Capsule Summary:
Blood eosinophil progenitors (EoPs) correlate with tissue pathology during active eosinophilic esophagitis (EoE), providing additional evidence for blood EoP levels as a biomarker for disease activity and suggesting a role for EoPs in EoE pathogenesis.
Keywords: eosinophil, eosinophil progenitor, eosinophilic esophagitis histologic scoring system, eosinophilic esophagitis, biomarker
To the Editor:
Eosinophilic esophagitis (EoE) is a chronic inflammatory disease of the gastrointestinal tract characterized by esophageal eosinophilia. Current EoE management comprise serial, invasive endoscopies with esophageal biopsy to diagnose and subsequently monitor treatment efficacy. A less invasive, blood-based biomarker to monitor EoE disease activity would substantially advance EoE management; however, such a biomarker has yet to be identified.
Peak eosinophil count (PEC) ≥ 15 intraepithelial eosinophils within at least one high-power field (hpf) of esophageal biopsy remains the consensus threshold for histologically diagnosing active disease. However, pathologic features other than eosinophilic inflammation are associated with active EoE. Recently, Collins et al. described the EoE histologic scoring system (EoEHSS), which provides a standardized method to objectively assess the severity and extent of multiple pathologic features of EoE within esophageal biopsies.1 The EoEHSS composite score outperformed the PEC in differentiating patients with treated and untreated EoE.1 Additionally, various EoEHSS elements were associated with distinct disease endotypes.2 These data demonstrate the importance of pathologic features distinct from eosinophil infiltration in assessing EoE disease activity.
Eosinophil progenitor cells (EoPs) are eosinophil lineage–committed, CD34+ progenitor cells that primarily reside in the bone marrow. Growing evidence demonstrates that EoPs mobilize into the peripheral blood during allergic responses and accumulate within allergen-exposed tissues. The functional significance of mobilized EoPs remains unclear, but it has been postulated that these cells propagate local tissue eosinophilia through in situ proliferation and maturation into eosinophils and secretion of IL-5.3–5 Moreover, studies using isolated peripheral blood CD34+ hematopoietic stem/progenitor cells have demonstrated that these cells can be potent produces of T helper type 2 (Th2) cytokines, particularly IL-13.6 Consequently, EoPs may be participating in propagating Th2 inflammatory responses.
We previously demonstrated that EoP levels in the peripheral blood were significantly increased in patients with active EoE.7 Notably, a cutoff value of < 15.5 absolute EoPs per mL was associated with 100% sensitivity for differentiating EoE disease activity, suggesting that peripheral blood EoP levels may represent a novel disease-monitoring biomarker for pediatric patients with EoE.7 No clinical studies to date have evaluated the relationship between peripheral blood EoP levels and the pathologic changes assessed by the EoEHSS. We hypothesized that EoP levels would correlate with the histologic changes observed in the esophagus during active EoE and would therefore correlate with EoEHSS disease scores.
To test our hypothesis, biopsies from our previously published pediatric EoE cohort7 underwent blinded assessment by a pathologist using the EoEHSS, as previously described.1 The EoEHSS evaluates eight pathologic features commonly seen in EoE esophageal biopsies (Supplemental Table EI). The severity (grade) and extent (stage) of these pathologic changes within the esophageal biopsies were scored and ratios generated by dividing the score obtained by the maximum possible score. The grade and stage ratios were combined to generate a total EoEHSS composite ratio. The cohort included subjects 1 to 18 years of age with an EoE diagnosis and previous failure of proton pump inhibitor therapy to abate their esophageal eosinophilia.7 A total of 31 patients were divided into two groups based on their PEC: inactive EoE (PEC <15 eosinophils/hpf; n=14) and active EoE (PEC ≥15/hpf; n=17) (Supplemental Table EII). Patient age, sex, and atopic status were not significantly different between these groups. EoPs were identified within isolated peripheral blood mononuclear cells by flow cytometry as the live, CD45RA-, CD34High+, CD38+ and CD125+ (IL-5Rα) events. Notably, EoPs were significantly increased in the peripheral blood of patients with active EoE.7
We previously demonstrated that EoP levels significantly correlated with the maximum severity of esophageal eosinophilia (i.e., PEC), which remains the gold standard for assessing disease activity.7 Severity is a principal metric, but the extent of tissue involvement is also essential to accurately assess disease activity. Indeed, both grade (severity) and stage (extent) are measures routinely used to evaluate other inflammatory gastrointestinal diseases.8 There is a distinct advantage to using the EoEHSS, which evaluates grade and stage, compared to the PEC alone.1 To date, however, composite EoEHSS score thresholds have not been determined for active disease. Consequently, we determined whether EoP levels correlated with EoEHSS histologic metrics. The EoEHSS composite ratio (combined grade and stage scores) was significantly higher in active EoE than inactive EoE (P < 0.001; Mann-Whitney test, Fig. 1A). Next, we evaluated the relationship between EoP levels and the EoEHSS composite ratio. We observed a significant, positive correlation between the absolute blood EoP levels and the EoEHSS composite ratio (Spearman ρ = 0.53; P = 0.02; Fig.1B) and between EoP levels and both grade (Spearman ρ = 0.54; P = 0.002; Fig. 1C) and stage (Spearman ρ = 0.50; P = 0.005; Fig. 1D) ratio scores.
