Abstract
Human organoids provide constructive in vitro models of human development and disease, as these recapitulate important morphogenetic and functional features of the tissue and species of origin. However, organoid culture technologies often involve the use of biologically-derived materials (e.g. Matrigel™) that do not allow dissection of the independent contributions of the biochemical and biophysical matrix properties to organoid development. Additionally, their inherent lot-to-lot variability and, in the case of Matrigel™, tumor-derived nature limits their applicability as platforms for drug and tissue transplantation therapies. Here, we highlight recent studies that overcome these limitations through engineering of novel biomaterial platforms that (1) allow to study the independent contributions of physicochemical matrix properties to organoid development and their potential for translational therapies, and (2) better recreate the tumor microenvironment for high-throughput, pre-clinical drug development. These studies illustrate how innovative biomaterial constructs can contribute to the modeling of human development and disease using organoids, and as platforms for development of organoid-based therapies. Finally, we discuss the current limitations of the organoid field and how they can potentially be addressed using engineered biomaterials.
Introduction
The establishment of the first permanent cell line in culture in 1943 revolutionized science through 2D in vitro cell culture techniques1. Controlled and systematic expansion of cells on planar surfaces has allowed diverse scientific advances ranging from vaccine development (e.g. rabies or measles vaccine) to large-scale protein production (e.g. monoclonal antibodies, plasminogen activator (tPA) for thrombolytic therapy). Subsequently, to better recreate the native environment of tissues, 3D organotypic in vitro cultures were developed to study morphogenetic developmental processes and disease progression2,3. These in vitro 3D culture techniques involve the encapsulation of cells using biologically-derived materials (e.g. collagen or Matrigel™) to better promote morphogenetic processes specific to their tissues of origin and that can only be recapitulated in a 3D environment2–4. For instance, encapsulation of human pancreatic epithelial cells in Matrigel™ results in 3D morphogenetic organization into cysts with apicobasal polarity, as opposed to disorganized colonies formed by pancreatic cancer cell lines5. However, these models typically involve one or two cell types which constrains the histological complexity and morphogenetic dynamics of the tissues6. Consequently, in order to overcome these limitations, recent novel in vitro culture techniques that sustain the stem cell-driven formation and organization of self-renewing multicellular structures, termed organoids, have emerged as the state of the art in vitro models of human development and disease7,8.
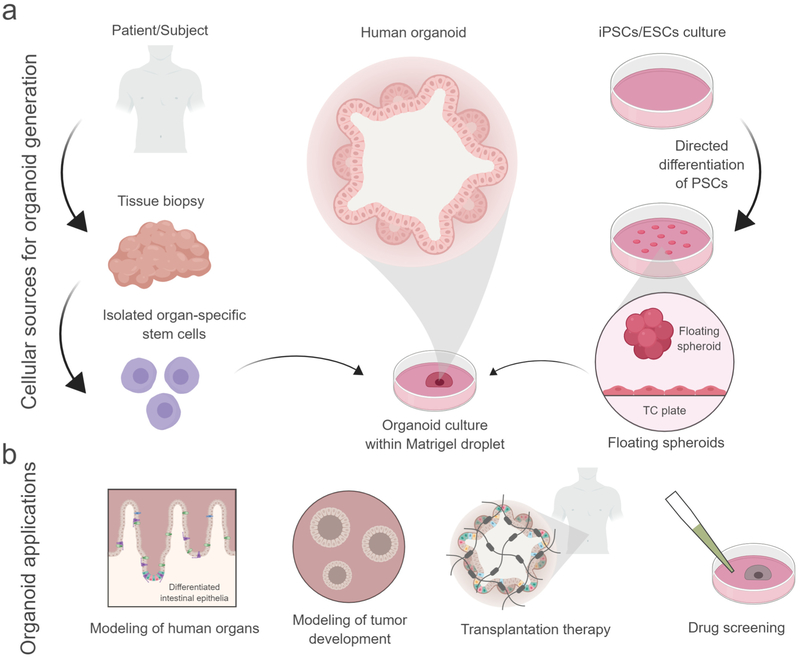
To generate organoids in vitro, human pluripotent stem cell (hPSC)-derived tissues or primary adult stem cells are typically embedded within collagen gels or Matrigel™ (Fig. 1a)7,8. Upon culture in specific growth factor cocktails, these materials support formations of multicellular structures that contain multiple cell types and exhibit functions that recapitulate many aspects of the tissue of origin. Protocols have been developed to generate different organoids (e.g., intestine, lung, brain, retina, and kidney) from these cellular sources that recapitulate important morphogenetic and functional features of the tissue and species of origin7,9. For instance, McCracken et al.10 developed an efficient method to generate pluripotent stem cell (PSC)-derived human intestinal organoids (HIOs), which closely mimic embryonic intestinal development by giving rise to major intestinal epithelium lineages and a functional dipeptide transport system. In parallel, Boj et al. established mouse and human organoids derived from primary pancreatic cancer cells that recapitulate tumor development from early-stage neoplasms to invasive ductal adenocarcinoma, and allow characterization of the genetic and proteomic changes that occur during cancer progression7,11. Without a doubt, organoids have the potential to serve as powerful tools for modeling of organ development and disease (Fig. 1b).
