Howard SL, et al. Microbial symbionts regulate the primary Ig repertoire. J Exp Med. 2018;215(5):1397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunker JJ, Flynn TM, Koval JC, Shaw DG, Meisel M, McDonald BD, et al. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity. 2015;43(3):541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donaldson GP, Ladinsky MS, Yu KB, Sanders JG, Yoo BB, Chou WC, et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science. 2018;360(6390):795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158(5):1000–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadlallah J, El Kafsi H, Sterlin D, Juste C, Parizot C, Dorgham K, et al. Microbial ecology perturbation in human IgA deficiency. Sci Transl Med. 2018;10(439). [DOI] [PubMed] [Google Scholar]; ** This study demonstrates that IgA-deficient patients that are prone to autoimmunity carry a dysbiotic gut microbiome with aberrant IgM coating.
- 17.Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325(5940):617–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagan AJ, Pepper M, et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337(6101):1553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greiling TM, Dehner C, Chen X, Hughes K, Iniguez AJ, Boccitto M, et al. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci Transl Med. 2018;10(434). [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study defines human Ro60 ortholog-expressing commensal bacteria in SLE patients and demonstrates cross-reactivity between Ro60 orthologs and the human SLE autoantigen Ro60.
- 20.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38. [DOI] [PubMed] [Google Scholar]
- 21.McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389(10086):2328–37. [DOI] [PubMed] [Google Scholar]
- 22.Brusca SB, Abramson SB, Scher JU. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr Opin Rheumatol. 2014;26(1):101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holers VM, Demoruelle MK, Kuhn KA, Buckner JH, Robinson WH, Okamoto Y, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol. 2018;14(9):542–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith R The Surgical Relief of Intestinal Foci of Infection in Cases of Arthritis Deformans. Ann Surg. 1922;76(4):515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng F, Klinger CN, Felix KM, Bradley CP, Wu E, Tran NL, et al. Gut Microbiota Drive Autoimmune Arthritis by Promoting Differentiation and Migration of Peyer’s Patch T Follicular Helper Cells. Immunity. 2016;44(4):875–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block KE, Zheng Z, Dent AL, Kee BL, Huang H. Gut Microbiota Regulates K/BxN Autoimmune Arthritis through Follicular Helper T but Not Th17 Cells. J Immunol. 2016;196(4):1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim K, Bang SY, Lee HS, Bae SC. Update on the genetic architecture of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(1):13–24. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21(8):895–905. [DOI] [PubMed] [Google Scholar]
- 30.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda Y, Kurakawa T, Umemoto E, Motooka D, Ito Y, Gotoh K, et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016;68(11):2646–61. [DOI] [PubMed] [Google Scholar]; * This study is the first demonstration that P. copri, implicated in human RA pathogenesis, promotes arthritis and Th17 cells in a gnotobiotic RA model.
- 32.Pianta A, Arvikar S, Strle K, Drouin EE, Wang Q, Costello CE, et al. Evidence of the Immune Relevance of Prevotella copri, a Gut Microbe, in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2017;69(5):964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikuls TR, Thiele GM, Deane KD, Payne JB, O’Dell JR, Yu F, et al. Porphyromonas gingivalis and disease-related autoantibodies in individuals at increased risk of rheumatoid arthritis. Arthritis Rheum. 2012;64(11):3522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8(369):369ra176. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study identifies an oral pathobiont involved in periodontitis as a key driver of hypercitrullination of autoantigens in neutrophils.
