Abstract
BACKGROUND:
This study tested if central obesity, hypertension, or depressive symptoms moderated the relationship between beta-amyloid (Aβ) and longitudinal cognitive performance in late middle-aged adults enriched for Alzheimer’s disease (AD) risk.
METHODS:
Participants (n=207; ages=40–70; 73% parental AD) in the Wisconsin Registry for Alzheimer’s Prevention study completed 3+ neuropsychological evaluations and a [11C]PiB PET scan or lumbar puncture. Linear mixed-effects regression models tested interactions of risk factor x Aβ x visit age on longitudinal Verbal Learning & Memory (VLM) and Speed & Flexibility (SF) factor scores.
RESULTS:
The relationship between Aβ and VLM decline was moderated by hypertension (χ2(1) = 3.85, p = .04) and obesity (χ2(1) = 6.12, p = .01); those with both elevated Aβ and the risk factor declined at faster rates than those with only elevated Aβ or elevated risk factors.
CONCLUSION:
In this cohort, hypertension and obesity moderated the relationship between Aβ and cognitive decline.
Keywords: preclinical Alzheimer’s disease, beta-amyloid, hypertension, obesity, depression, neuropsychology
1. INTRODUCTION
Although available treatments for Alzheimer’s disease (AD) may provide some short-term benefits, they have limited efficacy in terms of modifying the course of the disease [1, 2]. The lack of an effective disease-modifying therapy has led to increased efforts targeted at both early detection of AD and modifiable risk factors that may influence disease progression. Two commonly used ante-mortem biomarkers allowing for early detection of AD-related pathology are [11C]PiB positron emission tomography (PET), which allows for imaging of beta-amyloid (Aβ) plaques in vivo [3], and cerebrospinal fluid (CSF) levels of Aβ 1–42 (Aβ42), which are correlated with the formation of Aβ plaques in the brain [4, 5]. Prior investigations indicate that cognitively unimpaired adults with higher mean cortical [11C]PiB binding potential and/or lower CSF Aβ42 are at increased risk of developing dementia [6–9]. Furthermore, cognitively healthy adults with elevated Aβ deposition are more likely to exhibit cognitive decline than adults with lower Aβ levels [10–14]; however, up to 30% of older adults with elevated Aβ remain cognitively unimpaired in late life [15]. This finding suggests that not all adults with elevated Aβ accumulation will progress to dementia, and that other factors may moderate the relationship between Aβ and cognitive decline.
Supporting this hypothesis, epidemiological studies suggest that seven potentially modifiable risk factors for AD, including midlife hypertension, midlife obesity, smoking, depression, low educational attainment, physical inactivity, and diabetes may account for up to half of dementia cases in the United States [16]. Several studies report greater risk for dementia and/or longitudinal decline on neuropsychological measures in adults with depressive symptomatology [17–24], obesity [24–30], or hypertension [28, 31–35]. Moreover, prior investigations revealed elevated Aβ deposition in non-demented older adults with depression [36] or hypertension [37–38]. Two recent studies also observed a relationship between midlife obesity and increased Aβ in later life [39, 40]. Finally, one prior investigation demonstrated that adults with abnormal plasma Aβ levels and elevated blood pressure at midlife were at greatest risk of developing AD [41]; however, no study to date has examined if the relationships between these modifiable risk factors and Aβ deposition interact to affect longitudinal cognitive performance in cognitively unimpaired adults.
Therefore, the purpose of the current study was to determine if the presence of modifiable risk factors moderates the relationship between Aβ level and longitudinal performance on neuropsychological measures in cognitively normal late middle-aged adults. This study focuses on three midlife modifiable risk factors that were well-characterized in the Wisconsin Registry for Alzheimer’s Prevention (WRAP) cohort: hypertension, obesity, and depression. We investigated the moderating effects of these risk factors on the relationship between Aβ deposition (as assessed via [11C]PiB PET imaging or CSF Aβ42) and longitudinal neuropsychological performance. We hypothesized that each risk factor would moderate the relationship between Aβ burden and longitudinal cognitive performance (i.e., three-way interaction among visit age (time-varying) x risk factor group (time-invariant) x Aβ group (time-invariant)) would account for a significant amount of variance in longitudinal neuropsychological performance.
2. MATERIALS AND METHODS
2.1. Participants
Data were from 207 participants enrolled in the WRAP study, which consists of a cohort of ~1550 asymptomatic (at study entry) late middle-aged adults enriched for parental history of AD [42]. The ongoing parent study includes biennial evaluations (with the exception of a four-year interval between visits 1 and 2) that involve a physical exam, labs, a neuropsychological evaluation, and optional linked studies for acquisition of neuroimaging and CSF biomarkers of AD pathophysiology. Inclusion criteria for this study were as follows: cognitive data for at least three longitudinal visits, no diagnosis of dementia as determined via consensus conference review using NIA-AA diagnostic criteria [43], and completion of either a [11C]PiB PET scan or lumbar puncture. The inclusion of human subjects in this study was approved by the University of Wisconsin-Madison Institutional Review Board and all participants provided informed consent.
2.2. Beta-amyloid status determination
The biomarker data were collected as part of linked study protocols and did not occur at the same study visit for all participants (see Figure 1). Biomarker data were collected at visit 2 for n=61 participants, at visit 3 for n=111 participants, and at visit 4 for n=35 participants. Elevated or non-elevated Aβ status was determined by mean [11C]PiB distribution volume ratio (DVR) or by CSF Aβ42 or Aβ42/Aβ40 levels.
Figure 1.
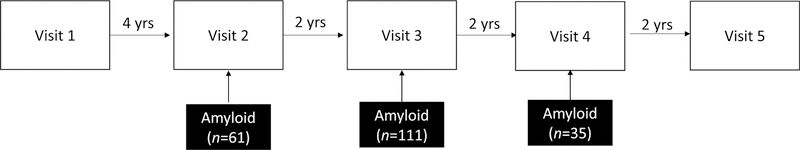
Flow diagram of study visits. Neuropsychological evaluations are conducted at each visit. Beta-amyloid data was acquired as part of linked studies near visits 2, 3, or 4. Modifiable risk factor data were examined at the study visit closest to the biomarker acquisition visit.