Fig 1: Cellular sources for organoid generation and their current and potential applications.
(a) For in vitro generation of organoids, primary tissue containing organ-specific stem cells, ESCs-derived or iPSCs-derived spheroids are embedded in naturally-derived materials. Upon culture in specific growth factor cocktails, these materials promote formation of multicellular structures that contain multiple cell types and exhibit functions that recapitulate many aspects of the tissue of origin. (b) In vitro organoids are commonly used for modeling of organ development and disease (e.g. cancer). Potential future applications for organoids using biomaterials are organoid-based transplantation therapies and high-throughput drug screenings. Created with BioRender.
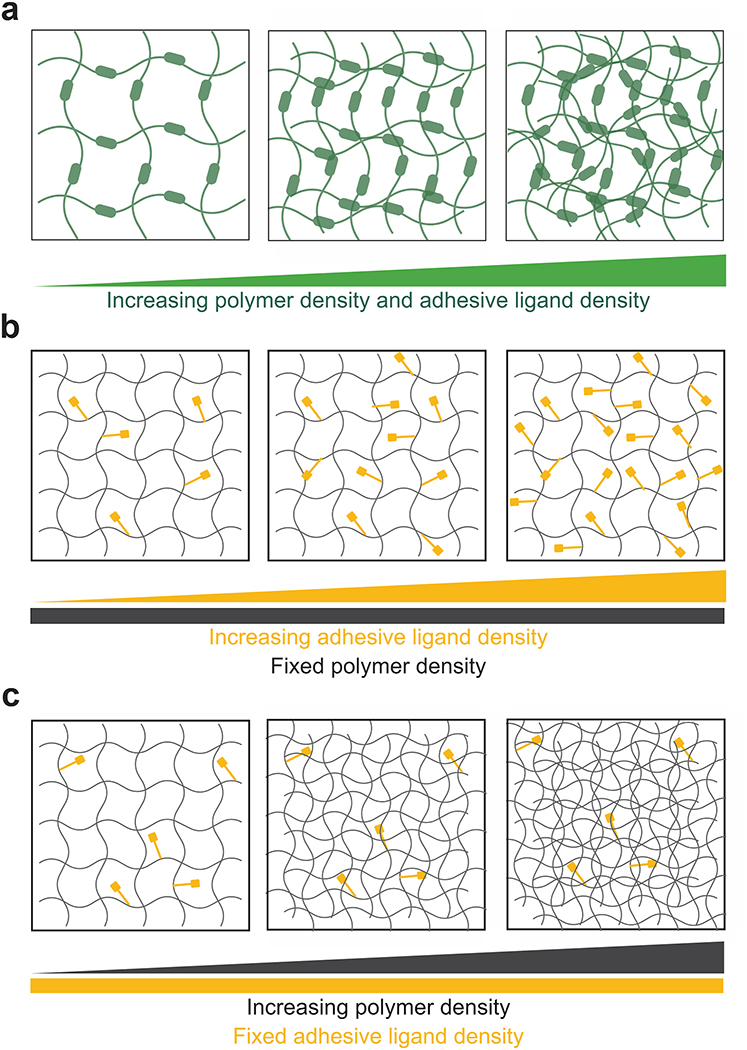
However, differences between organoids and the native organ still exist suggesting that biologically-derived culture materials do not yet offer the complete native environmental signals that promote organ development and maturation6. Achieving organoid maturation from fetal to adult stage in vitro remains a challenge for many hPSC-derived organoid models, which could be attributed to the lack of supporting physicochemical matrix properties from culture materials, the absence of paracrine signals12 and/or other cellular systems (e.g. immune cells, functional vasculature)13. Although these 3D culture materials have revealed important signals that regulate morphogenesis and tumorigenesis, the inability to uncouple biophysical and biochemical matrix properties does not allow to dissect their contributions to morphogenesis14 and to optimize matrix properties to promote enhanced organoid development and maturation. For instance, changes to the bulk density of collagen gels is a common approach to vary its mechanical properties (Fig. 2a). However, these changes in collagen concentration unavoidably alter other matrix properties, such as density of integrin-binding sites and fiber density/structure14. Moreover, the lot-to-lot structural and compositional variability of these biologically-derived materials reduces their reliability and, in the case of Matrigel™, its tumor-derived nature limits organoid potential as tissue sources for regenerative therapies14–16.
