Abstract
IL-6 signals through the ubiquitously expressed glycoprotein 130 (gp130) transmembrane protein to activate intracellular signaling that includes signal transducer and activator of transcription 3 (STAT3) and extracellular signal-regulated kinase 1/2 (ERK1/2). Dynamin-1-like protein (DRP-1) and mitochondrial fission 1 protein (FIS-1) are key proteins in the process of mitochondrial fission and have emerged as IL-6-sensitive targets. The purpose of this study was to examine the regulation of DRP-1 and FIS-1 expression by IL-6 and gp130 signaling in myotubes and skeletal muscle. Fully differentiated C2C12 myotubes were treated with 100 ng of IL-6 for 24 hours in the presence of gp130siRNA, C188-9 (STAT3 inhibitor), or PD98059 (ERK1/2 inhibitor). Male C57BL/6 (B6) and muscle-specific gp130 knockout mice (KO) had IL-6 systemically overexpressed for 2 weeks by transient transfection with 50 ng of an IL-6-expressing or control plasmid in the quadriceps muscles, and the tibialis anterior muscle was analyzed to determine systemic effects of IL-6. IL-6 induced DRP-1 and FIS-1 expression in myotubes 124% and 82% (p = .001) and in skeletal muscle 97% and 187% (p = .001). Myotube gp130 knockdown suppressed the IL-6 induction of DRP-1 68% (p = .002) and FIS-1 65% (p = .001). Muscle KO suppressed the IL-6 induction of DRP-1 220% (p = .001) and FIS-1 121% (p = .001). ERK1/2 inhibition suppressed the IL-6 induction of DRP-1 59% (p = .0003) and FIS-1 102% (p = .0001) in myotubes, while there was no effect of STAT3 inhibition. We report that chronically elevated IL-6 can directly induce DRP-1 and FIS-1 expression through gp130 signaling in cultured myotubes and skeletal muscle. Furthermore, ERK 1/2 signaling is necessary for the IL-6 induction of DRP-1 and FIS-1 expression in myotubes.
1. Introduction
Chronic inflammation is a hallmark of many illnesses, `including cancer, diabetes, and cardiovascular disease. Furthermore, skeletal muscle glucose metabolism and mass regulation are disrupted by these conditions [1, 2]. The interleukin-6 (IL-6) cytokine family has been investigated extensively as a critical driver of inflammation during chronic disease and is an established effector of skeletal muscle dysfunction [2–6]. IL-6 is a pleiotropic cytokine capable of serving as both pro- and anti-inflammatory. Classically, intracellular IL-6 signaling is induced through binding with a specific IL-6 cytokine receptor that dimerizes with glycoprotein 130 (gp130), a ubiquitously expressed transmembrane protein [7–9]. IL-6 signaling can also be initiated through trans-signaling, whereby IL-6 binds to the soluble form of the IL-6 receptor to initiate cellular signaling through interaction with gp130 on the cell membrane [10]. IL-6 is capable of inducing several intracellular signaling pathways that can regulate skeletal muscle mass and metabolism. IL-6 can induce skeletal muscle signal transducer and activator of transcription 3 (STAT3) and extracellular regulated kinase (ERK1/2) in several preclinical cancer cachexia models [2, 3, 11–15]. While IL-6 signaling has established a role in the regulation of muscle mass and metabolism; a role for regulating skeletal muscle mitochondria homeostasis has not been clearly established.
Skeletal muscle mitochondria are essential for maintaining metabolic plasticity and function [4, 16, 17]. Mitochondrial quality control encompasses the biogenesis, turnover (mitophagy), and remodeling (dynamics) of mitochondria [18–22]. Chronic disease can disrupt all of these skeletal muscle mitochondria quality control components, and they have been connected to the skeletal muscle proteostasis that occurs with these conditions [18]. Mitochondrial remodeling (dynamics) is a process that consists of constant fission and fusion of mitochondria in response to metabolic stressors [19, 21, 23]. Fission is controlled by GTPase cytosolic dynamin-related protein-1 (DRP-1), which will translocate to the outer mitochondrial membrane and develop active fission sites [17, 23–26]. Fission protein 1 (FIS-1) recruits DRP-1 to the mitochondria [21, 22]. The acceleration of fission can result in the isolation of mitochondria from the network and reduced ATP efficiency, resulting in turnover or apoptosis [17, 22, 27]. Altered mitochondrial fission has been linked to skeletal muscle mass regulation [19, 21, 28]. Since accelerated fission can result in muscle metabolic dysfunction, and the attenuation of fission can result in muscle atrophy, mitochondrial remodeling processes appear necessary for overall muscle homeostasis [18, 29].
STAT3 and ERK1/2 are downstream effectors of IL-6 that have established roles in skeletal skeletal muscle mass regulation. STAT3 is a widely investigated downstream effector of IL-6 in skeletal muscle [10, 14, 30–34], and chronic STAT3 activation can drive muscle atrophy through accelerated protein degradation [3, 4, 33, 35]. STAT3 can target mitochondrial function through complex I suppression [36]. ERK1/2 signaling is also an established regulator of skeletal muscle dysfunction during cancer cachexia, chemotherapy, and exercise [37–41]. ERK1/2 activation coincides mitochondrial content loss and biogenesis suppression during chemotherapy-induced cachexia [41]. Furthermore, ERK1/2 activation can promote DRP-1-mediated fission in MEF cells [42], which has not been established in skeletal muscle. While STAT3 and ERK1/2 are well-characterized signaling pathways in skeletal muscle, their regulation of skeletal muscle mitochondrial fission warrants further investigation.
Chronically elevated circulating IL-6 has been reported in cancer patients and preclinical models of cancer cachexia. Muscle wasting is also often associated with increased skeletal muscle expression of mitochondrial fission regulating proteins [4, 20, 22, 24]. Furthermore, increased FIS-1 expression in cachectic skeletal muscle from tumor-bearing mice is dependent on IL-6; tumor-bearing mice treated with an IL-6 receptor antibody demonstrated an attenuation of muscle STAT3 and FIS-1 expression [15]. Our laboratory has also demonstrated that chronically elevated IL-6 can increase STAT3 activation and FIS-1 expression in myotubes and skeletal muscle independent of a cancer environment [15]. While these findings suggest that circulating IL-6 can regulate skeletal muscle mitochondrial fission [4, 15], the intracellular signaling that links IL-6 to increased mitochondrial fission requires further investigation. Therefore, the purpose of this study was to examine the regulation of DRP-1 and FIS-1 expression by IL-6 and gp130 signaling in skeletal muscle. Additionally, we investigated the role of intracellular STAT3 and ERK 1/2 activation in myotubes. We report that chronically elevated IL-6 can directly induce DRP-1 and FIS-1 expression through gp130 signaling in cultured myotubes and skeletal muscle. Furthermore, ERK 1/2 signaling is necessary for the IL-6 induction of DRP-1 and FIS-1 expression in myotubes.
