Abstract
Aim.
Identifying kidney transplant patients at highest risk for graft loss prior to loss may allow for effective interventions to improve 5-year survival.
Methods.
We performed a ten-year retrospective cohort study of adult kidney transplant recipients (n=1,747). We acquired data from electronic health records, United Network of Organ Sharing, social determinants of health, natural language processing data extraction, and real-time capture of dynamically evolving clinical data obtained within 1-year of transplant; from which we developed a 5-year graft survival model.
Results.
1,439 met eligibility; 265 (18.4%) of which experienced graft loss by 5-years. Graft loss patients were characterized by: older age, being African-American, diabetic, unemployed, smokers, having marginal donor kidneys, and cardiovascular comorbidities. Predictive dynamic variables included: low mean blood pressure, higher pulse pressures, higher heart rate, anemia, lower eGFR peak, increased tacrolimus variability, rejection, and readmissions. This Big Data analysis generated a 5-year graft loss model with an 82% predictive capacity, vs 66% using baseline United Network of Organ Sharing data alone.
Conclusion.
Our analysis yielded a 5-year graft loss model demonstrating superior predictive capacity compared to United Network of Organ Sharing data alone, allowing post-transplant individualized risk-assessed care prior to transitioning back to community care.
Keywords: data analysis, decision support techniques, kidney, graft survival, transplants
INTRODUCTION
The current regulatory environment places a high priority on short-term graft survival, with corresponding improvements in the one-year renal graft survival (1, 2). Current induction and maintenance immunosuppressive regimens have resulted in one year graft survival reaching 90 – 97%, dependent upon deceased or living donor organ source (3–5). In the United States, one-year graft survival is comparable to other western countries, whereas long-term graft survival (3–10 years) is significantly worse (4). The etiology of this outcome discrepancy is not clearly understood, but may be due to less rigorous long-term post-transplant care provided by transplant centers in concert with increased reliance upon primary care physicians (6, 7). To improve long-term outcomes within the US, there is need to accurately identify patients at higher risk of graft loss and focus interventions on this vulnerable population (3, 4). Reliable predictors of long-term graft loss are the first step in developing and testing effective interventions aimed at improving long-term graft survival.
We previously developed predictive models for 1- and 3-year graft survival utilizing a Big Data approach (8). This model utilized dynamically evolving clinically relevant patient level information along with administrative data; taking advantage of the vast array of information available in the electronic health record (EHR) by using real-time electronic capture and abstraction of unstructured data fields through natural language processing (NLP) to improve predictability of early patient and graft survival. While this Big Data approach to analysis improved predictability, many 1- and 3-year graft loss and patient mortality were random events thus the clinical application of the model was limited. In the current study, we incorporated a Big Data approach into an analytical model using data obtained up to 1-year post transplant to develop robust predictive models for 5-year graft loss. Our hypothesis is that graft loss 1–5 years post renal transplant is a predictable event involving mutable factors.
MATERIALS and METHODS
Patient Population
This is a retrospective cohort study of adult (>18 years of age) solitary kidney transplant recipients at the Medical University of South Carolina (MUSC), Charleston, SC during the time period from January 1, 2007 to June 30, 2017. We chose this period of time as a consequence of the accuracy and the availability of EHR and administrative records for data capture. Patients were excluded if they were less than 18 years old, non-renal transplant, experienced graft loss or death in the first year or had less than one-year follow-up time. Patients with graft failure during the first year post-transplant were excluded from this study since the aim of this study was to predict late graft failure and since early graft loss is rare and usually due to very early post-operative complications related to the donor, surgery or CV events that occurred in the peri-operative time period. Patients with less than one-year follow-up were excluded due to the necessary construction of the dynamic variables. The MUSC Institutional Review Board for Human Research approved this study (#00064075).
Data Sources
Structured data were acquired from EHR using Practice Partner (McKesson, Seattle, WA) prior to May 2012 and Epic® (Epic Corp, Madison, WI) from July 2011 onwards. Elements from the (UNOS) database containing Organ Procurement and Transplantation Network (OPTN) data were acquired since 1986. The key social determinants of health were obtained from the transplant database Velos® (Velos, Inc., Fremont, CA) before September 2014 and Epic® afterwards. Natural language processing (NLP) was used to extract text Banff data from biopsies and vital signs found in records that predated electronic capture.
Primary outcome measures
The primary outcome for this study was five-year graft loss. Graft loss data were retrieved from internal records and UNOS files. A graft loss event was defined as a return to chronic dialysis, re-transplantation, or death. For patients who received more than one transplant, multiple graft losses per patient were considered unique observations. However, death, which was considered a graft loss, was linked to the most recent transplant event. A 1-year exposure period was used to derive 5-year graft loss model (i.e. the model was run only with cumulative data up to 365 days post-transplant).
