Abstract
Combination of natural products with chemodrugs is becoming a trend in discovering new therapeutics approach for enhancing the cancer treatment process. In the present study, we aimed to investigate the cytotoxic and apoptosis induction of Gelam honey (GH) combined with or without 5-Fluorouracil (5-FU) on HT-29 cells. The cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay to assess cytotoxicity. Morphological changes and apoptosis were determined by the inverted microscope, Annexin V-FITC, and DNA fragmentation via flow cytometric analysis, respectively. Our results demonstrate that combined treatment revealed a remarkable and concentration-dependent cytotoxic effect on HT-29 cells in comparison with GH and 5-FU alone. Flow cytometry analysis showed that early apoptosis event was more pronounced in combined treatment. In addition, compared to 5-FU alone, apoptosis of HT-29 cells treated with combinations of GH and 5-FU demonstrated increasing percentages of fragmented DNA. Our results suggest that GH has a synergistic cytotoxic effect with 5-FU in HT-29 cell lines in vitro. Although the actions of the molecular mechanisms are not yet clear, the results reveal that the combination of GH and 5-FU could have the potential as a therapeutic agent.
1. Introduction
Since antiquity, honey has been consumed as a daily nutritional supplement. Its major constituent is carbohydrates such as glucose, fructose, and sucrose. Honey bees collect pollen from flowers and later convert them into honey via regurgitations and evaporations. There are several varieties of honey in Malaysia, such as Honey, Tualang Honey, and Pineapple Honey. All of these are differentiated based on their dominant quantity of pollen, which can be identified by pollen analysis as the pollen is species-specific [1]. Honey is scientifically proven to have several medicinal properties such as antimicrobial [2, 3], antioxidant [4], anti-inflammatory [5], antitumour [6], and wound healing abilities [7]. Phenolic compounds inside honey, such as gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, ellagic acid, quercetin, hesperetin, and chrysin, are the major contributors to the anti-inflammatory and antitumour effects of honey [6, 8].
During the initial treatment of colorectal cancer, a chemotherapeutic drug known as 5-Fluorouracil (5-FU) is usually used. Its main mechanism involves the disruption of the normal functions of DNA and RNA via the misincorporation of fluoronucleotide into sequence, apart from inhibiting the function of thymidylate synthase [9]. However, 5-FU has been reported to be of low availability within the cells due to its degradation in the liver by the enzyme dipyrimidine dehydrogenase (DPD). Thus, a large dose is required during treatment [10]. Higher doses of this drug can cause severe side effects to the patients in addition to being very toxic to the human body. Past studies have found that combining the drug with natural substances such as honey can enhance its effect on cancerous cells and minimise its toxicity [11]. The usage of GH in combination with 5-FU has been shown to significantly reduce the growth of HCT-116 cells, unlike treatment with 5-FU alone [12]. In addition, GH plus ginger extracts has exhibited synergistic effects on HT-29 cells in terms of the upregulation of caspase-9 expression [13].
In this study, Gelam honey, 5-Fluorouracil, and their combination were used to determine the cytotoxic and apoptotic effects on HT-29 cells. Observations were made in terms of changes in the membrane integrity, fragmentation of DNA, and early events of apoptosis. These are useful for the creation of new strategies for the future treatment of colorectal cancer.
2. Materials and Methods
2.1. Honey Sample
The Gelam honey used in this study was obtained from Gelam Forest, Besut, Terengganu, Malaysia. Stock solution of honey was prepared by mixing the honey with RPMI-1640 medium and filter-sterilising using a 0.22-μm syringe filter. The Gelam honey used in this study has been tested by an accredited laboratory and confirmed to be pure honey.
2.2. Cell Line
Human colorectal adenocarcinoma HT-29 cells were obtained from American Tissue Culture Collection (Manassas, VA, USA). The cells were grown in RPMI-1640 medium (Sigma, St. Louis, USA), supplemented with 10% foetal bovine serum (GIBCO, USA) and antibiotics (i.e., 100.0 units/mL penicillin and 100.0 μg/mL streptomycin) (PAA, Austria). They were maintained in an incubator at 37°C with 5% CO2 and a humidified environment. The HT-29 cells were subcultured every 2 to 3 days in a semiconfluent condition in which they were treated with a trypsin-like enzyme and phenol red (GIBCO, USA) for 5 minutes. The cells were then resuspended in the medium with serum before being transferred into 2 or 3 new flasks. Samples with cell viability of 95% and above were selected for use throughout this study.
2.3. MTT Cytotoxicity Assay
The MTT assay was carried out in a 96-well plate, as described by Ali et al. [14]. A 100.0 μL of complete growth medium was placed into 96 flat-bottom microtiter plate (Nunclon, USA). This was followed by the addition of 100.0 μL of HT-29 cells at concentrations of 1-2 × 105 cells/mL that have been seeded for 24 h prior to usage. Honey samples (400 mg/ml) in RPMI-1640 medium were aliquoted into the wells in triplicate and serially diluted. Untreated cells were used as a control. The plate was incubated for 72 h in a CO2 incubator at 37°C. After incubation, 20.0 μL of MTT solution (5.0 mg/mL) was added to each well and further incubated for 4 h. The culture medium was then removed from the wells and 100.0 μL of dimethylsulphoxide (DMSO) added to each well to solubilise the resulting formazan [15]. The optical densities (OD) of the wells were analysed at 570 nm using a plate reader (BIOTEK, USA), with a reference at 630 nm. A dose-response curve of cell viability versus sample concentration was subsequently plotted.