Figure 1.
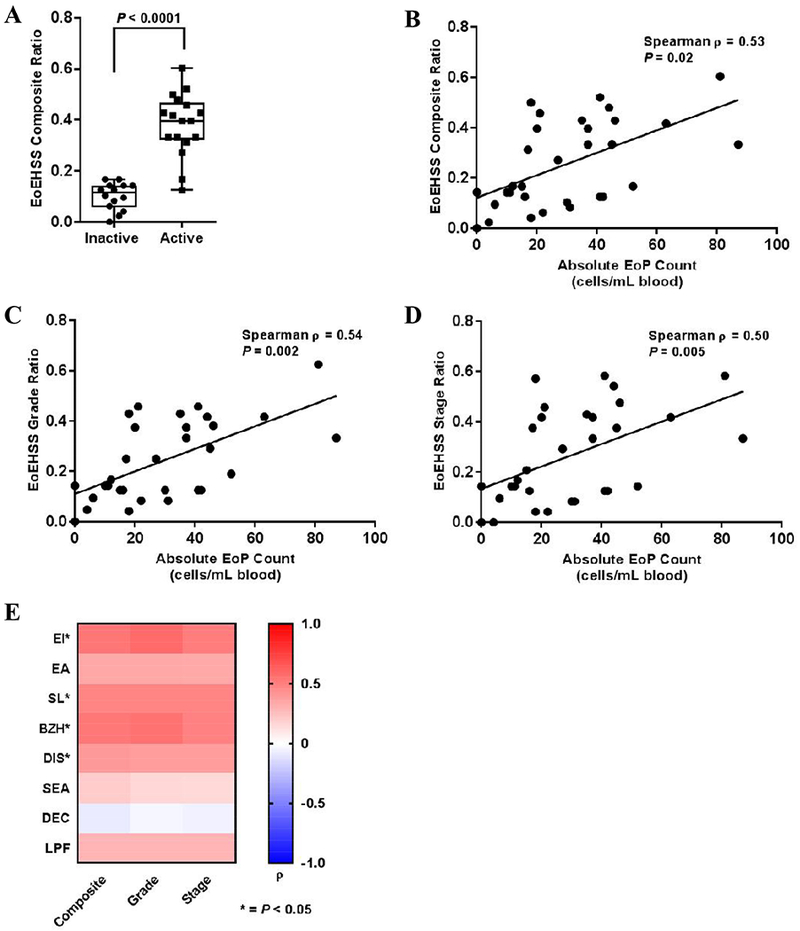
Cohort analysis of patients with inactive EoE (PEC < 15 eosinophils/hpf; n = 14) and active EoE (PEC < 15/hpf; n = 17). A. Median EoEHSS composite ratio scores in patients with inactive versus active EoE. Boxes indicate the 25–75th percentile, whiskers indicate the minimum to maximum values, and each point represents an individual patient. Correlation between the absolute EoP level in the peripheral blood and the EoEHSS composite ratio (B), grade score (C), and stage score (D). Significance was determined using nonparametric Spearman analysis, and all points are shown with each point representing an individual patient. E. Heat map demonstrating the Spearman correlation values for each pathologic feature between peripheral EoP levels and the EoEHSS composite, grade, and stage scores. Red indicates a higher degree of positive correlation (Spearman ρ closer to 1.0) and blue represents an inverse correlation (Spearman ρ closer to −1.0). Significance is demonstrated by an asterisk (*P < 0.05). EoE, eosinophilic esophagitis; PEC, peak eosinophil count; hpf, high-power field; EoEHSS, eosinophilic esophagitis histologic scoring system; EoP, eosinophil progenitor cell.