Covariates
UNOS data elements were utilized for key donor and recipient demographic and transplant related variables in accordance with published methodology used by the Scientific Registry of Transplant Recipients (SRTR). These data, in concert with the MUSC EHR, were used to extract key social determinants of health. EHR data were also utilized to identify patient comorbidities, vital signs, cardiovascular events, laboratory data, transplant length of stay, and post-transplant acute care utilization data (including both inpatient and emergency department visits).
Using enhanced International Classification of Diseases (ICD-9-CM and ICD-10-CM) codes, patient comorbidities were derived from a modified Elixhauser coding algorithm utilizing select Charlson comorbidities (9). Post-transplant cardiovascular events such as arrhythmias and myocardial infarction were included collectively in determination of cardiovascular risk. Immunologic risk was captured by rejection rates (Banff scores; defined as ≥1A), cytomegalovirus (CMV) infection, BK virus (BK) infection (viral load ≥500 copies/ml), and tacrolimus trough concentrations. Social determinants of health were summarized as demographics, education level, and income variables.
Statistical analysis
Event data were captured beginning at transplant date and extending to 365 days post-transplant, which included data up to 24 hours prior to a death and/or GL event. Means, standard deviations, maximums, and the slope of the regression lines were used to represent dynamic variables, capturing effects of change, direction of change, and magnitude of change throughout the 1-year post-transplant exposure period. This treatment was applied to estimated GFRs (eGFRs), pulse rates, blood pressures, glucose, tacrolimus, and hemoglobin (HGB) levels. eGFR and HGB within the first week post-transplant were excluded because of inherent post-transplant fluctuations in values prior to patient stabilization. A multivariable Cox regression model was developed using baseline and follow-up data obtained up to 365 days post-transplant exposure period to develop a 5-year graft loss model. Statistical significance was determined at the two-sided 5% level. The Harrell’s concordance (10, 11) and time dependent ROC and AUC (12) were used to summarize the predictive accuracy of the fitted model. IBM Watson Content Analytics Suite IBM SPSS Modeler (Version 17), IBM SPSS Statistics - Essentials for R (IBM Corporation) and SAS 9.4 were used for the statistical analysis.
RESULTS
Study population and baseline characteristics
Of the 1,747 kidney transplants performed between January 1, 2007 and June 30, 2017, 1,439 unique transplant events met eligibility criteria to be included in the analysis. There were 265 graft loss events (18.4%) during the five-year post-transplant follow-up time period (See Figure 1 for the Consort diagram depicting how the cohort was developed). Demographic and clinical characteristics of patients included in the study, stratified by graft loss, are summarized in Table 1. Adjusted risk factors for graft loss are displayed in Table 2. Significant baseline risk factors for graft loss included: male gender, unemployment, smokers, comorbidities, receiving a marginal donor kidney, and living >200 miles from the transplant center.
Figure 1.
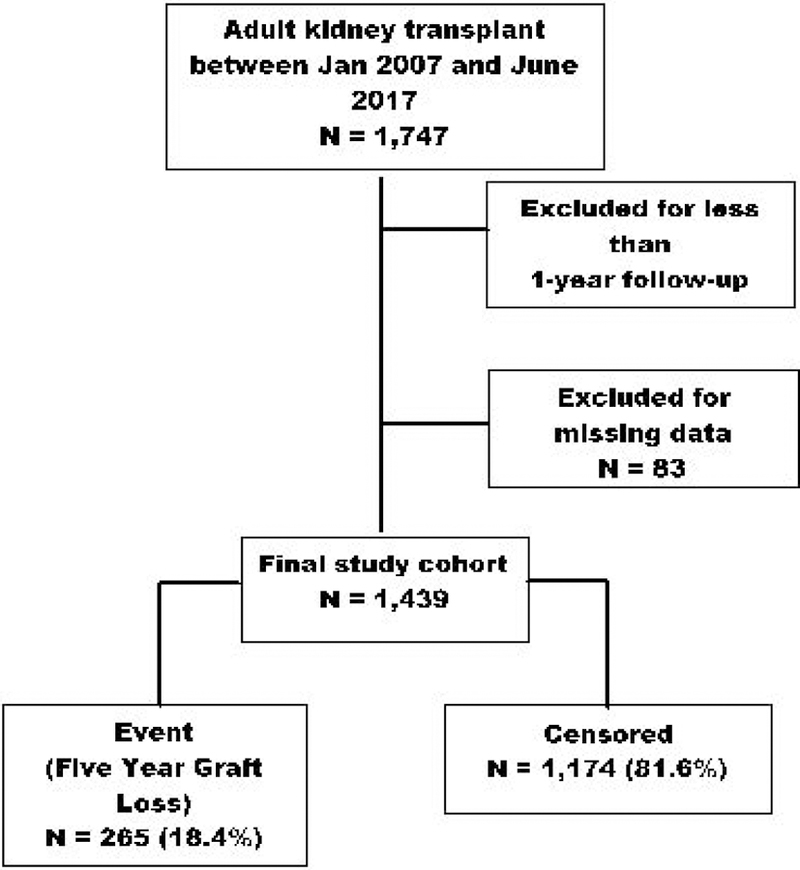
Consort diagram for study enrollment.