2.4. Acridine Orange and Propidium Iodide Staining (AO/PI) Analysis
The HT-29 cells were treated with GH, 5-FU, and a combination of both for 24, 48, and 72 h. After being incubated for 24 h, the cells were harvested into centrifuge tubes and pelleted down at 300 × g for 10 min. The cell pellets were washed with PBS by centrifuging as mentioned above. The pellets were then suspended in 50.0 μL of acridine orange (10.0 μg/mL) and 50.0 μL of propidium iodide (10.0 μg/mL) for 5 min. A volume of 10.0 μL of stained cells was pipetted onto a glass slide and covered with a cover slip. The viable, apoptotic, and necrotic cells were scored in populations of more than 100 cells using an inverted fluorescence microscope (Nikon TE2000-U, Nikon, Japan), as described by Ali et al. [16]
2.5. Phosphatidylserine Externalisation Analysis by Flow Cytometry
An Apoptosis Detection Kit (BD Annexin V-FITC) was used for flow cytometry analysis. The kit contained Annexin V conjugated with fluorochrome FITC, propidium iodide, and a binding buffer. HT-29 cells (2 × 105 cells/mL) were treated with GH, 5-FU, and a combination of both in 6 wells for 3, 6, and 12 h. Following the completion of treatment, the cells were harvested into 5-mL tubes and centrifuged at 300 × g in a swing rotor for 10 minutes. The cell pellets were washed twice with PBS, after which 100 μl of binding buffer was added to the tubes. A volume of 5 μl of Annexin V FITC and 5 μl of PI were added to the tubes as a staining solution. The mixture was then incubated in the dark for 15 minutes. This was followed by addition of 400 μl of binding buffer to each tube. The tubes were gently vortexed prior to analysis using a CytoFLEX flow cytometer (Beckman Coulter, USA). Approximately 10,000 events were sorted accordingly into viable, early apoptotic, late apoptotic, and necrotic cells stages [17, 18].
2.6. DNA Fragmentation Analysis by Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labelling (TUNEL)
TUNEL assay was carried out using APODIRECT™ Kit in accordance with the manufacturer's protocol (Becton Dickinson, USA). Cells at the concentration 1 × 106 cells/mL were fixed in 1% (w/v) paraformaldehyde with PBS (pH 7.4) for 60 minutes on ice. The cells were then pelleted by centrifugation at 300 × g for 5 minutes before being washed twice with 5 mL of PBS. The cells were then resuspended in 70% (v/v) ice-cold ethanol and stored for 1 week at -20°C. After incubation, the ethanol was removed and the cells were washed twice using a washing buffer. The cells were then subjected to end-labelling by incubation in 50 μl of a DNA Labelling Solution (containing TdT Enzyme and FITC dUTP dissolved in the reaction buffer) for 60 minutes at 37°C. Following incubation, the cells were washed twice in 1 mL of rinse buffer prior to staining with 0.5 mL of PI/RNase Staining Buffer. The staining process was carried out for 30 minutes in a dark environment. After that, the cells were then analysed in CytoFLEX (Beckman Coulter, USA) using the CytExpert software.
2.7. Statistical Analysis
The numerical parameters were expressed as means ± standard error means (S.E.M.). All experiments were performed in triplicate and analysed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. Values with confidence levels of p ≤0.05 were considered to be statistically significant.
3. Results
3.1. Cytotoxicity of Gelam Honey, 5-Fluorouracil, and Their Combination in HT-29 Cells and Normal Colon Cells
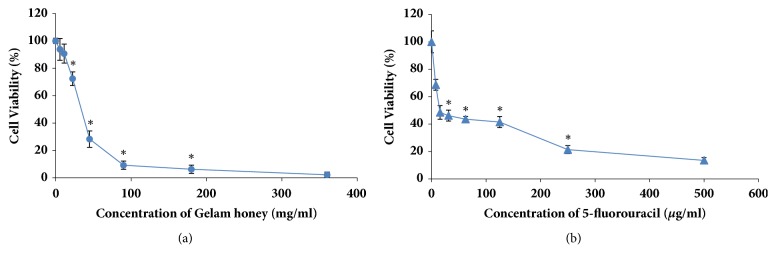
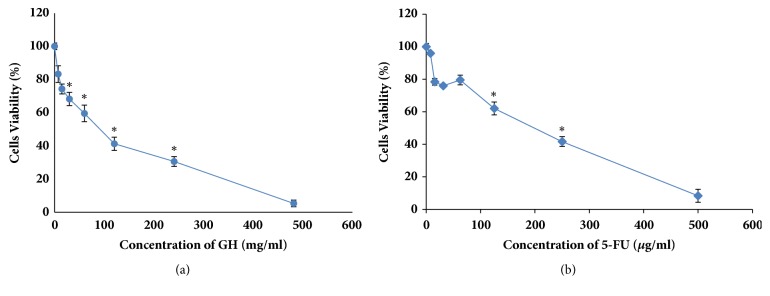