Given that the EoEHSS evaluates both eosinophil inflammation (i.e., EI, EA, SL, SEA) and pathologic features independent of tissue eosinophilia (i.e., BZH, DIS, DEC and LPF), we investigated the relationship between blood EoP levels and each pathologic feature assessed by the EoEHSS (Fig. 1E). EoP levels correlated most significantly with measures of eosinophil tissue infiltration (EI composite [Spearman ρ = 0.54, P = 0.002] and SL composite [Spearman ρ = 0.48, P = 0.007]), but were also significantly correlated with pathologic features separate from tissue eosinophils (BZH composite [Spearman ρ = 0.53, P = 0.002] and DIS composite [Spearman ρ = 0.40, P = 0.03]). Combined with the finding that the levels of esophageal eosinophilia correlate most strongly with BZH and EI2, these data suggest a potential role for EoPs in contributing to tissue pathologic changes and disease pathogenesis.
Peripheral blood absolute eosinophil counts (AEC) positively correlate with tissue eosinophilia in patients with EoE.9 Consequently, we compared blood EoP and AEC levels to EoEHSS scores in a subset of patients that had a documented AEC at the time of biopsy (n = 10; inactive = 4, active = 6; Fig. 2). The peripheral blood EoP levels were significantly increased in patients with active disease and correlated with the EoEHSS composite ratio (Fig.2A and 2B, respectively). In contrast, although there was a trend towards increased AECs in patients with active disease and increased EoEHSS ratios in patients with higher AECs, no statistical significance was observed (Fig.2C-D). These findings suggest that peripheral blood EoP levels may outperform the AEC in association with histologic changes observed in active EoE, although the small sample size makes definitive conclusions difficult.
Figure 2.
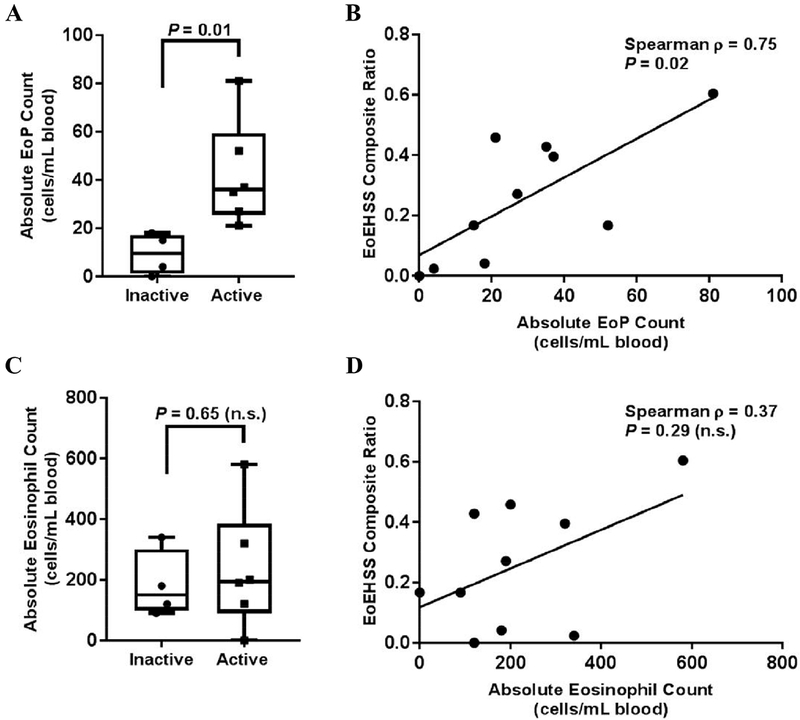
Subgroup analysis of patients with EoE and a documented AEC at time of biopsy (total n = 10; inactive = 4, active = 6). A. Median EoP levels in patients with inactive versus active EoE. Boxes indicate the 25–75th percentile, whiskers indicate the minimum to maximum values, and each point represents an individual patient. B. Correlation between the absolute EoP level in the peripheral blood and the EoEHSS composite ratio. Significance was determined using nonparametric Spearman analysis, and each point represents an individual patient. C. Median AEC in patients with inactive versus active EoE. D. Correlation between the AEC in the peripheral blood and the EoEHSS composite ratio. EoE, eosinophilic esophagitis; AEC, absolute eosinophil count; EoP, eosinophil progenitor cell; EoEHSS, eosinophilic esophagitis histologic scoring system.