Table 1.
Patient characteristic comparison table. United Network of Organ Sharing, UNOS; Kidney Donor Profile Index, KPDI; Body Mass Index, BMI; Medical University of South Carolina, MUSC; Electronic Health Record, EHR; Length of Stay, LOS; Systolic Blood Pressure, SBP; Myocardial Infarction, MI; Hemoglobin, HGB; Cytomegalovirus, CMV; estimated Glomerular Filtration Rate, eGFR; Inpatient, IP; Emergency Department, ED; Natural Language Processing, NLP; BK virus, BK; Standard Deviation = SD
| Variable | 5Yr Graft Loss (N=265) |
Censored (N=1,174) |
P-value |
|---|---|---|---|
| UNOS | |||
| Age at Transplant (yrs; mean ± SD) | 54 ± 14 | 51 ± 14 | 0.005 |
| Categorical Age at Transplant | 0.005 | ||
| Age <= 40 | 20% | 23% | 0.202 |
| Age = 41–60 | 41% | 47% | 0.049 |
| Age > 60 | 40% | 29% | 0.001 |
| Female | 38% | 40% | 0.428 |
| African American | 57% | 54% | 0.521 |
| Deceased Donor Type | 89% | 83% | 0.018 |
| African American Donor | 33% | 26% | 0.020 |
| KDPI (mean ± SD) | 52 ± 31 | 39 ± 29 | <.001 |
| Categorical KDPI | <.001 | ||
| KDPI = 1% - 20% | 20% | 34% | <.001 |
| KDPI = 21% - 84% | 61% | 60% | 0.633 |
| KDPI = 85% - 100% | 19% | 7% | <.001 |
| Blood Type B | 17% | 16% | 0.708 |
| Waiting Time (Years) | 1.8 ± 1.7 | 1.7 ± 1.6 | 0.337 |
| Delayed Graft Function | 18% | 15% | 0.117 |
| Diabetes | 41% | 34% | 0.033 |
| BMI (mean ± SD) | 28.8 ± 5.7 | 29.0 ± 5.4 | 0.497 |
| Categorical BMI | 0.907 | ||
| BMI < 20 | 5% | 4% | 0.689 |
| BMI = 20–35 | 80% | 81% | 0.732 |
| BMI >= 35 | 15% | 15% | 0.882 |
| Private Insurance | 22% | 28% | 0.072 |
| Distance to MUSC_km (mean ± SD) | 249 ± 172 | 238 ± 196 | 0.358 |
| Categorical Distance to MUSC | 0.295 | ||
| Distance < 80 km | 23% | 26% | 0.399 |
| Distance = 80 – 161 km | 9% | 11% | 0.484 |
| Distance = 161 – 322 km | 38% | 40% | 0.661 |
| Distance >= 322 km | 29% | 23% | 0.063 |
| Previous Kidney Transplant | 11% | 8% | 0.135 |
| Velos+EHR | |||
| Finish High School | 85% | 87% | 0.243 |
| Employed | 25% | 33% | 0.013 |
| Received Disability | 50% | 47% | 0.338 |
| Married | 59% | 62% | 0.480 |
| Smoker | 12% | 6% | 0.001 |
| EHR | |||
| Congestive Heart Failure | 17% | 12% | 0.036 |
| Peripheral Vascular Disorders | 14% | 11% | 0.231 |
| Cerebrovascular Disease | 5% | 6% | 0.644 |
| Cardiac Arrhythmias | 30% | 22% | 0.005 |
| Valvular Disease | 9% | 9% | 0.767 |
| Hypertension | 96% | 96% | 0.931 |
| Alcohol Abuse | 5% | 3% | 0.201 |
| Drug Abuse | 4% | 3% | 0.328 |
| Depression | 13% | 12% | 0.891 |
| Myocardial Infarction | 12% | 5% | <.001 |
| Transplant LOS_Days (mean ± SD) | 4.0 ± 3.9 | 3.4 ± 2.3 | 0.024 |
| Transplant LOS > 3 days | 26% | 20% | 0.057 |
| Acute MI ‡ | 3.4% | 0.9% | 0.002 |
| Cardiac or Vascular Event | 41% | 20% | <.001 |
| Any CMV > 500 | 14% | 12% | 0.338 |
| SBP Mean (mm Hg mean ± SD) | 141 ± 13 | 142 ± 12 | 0.357 |
| Categorical SBP Mean | 0.172 | ||
| SBP Mean < 110 | 3% | 1% | 0.061 |
| SBP Mean = 110–159 | 92% | 93% | 0.455 |
| SBP Mean >=160 | 6% | 6% | 0.946 |
| Pulse Pressure SDev | 14.8 ± 3.7 | 13.8 ± 3.8 | <.001 |
| Pulse Mean | 81 ± 9 | 80 ± 9 | 0.095 |
| Glucose Mean (g/dL) | 140 ± 33 | 134 ± 32 | 0.002 |
| HGB Mean (g/dL) | 10.7 ± 1.6 | 11.3 ± 1.4 | <.001 |
| HGB Slope per30days (g/dL/30 days)† | −0.21 ± 3.72 | 0.33 ± 0.60 | 0.021 |
| Maximum eGFR (ml/min/1.73 m2) | 64 ± 41 | 69 ± 23 | 0.043 |
| eGFR SDev (ml/min/1.73 m2) | 11.0 ± 8.4 | 10.1 ± 5.3 | 0.073 |