Detailed methods for [11C]PiB radiochemical synthesis, PET imaging parameters, and parametric DVR map generation were implemented as described previously [44]. Eight bilateral regions of interest (angular gyrus, anterior cingulate gyrus, posterior cingulate gyrus, frontal medial orbital gyrus, precuneus, supramarginal gyrus, middle temporal gyrus, and superior temporal gyrus) were selected from the automated anatomic labeling atlas, standardized, and reverse warped to native space. The mean DVR across these eight regions of interest was calculated for determination of Aβ status (elevated or non-elevated) using a cutoff of 1.19 as previously reported for the WRAP cohort [45].
CSF was collected in the morning after a minimum 12-hour fast. A Sprotte spinal needle was used to extract 22 mL of CSF from the L3-L4 or L4-L5 vertebral interspace via gentle extraction into polypropylene syringes. Within 30 minutes of collection, the CSF was combined, gently mixed, centrifuged to remove red blood cells or other debris, aliquoted into 0.5-mL polypropylene tubes, and stored at −80°C. Samples were analyzed at the Clinical Neurochemistry Laboratory at the Sahlgrenska Academy of the University of Gothenburg, Sweden for Aβ 1–40 and Aβ 1–42 using commercially available enzyme-linked immunosorbent assay methods (INNOTEST assays, Fujirebio, Ghent, Belgium; Triplex assays, MSD Human Aβ peptide ultra-sensitive kit, Meso Scale Discovery, Gaithersburg, MD). An Aβ42 (innotest) value < 471.54 pg/ml or an Aβ42/Aβ40 (triplex) ratio < .09 was used to define elevated Aβ, based on prior receiver operating characteristic analyses showing these values best discriminated cognitively healthy adults from individuals with dementia[46].
2.3. Modifiable Risk Factor Assessment
The modifiable risk factors included in this study were hypertension, obesity, and depression, which were chosen because they were midlife modifiable risk factors included in the epidemiological study previously described [16] and were present in >10% of the entire WRAP cohort (diabetes was present in only 5% of the cohort and current tobacco use was present in only 9% of the cohort). The data included in this study were collected at the closest point in time to the biomarker visit (mean time between risk factor data and biomarker data = 0.65 years, range = 0–2 years). Blood pressure and anthropometric measures were obtained according to the Atherosclerosis Risk in Communities Study protocol. Prior to cognitive test administration, participants were instructed to sit for 10 minutes and then have blood pressure readings obtained. Blood pressure was measured up to three times within an examination visit to obtain a stable measure, with the participant seated using a random-zero sphygmomanometer. Cuff size was chosen appropriate to the participant’s arm circumference. Hypertension was defined according to the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure 8 guidelines [47] (i.e., systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg). Use of antihypertensive medication was included as a covariate in primary analyses and examined further in post-hoc analyses. Waist circumference was measured with an anthropometric tape to the nearest centimeter with the participant standing. The waist circumference was taken at the level of the natural waist (narrowest part). Waist measurements were taken twice by trained staff and the smallest measurement was used. Waist circumference measurements greater than 88 cm for women and 102 cm for men were coded as obese based on standard guidelines [48]. Depressive symptoms were assessed using the Center for Epidemiologic Studies of Depression scale (CES-D), with total scores ≥ 16 coded as depressed [49]. Risk factor variables were dichotomized using clinically-defined cut-points to improve interpretability, generalizability, and clinical relevance.
2.4. Cognitive Assessment
A comprehensive neuropsychological assessment was completed at each visit [42]. A prior factor analysis indicated that learning trials 3–5 and the delayed recall trial (trial 7) on the Rey Auditory Verbal Learning Test loaded onto a Verbal Learning & Memory factor and that measures of speed and executive function (Trailmaking Test Parts A & B, Stroop Color-Word Interference condition) loaded onto a Speed & Flexibility factor [50]. Each factor was calculated as a weighted composite of the contributing tests and then standardized around the baseline mean and standard deviation of the composite (i.e., each factor is a z-score) [51]. These factors were selected for analyses because they represent cognitive domains that have been shown to exhibit early decline, are associated with Aβ in preclinical AD based on prior meta-analyses [52–54], and include measures that were given at all study visits.
2.5. Statistical Analysis
To test for the presence of relationships between Aβ status and modifiable risk factors, chi-square analyses were conducted for the dichotomous risk factor variables and ANOVAs were conducted for the continuous risk factor variables in SPSS version 24. To test the hypothesis that longitudinal cognitive performance would vary by risk factor and Aβ status, linear mixed-effects regression analysis was conducted in R version 3.3.1 using the lme4 package version 1.1–12 [55]. Linear mixed-effects models were used due to their ability to handle unbalanced and missing data as well as a continuous variable for time [56]. Outcome variables were Verbal Learning & Memory and Speed & Flexibility factor scores. Random effects included intercept and slope nested within-subject. Fixed effects for model 1 included main effects of hypertension status, Aβ status, and age at each visit (time-varying variable; centered on the sample mean) and interaction terms of hypertension status x Aβ status, age at each visit x hypertension status, age at each visit x Aβ status, and age at each visit x hypertension status x Aβ status. Fixed effects for models 2 and 3 were identical to model 1 except for obesity status and depression status instead of hypertension. Covariates included age at biomarker visit, sex, education, practice effects (time-varying variable; number of prior exposures to cognitive test [total visits completed – 1]), and treatment status (e.g., antihypertensive medication use (yes vs. no) for model 1 or antidepressant medication use (yes vs. no) for model 3). The overall significance of the three-way interaction term was assessed by likelihood ratio tests comparing the full model and a nested model that did not include the three-way interaction term. Statistical comparison of model coefficients to determine direction of group differences was performed using the Wald test. Statistical tests were two-tailed and an alpha-level of p < .05 was used to determine statistical significance. Model fit was evaluated by visual inspection of the residuals and a Pearson goodness-of-fit test.