Fig 2: Engineered materials with tunable biophysical and biochemical matrix properties.
(a). Illustration of biological matrices such as collagen gels and Matrigel™ where changes in polymer density to vary mechanical properties invariably alter adhesive site density and organization through changes to fiber structure and density. (b,c) Illustration of independent control of polymer density and adhesive ligand density in multi-armed PEG-based hydrogels. (b) Changes in adhesive ligand density is independent of polymer density; conversely, (c) changes in polymer density to vary mechanical properties does not alter adhesive ligand density and organization. Created with BioRender.
In this Review, we highlight recent biomaterial advances addressing current limitations in organoid culture technologies. First, we discuss the development of synthetic materials for organoid culture as a new avenue to understand the independent contributions of extracellular matrix (ECM) properties to organoid development, and as a vehicle for organoid transplantation (Fig. 1b). We then cover the use of engineered biomaterials to better model the microenvironmental signals that drive cancer progression as potential platforms for screening of drug therapies (Fig. 1b). Finally, we provide an outlook on organoid research and how biomaterial approaches might contribute new insights to that field.
Recent advances in biomaterials for organoid generation, maturation and transplantation
The presence of parental stem cells and the self-renewing capacity of organoids underscore their potential as tissue sources for regenerative therapies8. In their native environment, stem cells receive specific biochemical and biophysical signals from the microenvironmental niche to replicate, differentiate and assemble into tissues and organs. Researchers have recreated some of these signals in vitro through tissue embedding in biologically-derived materials and culture in growth-factor cocktails that mimic cell niches. When stem cells are cultured in these conditions, they differentiate into different organ-specific cell types and self-assemble into structures (organoids) that bear a morphological resemblance to the organ to which they are predetermined.
Matrigel™ is the commonly used material for organoid generation. Nevertheless, the inability to uncouple its physicochemical matrix properties, its lot-to-lot variability and tumor-derived nature limit its use to study organoid development and the potential use of organoids as tissue sources for regenerative therapies. In this context, researchers have addressed the limitations of Matrigel™ by engineering synthetic materials that allow independent control over its physicochemical properties to understand the independent contributions of matrix properties to organoid development (Fig. 2b,c). Gjorevski et al.17 characterized the morphogenetic program of Lgr5+ intestinal stem cells (ISCs)-derived mouse organoids as a two-stage process using a set of defined poly(ethylene glycol) (PEG)-based hydrogels with independently tunable physicochemical properties. A particular range of matrix stiffness (1.3 kPa) and fibronectin-based adhesion was optimal for ISC expansion and proliferation, whereas a softer matrix (190 Pa) and laminin-based adhesion was required for subsequent ISC differentiation and organoid formation. These observations demonstrate that, within a minimal 3D environment, separate stages of the organoid morphogenesis program require different microenvironments. Also, this study establishes the potential of synthetic materials to mimic the dynamic character of native microenvironments, which may help to enhance the similarity of organoids to native organs in a way that may be unachievable using biologically-derived materials.