Gelam honey was examined for its cytotoxicity towards HT-29 cells using a MTT assay. The optical density of the resulting formazan blue was determined, which reflected the normal function of mitochondrial dehydrogenase in viable cells [15]. The degrees of toxicities of GH, 5-FU, and their combination after 72 h treatment (with respect to the untreated cells) were determined according to their respective concentrations that reduced the cell populations to 50% (CD50). Gelam honey reduced the number of viable HT-29 cells at a CD50 of 36.2 mg/ml. Meanwhile, the CD50 of 5-FU was 15.5 μg/ml (Figure 1). From the results, the CD25 and CD75 of 5-FU were 6.25 μg/ml and 227.4 μg/ml, respectively. The combination of CD50 concentration of GH with three different concentrations (CD25, CD50, and CD75) of 5-FU reduced the CD50 of GH to 30.4 mg/ml, 13.2 mg/ml, and 2.4 mg/ml, respectively (Figure 2). The cytotoxicities of GH and 5-FU were also tested on a normal colon cell line, CCD-18Co. The CD50 value of GH in CCD-18Co cells was 89.8 mg/ml. Meanwhile the CD50 of 5-FU in CCD-18Co cells was 203.1 μg/ml (Figure 3). Therefore, the combination of 36.2 mg/ml GH and 15.5 μg/ml 5-FU was chosen as both values were below the CD50 values of GH and 5-FU in normal colon cells. The combination index was calculated to ascertain the synergistic activity of the combination of GH and 5-FU [19]. The combination index (CI) analysis value obtained was less than 1, indicating that there was a synergistic effect between GH and 5-FU. The CD50 values of GH, 5-FU, and their combination (all at CD50 concentrations) were used in every subsequent assay of this study.
Figure 1.
Cytotoxicity of GH and 5-FU against HT-29 cells after 72 h of treatment. The CD50 of GH was 36.2 mg/ml (a) and the CD50 of 5-FU was 15.5 μg/ml (b). Each point represents the mean of the results of three independent experiments. Error bars represent means ± S.E.M. of the three independent experiments.∗Statistical significance (P<0.05) between control cells and treatment groups.
Figure 2.
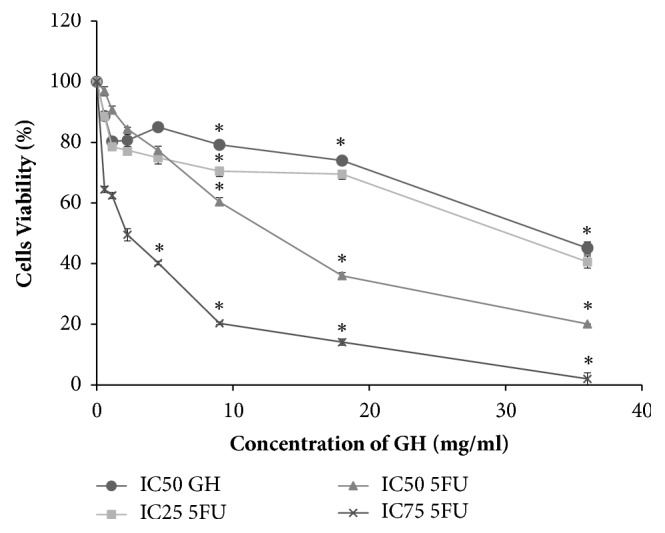
Effects of GH at CD50 concentration on the viability of HT-29 cells after incubation in different concentrations of 5-FU for 72 h. Each point represents the mean of the results of three independent experiments. Error bars represent means ± S.E.M. of the three independent experiments. ∗Statistical significance (P<0.05) between control cells and treatment groups.
Figure 3.
Cytotoxicity of GH and 5-FU against normal colon cells (ATCC® CRL-1459) after 72 h of treatment. The CD50 of GH was 89.8 mg/ml (a) and the CD50 of 5-FU was 203.1 μg/ml (b). Each point represents the mean of the results of three independent experiments. Error bars represent means ± S.E.M. of the three independent experiments. ∗Statistical significance (P<0.05) between control cells and treatment groups.
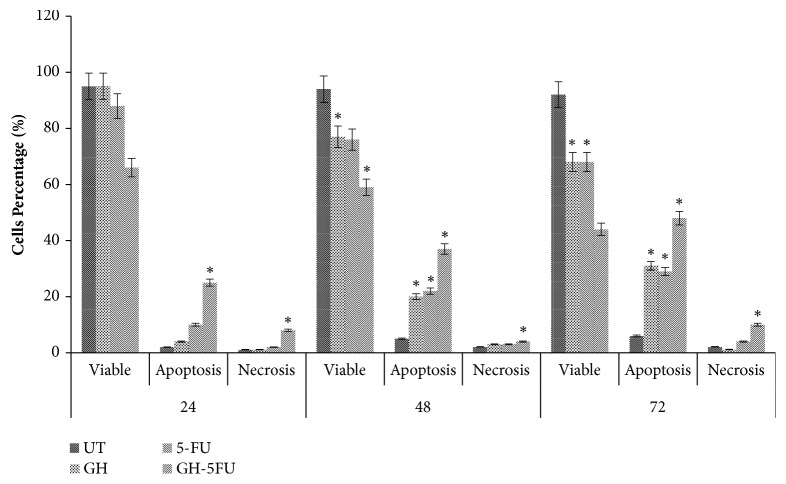
3.2. Mode of Cell Death by Dual-Staining with Acridine Orange and Propidium Iodide
Morphological assessments of the cells (using acridine orange and propidium iodide) were conducted to determine the modes of death of the treated HT-29 cells at CD50 doses. As shown in Figure 4, the number of apoptotic cells increased significantly after treatment with GH from 20.67% at 24 h to 34.67% at 48 h and 41.03% at 72 h. Similarly, in the positive control (5-FU-treated cells), the corresponding values were 7.67% at 24 h, 22.02% at 48h, and 23.01% at 72 h. The highest percentages of apoptotic cells were recorded for the combined treatment of GH + 5-FU, with values of 25.33% at 24 h, 33.05% at 48 h, and 46.67% at 72 h. A low percentage of necrotic cells was detected in the treatment groups, which did not differ significantly from the untreated group.