| eGFR Slope perWK - Maximum Value (ml/min/1.73 m2/week) | −5.5 ± 22.1 | −1.4 ± 3.7 | 0.003 |
| Tacrolimus Mean (ng/ml) | 8.6 ± 1.6 | 8.8 ± 1.3 | 0.043 |
| Tacrolimus SDev (ng/ml) | 4.0 ± 1.4 | 3.7 ± 1.1 | <.001 |
| INP Readm Count | 1.6 ± 1.7 | 0.9 ± 1.3 | <.001 |
| ED Readm Count | .17 ± .74 | .15 ± .51 | 0.666 |
| EHR+NLP | |||
| BK plus Rejection | <.001 | ||
| Any BK > 500 and Rejection | 4% | 3% | 0.413 |
| Any BK > 500 Only | 11% | 14% | 0.124 |
| Rejection Only | 11% | 3% | <.001 |
| No BK > 500 and No Rejection | 74% | 79% | 0.057 |
HGB slope determined between 7 days post-transplant until the end of the exposure.
Exposure period for 5-year model is 365 days.
Table 2.
Cox proportional regression hazard ratio and 95% confidence intervals for 5-year graft survival model.For abbreviations, see Table 1.
| Variable | Hazard Ratio |
HR | 95% CI | P-value |
|---|---|---|---|---|
| Female | 0.665 | 0.511 | 0.866 | 0.003 |
| KDPI (ref=‘1–20’) | ||||
| 21–84 | 1.541 | 1.119 | 2.122 | 0.008 |
| 85–100 | 2.285 | 1.522 | 3.428 | <.001 |
| Distance to MUSC_km (ref=‘>322’) | ||||
| 161–322 | 0.706 | 0.519 | 0.959 | 0.026 |
| 80–161 | 0.569 | 0.356 | 0.908 | 0.018 |
| < 80 | 0.470 | 0.324 | 0.680 | <.001 |
| Employed | 0.757 | 0.550 | 1.042 | 0.088 |
| Receive Disability | 0.708 | 0.538 | 0.932 | 0.014 |
| Smoker | 1.954 | 1.323 | 2.884 | 0.001 |
| Myocardial Infarction | 1.713 | 1.151 | 2.548 | 0.008 |
| Cardiac Arrhythmias | 1.450 | 1.080 | 1.947 | 0.014 |
| Drug Abuse | 1.737 | 0.929 | 3.250 | 0.084 |
| Cardiac or Vascular Event | 2.069 | 1.550 | 2.761 | <.001 |
| BK>500 and Rejection (ref = ‘No BK No REJ) | ||||
| BK>500 and Rejection | 0.999 | 0.538 | 1.856 | 0.998 |
| BK>500 Only | 1.050 | 0.701 | 1.573 | 0.812 |
| Rejection Only | 2.189 | 1.453 | 3.298 | <0.001 |
| SBP Mean (ref = ‘110 – 159’) | ||||
| < 110 | 4.274 | 1.702 | 10.731 | 0.002 |
| >= 160 | 0.714 | 0.425 | 1.197 | 0.201 |
| Pulse Pressure SDev | 1.065 | 1.029 | 1.103 | <0.001 |
| HGB Mean | 0.858 | 0.774 | 0.951 | 0.004 |
| HGB Slope per Month | 0.719 | 0.630 | 0.822 | <.001 |
| Heart Rate Mean | 1.023 | 1.008 | 1.038 | 0.002 |
| Max eGFR | 0.994 | 0.988 | 0.999 | 0.017 |
| eGFR Slope perWeek | 0.978 | 0.972 | 0.983 | <.001 |
| Tacrolimus Mean | 0.913 | 0.828 | 1.007 | 0.069 |
| Tacrolimus SDev | 1.176 | 1.065 | 1.299 | 0.001 |
| Inpatient Readm Count | 1.101 | 1.011 | 1.199 | 0.027 |
Clinical variables during the first year follow-up as predictors of graft loss
Several clinical variables obtained from the EHRs during the first year post-transplant were significant risk factors for 5-year graft loss (Table 2). A low mean blood pressure, higher pulse pressures, a higher mean heart rate, anemia, decreasing HGB slope, lower eGFR peak, reduced eGFR slope, increased tacrolimus variability and number of post-transplant readmissions were significant and independent predictors of 5-year graft loss. A positive unit rate of HGB change was associated with a 28% decreased risk of graft loss. Acute rejection within one-year post-transplant was significantly more common in those that developed graft loss within 5 years of transplant (11% vs 3%, p < 0.001), and associated with more than twice the risk of graft loss. The 5 yr graft loss group also demonstrated higher rates of rejection for each of the acute rejection classifications (Supplemental Table 1).