2. Methods
2.1. Animals
Male C57BL/6 (Bar Harbor, ME, USA) were bred at the University of South Carolina's animal resource facility, as previously described [7, 43, 44]. Male mice on a C57BL/6 background were bred with gp130 floxed mice provided by Dr. Colin Stewart's laboratory [Laboratory of Cancer and Developmental Biology, National Cancer Institute, US National Institutes of Health (NIH), Frederick, MD, USA] in collaboration with Dr. Lothar Hennighausen (Laboratory of Genetics and Physiology, National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Bethesda, MD, USA) and crossed with a myosin light chain cre promoter, as previously published [7, 43, 44]. All mice were group-housed (five to a cage) and were sacrificed at 12 weeks of age. All experiments and methods performed were done in accordance with relevant guidelines and regulations at the University of South Carolina. The Institutional Animal Care and Use Committee at the University of South Carolina approved all experiments.
2.2. IL-6 Overexpression
To increase circulating IL-6 levels, a total of 32 mice, 16 WT and 16 KO, were divided into two subgroups (vector; n = 16 and + IL-6; n = 16) for IL-6 overexpression experiments. In vivo intramuscular electroporation of an IL-6 plasmid into the quadriceps muscle was used to increase circulating IL-6 levels in mice, as previously described [15]. The quadriceps muscle was used as a vessel to produce IL-6 and secrete it into circulation and was not used for any analyses in this study. In order to test the systemic effects of our IL-6 treatment, the tibialis anterior muscle was used for analysis in this study and was not subjected to electroporation or any kind of electrical stimulation. Briefly, mice were anesthetized with a 2% mixture of isoflurane and oxygen (1 L/minute). The leg was shaved, and a small incision was made over the quadriceps muscle. Fat was dissected away from the quadriceps muscle, which was then injected with 50 μg of the IL-6 plasmid driven by the CMV promoter or empty control vector [44]. A series of eight 50 ms, 100 V pulses was used to promote uptake of the plasmid into myofibers, and then the incision was closed with a wound clip [5, 44]. Both vector control and IL-6 groups received the appropriate plasmid starting at 10 weeks of age; at 11 weeks, the mice underwent the same procedure on the opposite quadriceps muscle in order to maintain elevated IL-6 levels over a two-week period. All mice were euthanized 7 days after their second electroporation.
2.3. C2C12 Myotube Cell Culture
C2C12 myoblasts (American Type Culture Collection, Manassas, VA, USA) were cultured in DMEM, supplemented with 10% FBS, 50 U/mL penicillin, and 50 μg/mL streptomycin [7]. At >90% confluency, C2C12 myoblasts were incubated in a differentiation medium (DMEM supplemented with 2% heat-inactivated horse serum, 50 U/mL penicillin, and 50 μg/mL streptomycin) for 72 h to induce differentiation into myotubes, and experiments were performed. Each experiment was replicated, and all analyses included six replicates per control and treatment group.
2.4. C2C12 IL-6 Cytokine Treatment
After a 72 h differentiation period, IL-6 (Sigma, St. Louis, MO, USA) was added to serum-free DMEM and incubated for 24 h. Cells were harvested by washing with ice-cold PBS and then scraped in ice-cold lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 5 mM NaF, 1 mM β-glycerophosphate, 1 mM NaVO3, and 1/200 protease inhibitor cocktail (Sigma, P8340), pH 8.0). After sonication, cell debris was removed by centrifugation, and the supernatant was stored at −80°C. Protein concentrations were measured by the Bradford assay (Bio-Rad, Hercules, CA, USA), and the samples were used for Western blot analysis. Each experiment was replicated, and all analyses included six replicates per control and treatment groups.
2.5. RNA Interference
C2C12 myotubes were transfected with scramble siRNA (GE Dharmacon, Lafayette, CO, USA) or gp130 siRNA (Santa Cruz Biotech, Santa Cruz, CA, USA) 3 days post-differentiation using DharmaFECT 3 transfection reagent (GE Dharmacon, Lafayette, CO, USA), according to the manufacturer's instructions and as previously described [43]. Briefly, siRNA and transfection reagent were separately diluted in serum-free and antibiotics-free DMEM and incubated at room temperature for 5 min [43]. The diluted transfection reagent was then added to the siRNA mixture and allowed to complex with siRNA for 20 min. siRNA-transfection reagent complexes were then added to the antibiotics-free differentiation medium, and myotubes were incubated for 24 hours in a transfection-containing medium (100 nM siRNA concentration). Transfection efficiency was validated by cotransfecting 20 nM (final concentration) of siGLO RISC-Free Control siRNA (GE Dharmacon, Lafayette, CO, USA). Fluorescence was visualized by a Cy3 filter to determine the transfection efficiency. Validated transfected myotubes were collected for protein analysis.
2.6. C2C12 Myotube STAT3 and ERK 1/2 Inhibition
Following differentiation, a STAT3 inhibitor, C188-9 (10 μM) (BioVision, Milpitas, CA, USA), was added to a culture medium for a duration of 2 hours [35]. Following 2 hours, myotubes were treated with or without IL-6 for a duration of 24 hours and collected for western blot analysis [35]. To inhibit ERK1/2 signaling, myotubes were treated with PD98059 (20 μM, Cell Signaling Technology, Danvers MA) with or without the presence of IL-6 (100 ng) for a duration of 24 hours. Following 24 hours of incubation, myotubes were collected for western blot analysis. Each experiment was replicated, and all analyses included six replicates per control and treatment groups.
2.7. Statistics
Utilizing previous experiments from our laboratory, we conducted a power analysis to determine the sample size needed to observe statistical significance [15]. Power (1 − β) was set to 0.8, and error of probability (α) was set at 0.05. Based on previous results using a similar treatment and protein of interest (FIS-1) [15], to achieve significance for an estimated 30 ± 10% difference between groups, a sample size of 8 animals is needed per group. Results are reported as means ± standard error of the mean. Analysis was completed using either a standard one-way analysis of variance (ANOVA), two-way ANOVA, or Student's t-test, as appropriate. Post hoc analyses were performed with the Tukey's multiple comparison test when appropriate. Significance was set at p < 0.05. Statistical analysis was performed using GraphPad Prism version 7.0 (La Jolla, CA, USA).
3. Results
3.1. IL-6 Induction of Inflammatory Signaling, DRP-1, and FIS-1 in C2C12 Myotubes
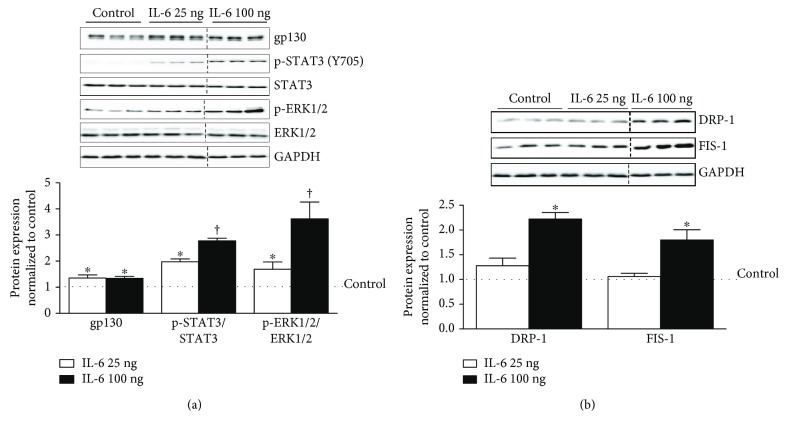
In order to examine and validate our IL-6 signaling pathway, fully differentiated myotubes were treated with a low and high dose (25 ng or 100 ng) of IL-6 for a duration of 24 hours and immediate downstream targets were analyzed. IL-6 treatment induced gp130 protein expression by 38% and was independent of IL-6 dose used (Figure 1(a)). The activation of STAT3 Y705 was induced 101% by 25 ng (p = .001) and further induced to 181% by 100 ng of IL-6 (p = .001), when compared to control (Figure 1(a)). ERK 1/2 activation followed a similar trend as STAT3, being induced by 73% with 25 ng and further induced by 266% with 100 ng of IL-6, when compared to control (Figure 1(a)). Next, we examined the activation of mitochondrial fission proteins DRP-1 and FIS-1. DRP-1 and FIS-1 were not altered with 25 ng of IL-6 but were induced by 124% and 82% above the control following 100 ng of IL-6 treatment (Figure 1(b)).