Figure 4.
Mode of cell death of untreated HT-29 cells and those which have been treated with GH, 5-FU, and both. Error bars represent means ± S.E.M. of the three independent experiments. ∗Statistical significance (p<0.05) between control cells and treatment groups.
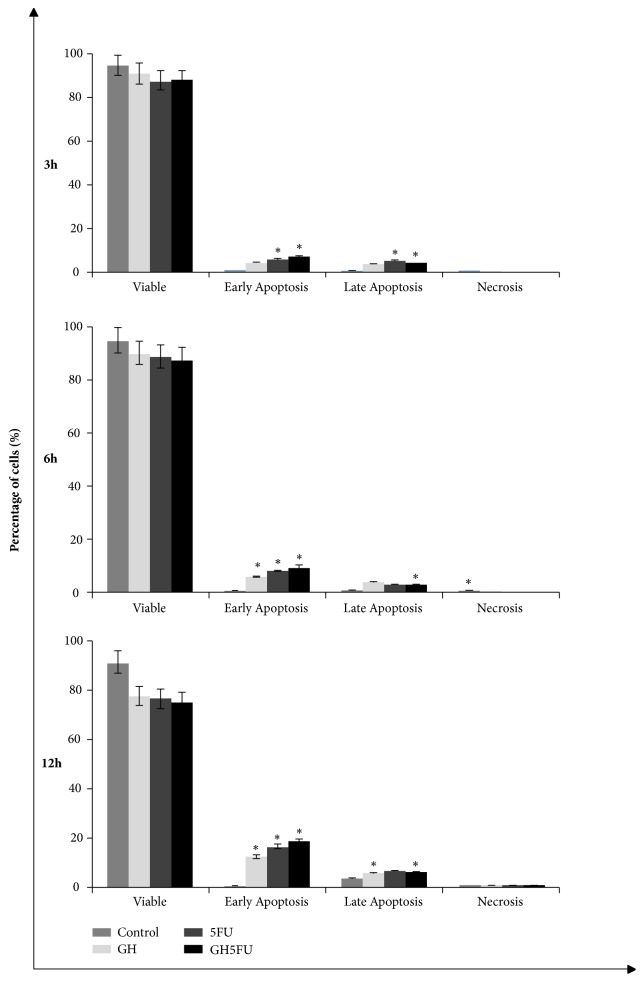
3.3. Phosphatidylserine Externalisation Analysis in Terms of Early Apoptotic Events
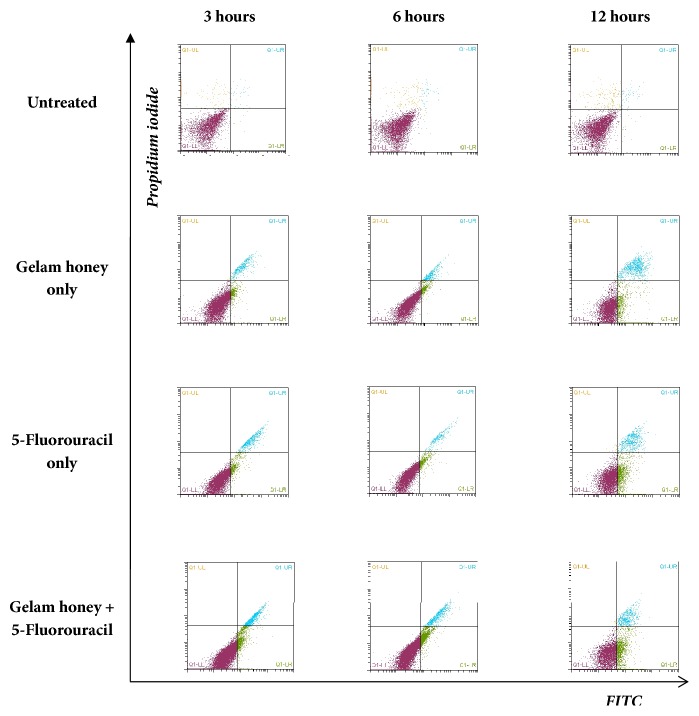
Phosphatidylserine externalisation is an early event during apoptosis, which can be evaluated by using Annexin V and PI staining. In this study, it was found that this early event of apoptosis was detected in all groups within 3 h of treatment at CD50 dose (Figures 6 and 7). For the GH-treated specimens, the percentages of early apoptotic events were 4.66% at 3 h, 6.20% at 6 h, and 12.31% at 12 h. The percentages of late events measured were 4.5% at 3 h, 3.85% at 6 h, and 10.16% at 12 h. No events of necrosis were detected in the GH-treated specimens. Similar measurements were conducted for the 5-FU-treated specimens. The percentages of early apoptotic events in the 5-FU-treated specimens increased over time (6.66% at 3 h, 8.11% at 6 h, and 16.56% at 12 h). However, there were lower percentages of late apoptotic events in the 5-FU-treated specimens as compared to the GH-treated groups (4.41% at 3 h, 3.05% at 6 h, and 6.79% at 12 h). Higher percentages of apoptotic cells were obtained in the combined (GH + 5-FU) treatment group as compared to the either GH or 5-FU alone. In the combined treatment, the percentages of early apoptotic events were 7.51% at 3 h, 9.37% at 6 h, and 18.64% at 12h.