Cruz-Acuña & Quirós et al.9,18 developed a fully defined, synthetic hydrogel that supports the in vitro growth and expansion of hPSC-derived intestinal spheroids into human intestinal organoids (HIOs). The tunable nature of this material allowed to identify the independent contributions of the biophysical and biochemical matrix properties to organoid survival, and to establish a soft (~100 Pa), protease-degradable hydrogel presenting RGD adhesive peptide as an optimal formulation for human organoid generation and culture of both hPSC-derived intestinal and lung organoids. Furthermore, hydrogel-generated HIOs differentiated into mature intestinal tissue when transplanted into an in vivo environment, presenting a crypt-like architecture and differentiated intestinal epithelial cells. Finally, injection of the hydrogel precursor solutions and HIOs to mucosal colonic wounds resulted in localized organoid engraftment and enhanced wound repair. This study establishes a well-defined synthetic material that not only can generate HIOs to similar levels as Matrigel™ and support other types of human organoids, but more importantly, has the potential to be used for HIO-based therapies to treat gastrointestinal diseases (e.g. inflammatory bowel disease), as opposed to Matrigel™ which is limited by lot-to-lot variabilities and tumor-derived nature (Fig 1b).
Regardless of the material used (biological or synthetic) to generate hPSC-derived intestinal organoids, the maturation status of HIOs only closely mimics embryonic intestinal tissue. Because HIOs are a powerful tool for the study of physiological interactions and have the potential to be used for patient-specific tissue transplantation therapy, methods are needed to generate mature HIOs with more similarities to adult human intestine. In this context, Poling et al.19 implemented a biomaterials approach to study how mechanical cues induce maturation and morphological complexity of fetal-state HIOs in an in vivo environment. In this study, a nitinol spring was used as an endoluminal lengthening device that exerted uniaxial strain inside HIOs transplanted into the mesentery of mice. Exposing HIOs to strain enhanced maturation by presenting increased morphological, transcriptional and functional characteristics that are more similar to postnatal human intestine, as compared to HIOs that did not experience applied force.
Taken together, these studies have established the importance of characterizing and integrating biomechanical and biochemical signals that collectively contribute to organoid generation, maturation and transplantation. The tunable nature of these biomaterials allows to evaluate organoid responses to the independent effects of the physicochemical properties of the matrix with potential to be adapted for the study of other types of organoids (e.g. lung organoids9,18). The use of novel biomaterials for organoid generation has led us one step closer in developing HIO-based therapies to treat gastrointestinal diseases in humans (Fig. 1b).
Recent advances in biomaterials for cancer organoid modeling and drug testing
Monolayer cultures of adherent cancer cells have been traditionally used for studying the physiology of cancer, the signals that drive its progression, its metastatic characteristics and for drug screening20. For instance, cancer cell lines are used as a model to identify thousands of mutations that correspond to the tumor they are derived from21. Nevertheless, as a consequence of the passaging conditions, cancer cell lines acquire culture-induced mutations and genetic homogeneity that reduces their reliability to recapitulate the genomic aberrations and heterogeneity observed in tumors8. Additionally, despite the practicality of cancer cell line models, 2D culture systems fail to recapitulate important features of the complex, heterogeneous in vivo microenvironment of cancer. A non-physiologically stiff substrate, the lack of morphologically-relevant 3D cellular interactions, and absence of matrix proteins and other cell types are some of the discrepancies of 2D culture systems to in vivo tumor environment20. Therefore, patient-derived tumor organoids developed in vitro have become an attractive pre-clinical model to study cancer biology and response to anticancer drugs, as these have demonstrated to retain key biological characteristics of the primary tumor and to recapitulate patient’s drug responses in the clinic (Fig. 1b)8,22.