Figure 1.
IL-6 activation of gp130 signaling and mitochondrial fission in C2C12 myotubes. Fully differentiated C2C12 myotubes were treated with either 25 or 100 ng of IL-6 for a duration of 24 hours. (a). Upper: representative immunoblot of gp130, p-STAT3 (Y705), STAT3, p-ERK1/2, ERK, and GAPDH. Lower: quantification of above immunoblots. Dashed line indicates blot was cropped for representative purposes. (b). Upper: representative immunoblot DRP-1, FIS-1, and GAPDH. Lower: quantification of above immunoblots. All values are reported as means ± standard error. Six total replicates were used for all analysis. All values were normalized to control (dashed line on graph). Analysis was conducted using a standard one-way analysis of variance (ANOVA). Statistical significance was set at p < 0.05. ∗Statistically different from control. †Statistically different to all groups.
3.2. gp130 Regulation of DRP-1 and FIS-1 in IL-6-Treated C2C12 Myotubes
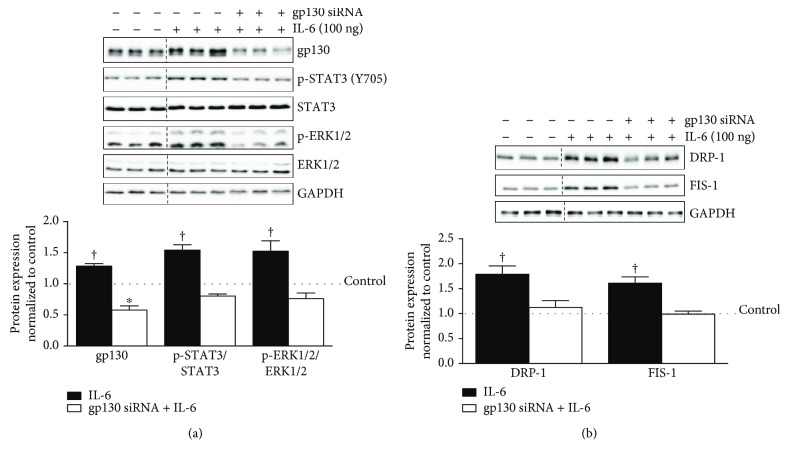
To examine gp130's role in mitochondrial fission, siRNA was used to knock down gp130 expression in C2C12 myotubes. Myotubes were treated with either a scrambled siRNA (Control) or a gp130 siRNA. The protein expression of gp130 was suppressed by 30% in the basal state with siRNA treatment (Supplemental Figure 1A). Moreover, gp130 siRNA treatment suppressed STAT3 and ERK1/2, while inducing DRP-1 and FIS-1 in the basal state (Supplemental Figure 1B). To mechanistically examine the role of gp130 in the IL-6 induction of DRP-1 and FIS-1, C2C12 myotubes were treated with either a scrambled siRNA (Control) or a gp130 siRNA in the presence of 100 ng of IL-6. IL-6 induced gp130 protein expression by 30% compared to control myotubes and was significantly different compared to all groups (p = .002). The IL-6 induction of gp130 was blocked with gp130 siRNA treatment 71% (p = .0004) and was actually 41% lower than control-treated myotubes (p = .0002) (Figure 2(a)). gp130 siRNA suppressed STAT3 and ERK 1/2 phosphorylation 75% (p = .004) in the presence of IL-6 (Figure 2(a)). The IL-6 induction of DRP-1 was suppressed by 68% (p = .002) and FIS-1 by 65% (p = .001) by gp130 siRNA treatment (Figure 2(b)). Collectively, these results demonstrate a role for IL-6-induced gp130 signaling in the regulation of DRP-1 and FIS-1 expression in C2C12 myotubes.
Figure 2.
gp130 regulation of mitochondrial fission proteins DRP-1 and FIS-1 in IL-6-treated C2C12 myotubes. We next examined the role of immediate downstream IL-6 target gp130 in the regulation of IL-6 signaling and mitochondrial fission proteins DRP-1 and FIS-1 in fully differentiated C2C12 myotubes. (a). Upper: representative immunoblot of gp130, p-STAT3 (Y705), STAT3, p-ERK1/2, ERK, and GAPDH in myotubes treated with 100 ng of IL-6 or the combination of IL-6 and gp130 siRNA. Lower: quantification of above immunoblots. Dashed line indicates blot was cropped for representative purposes. (b). Upper: representative immunoblot DRP-1, FIS-1, and GAPDH in myotubes treated with 100 ng of IL-6 or the combination of IL-6 and gp130 siRNA. Lower: quantification of above immunoblots. All values are reported as means ± standard error. Six total replicates were used for all analysis. All values were normalized to control (dashed line on graph). Analysis was conducted using a standard one-way analysis of variance (ANOVA). Statistical significance was set at p < 0.05. ∗Statistically different from control. †Statistically different to all groups.
3.3. STAT3 Regulation of DRP-1 and FIS-1 in IL-6-Treated C2C12 Myotubes
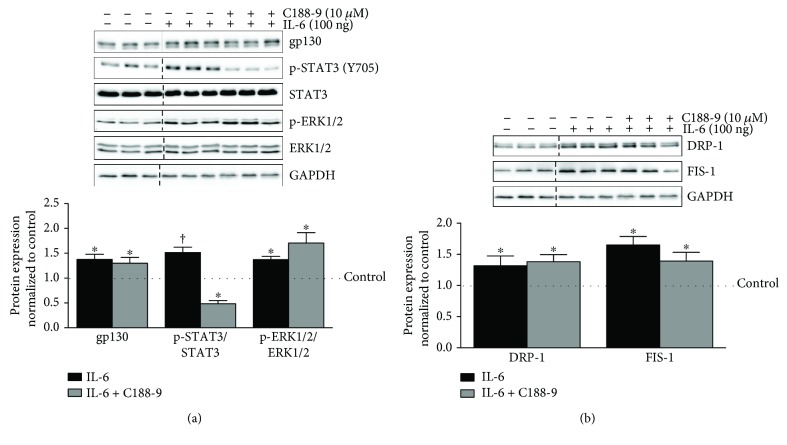
We continued our examination of IL-6 signaling by inhibiting STAT3 phosphorylation in differentiated myotubes treated with 100 ng of IL-6. STAT inhibitor C188-9 suppressed basal STAT3 phosphorylation by 66% without altering basal ERK 1/2 phosphorylation (Supplemental Figure 1C). Basal STAT3 inhibition did not alter DRP-1 and FIS-1 (Supplemental Figure 1D). The IL-6 induction of STAT3 (Y705) phosphorylation was suppressed by 103% (p = .0003) with C188-9 treatment and was 50% lower (p = .01) than that of control myotubes (Figure 3(a)). C188-9 did not alter the IL-6 induction of gp130 expression or ERK1/2 phosphorylation (Figure 3(a)). We next examined mitochondrial fission proteins DRP-1 and FIS-1 in the presence of high IL-6 with C188-9. STAT3 inhibition by C188-9 did not alter the IL-6 induction of DRP-1 or FIS-1, suggesting that the regulation of these proteins by IL-6 occurs via a STAT3-independent mechanism (Figure 3(b)).