Figure 6.
Analysis of phosphatidylserine externalisation in HT-29 cells via Annexin V and PI staining using a flow cytometer. Results are presented as means ± S.E.M. of three independent experiments. ∗Statistical significance (P<0.05) between control cells and treatment groups.
Figure 7.
Flow cytometry analysis of untreated and CD50-treated HT-29 cells via staining with Annexin V FITC/propidium iodide (PI). Viable cells are in the lower left quadrant, early apoptotic cells are in the lower right quadrant, late apoptotic cells are in the upper right quadrant, and nonviable necrotic cells are in the upper left quadrant. Each dot in the plot represents 10,000 cells in a single replicate.
3.4. DNA Fragmentation Analysis: Hallmark Apoptosis Detection
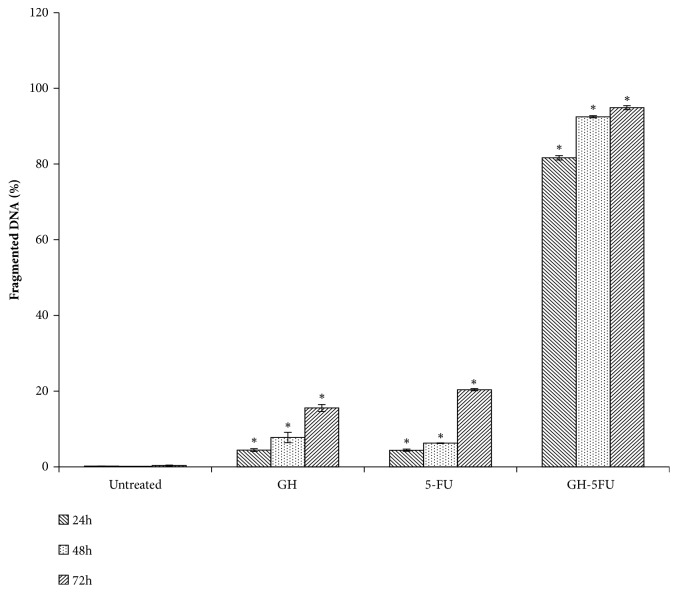
DNA fragmentation is one of the hallmark events of apoptosis. A quantitative evaluation of DNA fragmentation in the cells was performed using TUNEL assay. After a 24 h treatment period, the DNA inside the HT-29 cells started to undergo cleavage in all treatment groups (with the percentages of such cells being 4.44% in GH treatment, 4.21% in 5-FU treatment, and 71.67% in the combined GH + 5-FU treatment). Figure 8 shows a significant increase in the percentage of TUNEL-positive cells in the combined GH + 5-FU treatment group (92.49% after 48 h and 94.90% after 72 h). It was noted that all treatments at CD50 doses increased the percentages of TUNEL-positive cells with time. In contrast, the control groups recorded a very low incidence of DNA cleavage (less than 0.3%) throughout the treatment period. This showed that DNA cleavage was not a normal occurrence in healthy HT-29 cells, hence suggesting that the cause of the same was the cytotoxicities of GH and 5-FU.
Figure 8.
DNA fragmentation of HT-29 cells after treatment with GH, 5-FU, and both. Results are expressed as percentages of cells stained by terminal dUTP nick-end labelling (TUNEL positive). Error bars represent means ± S.E.M. of the three independent experiments. ∗Statistical significance (P<0.05) between control cells and treatment groups.