3. RESULTS
3.1. Sample characteristics
The sample was on average 55 years of age at study enrollment and 61 years of age at biomarker visit. The sample was 68% female, highly educated (mean 16 years of education), and enriched for Alzheimer’s disease risk (73% had at least one parent with Alzheimer’s disease; 38% apolipoprotein (APOE) ε4 genotype carriers; see Table 1). All 207 participants completed at least three neuropsychological evaluations. Twenty-five participants (12%) completed three study visits (six years follow-up), 82 (40%) completed four study visits (eight years follow-up), and 100 (48%) completed five study visits (ten years follow-up). There was a mean of 6 months between the neuropsychological evaluation and biomarker visit (range = 0–2 years) and this did not differ between Aβ groups (t(205) = 0.43, p=.67). Sixty-two participants (30%) exhibited elevated levels of Aβ. At the study visit closest to the biomarker assessment, n = 40 (19%) were hypertensive, n = 89 (43%) were obese, and n = 13 (6%) were depressed. Results of chi-square analyses indicated no significant association between hypertension and obesity status (χ2(1) = 0.99, p = .32), hypertension and depression status (χ2(1) = 0.14, p = .71), or obesity and depression status (χ2(1) = 1.95, p = .16).
Table 1.
Sample characteristics (mean (SD) or N (%))
| Characteristics | Total Sample | Aβ+ | Aβ− |
|---|---|---|---|
| Total Participants | 207 | 62 | 145 |
| Baseline age | 54.6 (6.3) | 56.7 (5.4) | 53.7 (6.5) |
| Biomarker (PET or CSF) age | 60.7 (6.4) | 63.0 (5.0) | 59.8 (6.7) |
| Biomarker visit to WRAP visit interval (yrs) | 0.6 (0.5) | 0.6 (0.5) | 0.7 (0.5) |
| Sex (Female) | 140 (68%) | 43 (69%) | 97 (67%) |
| Education (years) | 16.1 (2.4) | 16.5 (2.4) | 15.9 (2.3) |
| APOE (ε4 carrier) | 78 (38%) | 35 (57%) | 43 (30%) |
| Parental history of AD (positive) | 151 (73%) | 48 (77%) | 103 (71%) |
| Baseline Verbal Learning & Memory (z-score) | .08 (1.0) | 0.17 (0.9) | 0.04 (1.0) |
| Baseline Speed & Flexibility (z-score) | −0.003 (0.9) | −0.19 (0.9) | 0.07 (1.0) |
| Beta-amyloid modality | CSF: 26 (13%) | CSF: 5 (8%) | CSF: 21 (15%) |
| PiB: 75 (36%) | PiB: 19 (31%) | PiB: 56 (39%) | |
| Both: 106 (51%)a | Both: 38 (61%) | Both: 68 (47%) | |
| Hypertensionb | 40 (19%) | 13 (21%) | 27 (19%) |
| Obesityc | 89 (43%) | 22 (36%) | 67 (46%) |
| Depressiond | 13 (6%) | 4 (7%) | 9 (6%) |
| Diabetes | 4 (2%) | 0 (0%) | 4 (3%) |
| Tobacco use (current smoker) | 8 (4%) | 2 (3%) | 6 (4%) |
| Systolic blood pressure | 125.8 (15.7 | 126.2 (16.1) | 125.6 (15.6) |
| Diastolic blood pressure | 74.4 (9.7) | 74.7 (9.9) | 74.4 (9.6) |
| Waist circumference (cm) | 92.3 (14.9) | 90.3 (15.1) | 93.2 (14.8) |
| Center for Epidemiologic Studies of Depression scale |
5.9 (5.9) | 5.6 (5.1) | 6.0 (6.2) |
| Anti-hypertensive medication use | 45 (22%) | 18 (29%) | 27 (19%) |
Of these 106, n=85 (80%) were congruent across modalities and n=21 (20%) were incongruent (n=9 were classified as beta-amyloid positive by PiB, but negative by CSF and n=12 were classified as beta-amyloid positive by CSF, but negative by PiB). Positivity status using either modality resulted in classification in the beta-amyloid positive group.
(systolic blood pressure > 139 or diastolic blood pressure > 89)
(waist circumference > 102 cm for males or > 88 cm for females)
(Center for Epidemiologic Studies of Depression scale ≥ 16)
3.2. Relationship between modifiable risk factors and beta-amyloid status
Results of chi-square analyses indicated that risk factor status did not differ by Aβ status (see Table 2). Specifically, there were no differences in proportion of participants with elevated Aβ by hypertension status (χ2(1) = .15, p = .70), obesity status (χ2(1) = 2.04, p = .15), or depression status (χ2 = .004, p = .95). Similarly, there were no differences between Aβ groups in systolic blood pressure (F = 0.06, p = .80; estimated marginal means: Aβ-= 125.6 [95% CI: 123.0–128.2], Aβ+ = 126.2 [95% CI: 122.2–130.1]), diastolic blood pressure (F = 0.04, p = .84; estimated marginal means: Aβ-= 74.4 [95% CI: 72.8–76.0], Aβ+ = 74.7 [95% CI: 72.2–77.1]), waist circumference (F=1.62, p=.21; estimated marginal means: Aβ-= 93.2 [95% CI: 90.8–95.6], Aβ+ = 90.32 [95% CI: 86.6–94.1]), and CES-D depressive symptoms (F = 0.14, p = .71; estimated marginal means: Aβ-= 5.96 [95% CI: 5.0–6.9], Aβ+ = 5.6 [95% CI: 4.2–7.1]).
Table 2.
Beta-amyloid (Aβ) status by risk factor status (n (%))
| Aβ status | Hypertension | Obesity | Depression | Total | |||
|---|---|---|---|---|---|---|---|
| Normal | Elevateda | Normal | Elevatedb | Normal | Elevatedc | ||
| Non-elevated | 118 (81) | 27 (19) | 78 (54) | 67 (46) | 136 (94) | 9 (6) | 145 |
| Elevated | 49 (79) | 13 (21) | 40 (65) | 22 (35) | 58 (94) | 4 (6) | 62 |
| Total | 167 (81) | 40 (19) | 118 (57) | 89 (43) | 194 (94) | 13 (6) | 207 |
| χ2(1), p | .15 | .70 | 2.04 | .15 | .004 | .95 | |
(systolic blood pressure > 139 or diastolic blood pressure > 89)
(waist circumference > 102 cm for males or > 88 cm for females)
(Center for Epidemiologic Studies of Depression scale ≥ 16)
Note: n=20 participants are in both elevated hypertension and elevated obesity groups, n=8 participants are in both elevated obesity and elevated depression groups, n=2 participants are in both elevated hypertension and elevated depression groups, n=1 participant was elevated on all 3 risk factors.