Figure 3.
STAT3 regulation of mitochondrial fission proteins DRP-1 and FIS-1 in IL-6-treated C2C12 myotubes. We moved further downstream of gp130 and examined the role of STAT3 in the regulation of IL-6 signaling and mitochondrial fission proteins DRP-1 and FIS-1 in IL-6-treated C2C12 myotubes. (a). Upper: representative immunoblot of gp130, p-STAT3 (Y705), STAT3, p-ERK1/2, ERK, and GAPDH in myotubes treated with 100 ng of IL-6 or the combination of IL-6 and C188-9 (STAT3 small molecule inhibitor). Lower: quantification of above immunoblots. Dashed line indicates blot was cropped for representative purposes. (b). Upper: representative immunoblot DRP-1, FIS-1, and GAPDH in myotubes treated with 100 ng of IL-6 or the combination of IL-6 and C188-9. Lower: quantification of above immunoblots. All values are reported as means ± standard error. Six total replicates were used for all analysis. All values were normalized to control (dashed line on graph). Analysis was conducted using a standard one-way analysis of variance (ANOVA). Statistical significance was set at p < 0.05. ∗Statistically different from control. †Statistically different to all groups.
3.4. ERK 1/2 Regulation of Mitochondrial Fission Proteins DRP-1 and FIS-1 in IL-6-Treated C2C12 Myotubes
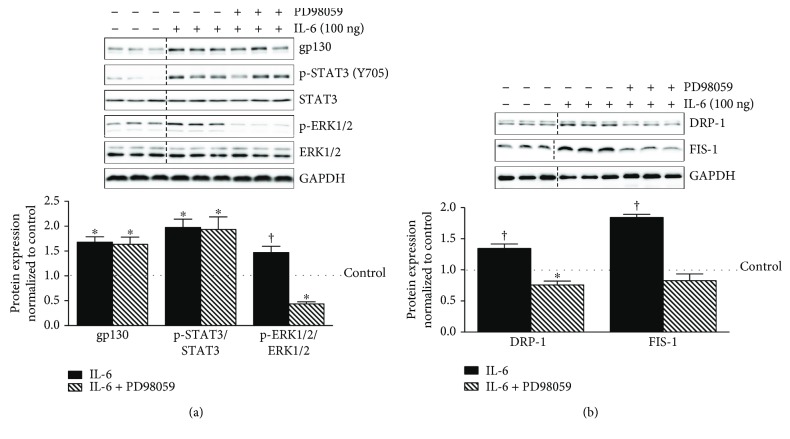
We next examined ERK 1/2 signaling using inhibitor PD98059. ERK 1/2 inhibition suppressed basal ERK 1/2 phosphorylation 44%, DRP-1 52%, and FIS-1 47% (Supplemental Figures 1E and F). IL-6 treatment induced ERK 1/2 phosphorylation 49% above control level (p = .01), which was reduced by 103% (p = .0004) by PD98059 (Figure 4(a)). PD98059 did not alter the IL-6 induction of gp130 or STAT3 (Figure 4(a)). The IL-6 induction of DRP-1 and FIS-1 protein expression was reduced by 59% (p = .0003) and 102% (p = .0001) by PD98059 treatment (Figure 4(b)). These results collectively demonstrate that the IL-6 regulation of DRP-1 and FIS-1 protein expression occurs through a gp130/ERK1/2 signaling axis.
Figure 4.
ERK1/2 regulation of mitochondrial fission proteins DRP-1 and FIS-1 in IL-6-treated C2C12 myotubes. Continuing our mechanistic investigation of IL-6 signaling, we next examined the role of ERK1/2 in the regulation of mitochondrial fission proteins DRP-1 and FIS-1 in IL-6-treated C2C12 myotubes. (a). Upper: representative immunoblot of gp130, p-STAT3 (Y705), STAT3, p-ERK1/2, ERK, and GAPDH in myotubes treated with 100 ng of IL-6 or the combination of IL-6 and PD98059. Lower: quantification of above immunoblots. Dashed line indicates blot was cropped for representative purposes. (b). Upper: representative immunoblot DRP-1, FIS-1, and GAPDH in myotubes treated with 100 ng of IL-6 or the combination of IL-6 and PD98059. Lower: quantification of above immunoblots. All values are reported as means ± standard error. Six total replicates were used for all analysis. All values were normalized to control (dashed line on graph). Analysis was conducted using a standard one-way analysis of variance (ANOVA). Statistical significance was set at p < 0.05. ∗Statistically different from control. †Statistically different to all groups.