Invasive endoscopy with esophageal biopsy remains the gold-standard for disease monitoring in EoE. The development of the EoEHSS has improved the ability of pathologists to evaluate disease activity in biopsies, but the development of less-invasive biomarkers remains a priority in EoE management. Our data suggest that peripheral blood EoP levels correlate sufficiently with the histologic changes observed in active EoE and provide additional evidence of their utility as a relevant biomarker for monitoring disease activity and suggest a potential role for EoPs in EoE pathogenesis. However, future studies with larger patient cohorts and longitudinal assessments of EoP levels within individuals are needed before this potential biomarker is ready for clinical application.
Supplementary Material
Funding Sources
This work was supported by the National Institute of Allergy and Infectious Diseases, Grant/Award Number: R01 AI130033, T32 AI60515 and CEGIR (U54 AI117804). CEGIR (U54 AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED CURED and EFC. This project was also supported in part by the NIH grant P30 DK078392 (Gene and Protein Expression and Flow Cytometry Cores) of the Digestive Disease Research Core Center in Cincinnati.
Abbreviations:
- EoP
eosinophil lineage–committed progenitor
- EoE
eosinophilic esophagitis
- EoEHSS
eosinophilic esophagitis histologic scoring system
- hpf
high-power microscopic field
- BZH
basal zone hyperplasia
- EI
eosinophil inflammation
- EA
eosinophil abscesses
- SL
eosinophil surface layering
- DIS
dilated intercellular spaces
- SEA
surface epithelial alteration
- DEC
dyskeratotic epithelial cells
- LPF
lamina propria fibrosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statements
P.C.F. has received grants from the National Institutes of Health, has served as a consultant for Genentech, Inc., and has received research funding from Knopp Biosciences, LLC. M.E.R. is a consultant for Pulm One, Spoon Guru, ClostraBio, Celgene, Shire, Astra Zeneca, GlaxoSmithKline, Allakos, Adare, Regeneron and Novartis and has an equity interest in the first four listed and Immune Pharmaceuticals, and royalties from reslizumab (Teva Pharmaceuticals) and UpToDate. M.E.R. is an inventor of patents, owned by Cincinnati Children’s. M.H.C. has received research funding from Biogen Idec., Meritage, Receptos, Regeneron, and Shire Pharmaceuticals; and is a consultant for Allakos, Receptos, Regeneron and Shire Pharmaceuticals. The remaining authors declare no relevant conflicts of interest.
References
- 1.Collins MH, Martin LJ, Alexander ES, Boyd JT, Sheridan R, He H, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus 2017; 30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoda T, Wen T, Aceves SS, Abonia JP, Atkins D, Bonis PA, et al. Eosinophilic oesophagitis endotype classification by molecular, clinical, and histopathological analyses: a cross-sectional study. Lancet Gastroenterol Hepatol 2018; 3:477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron L, Christodoulopoulos P, Lavigne F, Nakamura Y, Eidelman D, McEuen A, et al. Evidence for local eosinophil differentiation within allergic nasal mucosa: inhibition with soluble IL-5 receptor. J Immunol 2000; 164:1538–45. [DOI] [PubMed] [Google Scholar]
- 4.Kim YK, Uno M, Hamilos DL, Beck L, Bochner B, Schleimer R, et al. Immunolocalization of CD34 in nasal polyposis. Effect of topical corticosteroids. Am J Respir Cell Mol Biol 1999; 20:388–97. [DOI] [PubMed] [Google Scholar]
- 5.Sehmi R, Smith SG, Kjarsgaard M, Radford K, Boulet LP, Lemiere C, et al. Role of local eosinophilopoietic processes in the development of airway eosinophilia in prednisone-dependent severe asthma. Clin Exp Allergy 2016; 46:793–802. [DOI] [PubMed] [Google Scholar]
- 6.Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol 2009; 123:472–8. [DOI] [PubMed] [Google Scholar]
- 7.Morris DW, Stucke EM, Martin LJ, Abonia JP, Mukkada VA, Putnam PE, et al. Eosinophil progenitor levels are increased in patients with active pediatric eosinophilic esophagitis. J Allergy Clin Immunol 2016; 138:915–8 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine A Pediatric inflammatory bowel disease: is it different? Dig Dis 2009; 27:212–4. [DOI] [PubMed] [Google Scholar]
- 9.Min SB, Nylund CM, Baker TP, Ally M, Reinhardt B, Chen YJ, et al. Longitudinal Evaluation of Noninvasive Biomarkers for Eosinophilic Esophagitis. J Clin Gastroenterol 2017; 51:127–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