4. Discussion
Over the past few years, cancer chemoprevention and therapy using traditional medicines have garnered a great deal of research attention globally. In this study, the cytotoxic and apoptosis induction of Gelam honey (GH) combined with or without 5-Fluorouracil (5-FU) on HT-29 cell were investigated. The cytotoxic result revealed that GH exhibited the ability to reduce the viability of HT-29 cells, and in combined treatment the cytotoxic dose reduced compared with GH and 5-FU alone in time and dose dependent manner. The results also indicated synergistic effect of GH and chemoagent 5-FU in altering growth of HT-29 cells, which was quantitatively analysed according to Chou-Talalay method [19]. Previous study showed that GH has high levels of total phenolic compound which correlated significantly to its bioactivity [20]. It is also found that phenolic compounds in honey contribute directly to the reduction of the colon cancer cells viability [21]. Similar effects could not be seen in normal colon cells, suggesting that GH activity is cell dependent and therefore has the potential to be used as alternative for colon cancer therapy in combinations with 5-FU. 5-FU is a well-known drug and widely used in colon chemotherapy. Numerous strategies have been established to improve the activity of 5-FU, one of which is the use of combination therapy [22–24]. In common clinical practice, the combination of 5-FU with leucorin, irinotecan, or oxaliplatin has shown improvements of this drug activity [25] However, side effects associated with 5-FU might hamper the effort to treat cancer patients [26]. With the aim of highlighting the hypothesis that GH could possibly enhance the 5-FU activity, the combinations of GH and 5-FU initiated by Hakim et al. revealed that GH can be a potential candidate to work synergistically with 5-FU [12]. However, the discovery of its potential value was not sufficient and more data need to be acquired to reveal its benefits. To the best of our knowledge, no experimental evidence to date has shown the association of Gelam honey and 5-FU in inducing apoptosis in HT-29 cells, respectively. Nevertheless, researches on the bioactivity of Gelam honey alone were intensively being done. Gelam honey is a well-known prominent substance, inexpensive, relatively nontoxic, and easily available in marketplace. Furthermore, honey as regards its origin has been used since the ancient times. Gelam honey has been considered as a potential cancer therapy that has the ability to reduce the viability various cancerous cells line such as MCF-7, HCT-116, HepG2, A549, and HT-29 [12, 27–30].
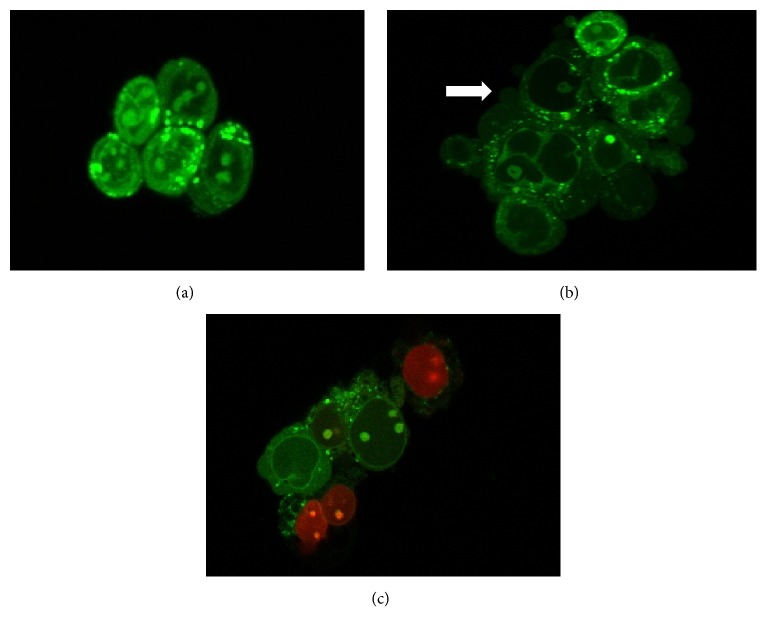
Many studies in vitro and in vivo showed that GH alone has significant antiproliferative and apoptotic effects observed in cancer cells treated by GH [13, 27, 28, 30, 31]. In cancer cells, the induction of apoptosis is known to be an efficient strategy for cancer therapy. Apoptosis is a programmed cell death that becomes focused in the field of cell death which involved either intrinsic (mitochondria dependent) or extrinsic (death receptor dependent) pathway [32–34]. During early event of apoptosis, translocation of phosphatidylserine (PS) from inner surface of the cell membrane to outer cell membrane occurred [35, 36]. We demonstrated that the phosphatidylserine (PS) was translocated as early as 3 hours after exposing the HT-29 cells to the treatment. Interestingly, the combined treatment showed that the highest percentage of PS was translocated in cells. Even though the translocation of PS has been reported in reversible process [37], our morphology examination showed that the cells treated in the combination treatment experienced blebbing characteristics with an increasing number of cells compared to a single treatment (Figure 5). These confirm that HT-29 cells undergo apoptosis and are not reversible. In addition, our result in DNA fragmented analysis showed that the combination of GH and 5-FU increased the fragmented DNA in HT-29 cells. DNA fragmentation is one of the late stage processes occurring in apoptosis pathway [38, 39]. Collectively, these findings further support the idea that cytotoxic effect of GH and 5-FU and combinations of both induced apoptosis in HT-29 cells in a time-dependent manner.
Figure 5.
Fluorescent photomicrographic evidence of HT-29 cells 24 h after treatment. Morphological changes following exposure to treatment are typical of apoptosis; A: viable cell, B: apoptotic cell, C: necrotic cell. The arrow ( ) shows membrane blebbing—one of the characteristics of apoptosis. This was viewed using a laser confocal inverted microscope at magnification of 630X.
) shows membrane blebbing—one of the characteristics of apoptosis. This was viewed using a laser confocal inverted microscope at magnification of 630X.
5. Conclusion
In conclusion, the combination of GH and 5-FU had a synergistic outcome with respect to the cytotoxic and apoptotic effects on human colorectal cancer HT-29 cells. The synergistic interaction of 5-FU and GH on HT-29 cells warrants further investigation of the underlying molecular mechanism on HT-29 cells where focus should be on the molecular interaction to find out specifically in which pathway GH enhances the efficacy of 5-FU.