3.5. In Vivo Systemic IL-6 Overexpression Induces Muscle gp130 Signaling, DRP-1, and FIS-1 In Vivo
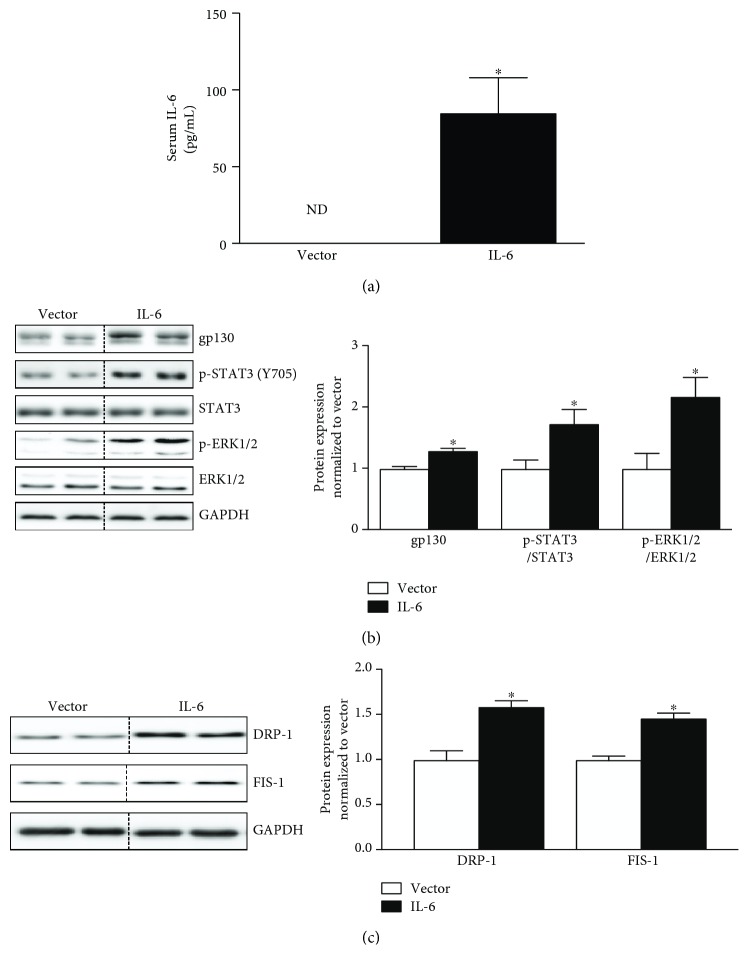
IL-6 was systemically overexpressed in vivo through electroporation of the quadriceps muscle with either 50 μg of IL-6 overexpression plasmid or control plasmid in 10-week-old C57BL/6 mice once a week for two weeks, alternating the quadriceps muscle electroporated. Serum IL-6 was elevated following 2 weeks of treatment (Figure 5(a)). Tibialis anterior muscle gp130 protein expression was induced by 31% (p = .001) above vector control. Immediate downstream targets STAT3 (Y705) and p-ERK1/2 (Figure 5(b)) were induced by 78% (p = .02) and 101% (p = .01) with 2 weeks of systemic IL-6 overexpression. Similar to our in vitro analysis, the induction of gp130 signaling was also accompanied by a 51% induction of DRP-1 (p = .004) and a 48% induction of FIS-1 (p = .001) in the tibialis anterior muscle (Figure 5(c)). These results suggest that two weeks of elevated circulating IL-6 induces skeletal muscle mitochondrial fission proteins DRP-1 and FIS-1 in-vivo.
Figure 5.
Systemic IL-6 overexpression induces muscle gp130 signaling and mitochondrial fission proteins DRP-1 and FIS-1 in vivo. We next examined if systemic IL-6 overexpression is capable of inducing muscle gp130 signaling and mitochondrial fission proteins in vivo. (a). Circulating serum IL-6 levels in vector and IL-6-treated mice following 2 weeks of IL-6 overexpression. (b). Left: representative immunoblot of gp130, p-STAT3 (Y705), STAT3, p-ERK1/2, ERK, and GAPDH in tibialis anterior muscle of C57BL/6 mice treated with either vector or IL-6. Right: quantification of immunoblots. Dashed line indicates blot was cropped for representative purposes. (c). Left: representative immunoblot DRP-1, FIS-1, and GAPDH in tibialis anterior muscle of C57BL/6 mice treated with either vector or IL-6. Right: quantification of immunoblots. All values are reported as means ± standard error. N = 8 mice per treatment group. All values were normalized to Vector. Analysis was conducted using an un-paired students t-test. Statistical significance was set at p < 0.05. ∗statistically different from Control.
3.6. IL-6 Regulation of DRP-1 and FIS-1 in gp130 KO Mice
We next examined if systemic IL-6 directly regulated DRP-1 and FIS-1 expression in the skeletal muscle through gp130 signaling using a muscle-specific gp130 knockout mouse. Muscle gp130 loss suppressed basal gp130 expression 73%, STAT3 (Y705) phosphorylation 81%, and ERK1/2 phosphorylation 48% (p = .0001 for all proteins). The IL-6 induction of tibialis anterior muscle gp130 expression, STAT3 (Y705) phosphorylation, and ERK1/2 phosphorylation were blocked by muscle gp130 loss (Figure 6(a)). Similar to our in vitro results, IL-6 induced DRP-1 97% (p = .001) and FIS-1 187% (p = .001) in the tibialis anterior muscle of the control mice. Muscle gp130 loss was sufficient to suppress the IL-6 induction of DRP-1 220% and FIS-1 121%, (p = .0001) in the tibialis anterior muscle (Figure 6(b)). These results demonstrate that systemic IL-6 directly regulates DRP-1 and FIS-1 expression through muscle gp130 signaling.
Figure 6.
IL-6 regulation of gp130 signaling and mitochondrial fission proteins DRP-1 and FIS-1 in vivo in muscle gp130 KO mice. We examined the IL-6 regulation of gp130 and mitochondrial fission proteins DRP-1 and FIS-1 in muscle gp130 KO mice. (a). Left: representative immunoblot of gp130, p-STAT3, STAT3, p-ERK1/2, ERK1/2, and GAPDH in tibialis anterior muscle of WT and KO mice treated with either vector or IL-6. Right: quantification of immunoblot. Dashed line indicates blot was cropped for representative purposes. (b). Left: representative immunoblot DRP-1, FIS-1, and GAPDH in tibialis anterior muscle of WT or KO mice treated with either vector or IL-6. Right: quantification of immunoblots. All values are reported as means ± standard error. N = 8 mice per treatment group. All values were normalized to vector. Analysis was conducted using a two-way analysis of variance (ANOVA). Statistical significance was set at p < 0.05. †Statistically different to all groups. #Statistically different to WT vector.
4. Discussion
Mitochondria organelles play the critical role of maintaining skeletal muscle energy balance in [22, 26, 45], and chronic inflammation is a recognized mediator of mitochondrial dysfunction [5, 19, 21, 46, 47]. Chronically elevated systemic IL-6 can alter muscle mitochondrial morphology and negatively regulate oxidative genes [36, 46–48]. While the IL-6 family of cytokines has been associated with the regulation of muscle mitochondrial quality control [7, 43], the direct regulation of systemic IL-6 on intracellular pathways that regulate these mitochondrial processes has not been firmly established. IL-6 could exert an indirect effect on skeletal muscle homeostasis through immune signaling or metabolic processes in other tissues [2, 8]. Our results suggest that IL-6 can directly regulate the expression of skeletal muscle mitochondrial fission proteins DRP-1 and FIS-1. We report the novel finding that suppressing gp130 both in vitro and in vivo inhibits the IL-6 induction of DRP-1 and FIS-1. Additionally, we provide evidence that ERK1/2 signaling in C2C12 myotubes is sufficient to block the IL-6 induction of DRP-1, while STAT3 signaling was not necessarily the IL-6 induction of DRP-1 and FIS-1 in vitro. Collectively, we demonstrate that IL-6 can directly regulate muscle DRP-1 and FIS-1 expression through gp130 signaling, involving ERK1/2 activation. However, further work is needed to establish a role for ERK 1/2 signaling in skeletal muscle.