Model predictability performance
The 5-year Cox survival model was tested for its discriminatory ability using the C-statistic and construction of time-dependent ROC curves. The findings of this assessment indicate that the model has strong predictive ability and is capable of identifying those at high risk of graft loss in a sensitive manner. The overall Harrell’s concordance from this model was 0.759 (SE = 0.018) and the integrated AUCs (iAUC) over time was 0.819. Thus, by using baseline and 1-year post-transplant clinical data, the model was capable of accurately identifying 8 out of every 10 patients that developed graft loss within 5 years of transplant. The utilization of clinical data electronically captured and abstracted through the EHR using NLP significantly improved the predictive performance of the model, as compared to a model utilizing only baseline UNOS data (Figure 2). Finally, internal validation was conducted for the final model using bootstrapping (1,000 replicates) and an unrestricted random sampling method. The mean of bootstrapped iAUC was 0.823 (95% CI 0.791 – 0.852), indicating this model was stable with minimal bias.
Figure 2.

Comparison of time-dependent Area Under Curve (AUC) for 5-year graft survival model. Timedependent Receiver Operating Characteristic (ROC) and AUC used to determine predictive accuracy of the Big Data (UNOS+EHR+NLP) and the UNOS only models. UNOS iAUC =0.660 [95% CI 0.601 – 0.706]; Big Data iAUC = 0.819 [95% CI 0.771 – 0.857]. Time (t) expressed in years. United Network of Organ Sharing, UNOS; Electronic Health Record, EHR; Natural Language Processing, NLP.
DISCUSSION
The significant findings of this study demonstrate that combining donor and recipient baseline variables (i.e. UNOS model variables) with granular EHR data generated during the first year post-transplant produced a robust model capable of predicting more than 80% of patients that will have graft loss within 5 years of transplant. This Big Data approach utilized traditional transplant registry donor and recipient variables along with detailed sociodemographic and clinical elements obtained from EHR data, including comorbidities, complications, vital signs, change in lab values, health care utilization, and natural language processing for Banff scores.
In lieu of a “one size fits all” approach to follow-up patient management, post-transplant care has the potential to become truly personalized for patients via estimating risk of graft loss with the use of Big Data to develop and autonomously deploy sensitive risk models. It may be most appropriate to closely follow patients at highest risk of graft loss and apply center-level resources in a more intense manner to these vulnerable populations (7). As an example of how to use this approach, let us compare two individual patients in our cohort with similar baseline variables that presented during the one-year follow-up with distinctly different HGBs, eGFR slopes and tacrolimus variability with the end result of one patient suffering graft loss three-years post-transplant. These two non-smoking female African American patients received kidneys with KPDI of <20%, lived >200 miles from the transplant center, received disability income, had neither cardiovascular comorbidities at transplant nor demonstrated BK viral infection or acute rejection, and had similar mean SBP values (110 – 159 mm Hg). Immediate post-transplant mean HGB levels and maximum eGFR values were also similar. However, in the patient suffering from graft loss at 3 years, her 30-day HGB slope demonstrated a change of −0.25 g/dl per month vs. a 1.66 g/dl per month increase for the non-graft loss patient; an eGFR slope of −1.92 ml/min/1.73m2 per week vs. a flat 0.00 ml/min/1.73m2 and a mean tacrolimus value of 4.7 ng/mL vs. 8.6 ng/mL, respectively. In this example, these two patients have identical baseline risk, yet their post-transplant clinical trajectories were vastly different. Theoretically, our model would provide information allowing clinicians to focus on the high risk patient.
