Abstract
Aim of the Study. To verify the effect of modified sini decoction on patients with hepatitis B virus related acute-on-chronic liver failure. Materials and Methods. A retrospective cohort study was conducted. Patients who had been treated with modified sini decoction and standard comprehensive internal medicine were assigned to an observation group, and patients who had been treated with standard comprehensive internal medicine were selected as a control group. The total bilirubin (TBIL), albumin (ALB), alanine aminotransferase (ALT), prothrombin activity (PTA), CTP, and MELD scores were analyzed at weeks 4, 8, and 12 after treatment, respectively. Meanwhile, the 12-week survival rate was analyzed. Results. The levels of TBIL and ALT were remarkably decreased, while the levels of ALB and PTA were remarkably increased in both groups at weeks 4, 8, and 12 after treatment, respectively, but the effects in the observation group were greater (P < 0.05). The CTP and MELD scores at 8-week and 12-week were lower in the observation group than in the control group (P < 0.05). At 12 weeks, the mean survival times of the observation group and the control group were 66.7 and 45.5 d, respectively. Significant improvement of 12-week survival rate [39/62 (62.9%) versus 18/50 (36.0%), P = 0.001] was observed in the observation group after treatment. Conclusions. Modified sini decoction could protect the liver function and improve the survival rates of patients with hepatitis B virus related acute-on-chronic liver failure.
1. Introduction
Liver failure has various clinical types, a complex pathogenesis, and rapid disease progression, as well as a high mortality rate [1]. Acute-on-chronic liver failure (ACLF) is one type of it. ACLF is characterized by an acute deterioration with preexisting chronic liver diseases and ultimately results in increased mortality due to multisystem organ failure [2]. In most Asian countries, the aetiology of ACLF is primarily due to hepatitis B virus (HBV) infection because of the high prevalence of it [3]. As we all know, HBV has a worldwide distribution and is endemic in many populations [4]. 90% of ACLF is caused by chronic HBV infection in China [5]. So hepatitis B virus related acute-on-chronic liver failure (HBV-ACLF) caused by HBV infection is the most common type in China with various complications and a mortality rate as high as 40%-90% [1, 6, 7]. At present, liver transplantation is the most effective treatment, which can increase the survival rates of patients with liver failure [5]. But it is severely limited by high costs and donor shortage [8, 9]. Therefore, it is time to explore a new strategy to cure HBV-ACLF.
Previous study has reported the effects of traditional Chinese medicine (TCM) on HBV-ACLF, including improvement of symptoms, amelioration of liver function, and reduction of the risk of adverse reactions [10]. So we can continue to identify a novel strategy to cure HBV-ACLF from TCM. Sini decoction is a well-known traditional Chinese herbal formulation, which has been used to treat cardiovascular diseases and liver diseases [11–13] since the Han Dynasty, 200–210A.D. According to traditional Chinese medical theory, we created the formula “modified sini decoction” (MSND) to treat liver failure in the Hospital of Chengdu University of Traditional Chinese Medicine [14]. In our previous study [14], MSND was used to treat acute liver failure induced by D-galactosamine in rats. It was useful to protect the liver function and improve the survival rates of acute liver failure rats. So in order to investigate the effect of MSND on patients with hepatitis B virus related acute-on-chronic liver failure, we conducted this clinical trial.
2. Materials and Methods
2.1. Study Design
The retrospective cohort study was conducted at the Department of Infectious Diseases, Hospital of Chengdu University of Traditional Chinese Medicine from October 2011 to June 2015. Patients with HBV-ACLF formed an observation cohort or a control cohort according to whether they took TCM therapy or not. Patients in the observation cohort were given MSND and standard comprehensive internal medicine, while patients in the control cohort were only given standard comprehensive internal medicine. There were no artificial liver support system and liver transplantation. All patients or their immediate family signed the informed consent. According to the previous studies [10, 15], the survival rate in the control group was set to be 35%, while the survival rate in the observation was set to be 60% in this study. The match ratio, type I error, and power of test were 1:1, 5%, 80%, respectively [10, 16]. The sample size was 50 in each group, and the target sample size was 112 with an expulsion rate of 10%.