The mitochondrial fission process in skeletal muscle is a critical component of mitochondrial quality control regulation [15, 18, 20, 22, 24, 25, 43]. FIS-1 and DRP-1 proteins are critical regulators of skeletal muscle fission regulation by either exercise or chronic inflammation [20]. The metabolic consequences of accelerated fission have been demonstrated by selective targeting of either DRP-1 or FIS-1 proteins. However, fission inhibition can also be detrimental for muscle metabolic quality [17, 20, 25]. While FIS-1 and DRP-1 have been associated with the fission process, the mechanisms regulating these proteins are just beginning to be understood [4, 21]. Inflammation functions as an effector of mitochondrial fission through DRP-1 and FIS-1 [15, 19, 21]. Proinflammatory cytokines such as IL-6, TNF-α, and TGF-β have been widely investigated for their roles in chronic inflammation and have now been implicated in disrupting mitochondrial quality control through the aberrant regulation of fission [2, 49]. Circulating IL-6 is a driver of muscle wasting in the ApcMin/+ mouse model of cancer cachexia, and cachexia is accompanied by altered mitochondrial dynamics and increased FIS-1 protein expression [15]. The administration of an IL-6 receptor antibody systemically can attenuate muscle mass loss and suppressed muscle FIS-1 expression in tumor-bearing mice [15]. As previously reported, our results confirm that IL-6 is sufficient to induce muscle FIS-1 expression in the absence of a cancer environment in vivo. We have extended these findings to demonstrate that DRP-1 expression in also sensitive to circulating IL-6, which provides additional evidence for muscle mitochondrial fission processes being targeted by IL-6. Furthermore, we provide in vitro and in vivo evidence that the gp130 transmembrane protein in myotubes and skeletal muscle fibers is necessary for the IL-6 induction of DRP-1 and FIS-1 protein expression. Further work is warranted to determine if the suppression of specific gp130 downstream effectors could benefit aberrant skeletal muscle mitochondrial fission induced by chronic disease and cancer.
There is evidence that aberrant mitochondrial fission can negatively impact muscle mitochondrial quality control and lead to disrupted muscle metabolic homeostasis [19, 20, 29, 50]. While STAT3 activation is classically associated with IL-6 signaling [10], ERK1/2 is also activated through IL-6/gp130 signaling [29, 38, 42, 47, 51, 52]. ERK1/2 is commonly viewed as a stress response signal and has been investigated for a regulatory role in inflammation caused by cancer and chemotherapeutics [38, 47]. The IL-6 activation of ERK1/2 can suppresses PGC-1α gene expression in skeletal muscle of aged rats and in C2C12 myotubes [53]. Mitochondrial depletion during chemotherapy-related cachexia has also been associated with ERK1/2 signaling [47]. In the current study, IL-6 induced ERK1/2 phosphorylation in skeletal muscle and cultured myotubes. Knockdown of gp130 using either siRNA or muscle specific knockout inhibited the IL-6 induction of ERK1/2 phosphorylation and the subsequent induction of DRP-1 and FIS-1. Specifically, ERK1/2 inhibition in cultured myotubes attenuated the IL-6 induction of DRP-1 and FIS-1 expression. While these findings suggest that IL-6 regulates muscle DRP-1 and FIS-1 expression through a gp130/ERK1/2 signaling axis in vitro, further examination is needed to link gp130/ERK1/2 signaling to the mitochondrial fission process in skeletal muscle. Furthermore, it is interesting to speculate on whether this induction of fission is a driver of metabolic dysfunction or if this is a compensatory mechanism for other muscle homeostatic disruptions initiated by inflammation. Additional experimentation needs to examine if altered fission directly leads to muscle metabolic and functional decrements that accompany chronic disease.
Small molecule inhibitors have demonstrated a role for STAT3 in the regulation of muscle mass during cancer cachexia [3, 31]. Our laboratory has previously utilized a systemic IL-6 receptor antibody to attenuate muscle mass loss during cancer cachexia [15, 47, 54]. This antibody also suppressed the IL-6 activation of muscle STAT3 and FIS-1 protein expression during cachexia, suggesting that the intracellular IL-6 signaling targets such as STAT3 may contribute to the regulation of mitochondrial fission [15, 47, 54, 55]. However, use of a STAT3 siRNA demonstrated no effect on mitochondrial dynamics proteins FIS-1 and MFN-1 in C2C12 myotubes during basal conditions [43]. Interestingly, the inhibition of basal STAT3 in C2C12 myotubes using C188-9 induced autophagosome accumulation but was not further induced in the presence of bafilomycin A1, suggesting a role for STAT3 in the regulation of autophagy/mitophagy processes [43]. Furthermore, STAT3 has also been shown to have a role in the regulation of mitochondrial function [36]. Evidence in non-muscle cells revealed that STAT3 is capable of translocating to the mitochondria and binding to complex I in the electron transport chain and negatively regulating mitochondrial function; however, its role remains unclear related to DRP-1 and FIS-1 [36]. Furthermore, RNA sequencing of constitutively active STAT3 MEF cells demonstrated a suppression of oxidative genes and an induction of glycolytic genes with no significant alteration to mitochondrial morphology or mass, suggesting that STAT3 may contribute primarily to mitochondrial function and not dynamics [56]. C2C12 myotubes during basal conditions [43]. Our current findings suggest that STAT3 activation is not necessary for the IL-6 induction of DRP-1 and FIS-1 in C2C12 myotubes and extends our previous work demonstrating that inhibition of STAT3 in a basal state did not alter FIS-1 [43]. However, our findings related to STAT3 regulation of DRP-1 and FIS-1 in vitro require further investigation in vivo. There is the possibility that muscle STAT3 signaling is regulating other processes in skeletal muscle that influence mitochondrial quality control, including function and autophagy/mitophagy [43, 57].
While mitochondrial dysfunction has been implicated as a driver of muscle wasting [18, 19, 21, 57], further study is required to establish whether mitochondrial dysfunction is a secondary consequence of muscle wasting [17, 20, 22, 50, 58, 59]. Our laboratory previously demonstrated that 2 weeks of systemic IL-6 overexpression is sufficient to induce STAT3 and suppress basal muscle protein synthesis in non-tumor-bearing mice [44]. Interestingly, these changes were not associated with changes in muscle or fat mass and indicate that IL-6/STAT3 signaling has consequences in muscle beyond the regulation of muscle mass [44]. Systemic IL-6 overexpression is also sufficient to suppress mitochondrial proteins COXIV and cytochrome C expression without muscle mass loss [5]. These findings suggest that the inflammation-induced disruption of mitochondrial quality control and the suppression of protein synthesis can precede skeletal muscle mass loss. In Lewis lung carcinoma (LLC), tumor-bearing mouse mitochondrial dysfunction and degeneration also occur prior to muscle mass loss, which provides further evidence for disrupted mitochondrial quality control being an early event in the muscle wasting process [58].
Overall, our results provide novel and mechanistic insights into the IL-6 regulation of skeletal muscle DRP-1 and FIS-1 expression. Two weeks of systemically elevated IL-6 was sufficient to induce both DRP-1 and FIS-1 expression, and IL-6 regulated their expression through gp130 signaling in skeletal muscle and cultured myotubes. Collectively, our results suggest that IL-6 signals through the gp130 receptor to activate ERK1/2 and induce DRP-1 and FIS-1 protein expression. In conclusion, we provide evidence for the regulation of muscle DRP-1 and FIS-1 expression through a gp130/ERK1/2 signaling axis. Further investigation is warranted to establish if accelerated fission is occurring to compensate for metabolic dysfunction and improve mitochondrial quality and if targeting the regulation of mitochondrial quality control processes can benefit skeletal muscle subjected to chronic systemic inflammation.