It has yet to be determined whether utilization of this model to identify at-risk individuals can lead to better population outcomes overall. However, there are a number of significant mutable baseline and follow-up clinical variables that can be the focus of deliberate intervention. Patients that resided farther distances from the transplant center were at higher risk of graft loss. This may be a reflection of care coordination and logistical challenges in providing optimal follow-up at a substantial distance from the transplant center (7). Efforts to improve remote monitoring and follow-up may mitigate this risk. Active tobacco users at the time of transplant were at twice the risk of graft loss. Interventions to expand smoking cessation endeavors may help to mitigate this risk factor (13, 14). The predominant pre- and post-transplant risk factors were in the domain of cardiovascular (CV) events. CV health and risk factor control is a crucial part of optimizing long-term outcomes in all patient populations, but this is particularly the case within kidney transplantation. To significantly improve long-term outcomes, better management of CV disease and risk factors--particularly hypertension--is needed (15, 16).
A number of additional, potentially modifiable, post-transplant risk factors were identified through this model. Tacrolimus variability during the first year post-transplant was a significant risk factor for graft loss. Tacrolimus variability has previously been associated with graft loss in a number of studies, including within our own center; this may be a reflection of increased non-adherence (17–19). Acute rejection has traditionally been a strong risk factor for graft loss, which is consistent within our model--although it must be noted these events are quite rare. Acute rejection is not necessarily preventable in all cases, but transplant centers may need to closely follow these patients for several years to prevent downstream negative clinical sequelae associated with these events (20, 21).
Decreasing HGB slope was one of the strongest predictors of graft loss. Anemia is present in as many as 72% of patients one month post-transplant, decreasing to 40% of the patients by 3 months and 20% at 1 year (22). The early stages of anemia have been attributed to several factors, including: a consequence of end-stage renal disease pre-transplant nadir, surgical blood loss, inadequate nutrition, fluid overload, ischemic reperfusion injury, poor graft quality, delayed graft function, and known adverse effects from immunosuppressive therapy (22–24). Late (> 1-year post-transplant) anemia is thought to be attributable to impaired graft function, iron deficiency, female gender and adverse effects of pharmaceuticals (22). In two retrospective studies, HGB levels at 12-months post-transplant were correlated with long-term graft outcome. Anemia at 12 months, when stratified according to kidney function (i.e. eGFR), predicted graft loss in live donor recipients (25). Additionally, higher levels of HGB at 1-year were predictive of improved long-term graft survival (26). These studies also suggest that utilization of erythropoietin stimulating agents to counter late stage anemia may benefit graft survival. Such was the case in the CAPRIT trial, in which epoetin-β was used to correct post-transplant anemia (27). In this study, use of erythropoietin stimulating agent (ESAs) for 2 years normalized HGB levels and produced a 95% graft survival vs 80% for the partially normalized group. However, there has been inconsistencies in the utilization and reported outcomes of ESAs in long-term post-transplant anemia (22, 28), possibly as a consequence of the concerns regarding adverse cardiovascular events associated with the chronic use of these agents (29). Thus, further study into this potentially modifiable risk factor is clearly warranted.
There have been previous attempts at developing accurate predictive models for graft survival, designed to identify the combination of factors that result in poorer outcomes. Methodologies include data mining, computational forecasting, clinical markers, ‘Big Data’, and others (30–32). As a consequence of risk factor heterogeneity affecting graft survival, predictive models have met with mixed success. Krikov et al developed a tree-based learning model using United States Renal Data System (USRDS) data from 1990 – 1999 to predict kidney graft loss using donor, recipient, and transplant variables (30). More recently, a predictive model of 5-year graft survival was based solely upon pre-transplant data using ten predictors (30). Their results (www.transplantscore.com) gave a predictive value C statistic = 0.70 for patient mortality, 0.63 for graft failure and 0.63 for combined mortality/graft failure. A Korean group developed a combination model utilizing machine learning with survival statistics, immunological factors and specific donor and recipient variables to develop a long-term graft survival model (32). The ROC AUC values for their model ranged from 0.975 for 1-year, to 0.701 at 5-years, to 0.707 for 10-years post-transplant (32). There are other reports utilizing analytical forecasting models in conjunction with clinical outcomes to predict early allograft rejection (30), delayed graft function (31), 3-year deceased donor graft survival (33), cardiovascular-related deaths post-transplant (34, 35), and health care utilization post-transplant (36). As there are numerous examples of risk-prediction models in the transplant literature, future studies are now urgently required to determine if such models can be utilized as clinical-decision aids and lead to improved outcomes (20–26). Previously, we implemented such a model for 1- and 3- year graft survival (8). In that study, clinically relevant, daily patient and administrative data were used to improve predictability of early patient and graft survival. It is our intent in future studies to utilize a similar automated approach to demonstrate value of the current model in predicting 5 year graft survival.