3.3. Relationships among modifiable risk factors, beta-amyloid, and cognition
Regression diagnostics were performed and indicated that all models met the necessary assumptions. Specifically, model residuals appeared normally distributed, did not exhibit heteroscedasticity, and Pearson goodness-of-fit tests were non-significant. Random effects (e.g., intercept and slope) were not correlated with residuals and the random effect residuals were normally distributed.
3.3.1. Hypertension
Likelihood ratio tests comparing full and nested linear mixed-effects models indicated that the three-way interaction of hypertension status x Aβ status x visit age accounted for a statistically significant amount of variance in Verbal Learning & Memory performance (χ2(1) = 3.85, p = .04; see Table 3). This result indicates that the relationship between Aβ and age-related decline in verbal learning and memory was a function of hypertension status (see Figure 2 [Left]). Rates of cognitive change for each group were calculated from model coefficients (Table 5) and demonstrate greater rates of decline in the elevated Aβ group with hypertension compared to other groups. Statistical comparison of the model coefficients using Wald test indicated that those with elevated Aβ and hypertension exhibited significantly greater decline than those with elevated Aβ without hypertension (β = −0.05 (SE=.02), p = .046) and those with low Aβ with hypertension (β = −0.06 (SE=.03), p = .02). The interaction of hypertension status x Aβ status x visit age did not account for a significant amount of variability in Speed & Flexibility performance (χ2(1) = .04, p = .83; see Table 4).
Table 3.
Model parameter estimates for association between presence of modifiable risk factors and Verbal Learning & Memory outcome
| Risk Factor | |||
|---|---|---|---|
| Hypertension | Obesity | Depression | |
| β (SE); [95% CI] | β (SE); [95% CI] | β (SE); CI] | |
| Intercept | −2.3 (1.6); [−5.3, 0.7] | −2.2 (1.6); [−5.2, 0.9] | −2.3 (1.6); [−5.4, 0.7] |
| Risk factor group | −0.2 (0.2); [−0.5, 0.1] | −0.1 (0.1); [−0.4, 0.1] | −0.1 (0.3); [−0.7, 0.4] |
| Beta-amyloid (Aβ) group | 0.04 (0.1); [−0.2, 0.3] | −0.001 (0.2); [−0.3, 0.3] | 0.2 (0.1); [−0.1, 0.4] |
| Centered visit age (slope) | −0.04 (0.02); [−0.1, 0.01] | −0.04 (0.02); [−0.1, 0.002] | −0.04 (0.02); [−0.1, 0.01] |
| Biomarker age | 0.01 (0.02); [−0.04, 0.1] | 0.01 (0.02); [−0.04, 0.1] | 0.01 (0.02); [−0.04, 0.1] |
| Sex | 0.8*** (0.1); [0.6, 1.1] | 0.8*** (0.1); [0.6, 1.1] | 0.8*** (0.1); [0.6, 1.1] |
| Education | 0.1** (0.02); [0.03, 0.1] | 0.1** (0.02); [0.02, 0.1] | 0.1** (0.02); [0.02, 0.1] |
| Practice effect | 0.1 (0.1); [−0.03, 0.2] | 0.1 (0.1); [−0.02, 0.2] | 0.1 (0.1); [−0.02, 0.2] |
| Treatment status | 0.1 (0.1); [−0.2, 0.3] | -- | −0.1 (0.1); [−0.3, 0.1] |
| Risk factor group x Aβ group | 0.4 (0.3); [−0.2, 1.0] | 0.3 (0.2); [−0.1, 0.8] | −0.7 (0.5); [−1.6, 0.3] |
| Centered visit age x Risk factor group | 0.01 (0.01); [−0.02, 0.03] | 0.01 (0.01); [−0.01, 0.03] | −0.03 (0.02); [−0.1, 0.02] |
| Centered visit age x Aβ group | −0.01 (0.01); [−0.03, 0.02] | 0.01 (0.01); [−0.02, 0.03] | −0.02 (0.01); [−0.04, 0.004] |
| Centered visit age x Aβ group x Risk factor group |
−0.1* (0.03); [−0.1, −0.001] | −0.1 (0.02)**; [−0.1, −0.01] | 0.1 (0.04); [−0.02, 0.1] |
p≤.001
p≤.01
p≤.05
p-values for fixed effect coefficients were calculated using asymptotic properties of the estimates
Figure 2.
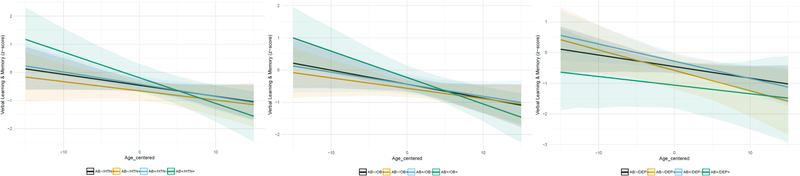
Graphs depict Verbal Learning & Memory z-scores on the y-axis, age at each visit (centered on mean age) on the x-axis, and estimated slopes for four beta-amyloid(Aβ)/risk factor groups adjusted for covariates of age at biomarker visit, sex, education, and practice effects. Risk factor groups are determined based on status at study visit closest to biomarker assessment. The left figure depicts the estimated slope for non-elevated Aβ and non-hypertensive (black; n=118), non-elevated Aβ and hypertensive (orange; n=27), elevated Aβ and non-hypertensive (blue; n=49), and elevated Aβ and hypertensive (green; n=13). The middle figure depicts the estimated slope for non-elevated Aβ and non-obese (black; n=78), non-elevated Aβ and obese (orange; n=67), elevated Aβ and non-obese (blue; n=40), and elevated Aβ and obese (green; n=22). The right figure depicts the estimated slope for non-elevated Aβ and non-depressed (black; n=136), non-elevated Aβ and depressed (orange; n=9), elevated Aβ and non-depressed (blue; n=58), and elevated Aβ and depressed (green; n=4).
Table 5.