- 35.Konig MF, Paracha AS, Moni M, Bingham CO 3rd, Andrade F. Defining the role of Porphyromonas gingivalis peptidylarginine deiminase (PPAD) in rheumatoid arthritis through the study of PPAD biology. Ann Rheum Dis. 2015;74(11):2054–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10(5):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajishengallis G Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Aquino SG, Abdollahi-Roodsaz S, Koenders MI, van de Loo FA, Pruijn GJ, Marijnissen RJ, et al. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J Immunol. 2014;192(9):4103–11. [DOI] [PubMed] [Google Scholar]
- 39.Konig MF, Giles JT, Teles RP, Moutsopoulos NM, Andrade F. Response to comment on “Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis”. Sci Transl Med. 2018;10(433). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkov M, Dekkers J, Loos BG, Bizzarro S, Huizinga TWJ, Praetorius HA, et al. Comment on “Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis”. Sci Transl Med. 2018;10(433). [DOI] [PubMed] [Google Scholar]
- 41.Rees F, Doherty M, Grainge MJ, Lanyon P, Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology (Oxford). 2017;56(11):1945–61. [DOI] [PubMed] [Google Scholar]
- 42.Lo MS, Tsokos GC. Recent developments in systemic lupus erythematosus pathogenesis and applications for therapy. Curr Opin Rheumatol. 2018;30(2):222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banchereau R, Hong S, Cantarel B, Baldwin N, Baisch J, Edens M, et al. Personalized Immunomonitoring Uncovers Molecular Networks that Stratify Lupus Patients. Cell. 2016;165(6):1548–50. [DOI] [PubMed] [Google Scholar]
- 44.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–33. [DOI] [PubMed] [Google Scholar]
- 45.Horai R, Zarate-Blades CR, Dillenburg-Pilla P, Chen J, Kielczewski JL, Silver PB, et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity. 2015;43(2):343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tai N, Peng J, Liu F, Gulden E, Hu Y, Zhang X, et al. Microbial antigen mimics activate diabetogenic CD8 T cells in NOD mice. J Exp Med. 2016;213(10):2129–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hebbandi Nanjundappa R, Ronchi F, Wang J, Clemente-Casares X, Yamanouchi J, Sokke Umeshappa C, et al. A Gut Microbial Mimic that Hijacks Diabetogenic Autoreactivity to Suppress Colitis. Cell. 2017;171(3):655–67 e17. [DOI] [PubMed] [Google Scholar]
- 48.Szymula A, Rosenthal J, Szczerba BM, Bagavant H, Fu SM, Deshmukh US. T cell epitope mimicry between Sjogren’s syndrome Antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin Immunol. 2014;152(1–2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Datta R, Dehner CA, Ruff WE, Greiling T, Kriegel MA. Gut commensal-dependent production of autoantibodies against the primordial lupus autoantigen Ro60 in the absence of mammalian Ro60. J Immunol 2018;Vol. 200(1):Supplement 162.11. [Google Scholar]
- 50.Schickel JN, Glauzy S, Ng YS, Chamberlain N, Massad C, Isnardi I, et al. Self-reactive VH4–34-expressing IgG B cells recognize commensal bacteria. J Exp Med. 2017;214(7):1991–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rollenske T, Szijarto V, Lukasiewicz J, Guachalla LM, Stojkovic K, Hartl K, et al. Cross-specificity of protective human antibodies against Klebsiella pneumoniae LPS O-antigen. Nat Immunol. 2018;19(6):617–24. [DOI] [PubMed] [Google Scholar]
- 52.Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359(6380):1156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study identifies E. gallinarum as a translocating pathobiont that induces pDCs, Th17, Tfh and autoantibodies in lupus-prone models and monocolonized mice. It further demonstrates E. gallinarum serum reactivity and DNA in human liver tissue of autoimmune patients.
- 53.Zegarra-Ruiz D, El Beidaq A, Iniguez A, Lubrano di Ricco M, Vieira S, Ruff W, et al. A diet-sensitive commensal Lactobacillus strain mediates TLR7-dependent systemic autoimmunity. Cell Host Microbe. 2018;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study demonstrates that L. reuteri exacerbates the pDC/type I IFN pathway in TLR7-mediated autoimmunity. It further links TLR7 to gut barrier integrity and shows that a starch diet prevents L. reuteri-related immunopathology via short-chain fatty acids.
- 54.Campisi L, Barbet G, Ding Y, Esplugues E, Flavell RA, Blander JM. Apoptosis in response to microbial infection induces autoreactive TH17 cells. Nat Immunol. 2016;17(9):1084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J, Wright K, Davis JM, Jeraldo P, Marietta EV, Murray J, et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016;8(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y, Chen B, Li S, Yang L, Zhu D, Wang Y, et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci Rep. 2018;8(1):14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciccia F, Guggino G, Rizzo A, Alessandro R, Luchetti MM, Milling S, et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. 2017;76(6):1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]