There are several limitations to our current study. The analysis that we developed utilized a retrospective cohort of patients from a single academic transplant center located in the Southeast. As such, our study population may not represent the larger cohort of transplant patients across the US. Distinct differences include a high proportion of African Americans (68% compared to 38% in the US), predominantly rural population, and relatively low socioeconomic status. Also, there were few patients with glomuleronephritis etiologies of ESRD. Furthermore, it is unclear whether or not the Big Data variables included in this algorithm are partially or fully generalizable across kidney transplant recipients in the US or are mutable through intervention. Although our cohort was small (n = 1445) as compared to the US registry databases, it did provide for development of a strong predictive 5-year graft loss model. Lastly, as a matter of policy, patients were not transplanted with pre-existing donor specific antibodies. Therefore, data on donor specific antibodies was not included in the modelling. This model would benefit from validation by an analysis of a larger patient cohort from additional academic transplant centers to ensure widespread applicability.
Utilizing Big Data predictive analytics, a 5-year graft loss model was developed from data autonomously captured during the first post-transplant year. The model resulted in strong predictability and discernment and substantially improves upon models utilizing only baseline patient and donor information. Through automation and utilization of this model, there is the strong potential for post-transplant care to be individualized for patients, with the goal of mitigating important risk factors in these patients to improve long-term graft survival.
Supplementary Material
Acknowledgements:
Support for these studies was provided by National Institutes of Health; K23DK091514 (DD) and R03DK106432 (DD). Additional thanks to Ms. Rachel Mehard for editorial assistance.
Abbreviations page:
- ROD
Registered Organ Donor
- AA
African American
- eGFR
Estimated Glomerular Filtration Rate
- UNOS
United Network of Organ Sharing
- EHR
Electronic Health Record
- NLP
Natural Language Processing
- SRTR
Scientific Registry of Transplant Recipients
- HGB
Hemoglobin
- AUC
Area under the curve
- ROC
Receiver operating characteristic
- SBP
Systolic blood pressure
- KPDI
Kidney donor profile index
- USRDS
United States Renal Data System
Footnotes
Conflict of Interest Statement: No actual or potential conflicts of interest reported by authors.
References Cited
- 1.Lodhi SA, Meier-Kriesche HU. Kidney allograft survival: the long and short of it. Nephrol Dial Transplant 2011;26(1):15–7. [DOI] [PubMed] [Google Scholar]
- 2.Wekerle T, Segev D, Lechler R, Oberbauer R. Strategies for long-term preservation of kidney graft function. Lancet 2017;389(10084):2152–62. [DOI] [PubMed] [Google Scholar]
- 3.Galichon P, Xu-Dubois YC, Finianos S, Hertig A, Rondeau E. Clinical and histological predictors of long-term kidney graft survival. Nephrol Dial Transplant 2013;28(6):1362–70. [DOI] [PubMed] [Google Scholar]
- 4.Wang JH, Skeans MA, Israni AK. Current Status of Kidney Transplant Outcomes: Dying to Survive. Adv Chronic Kidney Dis 2016;23(5):281–6. [DOI] [PubMed] [Google Scholar]
- 5.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant 2009;9(3):527–35. [DOI] [PubMed] [Google Scholar]
- 6.Gupta G, Unruh ML, Nolin TD, Hasley PB. Primary care of the renal transplant patient. Journal of general internal medicine 2010;25(7):731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schachtner T, Reinke P. Provision of Highly Specialized Aftercare by the Transplant Center Strongly Improves Patient and Allograft Survival in Long-term Follow-up After Kidney Transplantation. Transplantation 2017;101:S27.28437369 [Google Scholar]
- 8.Srinivas TR, Taber DJ, Su Z, et al. Big Data, Predictive Analytics, and Quality Improvement in Kidney Transplantation: A Proof of Concept. Am J Transplant 2017;17(3):671–81. [DOI] [PubMed] [Google Scholar]
- 9.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care 2005;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 10.Harrell FE Jr., Lee KL, Mark DB Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in medicine 1996;15(4):361–87. [DOI] [PubMed] [Google Scholar]
- 11.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Statistics in medicine 2011;30(10):1105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uno H, Cai T, Tian L, Wei LJ. Evaluating prediction rules for t-year survivors with censored regression models. Journal of the American Statistical Association 2007;102:527–37. [Google Scholar]
- 13.Yaribakht S, Malartic C, Grange G, Morel O. [Operative risk related to tobacco in gynecology]. Gynecol Obstet Fertil 2014;42(5):343–7. [DOI] [PubMed] [Google Scholar]