Historically, biologically-derived matrices have been extensively used to study tumor progression23,24 and for the generation of tumor organoids25,26. For instance, previous work using collagen I has revealed that the density of the stromal collagen I changes during breast cancer progression27,28, and that a higher density of collagen I at the tumor-stromal interface correlates with invasion29,30. However, changes in collagen density unavoidably alter other matrix properties (e.g. density of integrin-binding ECM components, fiber density/structure; Fig. 2a), preventing the examination of the role of individual matrix properties to tumor progression. Therefore, several studies have focused on the use of biomaterials as a physiologically-relevant 3D tumor microenvironment model that allows to understand the independent contributions of the physicochemical matrix properties to tumor progression and metastasis31,32. Motivated by their observations that mammary tumors preferentially disseminate into collagen I and not into Matrigel™, Beck et al.31 developed a composite hydrogel to characterize the material properties that induce mammary tumor cell dissemination from cancer organoids into Matrigel™. The hydrogel was composed of PEG networks with tunable mechanical properties, and adhesive peptide (RGD) density, in combination with Matrigel™ scaffolds that model the laminin-rich basement membrane. PEG-Matrigel™ composite hydrogel with a narrow range of matrix elasticity and adhesive peptide density induced tumor organoids to support dissemination of malignant mammary epithelial cells, revealing that a specific combination of biophysical and biochemical signals of PEG-Matrigel™ composite hydrogel can induce dissemination without requiring the fibrillar structure or specific adhesion sequences of type I collagen. These observations challenge our current knowledge regarding the role of collagen I in tumor progression and further suggest that breast cancer dissemination could be driven by molecular routes that do not depend on collagen I and can occur in collagen I-deficient tissues with the characteristic physicochemical matrix properties.
High-throughput sequencing techniques have been used to generate large-scale genomics data to predict cancer behavior and response to therapeutics20. Nevertheless, this data is conventionally generated using cancer cell lines which are not an adequate model to predict response to therapeutics as they cannot replicate the intra-tumoral heterogeneity in patient tumors22,33. Therefore, patient-derived xenograft (PDX) models have emerged as robust pre-clinical models to predict therapeutic responses, as PDXs preserve tumor heterogeneity, histopathological characteristics and drug response to patient tumors34,35. However, the labor-intensiveness and high cost of PDX animal models have fueled the in vitro generation of PDX-derived human tumor organoids using novel hydrogels as platforms for drug screening34. For instance, Fong et al.33 generated an in vitro system of human tumor organoids using cells derived from 14 different PDX lines from hepatocellular carcinoma (HCC) patients to study its potential as a model for drug testing. For the generation of tumor organoids, they used a hydroxypropyl cellulose hydrogel conjugated with galactose ligands that supported the viability, proliferation and intra-tumoral heterogeneity of the HCC-PDX organoids. This group had previously shown that this hydrogel is superior to other matrices (e.g. collagen I), as its macropores enable spheroid size control that prevents cell death of inner core due to diffusion limitations, presents in vivo-like mechanical stiffness, has minimal drug absorption and is readily scalable, establishing this hydrogel platform for high-throughput, large-scale drug safety testing applications33,36. Moreover, correlative studies demonstrated that the hydrogel-generated organoids had a strong genomic and transcriptomic resemblance to their in vivo counterparts and retained intra-tumoral heterogeneity. Finally, HCC-PDX organoids were sensitive to treatment of drugs used for HCC patients, suggesting that organoids generated in the biomaterial system can be used for pre-clinical drug development. However, the physical constraint exerted by the non-degradable hydrogel crosslinks may have limited the proliferation of the organoids, demonstrating the importance of designing materials that allow cell-directed matrix remodeling by incorporation of matrix metalloproteinase-sensitive moieties. Yet, this study demonstrates the potential of well-defined engineered biomaterials to overcome the limitations of biologically-derived materials by presenting in vivo-like properties and scalable features with potential to serve as pre-clinical models for high-throughput screening of anticancer drugs using organoids.
Since conventional methods to understand cancer biology and for drug testing continue to rely on 2D culture of cancer cell lines and biologically-derived matrices, these studies underscore their limitations and explore avenues to create new in vitro models that better represent the heterogeneity of tumors and their microenvironment with the use of engineered biomaterials. The well-defined, tunable design of these biomaterials allows to characterize matrix properties that more closely resemble the native tumor microenvironment and facilitate the formation of organoids that closely recapitulate human tumor biology and can be used for high-throughput, large-scale pre-clinical drug screenings (Fig. 1b).