Acknowledgments
The authors would like to thank the Faculty of Bioresources and Food Industry, Universiti Sultan Zainal Abidin; and the School of Fundamental Science, Universiti Malaysia Terengganu; and the Universiti Islam Malaysia for the technical and financial support of the project and Ministry of Education Malaysia for Ph.D. scholarship support.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding this paper publication.
References
- 1.Olusola A., Oluwatoyin O. Nectar sources for the honey bee (Apis mellifera adansonii) revealed by pollen content. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2009;37:211–217. [Google Scholar]
- 2.Nasir N.-A. M., Halim A. S., Singh K.-K. B., Dorai A. A., Haneef M.-N. M. Antibacterial properties of tualang honey and its effect in burn wound management: a comparative study. BMC Complementary and Alternative Medicine. 2010;10, article 31 doi: 10.1186/1472-6882-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aljadi A. M., Yusoff K. M. Isolation and identification of phenolic acids in Malaysian honey with antibacterial properties. Turkish Journal of Medical Sciences. 2003;33(4):229–236. [Google Scholar]
- 4.Erejuwa O. O., Sulaiman S. A., Ab Wahab M. S. Honey: a novel antioxidant. Molecules. 2012;17(4):4400–4423. doi: 10.3390/molecules17044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nooh H. Z., Nour-Eldien N. M. The dual anti-inflammatory and antioxidant activities of natural honey promote cell proliferation and neural regeneration in a rat model of colitis. Acta Histochemica. 2016;118(6):588–595. doi: 10.1016/j.acthis.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Wen C. T. P., Hussein S. Z., Abdullah S., Karim N. A., Makpol S., Yusof Y. A. M. Gelam and nenas honeys inhibit proliferation of HT 29 colon cancer cells by inducing DNA damage and apoptosis while suppressing inflammation. Asian Pacific Journal of Cancer Prevention. 2012;13(4):1605–1610. doi: 10.7314/APJCP.2012.13.4.1605. [DOI] [PubMed] [Google Scholar]
- 7.Lusby P. E., Coombes A., Wilkinson J. M. Honey: a potent agent for wound healing? Journal of Wound Ostomy & Continence Nursing. 2002;29(6):295–300. doi: 10.1067/mjw.2002.129073. [DOI] [PubMed] [Google Scholar]
- 8.Hussein S. Z., Mohd Yusoff K., Makpol S., Mohd Yusof Y. A. Gelam honey inhibits the production of proinflammatory, mediators NO, PGE2, TNF-α, and IL-6 in carrageenan-induced acute paw edema in rats. Evidence-Based Complementary and Alternative Medicine. 2012;2012:13. doi: 10.1155/2012/109636.109636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longley D. B., Harkin D. P., Johnston P. G. 5-Fluorouracil: mechanisms of action and clinical strategies. Nature Reviews Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 10.Diasio R. B., Harris B. E. Clinical pharmacology of 5-fluorouracil. Clinical Pharmacokinetics. 1989;16(4):215–237. doi: 10.2165/00003088-198916040-00002. [DOI] [PubMed] [Google Scholar]
- 11.Meregalli M., Martignoni G., Frontini L., Zonato S., Pavia G., Beretta G. Increasing doses of 5-fluorouracil and high-dose folinic acid in the treatment of metastatic colorectal cancer. Tumori. 1998;84(6):662–665. doi: 10.1177/030089169808400609. [DOI] [PubMed] [Google Scholar]
- 12.Hakim L., Alias E., Makpol S., Ngah W.Z. W., Morad N. A., Yusof Y. A. Gelam honey and ginger potentiate the anti cancer effect of 5-FU against HCT 116 colorectal cancer cells. Asian Pacific Journal of Cancer Prevention. 2014;15(11):4651–4657. doi: 10.7314/apjcp.2014.15.11.4651. [DOI] [PubMed] [Google Scholar]
- 13.Tahir A. A., Sani N. F. A., Murad N. A., Makpol S., Ngah W. Z. W., Yusof Y. A. M. Combined ginger extract & Gelam honey modulate Ras/ERK and PI3K/AKT pathway genes in colon cancer HT29 cells. Nutrition Journal. 2015;14:31–40. doi: 10.1186/s12937-015-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali A. M., Mackeen M. M., El-Sharkawy S. H., et al. Antiviral and cytotoxic activities of some plants used in Malaysian indigenous medicine. Pertanika Journal of Tropical Agricultural Science. 1996;19:129–136. [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Ali A. M., Umar N. T., Mohamed S., et al. Induction of apoptosis in leukaemic cells by goniothalamin. Journal of Biochemistry Molecular Biology and Biophysics. 2001;5:227–235. [Google Scholar]
- 17.Majno G., Joris I. Apoptosis, oncosis, and necrosis: an overview of cell death. The American Journal of Pathology. 1995;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- 18.Tajudin T.-J. S. A., Mat N., Siti-Aishah A. B., Yusran A. A. M., Alwi A., Ali A. M. Cytotoxicity, antiproliferative effects, and apoptosis induction of methanolic extract of Cynometra cauliflora linn. Whole fruit on human promyelocytic leukemia HL-60 cells. Evidence-Based Complementary and Alternative Medicine. 2012;2012:6. doi: 10.1155/2012/127373.127373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou T., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 20.Hussein S. Z., Yusoff K. M., Makpol S., Yusof Y. A. M. Antioxidant capacities and total phenolic contents increase with gamma irradiation in two types of Malaysian honey. Molecules. 2011;16(8):6378–6395. doi: 10.3390/molecules16066378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaganathan S. K. Honey Constituents and their apoptotic effect in colon cancer cells. Journal of ApiProduct and ApiMedical Science. 2009;1(2):29–36. doi: 10.3896/IBRA.4.01.2.02. [DOI] [Google Scholar]