Estimated annual rates of cognitive change (z-score units) across groups
| Verbal Learning & Memory | Speed & Flexibility | |
|---|---|---|
| Hypertension | β [95% CI] | β [95% CI] |
| Aβ−/HTN− | −0.039 [−0.083, 0.005] | 0.002 [−0.037, 0.041] |
| Aβ+/HTN− | −0.043 [−0.089, 0.002] | −0.011 [−0.051, 0.029] |
| Aβ−/HTN+ | −0.033 [−0.083, 0.017] | −0.001 [−0.044, 0.042] |
| Aβ+/HTN+ | −0.091 [−0.150, −0.032] | −0.020 [−0.073, 0.032] |
| Obesity | ||
| Aβ−/OB− | −0.043 [−0.089, 0.002] | 0.005 [−0.035, 0.045] |
| Aβ+/OB− | −0.038 [−0.084, 0.009] | −0.010 [−0.051, 0.030] |
| Aβ−/OB+ | −0.032 [−0.077, 0.013] | 0.001 [−0.039, 0.040] |
| Aβ+/OB+ | −0.082 [−0.134, −0.030] | −0.014 [−0.059, 0.031] |
| Depression | ||
| Aβ−/DEP− | −0.038 [−0.082, 0.007] | −0.0001 [−0.039, 0.038] |
| Aβ+/DEP− | −0.056 [−0.102, −0.011] | −0.014 [−0.053, 0.026] |
| Aβ−/DEP+ | −0.067 [−0.127, −0.006] | −0.030 [−0.085, 0.025] |
| Aβ+/DEP+ | −0.028 [−0.102, 0.046] | −0.035 [−0.095, 0.025] |
Aβ=beta-amyloid; HTN=hypertension; OB=obesity; DEP=depression
Table 4.
Model parameter estimates for association between presence of modifiable risk factors and Speed & Flexibility outcome
| Risk Factor | |||
|---|---|---|---|
| Hypertension | Obesity | Depression | |
| β (SE); [95% CI] | β (SE); [95% CI] | β (SE); [95% CI] | |
| Intercept | 4.3** (1.4); [1.6, 7.0] | 4.3** (1.4); [1.6, 7.0] | 4.1** (1.4); [1.4, 6.8] |
| Risk factor group | 0.1 (0.2); [−0.2, 0.4] | 0.1 (0.1); [−0.1, 0.4] | −0.4 (0.3); [−0.8, 0.1] |
| Beta−amyloid (Aβ) group | −0.1 (0.1); [−0.3, 0.2] | 0.02 (0.1); [−0.3, 0.3] | −0.1 (0.1); [−0.3, 0.2] |
| Age at each visit (centered) | 0.002 (0.02); [−0.04, 0.04] | 0.01 (0.02); [−0.03, 0.04] | −0.0001 (0.02); [−0.04, 0.04] |
| Biomarker age | −0.1*** (0.02); [−0.1, −0.03] | −0.1*** (0.02); [−0.1, −0.03] | −0.1*** (0.02); [−0.1, −0.03] |
| Sex | −0.02 (0.1); [−0.2, 0.2] | −0.04 (0.1); [−0.3, 0.2] | −0.01 (0.1); [−0.2, 0.2] |
| Education | −0.002 (0.02); [−0.04, 0.04] | −0.001 (0.02); [−0.04, 0.04] | 0.001 (0.02); [−0.04, 0.04] |
| Practice effect | 0.05 (0.1); [−0.1, 0.2] | 0.04 (0.1); [−0.1, 0.1] | 0.1 (0.1); [−0.1, 0.2] |
| Medication use | 0.005 (0.1); [−0.2, 0.3] | −− | −0.03 (0.1); [−0.2, 0.2] |
| Risk factor group x Aβ group | 0.04 (0.3); [−0.5, 0.6] | −0.2 (0.2); [−0.7, 0.2] | −0.2 (0.4); [−1.1, 0.7] |
| Centered visit age x Risk factor group | −0.003 (0.01); [−0.03, 0.02] | −0.005 (0.01); [−0.02, 0.01] | −0.03 (0.02); [−0.1, 0.01] |
| Centered visit age x Aβ group | −0.01 (0.01); [−0.03, 0.01] | −0.02 (0.01); [−0.04, 0.01] | −0.01 (0.01); [−0.03, 0.004] |
| Centered visit age x Aβ group x Risk factor group |
−0.01 (0.02); [−0.1, 0.04] | 0.001 (0.02); [−0.04, 0.04] | 0.01 (0.03); [−0.1, 0.1] |
p≤.001
p≤.01
p≤.05
p-values for fixed effect coefficients were calculated using asymptotic properties of the estimates
Post-hoc analyses were conducted to examine the effect of antihypertensive medication use on these results. Twenty-two percent (n=45) were treated with an antihypertensive medication. Of the sample of n=207, n=132 were normotensive/non-treated, n=35 were normotensive/treated (e.g., blood pressure in normal range and taking an antihypertensive medication), n=30 were hypertensive/non-treated, and n=10 were hypertensive/treated (e.g., elevated blood pressure despite antihypertensive medication use). The overall three-way interaction among blood pressure status/treatment status (4-level variable) x Aβ status x visit age did not account for a statistically significant amount of variance in Verbal Learning & Memory performance (χ2(3) = 5.82, p = .12; see Supplemental Table 1).
3.3.2. Obesity
Likelihood ratio tests comparing the full and nested linear mixed-effects models indicated that the three-way interaction of obesity status x Aβ status x visit age was statistically significant for the Verbal Learning & Memory factor (χ2(1) = 6.12, p = .01; Table 3), indicating that the relationship between Aβ status and rate of age-related decline in verbal learning and memory was dependent on obesity status (see Figure 2 [Middle]). Rates of change in Table 5 show greater decline in those with elevated Aβ and obesity. Statistical comparison of model coefficients indicated that obese participants with elevated Aβ exhibited greater decline than non-obese participants with elevated Aβ (β = −0.04 (SE=.02), p = .02) and obese participants with low Aβ (β = −0.05 (SE=.02), p = .006). Similar to analyses with hypertension, the three-way interaction of obesity status x Aβ status x visit age was not associated with performance on the Speed & Flexibility factor (χ2(1) = .004, p = .95; Table 4).