Outlook for biomaterial approaches for organoid research
These recent studies present novel biomaterial approaches to overcome some of the current limitations of the conventional culture matrices of organoids for the modeling of human development and disease. Conventional 2D culture systems and biologically-derived materials do not fully recapitulate native cellular developmental processes, have restricted control over microenvironmental cues in development and disease, and their inherent variability limits their reliability as platforms for drug screenings and tissue transplantation therapy. Whereas, well-defined, engineered materials highlighted in this review allow to understand the independent contributions of physicochemical matrix properties to organoid development, and can better characterize the heterogeneous tumor microenvironment and serve as scalable platforms to predict cancer patient drug response. However, although engineered systems provide superior tunability of matrix properties over biologically-derived matrices, there are still several challenges that need to be addressed in order to recapitulate the structural properties of the native tissue of interest. For instance, crosslinking of multi-armed PEG macromers yields an amorphous structure with a nanoscale mesh size, as opposed to the fibrillar architecture with micrometer-sized pores of stromal collagen. Additionally, in many synthetic systems, it is not possible to uncouple mechanical properties from diffusional properties, such that varying polymer density also alters the hydrogel mesh size, which can impact diffusional properties of these synthetic networks. Although researchers have demonstrated that several morphogenetic programs recapitulated in vitro are insensitive to differences in matrix architecture and diffusion properties18,37, it is important to acknowledge and address these implications when trying to develop a synthetic matrix that resembles the characteristics of the native tissue. In that context, others have addressed these implications with other hydrogel systems (e.g. hyaluronic acid-based, poly(lactic-co-glycolic acid)-based hydrogels) by applying electrospinning to the synthesis process, fabricating a porous, fibrillar hydrogel for tissue engineering applications and 3D culture techniques38,39.
Biomaterial approaches still need to address several important implications towards their capacity to model human development and disease using organoids. For instance, current in vitro protocols to generate hPSC-derived organoids fail to recapitulate the morphogenetic maturity and differentiation of adult human tissue without subsequent implantation into animals. Therefore, to further explore the potential use of human organoids for patient-specific transplantation therapy, synthetic biomaterial designs need to be further engineered to generate tissues with increased size, maturity and function. Perhaps materials that present multiple adhesive ligand types at specific densities and mimic the structural properties of ECM proteins (e.g. fibrillar structure) can orchestrate such complex organoid morphogenetic programs. In addition, co-culture of organoids with supporting cellular systems (e.g. immune, mesenchymal cells) and/or exposure to paracrine signals (e.g. STAT3 signaling) could help induce organoid maturation, circumventing the need for in vivo implantation13. Finally, considering that each organ requires different physicochemical signals during development, researchers should take advantage of the user-defined tunability of engineered biomaterials to demonstrate their adaptability to model other complex developmental processes (e.g. tubular morphogenesis of kidney and blood vessels, folding process of mucosa) and pathologies (e.g. polycystic kidney disease) using organoids40–42.
Although novel biomaterials can better recreate the tumor microenvironment as compared to traditional culture techniques, these do not fully recapitulate its heterogeneity and its variations during the different stages of cancer. Biomaterial-based in vitro models that recreate the complexity and changes in physicochemical signals over time can serve as platforms to model the different stages of a specific type of cancer and for screening of drug therapies. In this context, perhaps exploring new biomaterial designs that allow controlled changes to the physicochemical matrix properties via external stimuli (e.g. by incorporation of light-sensitive moieties) during organoid culture may better model changes in the native tumor microenvironment43,44. For instance, materials that allow temporal control over its biophysical properties via external stimuli have demonstrated to be advantageous to study the progression of matrix-stiffness dependent pathological states, such as tissue fibrosis and cardiac remodeling after myocardial infarction45–49, demonstrating the potential of engineered biomaterials to study different pathological states.
Whereas innovative ways of integrating and regulating bioactivity into biomaterials are developed, engineered materials will continue to offer new developments in translational medicine and tumor models using organoids, and promise innovative therapeutic options that biological matrices cannot provide.