- 14.Khullar D, Maa J. The impact of smoking on surgical outcomes. Journal of the American College of Surgeons 2012;215(3):418–26. [DOI] [PubMed] [Google Scholar]
- 15.Taber DJ, Hunt KJ, Fominaya CE, et al. Impact of Cardiovascular Risk Factors on Graft Outcome Disparities in Black Kidney Transplant Recipients. Hypertension 2016;68(3):715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bostom AD, Brown RS Jr., Chavers BM, et al. Prevention of post-transplant cardiovascular disease--report and recommendations of an ad hoc group. Am J Transplant 2002;2(6):491–500. [DOI] [PubMed] [Google Scholar]
- 17.Borra LC, Roodnat JI, Kal JA, Mathot RA, Weimar W, van Gelder T. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant 2010;25(8):2757–63. [DOI] [PubMed] [Google Scholar]
- 18.Sapir-Pichhadze R, Wang Y, Famure O, Li Y, Kim SJ. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int 2014;85(6):1404–11. [DOI] [PubMed] [Google Scholar]
- 19.van Gelder T Within-patient variability in immunosuppressive drug exposure as a predictor for poor outcome after transplantation. Kidney International 2014;85(6):1267–8. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson R Acute rejection episodes--best predictor of long-term primary cadaveric renal transplant survival. Clin Transplant 1994;8(3 Pt 2):328–31. [PubMed] [Google Scholar]
- 21.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004;4(3):378–83. [DOI] [PubMed] [Google Scholar]
- 22.Bamgbola OF. Spectrum of anemia after kidney transplantation: pathophysiology and therapeutic implications. Clin Transplant 2016;30(10):1185–94. [DOI] [PubMed] [Google Scholar]
- 23.Parajuli S, Clark DF, Djamali A. Is Kidney Transplantation a Better State of CKD? Impact on Diagnosis and Management. Adv Chronic Kidney Dis 2016;23(5):287–94. [DOI] [PubMed] [Google Scholar]
- 24.Joist H, Brennan DC, Coyne DW. Anemia in the kidney-transplant patient. Adv Chronic Kidney Dis 2006;13(1):4–10. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Song T, Fu L, et al. Post-renal transplantation anemia at 12 months: prevalence, risk factors, and impact on clinical outcomes. International urology and nephrology 2015;47(9):1577–85. [DOI] [PubMed] [Google Scholar]
- 26.Lofaro D, Greco R, Papalia T, Bonofiglio R. Increasing levels of hemoglobin improve renal transplantation outcomes. Transplant Proc 2011;43(4):1036–8. [DOI] [PubMed] [Google Scholar]
- 27.Choukroun G, Kamar N, Dussol B, et al. Correction of postkidney transplant anemia reduces progression of allograft nephropathy. J Am Soc Nephrol 2012;23(2):360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molnar MZ, Mucsi I, Macdougall IC, et al. Prevalence and management of anaemia in renal transplant recipients: data from ten European centres. Nephron Clinical practice 2011;117(2):c127–34. [DOI] [PubMed] [Google Scholar]
- 29.Molnar MZ, Tabak AG, Alam A, et al. Serum erythropoietin level and mortality in kidney transplant recipients. Clin J Am Soc Nephrol 2011;6(12):2879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furness PN, Kazi J, Levesley J, Taub N, Nicholson M. A neural network approach to the diagnosis of early acute allograft rejection. Transplant Proc 1999;31(8):3151. [DOI] [PubMed] [Google Scholar]
- 31.Decruyenaere A, Decruyenaere P, Peeters P, Vermassen F, Dhaene T, Couckuyt I. Prediction of delayed graft function after kidney transplantation: comparison between logistic regression and machine learning methods. BMC Med Inform Decis Mak 2015;15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoo KD, Noh J, Lee H, et al. A Machine Learning Approach Using Survival Statistics to Predict Graft Survival in Kidney Transplant Recipients: A Multicenter Cohort Study. Scientific reports 2017;7(1):8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldfarb-Rumyantzev AS, Scandling JD, Pappas L, Smout RJ, Horn S. Prediction of 3-yr cadaveric graft survival based on pre-transplant variables in a large national dataset. Clin Transplant 2003;17(6):485–97. [DOI] [PubMed] [Google Scholar]
- 34.Mansell H, Stewart SA, Shoker A. Validity of cardiovascular risk prediction models in kidney transplant recipients. TheScientificWorldJournal 2014;2014:750579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pita-Fernandez S, Pertega-Diaz S, Valdes-Canedo F, et al. Incidence of cardiovascular events after kidney transplantation and cardiovascular risk scores: study protocol. BMC cardiovascular disorders 2011;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keong FM, Afshar YA, Pastan SO, Chowdhury R, Binongo JN, Patzer RE. Decreasing Estimated Glomerular Filtration Rate Is Associated With Increased Risk of Hospitalization After Kidney Transplantation. Kidney international reports 2016;1(4):269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