3.3.3. Depression
Likelihood ratio tests comparing the full and nested linear mixed-effects models indicated that the three-way interaction of depression status x Aβ status x visit age was not significantly associated with Verbal Learning & Memory (χ2(1) = 2.01, p = .16; see Table 3) or Speed & Flexibility (χ2(1) = 0.07, p = .80; see Table 4) performances (see Figure 2 [right]).
3.3.4. Post-hoc sensitivity analysis
Due to lack of three-way interaction effects for any of the risk factors on the Speed & Flexibility outcome, post-hoc analyses were conducted to test if the risk factors were associated with Speed & Flexibility performance in the absence of an interaction with Aβ. To test this hypothesis, the three-way interaction term (risk factor status x Aβ status x visit age) was removed from each model and the effect of each two-way interaction (risk factor x age, Aβ status x age, risk factor status x Aβ) was examined – results indicated that the two-way interaction effects were not significantly related to Speed & Flexibility performance. To further test this hypothesis, the Aβ terms were removed from the models and the effect of the two-way interactions (risk factor x age) and main effects of the risk factors were examined These results indicated non-significant interactions and main effects for hypertension and obesity factors, but a near-significant interaction effect of depression x age (p = .08) and a significant main effect of depression (p = .04) on Speed & Flexibility performance.
4. DISCUSSION
In a sample of 207 late middle-aged adults enriched for Alzheimer’s disease risk, presence of hypertension or obesity moderated the relationship between Aβ (on PET scan or in CSF) and longitudinal verbal memory, but not speed & flexibility, performance. These findings suggest that the presence of hypertension or obesity in midlife may exacerbate subtle cognitive decline associated with Aβ deposition. These results have potentially important implications for asymptomatic adults with elevated Aβ, which included 30% of the current sample, similar to prior reports of prevalence of elevated Aβ in cognitively unimpaired adults [57, 58]. The results suggest that the increased risk for cognitive decline in those with elevated Aβ [13, 14, 59] may be driven by those with both elevated Aβ and vascular risk factors. Specifically, in this sample, the annual estimated rate of memory decline was doubled in those with elevated Aβ and hypertension or obesity compared to those with elevated Aβ without hypertension or obesity. Based on this estimate, adults in the high-risk groups (Aβ+ and hypertensive or obese) would be expected to exhibit a one standard deviation decline on the memory composite within 10 years, thus potentially crossing the threshold into the clinically impaired range earlier than those in other groups. Although the estimated rate of change is subtle, the potential to identify and modify this expected trajectory by addressing and effectively treating modifiable risk factors of hypertension and obesity could possibly result in delaying the onset of clinically significant cognitive impairment.
In contrast, the interaction among age x Aβ x depression was non-significant. A smaller proportion of the sample endorsed clinically significant depressive symptoms (6%) compared to those who met criteria for hypertension or obesity; it is possible that participants with depression in the larger WRAP cohort are less likely to participate in biomarker study procedures and therefore these results may reflect a biased sample of depressed individuals. Additionally, the small sample size may have reduced the power to detect a modifying effect. Further studies in larger sample sizes, as well as additional examination of types of depressive symptoms endorsed (e.g., somatic, emotional, cognitive) and the onset period of symptoms (e.g., chronically depressed vs new onset depression in mid or late life) are needed to further evaluate this relationship.
Effects of antihypertensive medication use on cognitive decline were not observed in this sample; however, it is possible that the small sizes of each cell in these post-hoc analyses may have limited statistical power to detect potential relationships. Examinations of model coefficients suggest that the hypertensive/untreated group exhibited greater rates of cognitive decline compared to the normotensive/treated group. This result should be interpreted with significant caution since the overall interaction term was non-significant; however, follow-up of this finding is warranted in a larger sample to confirm that there is no effect of antihypertensive medication use on cognitive decline in individuals with elevated levels of Aβ. Additionally, if treatment of midlife hypertension in those with elevated Aβ is associated with slowed rates of decline, clinical trials are needed to test hypotheses generated from the results of this study. Ultimately, a multi-modal intervention (i.e., similar to the FINGER study; [60]) that targets appropriate management of vascular risk factors along with physical, cognitive, and social activities may prove most effective in reducing risk for cognitive decline.
The exact cause of accelerated decline in adults with amyloid and hypertension or obesity is unknown and will require further investigation. One hypothesis is that hypertension and obesity lead to reduced cerebral blood flow and blood-brain barrier dysfunction which may reduce Aβ clearance, increase Aβ production, and result in neuronal dysfunction and ultimately cognitive decline [61]. However, in this sample there were no differences in the proportion of participants with elevated Aβ by hypertension, obesity, or depression status. These findings differ from some prior reports suggesting that vascular risk factors are associated with elevated Aβ [36–40]. These differences may be due to the use of different analytic methods (e.g., prior studies have found that higher blood pressure is associated with greater regional Aβ burden [37]), the timing of the assessments (e.g., a prior study found that multiple vascular risk factors in midlife was associated with greater Aβ accumulation in later life [40]), or sample age (e.g., mean age of ~60 in our study compared to mean ages of 69.4 [38] and 86.9 [36]). Another hypothesis is that hypertension or obesity is associated with cerebrovascular disease and that the additive effect of Aβ burden and cerebrovascular disease results in accelerated decline. Our future investigations will build on the current study by incorporating markers of cerebrovascular function (e.g., white matter lesion, cerebral blood flow) and neurodegeneration (e.g., atrophy) to further investigate the potential underlying mechanisms of the decline observed in this sample.
There are several limitations of the current study that require consideration. The Aβ data were not collected at the same study visit for all participants which makes interpretation of the time course of biomarker positivity and vascular risk factor development on cognitive decline challenging; however, as Aβ is thought to accumulate gradually over the course of many years this is less likely to threaten the validity of these results. The use of dichotomous rather than continuous variables for each vascular risk factor may reduce power to detect significant effects; however, use of clinically-defined cut-points may have stronger ecological validity. Significant associations were only observed on Verbal Learning & Memory and not Speed & Flexibility performances, with the exception of a main effect of depression; exploration of individual measures that comprise this construct and evaluation across participants at varying ages may be helpful to generate additional hypotheses to test in future investigations. The sample size was relatively small for testing three-way interactions with several covariates and although the mean age was 61 there was a wide age gap across the sample (ages 40–70). Model diagnostics suggested that model assumptions were not violated and there were no influential data points; however, an initiative to increase biomarker participation in the WRAP cohort is ongoing and we plan to replicate and extend these models as more data become available. Future studies are also needed to determine whether longitudinal changes in vascular risk factors (e.g., changes in blood pressure or waist circumference) modify the effect of Aβ on cognitive decline and whether comorbid conditions (e.g., hypertension and obesity) or other potential modifiable factors (e.g., physical activity) are associated with longitudinal cognitive trajectories. Lastly, these studies were conducted on a sample at higher risk for AD than the general public and may not generalize to non-risk populations.