Acknowledgments
This research was supported by the NIH (R01 AR062368, R01 AR062920 to A.J.G), the National Science Foundation Graduate Research Fellowship (DGE-1650044 to R.C.A.) and the Alfred P. Sloan Foundation’s Minority Ph.D. (MPHD) Program (G-2016–20166039 to R.C.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Doelle HW, Rokem JS & Berovic M BIOTECHNOLOGY - Volume I: Fundamentals in Biotechnology (Eolss Publishers, 2009). [Google Scholar]
- 2.Mroue R & Bissell MJ Three-Dimensional Cultures of Mouse Mammary Epithelial Cells. Methods in molecular biology (Clifton, N.J.) 945, 221–250, doi: 10.1007/978-1-62703-125-7_14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien LE, Zegers MMP & Mostov KE Building epithelial architecture: insights from three-dimensional culture models. Nature Reviews Molecular Cell Biology 3, 531, doi:10.1038/nrm859 10.1038/nrm859https://www.nature.com/articles/nrm859#supplementary-informationhttps://www.nature.com/articles/nrm859#supplementary-information (2002). [DOI] [PubMed] [Google Scholar]
- 4.Bryant DM & Mostov KE From cells to organs: building polarized tissue. Nature Reviews Molecular Cell Biology 9, 887, doi: 10.1038/nrm2523 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutierrez-Barrera AM, Menter DG, Abbruzzese JL & Reddy SAG Establishment of three-dimensional cultures of human pancreatic duct epithelial cells. Biochemical and biophysical research communications 358, 698–703, doi: 10.1016/j.bbrc.2007.04.166 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gjorevski N, Ranga A & Lutolf MP Bioengineering approaches to guide stem cell-based organogenesis. Development 141, 1794–1804, doi: 10.1242/dev.101048 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Clevers H Modeling Development and Disease with Organoids. Cell 165, 1586–1597, doi: 10.1016/j.cell.2016.05.082 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Fatehullah A, Tan SH & Barker N Organoids as an in vitro model of human development and disease. Nature cell biology 18, 246–254, doi: 10.1038/ncb3312 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Acuña R et al. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nature Protocols, doi: 10.1038/s41596-018-0036-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCracken KW, Howell JC, Wells JM & Spence JR Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc 6, 1920–1928, doi: 10.1038/nprot.2011.410 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boj Sylvia F. et al. Organoid Models of Human and Mouse Ductal Pancreatic Cancer. Cell 160, 324–338, doi: 10.1016/j.cell.2014.12.021 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asai A et al. Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development 144, 1056, doi: 10.1242/dev.142794 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung KB et al. Interleukin-2 induces the in vitro maturation of human pluripotent stem cell-derived intestinal organoids. Nature Communications 9, 3039, doi: 10.1038/s41467-018-05450-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz-Acuña R & García AJ Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. Matrix Biology, doi: 10.1016/j.matbio.2016.06.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes CS, Postovit LM & Lajoie GA Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886–1890, doi: 10.1002/pmic.200900758 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Madl CM, Heilshorn SC & Blau HM Bioengineering strategies to accelerate stem cell therapeutics. Nature 557, 335–342, doi: 10.1038/s41586-018-0089-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gjorevski N et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564, doi: 10.1038/nature20168 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Cruz-Acuña R et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nature cell biology, doi: 10.1038/ncb3632 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poling HM et al. Mechanically induced development and maturation of human intestinal organoids in vivo. Nature Biomedical Engineering 2, 429–442, doi: 10.1038/s41551-018-0243-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakuri PS, Liu C, Luker GD & Tavana H Biomaterials-Based Approaches to Tumor Spheroid and Organoid Modeling. Advanced Healthcare Materials 7, 1700980, doi: 10.1002/adhm.201700980 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodspeed A, Heiser LM, Gray JW & Costello JC Tumor-Derived Cell Lines as Molecular Models of Cancer Pharmacogenomics. Molecular cancer research : MCR 14, 3–13, doi: 10.1158/1541-7786.MCR-15-0189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlachogiannis G et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickup MW, Mouw JK & Weaver VM The extracellular matrix modulates the hallmarks of cancer. EMBO Reports 15, 1243–1253, doi: 10.15252/embr.201439246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark AG & Vignjevic DM Modes of cancer cell invasion and the role of the microenvironment. Current Opinion in Cell Biology 36, 13–22, doi: 10.1016/j.ceb.2015.06.004 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Praharaj PP, Bhutia SK, Nagrath S, Bitting RL & Deep G Circulating tumor cell-derived organoids: Current challenges and promises in medical research and precision medicine. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1869, 117–127, doi: 10.1016/j.bbcan.2017.12.