Acknowledgments
This work was supported by National Cancer Institute Grant R01 CA121249 (J. A. Carson). The authors thank Gaye Christmus, MPH, for the editorial review of the manuscript.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Authors' Contributions
J.A.C and D.K.F. conceived and designed the research; D.K.F. and B.N.V. performed the experiments; D.K.F. and B.N.V. analyzed the data; J.A.C. and D.K.F. interpreted the results of the experiments; D.K.F. prepared the figures; D.K.F. drafted manuscript; J.A.C., B.R.C., and B.N.V. edited and revised the manuscript; J.A.C. approved the final version of the manuscript.
Supplementary Materials
Supplemental Figure 1: basal inhibitor data.
References
- 1.Baltgalvis K. A., Berger F. G., Peña M. M. O., Mark Davis J., White J. P., Carson J. A. Activity level, apoptosis, and development of cachexia in Apcmin/+ mice. Journal of Applied Physiology. 2010;109(4):1155–1161. doi: 10.1152/japplphysiol.00442.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narsale A. A., Carson J. A. Role of interleukin-6 in cachexia: therapeutic implications. Current Opinion in Supportive and Palliative Care. 2014;8(4):321–327. doi: 10.1097/SPC.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonetto A., Aydogdu T., Jin X., et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. American Journal of Physiology. Endocrinology and Metabolism. 2012;303(3):E410–E421. doi: 10.1152/ajpendo.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carson J. A., Hardee J. P., VanderVeen B. N. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Seminars in Cell & Developmental Biology. 2016;54:53–67. doi: 10.1016/j.semcdb.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puppa M. J., White J. P., Velázquez K. T., et al. The effect of exercise on IL-6-induced cachexia in the Apcmin/+mouse. Journal of Cachexia, Sarcopenia and Muscle. 2012;3(2):117–137. doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gudiksen A., Schwartz C. L., Bertholdt L., Joensen E., Knudsen J. G., Pilegaard H. Lack of skeletal muscle IL-6 affects pyruvate dehydrogenase activity at rest and during prolonged exercise. PLoS One. 2016;11(6, article e0156460) doi: 10.1371/journal.pone.0156460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puppa M. J., Gao S., Narsale A. A., Carson J. A. Skeletal muscle glycoprotein 130’s role in Lewis lung carcinoma-induced cachexia. The FASEB Journal. 2014;28(2):998–1009. doi: 10.1096/fj.13-240580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst M., Jenkins B. J. Acquiring signalling specificity from the cytokine receptor gp130. Trends in Genetics. 2004;20(1):23–32. doi: 10.1016/j.tig.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Cron L., Allen T., Febbraio M. A. The role of gp130 receptor cytokines in the regulation of metabolic homeostasis. The Journal of Experimental Biology. 2016;219(2):259–265. doi: 10.1242/jeb.129213. [DOI] [PubMed] [Google Scholar]
- 10.Garbers C., Aparicio-Siegmund S., Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Current Opinion in Immunology. 2015;34:75–82. doi: 10.1016/j.coi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Baltgalvis K. A., Berger F. G., Peña M. M. O., Davis J. M., Carson J. A. Effect of exercise on biological pathways in ApcMin/+ mouse intestinal polyps. Journal of Applied Physiology. 2008;104(4):1137–1143. doi: 10.1152/japplphysiol.00955.2007. [DOI] [PubMed] [Google Scholar]
- 12.Fujita J., Tsujinaka T., Jano M., et al. Anti-interleukin-6 receptor antibody prevents muscle atrophy in colon-26 adenocarcinoma-bearing mice with modulation of lysosomal and ATP-ubiquitin-dependent proteolytic pathways. International Journal of Cancer. 1996;68(5):637–643. doi: 10.1002/(SICI)1097-0215(19961127)68:5<637::AID-IJC14>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Petersen E. W., Carey A. L., Sacchetti M., et al. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. American Journal of Physiology. Endocrinology and Metabolism. 2005;288(1):E155–E162. doi: 10.1152/ajpendo.00257.2004. [DOI] [PubMed] [Google Scholar]
- 14.Serrano A. L., Baeza-Raja B., Perdiguero E., Jardí M., Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metabolism. 2008;7(1):33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 15.White J. P., Puppa M. J., Sato S., et al. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skeletal Muscle. 2012;2(1):p. 14. doi: 10.1186/2044-5040-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake J. C., Wilson R. J., Yan Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. The FASEB Journal. 2016;30(1):13–22. doi: 10.1096/fj.15-276337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jheng H. F., Tsai P. J., Guo S. M., et al. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Molecular and Cellular Biology. 2012;32(2):309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romanello V., Guadagnin E., Gomes L., et al. Mitochondrial fission and remodelling contributes to muscle atrophy. The EMBO Journal. 2010;29(10):1774–1785. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romanello V., Sandri M. Mitochondrial quality control and muscle mass maintenance. Frontiers in Physiology. 2016;6:p. 422. doi: 10.3389/fphys.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suliman H. B., Piantadosi C. A. Mitochondrial quality control as a therapeutic target. Pharmacological Reviews. 2016;68(1):20–48. doi: 10.1124/pr.115.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanderVeen B. N., Fix D. K., Carson J. A. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: a role for inflammation. Oxidative Medicine and Cellular Longevity. 2017;2017:13. doi: 10.1155/2017/3292087.3292087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wai T., Langer T. Mitochondrial dynamics and metabolic regulation. Trends in Endocrinology and Metabolism. 2016;27(2):105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Iqbal S., Ostojic O., Singh K., Joseph A. M., Hood D. A. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle & Nerve. 2013;48(6):963–970. doi: 10.1002/mus.23838. [DOI] [PubMed] [Google Scholar]
- 24.Touvier T., de Palma C., Rigamonti E., et al. Muscle-specific Drp1 overexpression impairs skeletal muscle growth via translational attenuation. Cell Death & Disease. 2015;6(2, article e1663) doi: 10.1038/cddis.2014.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iqbal S., Hood D. A. The role of mitochondrial fusion and fission in skeletal muscle function and dysfunction. Frontiers in Bioscience. 2015;20(1):157–172. doi: 10.2741/4303. [DOI] [PubMed] [Google Scholar]
- 26.Yan Z., Lira V. A., Greene N. P. Exercise training-induced regulation of mitochondrial quality. Exercise and Sport Sciences Reviews. 2012;40(3):159–164. doi: 10.1097/JES.0b013e3182575599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding H., Jiang N., Liu H., et al. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochimica et Biophysica Acta. 2010;1800(3):250–256. doi: 10.1016/j.bbagen.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Still A. J., Floyd B. J., Hebert A. S., et al. Quantification of mitochondrial acetylation dynamics highlights prominent sites of metabolic regulation. The Journal of Biological Chemistry. 2013;288(36):26209–26219. doi: 10.1074/jbc.M113.483396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra P., Chan D. C. Metabolic regulation of mitochondrial dynamics. The Journal of Cell Biology. 2016;212(4):379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmers T. A., Fishel M. L., Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Seminars in Cell & Developmental Biology. 2016;54:28–41. doi: 10.1016/j.semcdb.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva K. A. S., Dong J., Dong Y., et al. Inhibition of Stat3 activation suppresses caspase-3 and the ubiquitin-proteasome system, leading to preservation of muscle mass in cancer cachexia. Journal of Biological Chemistry. 2015;290(17):11177–11187. doi: 10.1074/jbc.M115.641514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trenerry M. K., Carey K. A., Ward A. C., Cameron-Smith D. STAT3 signaling is activated in human skeletal muscle following acute resistance exercise. Journal of Applied Physiology. 2007;102(4):1483–1489. doi: 10.1152/japplphysiol.01147.2006. [DOI] [PubMed] [Google Scholar]