- 22.Tong J., Xie G., He J., Li J., Pan F., Liang H. Synergistic antitumor effect of dichloroacetate in combination with 5-fluorouracil in colorectal cancer. Journal of Biomedicine and Biotechnology. 2011;2011:7. doi: 10.1155/2011/740564.740564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang X.-Y., Zhu Y.-Q., Tao W.-H., Wei B., Lin X.-L. Synergistic effect of triptolide combined with 5-fluorouracil on colon carcinoma. Postgraduate Medical Journal. 2007;83(979):338–343. doi: 10.1136/pgmj.2006.055426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng P. L., Rajab N. F., Then S. M., et al. Piper betle leaf extract enhances the cytotoxicity effect of 5-fluorouracil in inhibiting the growth of HT29 and HCT116 colon cancer cells. Journal of Zhejiang University SCIENCE B. 2014;15(8):692–700. doi: 10.1631/jzus.B1300303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolpin B. M., Mayer R. J. Systemic treatment of colorectal cancer. Gastroenterology. 2008;134(5):1296–e1. doi: 10.1053/j.gastro.2008.02.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saif M. W., Choma A., Salamone S. J., Chu E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: A rational approach to improving therapeutic outcomes. Journal of the National Cancer Institute. 2009;101(22):1543–1552. doi: 10.1093/jnci/djp328. [DOI] [PubMed] [Google Scholar]
- 27.Wee L. H., Morad N. A., Aan G. J., Makpol S., Wan Ngah W. Z., Yusof Y. A. M. Mechanism of chemoprevention against colon cancer cells using combined Gelam honey and Ginger extract via mTOR and Wnt/β-catenin pathways. Asian Pacific Journal of Cancer Prevention. 2015;16(15):6549–6556. doi: 10.7314/APJCP.2015.16.15.6549. [DOI] [PubMed] [Google Scholar]
- 28.Abu M. N., Salleh M. A. M., Eshak Z., Hasan M. H., Hassan H. F., Ismail W. I. W. Anti-proliferative effect of Tinaspora crispa (L.) Hook. F. & Thompson and Gelam (Melaleuca sp.) honey on several cancer cell lines. Proceedings of the IEEE Symposium on Business, Engineering and Industrial Applications (ISBEIA '11); September 2011; pp. 545–548. [DOI] [Google Scholar]
- 29.Jubri Z., Narayanan N. N. N., Karim N. A., Ngah W. Z. W. Antiproliferative activity and apoptosis induction by Gelam honey on liver cancer cell line. International Journal of Applied Science and Technolog. 2012;2:135–141. [Google Scholar]
- 30.Fadhli M. R. M. N., Noorfathiah I., Hana M. A. N. F., et al. Gelam (Melaleuca sp.) honey demonstrates antiproliferative effect on colon cancer cell lines (HCT116). Proceedings of the IEEE Symposium on Business, Engineering and Industrial Applications (ISBEIA '12); September 2012; pp. 362–364. [Google Scholar]
- 31.Ahmed S., Othman N. H. Honey as a potential natural anticancer agent: a review of its mechanisms. Evidence-Based Complementary and Alternative Medicine. 2013;2013:7. doi: 10.1155/2013/829070.829070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elmore S. Apoptosis: a review of programmed cell death. Toxicologic Pathology. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen M., Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis. 2002;7(4):313–319. doi: 10.1023/A:1016167228059. [DOI] [PubMed] [Google Scholar]
- 34.Green D. R. Apoptotic pathways: Ten minutes to dead. Cell. 2005;121(5):671–674. doi: 10.1016/j.cell.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Bossy-Wetzel E., Green D. R. Detection of apoptosis by annexin V labeling. Methods in Enzymology. 2000;322:15–39. doi: 10.1016/S0076-6879(00)22004-1. [DOI] [PubMed] [Google Scholar]
- 36.Savill J., Fadok V., Henson P., Haslett C. Phagocyte recognition of cells undergoing apoptosis. Trends in Immunology. 1993;14(3):131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 37.Balasubramanian K., Mirnikjoo B., Schroit A. J. Regulated externalization of phosphatidylserine at the cell surface: Implications for apoptosis. The Journal of Biological Chemistry. 2007;282(25):18357–18364. doi: 10.1074/jbc.M700202200. [DOI] [PubMed] [Google Scholar]
- 38.Nagata S. Apoptotic DNA fragmentation. Experimental Cell Research. 2000;256(1):12–18. doi: 10.1006/excr.2000.4834. [DOI] [PubMed] [Google Scholar]
- 39.Collins J. A., Schandl C. A., Young K. K., Vesely J., Willingham M. C. Major DNA fragmentation is a late event in apoptosis. Journal of Histochemistry & Cytochemistry. 1997;45(7):923–934. doi: 10.1177/002215549704500702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.