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey SP, Martin KE & Reinhart-King CA Three-dimensional collagen matrix induces a mechanosensitive invasive epithelial phenotype. Scientific Reports 7, 42088, doi: 10.1038/srep42088 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egeblad M, Rasch MG & Weaver VM Dynamic interplay between the collagen scaffold and tumor evolution. Current Opinion in Cell Biology 22, 697–706, doi: 10.1016/j.ceb.2010.08.015 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levental KR et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 139, 891–906, doi: 10.1016/j.cell.2009.10.027 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Provenzano PP et al. Collagen density promotes mammary tumor initiation and progression. BMC Medicine 6, 11, doi: 10.1186/1741-7015-6-11 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Provenzano PP et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Medicine 4, 38, doi: 10.1186/1741-7015-4-38 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck JN, Singh A, Rothenberg AR, Elisseeff JH & Ewald AJ The independent roles of mechanical, structural and adhesion characteristics of 3D hydrogels on the regulation of cancer invasion and dissemination. Biomaterials 34, 9486–9495, doi: 10.1016/j.biomaterials.2013.08.077 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.John C et al. 3D hydrogel-based microwell arrays as a tumor microenvironment model to study breast cancer growth. Biomedical Materials 12, 025009 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Fong ELS et al. Generation of matched patient-derived xenograft in vitro-in vivo models using 3D macroporous hydrogels for the study of liver cancer. Biomaterials 159, 229–240, doi: 10.1016/j.biomaterials.2017.12.026 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Hidalgo M et al. Patient-Derived Xenograft Models: An Emerging Platform for Translational Cancer Research. Cancer Discovery 4, 998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao H et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nature medicine 21, 1318, doi:10.1038/nm.3954 10.1038/nm.3954https://www.nature.com/articles/nm.3954#supplementary-informationhttps://www.nature.com/articles/nm.3954#supplementary-information (2015). [DOI] [PubMed] [Google Scholar]
- 36.Nugraha B et al. Galactosylated cellulosic sponge for multi-well drug safety testing. Biomaterials 32, 6982–6994, doi: 10.1016/j.biomaterials.2011.05.087 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Enemchukwu NO et al. Synthetic matrices reveal contributions of ECM biophysical and biochemical properties to epithelial morphogenesis. The Journal of cell biology 212, 113–124, doi: 10.1083/jcb.201506055 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burdick JA & Prestwich GD Hyaluronic acid hydrogels for biomedical applications. Advanced materials (Deerfield Beach, Fla.) 23, H41–H56, doi: 10.1002/adma.201003963 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham QP, Sharma U & Mikos AG Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Engineering 12, 1197–1211, doi: 10.1089/ten.2006.12.1197 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Cruz NM et al. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nature materials 16, 1112, doi:10.1038/nmat4994 10.1038/nmat4994https://www.nature.com/articles/nmat4994#supplementary-informationhttps://www.nature.com/articles/nmat4994#supplementary-information (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wimmer RA et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510, doi: 10.1038/s41586-018-0858-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan HF et al. Folding artificial mucosa with cell-laden hydrogels guided by mechanics models. Journal Name: Proceedings of the National Academy of Sciences; Journal Volume: 115; Journal Issue: 29; Conference: null; Patent File Date: null; Patent Priority Date: null; Other Information: null; Related Information: null, Medium: X; Size: 7503 to 7508; Quantity: null; OS: null; Compatibility: null; Other: null (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee TT et al. Light-triggered in vivo Activation of Adhesive Peptides Regulates Cell Adhesion, Inflammation and Vascularization of Biomaterials. Nature materials 14, 352–360, doi: 10.1038/nmat4157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kloxin AM, Kasko AM, Salinas CN & Anseth KS Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science (New York, N.Y.) 324, 59–63, doi: 10.1126/science.1169494 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caliari SR et al. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Scientific Reports 6, 21387–21387, doi: 10.1038/srep21387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu F et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. The Journal of cell biology 190, 693–706, doi: 10.1083/jcb.201004082 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lampi MC & Reinhart-King CA Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Science Translational Medicine 10, eaao0475, doi: 10.1126/scitranslmed.aao0475 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Rodell Christopher B et al. Injectable Shear-Thinning Hydrogels for Minimally Invasive Delivery to Infarcted Myocardium to Limit Left Ventricular Remodeling. Circulation: Cardiovascular Interventions 9, e004058, doi: 10.1161/CIRCINTERVENTIONS.116.004058 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ifkovits JL et al. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proceedings of the National Academy of Sciences of the United States of America 107, 11507–11512, doi: 10.1073/pnas.1004097107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]