- 33.Guadagnin E., Mazala D., Chen Y. W. STAT3 in skeletal muscle function and disorders. International Journal of Molecular Sciences. 2018;19(8) doi: 10.3390/ijms19082265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGinnis G. R., Ballmann C., Peters B., et al. Interleukin-6 mediates exercise preconditioning against myocardial ischemia reperfusion injury. American Journal of Physiology-Heart and Circulatory Physiology. 2015;308(11):H1423–H1433. doi: 10.1152/ajpheart.00850.2014. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L., Pan J., Dong Y., et al. Stat3 activation links a C/EBPδ to myostatin pathway to stimulate loss of muscle mass. Cell Metabolism. 2013;18(3):368–379. doi: 10.1016/j.cmet.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wegrzyn J., Potla R., Chwae Y. J., et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323(5915):793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baracos V. E. Mitogen-activated protein kinases inhibitors: potential therapeutic agents for cancer cachexia. Molecular Cancer Therapeutics. 2017;16(2):263–264. doi: 10.1158/1535-7163.MCT-16-0753. [DOI] [PubMed] [Google Scholar]
- 38.Quan-Jun Y., Yan H., Yong-Long H., et al. Selumetinib attenuates skeletal muscle wasting in murine cachexia model through ERK inhibition and AKT activation. Molecular Cancer Therapeutics. 2017;16(2):334–343. doi: 10.1158/1535-7163.MCT-16-0324. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez A. M., Csibi A., Raibon A., et al. AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. Journal of Cellular Biochemistry. 2012;113(2):695–710. doi: 10.1002/jcb.23399. [DOI] [PubMed] [Google Scholar]
- 40.Widegren U., Wretman C., Lionikas A., Hedin G., Henriksson J. Influence of exercise intensity on ERK/MAP kinase signalling in human skeletal muscle. Pflügers Archiv. 2000;441(2-3):317–322. doi: 10.1007/s004240000417. [DOI] [PubMed] [Google Scholar]
- 41.Barreto R., Waning D. L., Gao H., Liu Y., Zimmers T. A., Bonetto A. Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget. 2016;7(28):43442–43460. doi: 10.18632/oncotarget.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prieto J., León M., Ponsoda X., et al. Early ERK1/2 activation promotes DRP1-dependent mitochondrial fission necessary for cell reprogramming. Nature Communications. 2016;7(1):p. 11124. doi: 10.1038/ncomms11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fix D. K., Hardee J. P., Gao S., VanderVeen B. N., Velázquez K. T., Carson J. A. Role of gp130 in basal and exercise-trained skeletal muscle mitochondrial quality control. Journal of Applied Physiology. 2018;124(6):1456–1470. doi: 10.1152/japplphysiol.01063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardee J. P., Fix D. K., Wang X., Goldsmith E. C., Koh H. J., Carson J. A. Systemic IL-6 regulation of eccentric contraction-induced muscle protein synthesis. American Journal of Physiology-Cell Physiology. 2018;315(1):C91–C103. doi: 10.1152/ajpcell.00063.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hood D. A., Tryon L. D., Vainshtein A., et al. Exercise and the regulation of mitochondrial turnover. Progress in Molecular Biology and Translational Science. 2015;135:99–127. doi: 10.1016/bs.pmbts.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Gough D. J., Corlett A., Schlessinger K., Wegrzyn J., Larner A. C., Levy D. E. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324(5935):1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White J. P., Baltgalvis K. A., Puppa M. J., Sato S., Baynes J. W., Carson J. A. Muscle oxidative capacity during IL-6-dependent cancer cachexia. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2011;300(2):R201–R211. doi: 10.1152/ajpregu.00300.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowes D. A., Webster N. R., Murphy M. P., Galley H. F. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. British Journal of Anaesthesia. 2013;110(3):472–480. doi: 10.1093/bja/aes577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VanderVeen B. N., Hardee J. P., Fix D. K., Carson J. A. Skeletal muscle function during the progression of cancer cachexia in the male ApcMin/+ mouse. Journal of Applied Physiology. 2018;124(3):684–695. doi: 10.1152/japplphysiol.00897.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vitorino R., Moreira-Goncalves D., Ferreira R. Mitochondrial plasticity in cancer-related muscle wasting: potential approaches for its management. Current Opinion in Clinical Nutrition and Metabolic Care. 2015;18(3):226–233. doi: 10.1097/MCO.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 51.Behera S., Kapadia B., Kain V., et al. ERK1/2 activated PHLPP1 induces skeletal muscle ER stress through the inhibition of a novel substrate AMPK. Biochimica et Biophysica Acta-Molecular Basis of Disease. 2018;1864(5):1702–1716. doi: 10.1016/j.bbadis.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 52.Williamson D., Gallagher P., Harber M., Hollon C., Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. The Journal of Physiology. 2003;547(3):977–987. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown J. L., Rosa-Caldwell M. E., Lee D. E., et al. PGC-1α4 gene expression is suppressed by the IL-6-MEK-ERK 1/2 MAPK signalling axis and altered by resistance exercise, obesity and muscle injury. Acta Physiologica. 2017;220(2):275–288. doi: 10.1111/apha.12826. [DOI] [PubMed] [Google Scholar]
- 54.White J. P., Baynes J. W., Welle S. L., et al. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apcmin/+ mouse. PLoS One. 2011;6(9, article e24650) doi: 10.1371/journal.pone.0024650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White J. P., Puppa M. J., Gao S., Sato S., Welle S. L., Carson J. A. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. American Journal of Physiology-Endocrinology and Metabolism. 2013;304(10):E1042–E1052. doi: 10.1152/ajpendo.00410.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demaria M., Giorgi C., Lebiedzinska M., et al. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging. 2010;2(11):823–842. doi: 10.18632/aging.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamada E., Bastie C. C., Koga H., Wang Y., Cuervo A. M., Pessin J. E. Mouse skeletal muscle fiber-type-specific macroautophagy and muscle wasting are regulated by a Fyn/STAT3/Vps34 signaling pathway. Cell Reports. 2012;1(5):557–569. doi: 10.1016/j.celrep.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown J. L., Rosa-Caldwell M. E., Lee D. E., et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. Journal of Cachexia, Sarcopenia and Muscle. 2017;8(6):926–938. doi: 10.1002/jcsm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fermoselle C., García-Arumí E., Puig-Vilanova E., et al. Mitochondrial dysfunction and therapeutic approaches in respiratory and limb muscles of cancer cachectic mice. Experimental Physiology. 2013;98(9):1349–1365. doi: 10.1113/expphysiol.2013.072496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: basal inhibitor data.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.