Despite these limitations, the current study adds to the literature by demonstrating that hypertension and obesity moderate relationships between Aβ and memory decline in late middle-age. This study supports the hypothesis that modifiable risk factors and Aβ interact to affect cognition, suggesting that vascular interventions in late middle-age in persons with elevated Aβ aggregation may mitigate effects of Aβ on cognition. Future work determining if treating hypertension or obesity in late middle-age slows the rate of cognitive decline or delays the onset of clinical symptoms of dementia is needed.
Supplementary Material
Research in Context.
Systematic Review:
A literature review was conducted using PubMed and Web of Science databases. While relationships among beta-amyloid and modifiable risk factors including hypertension, obesity, and depression have been observed, the interaction among beta-amyloid and modifiable risk factors on longitudinal cognitive performance in midlife was not well-characterized.
Interpretation:
In a sample of 207 late middle-aged adults enriched for Alzheimer’s disease risk, presence of hypertension or obesity moderated the relationship between Aβ (on PET scan or in CSF) and longitudinal verbal memory, but not speed & flexibility, performance. These findings suggest that hypertension or obesity in midlife may exacerbate subtle cognitive decline associated with Aβ deposition.
Future Directions:
Future studies are needed to determine if treatment of hypertension or obesity during midlife reduces the rate of cognitive decline and delays the onset of clinical symptoms of dementia. Further examination of physical activity level, diet, and antihypertensive medication use would be beneficial.
5. ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of researchers and staff at the Wisconsin Registry for Alzheimer’s Prevention and Wisconsin Alzheimer’s Disease Research Center for assistance in recruitment and data collection. We gratefully acknowledge Kaj Blennow, MD, PhD and Henrik Zetterberg, MD, PhD for assistance with cerebrospinal fluid sample assays. Most importantly, we thank the dedicated Wisconsin Registry for Alzheimer’s Prevention participants for their continued support and participation in this research. This project was supported by the Clinical Translational Science Award program through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences and grant UL1TR00427. This study was supported in part by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30 HD03352). Additional funding support was provided by NIH grants R01 AG021155 (SCJ), R01 AG027161 (SCJ), ADRC P50 AG033514 (SA), R01AG037639 (BBB), R01 AG054059, F30 AG054115 (SEB), T32 GM007507 (SEB), T32 GM008692 (SEB), and T32 CA009206 (TJB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- [1].Klafki H-W, Staufenbiel M, Kornhuber J, Wiltfang J. Therapeutic approaches to Alzheimer’s disease. Brain 2006;129:2840–55. [DOI] [PubMed] [Google Scholar]
- [2].Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F. New pharmacological strategies for treatment of Alzheimer’s disease: focus on disease modifying drugs. British journal of clinical pharmacology 2012;73:504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain 2008;131:1630–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Annals of neurology 2006;59:512–9. [DOI] [PubMed] [Google Scholar]
- [5].Strozyk D, Blennow K, White L, Launer L. CSF Aβ 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 2003;60:652–6. [DOI] [PubMed] [Google Scholar]
- [6].Chen X, Li M, Wang S, Zhu H, Xiong Y, Liu X. Pittsburgh compound B retention and progression of cognitive status–a meta-analysis. European journal of neurology 2014;21:1060–7. [DOI] [PubMed] [Google Scholar]
- [7].Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Archives of neurology 2009;66:1469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Roe C, Fagan A, Williams M, Ghoshal N, Aeschleman M, Grant E, et al. Improving CSF biomarker accuracy in predicting prevalent and incident Alzheimer disease. Neurology 2011;76:501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Soldan A, Pettigrew C, Li S, Wang M-C, Moghekar A, Selnes OA, et al. Relationship of cognitive reserve and cerebrospinal fluid biomarkers to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Neurobiology of aging 2013;34:2827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K. Cerebrospinal fluid β-amyloid 1–42 concentration may predict cognitive decline in older women. Journal of Neurology, Neurosurgery & Psychiatry 2007;78:461–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Petersen RC, Wiste HJ, Weigand SD, Rocca WA, Roberts RO, Mielke MM, et al. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA neurology 2016;73:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ossenkoppele R, van der Flier WM, Verfaillie SC, Vrenken H, Versteeg A, van Schijndel RA, et al. Long-term effects of amyloid, hypometabolism, and atrophy on neuropsychological functions. Neurology 2014;82:1768–75. [DOI] [PubMed] [Google Scholar]
- [13].Clark LR, Racine AM, Koscik RL, Okonkwo OC, Engelman CD, Carlsson CM, et al. Beta-amyloid and cognitive decline in late middle age: Findings from the Wisconsin Registry for Alzheimer’s Prevention study. Alzheimer’s & Dementia 2016;12:805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain 2014;137:221–31. [DOI] [PubMed] [Google Scholar]
- [15].Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Annals of neurology 2010;67:122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet Neurology 2011;10:819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Berger A-K, Fratiglioni L, Forsell Y, Winblad B, Bäckman L. The occurrence of depressive symptoms in the preclinical phase of AD A population-based study. Neurology 1999;53:1998-. [DOI] [PubMed] [Google Scholar]
- [18].Geda YE, Roberts RO, Mielke MM, Knopman DS, Christianson TJ, Pankratz VS, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. American Journal of Psychiatry 2014;171:572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Archives of neurology 2003;60:753–9. [DOI] [PubMed] [Google Scholar]
- [20].Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of general psychiatry 2006;63:530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Royall DR, Palmer RF. Alzheimer’s disease pathology does not mediate the association between depressive symptoms and subsequent cognitive decline. Alzheimer’s & Dementia 2013;9:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sachs-Ericsson N, Joiner T, Plant EA, Blazer DG. The influence of depression on cognitive decline in community-dwelling elderly persons. The American journal of geriatric psychiatry 2005;13:402–8. [DOI] [PubMed] [Google Scholar]
- [23].Verdelho A, Madureira S, Moleiro C, Ferro JM, T O’Brien J, Poggesi A, et al. Depressive symptoms predict cognitive decline and dementia in older people independently of cerebral white matter changes: the LADIS study. J Neurol Neurosurg Psychiatry 2013;84:1250–4. [DOI] [PubMed] [Google Scholar]
- [24].Xu W, Tan L, Wang H-F, Jiang T, Tan M-S, Tan L, et al. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2015:jnnp-2015–310548. [DOI] [PubMed]
- [25].Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of neurology 2005;62:1556–60. [DOI] [PubMed] [Google Scholar]
- [26].Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biological psychiatry 2010;67:505–12. [DOI] [PubMed] [Google Scholar]
- [27].Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Bmj 2005;330:1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Current Alzheimer Research 2007;4:111–6. [DOI] [PubMed] [Google Scholar]
- [29].Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass index over the adult life course and cognition in late midlife: the Whitehall II Cohort Study. The American journal of clinical nutrition 2009;89:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dahl AK, Hassing LB, Fransson EI, Gatz M, Reynolds CA, Pedersen NL. Body mass index across midlife and cognitive change in late life. International journal of obesity 2013;37:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gao S, Jin Y, Unverzagt FW, Liang C, Hall KS, Ma F, et al. Hypertension and cognitive decline in rural elderly Chinese. Journal of the American Geriatrics Society 2009;57:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA neurology 2014;71:1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Haring B, Wu C, Coker LH, Seth A, Snetselaar L, Manson JE, et al. Hypertension, dietary sodium, and cognitive decline: results from the women’s health initiative memory study. American journal of hypertension 2015;29:202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Current hypertension reports 2017;19:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–81. [DOI] [PubMed] [Google Scholar]
- [36].Harrington KD, Lim YY, Gould E, Maruff P Amyloid-beta and depression in healthy older adults: A systematic review. Australian & New Zealand Journal of Psychiatry 2015;49:36–46. [DOI] [PubMed] [Google Scholar]
- [36].Hughes TM, Kuller LH, Barinas-Mitchell EJ, McDade EM, Klunk WE, Cohen AD, et al. Arterial stiffness and β-amyloid progression in nondemented elderly adults. JAMA neurology 2014;71:562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Langbaum JB, Chen K, Launer LJ, Fleisher AS, Lee W, Liu X, et al. Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiology of aging 2012;33:827 e11-. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nation DA, Edland SD, Bondi MW, Salmon DP, Delano-Wood L, Peskind ER, et al. Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology 2013;81:2024–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chuang Y, An Y, Bilgel M, Wong D, Troncoso J, O’brien R, et al. Midlife adiposity predicts earlier onset of Alzheimer’s dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Molecular psychiatry 2016;21:910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. Jama 2017;317:1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shah NS, Vidal J-S, Masaki K, Petrovitch H, Ross GW, Tilley C, et al. Midlife Blood Pressure, Plasma β-Amyloid, and the Risk for Alzheimer Disease. Hypertension 2012:HYPERTENSIONAHA. 111.178962. [DOI] [PMC free article] [PubMed]
- [42].Johnson SC, Koscik RL, Jonaitis EM, Clark LR, Mueller KD, Berman SE, et al. The Wisconsin Registry for Alzheimer’s Prevention: A review of findings and current directions. Alzheimer’s & Dementia: Diagnois, Assessment, & Disease Monitoring 2018;In press. [DOI] [PMC free article] [PubMed]
- [43].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr. , Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2011;7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Johnson SC, Christian BT, Okonkwo OC, Oh JM, Harding S, Xu G, et al. Amyloid burden and neural function in people at risk for Alzheimer’s Disease. Neurobiology of Aging 2014;35:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Racine AM, Clark LR, Berman SE, Koscik RL, Mueller KD, Norton D, et al. Associations between Performance on an Abbreviated CogState Battery, Other Measures of Cognitive Function, and Biomarkers in People at Risk for Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD 2016;54:1395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Clark LR, Berman SE, Norton D, Koscik RL, Jonaitis E, Blennow K, et al. Age-accelerated cognitive decline in asymptomatic adults with CSF beta-amyloid. Neurology 2018. [DOI] [PMC free article] [PubMed]
- [47].James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8). JAMA 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- [48].WHO. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. 2011. [Google Scholar]
- [49].Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and aging 1997;12:277–87. [DOI] [PubMed] [Google Scholar]
- [50].Dowling NM, Hermann B, La Rue A, Sager MA. Latent Structure and Factorial Invariance of a Neuropsychological Test Battery for the Study of Preclinical Alzheimer’s Disease. Neuropsychology 2010;24:742–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Koscik RL, La Rue A, Jonaitis EM, Okonkwo OC, Johnson SC, Bendlin BB, et al. Emergence of mild cognitive impairment in late middle-aged adults in the wisconsin registry for Alzheimer’s prevention. Dementia and geriatric cognitive disorders 2014;38:16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology 2013;80:1341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology 2005;19:520–31. [DOI] [PubMed] [Google Scholar]
- [54].Duke Han S, Nguyen CP, Stricker NH, Nation DA. Detectable Neuropsychological Differences in Early Preclinical Alzheimer’s Disease: A Meta-Analysis. Neuropsychology review 2017. [DOI] [PMC free article] [PubMed]
- [55].Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. 2015. 2015;67:48. [Google Scholar]
- [56].Gibbons RD, Hedeker D, DuToit S. Advances in Analysis of Longitudinal Data. Annual review of clinical psychology 2010;6:79–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of Aging 2010; 31:1275–1283. [DOI] [PubMed] [Google Scholar]
- [58].Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of Cerebral Amyloid Pathology in Persons Without Dementia: A Meta-analysis. JAMA 2015;313:1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS. Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. Jama 2017;317:2305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. The Lancet 2015;385: 2255–2263. [DOI] [PubMed] [Google Scholar]
- [61].Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature reviews Neuroscience 2011;12:723–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