2.2. Inclusion Criteria
The inclusion criteria were [17, 18] (1) serum total bilirubin ≥ 171 μmol/L or a daily increase ≥ 17.1 μmol/L, (2) international normalized ratio (INR) ≥ 2.6 or prothrombin activity ≤ 20% without other reasons, (3) with hepatic encephalopathy or severe infection or hemorrhage of upper gastrointestinal tract or hepatorenal syndrome, (4) the presence of hepatitis B surface antigen in the serum for at least 6 mo, (5) age from 18 to 65 years, and (6) patients with Yang Deficiency of Spleen and Kidney who met diagnosis standards of Chinese medicine syndrome differentiation [19].
2.3. Exclusion Criteria
The exclusion criteria were (1) age < 18 years or age > 65 years; (2) patients with acute liver failure, subacute liver failure, or chronic liver failure; (3) superinfection or coinfection with hepatitis A, C, D, E viruses, or human immunodeficiency virus; (4) coexistence of any other serious systemic or psychiatric diseases; (5) coexistence of any other liver diseases, such as alcoholic liver disease, Wilson's disease, drug hepatitis, hepatocellular carcinoma, or autoimmune hepatitis; (6) women during pregnant stage and breast-feed stage; (7) prolonged prothrombin time induced by blood system disease; and (8) patients who had taken any illicit drugs or Chinese herbal products for any disease within the preceding 12 mo.
2.4. Treatments
All patients were given standard comprehensive internal medicine [20] in this study. Patients who accepted standard comprehensive internal medicine alone were selected as a control group. Patients who were treated by standard comprehensive internal medicine and TCM therapy (modified sini decoction) were considered an observation group. The composition of modified sini decoction consisted of Aconitum carmichaelii, Zingiber officinale, Glycyrrhiza uralensis, Panax ginseng, Prunus mume, etc. TCM clinicians tailored the formula based on changes in the syndrome and the patients' conditions.
2.5. Clinical and Laboratory Data
The patients' clinical and laboratory data were collected: (1) the 12-week survival rate; (2) the Child-Turcotte Pugh (CTP) score [21] and model for endstage liver disease (MELD) score [22] before and after treatment for 4, 8, and 12 weeks; (3) biochemical indicator: serum ALT, AST, total bilirubin (TBIL), and albumin (ALB) at 0, 4, 8, and 12 weeks after treatment, respectively; all assays used a colorimetric method (Automatic Analyzer 7170A, Hitachi, Japan); (4) PTA was performed following the manufacturer's instructions (STA-evolution, STAGO, France). It was tested at 0, 4, 8, and 12 weeks after treatment, respectively.
2.6. Safety Assessment
In this study, all patients were questioned about adverse events. Every adverse event including feelings of discomfort or unexpected symptoms was recorded. The start date, end date, and degree of each event were recorded. Patients with any adverse event received appropriate treatment.
2.7. Statistical Analysis
Patients' survival data were evaluated by the Kaplan–Meier method and compared by the log-rank test. Quantitative data were expressed as the means ± standard deviation. Differences between quantitative data were compared by independent-samples t-test or Mann–Whitney U tests. Differences between qualitative data were analyzed by χ2 test or Fisher's exact test. Statistical testing was performed with the SPSS software for Windows, version 17.0 (SPSS Inc., USA). All P values were two-sided, and P < 0.05 was considered to be statistically significant.
3. Results
3.1. Patients
One hundred and twelve patients were enrolled from 184 cases with hepatitis B virus related acute-on-chronic liver failure from Hospital of Chengdu University of Traditional Chinese Medicine. There were 62 patients in the observation group and 50 patients in the control group. The flowchart is shown in Figure 1.
Figure 1.
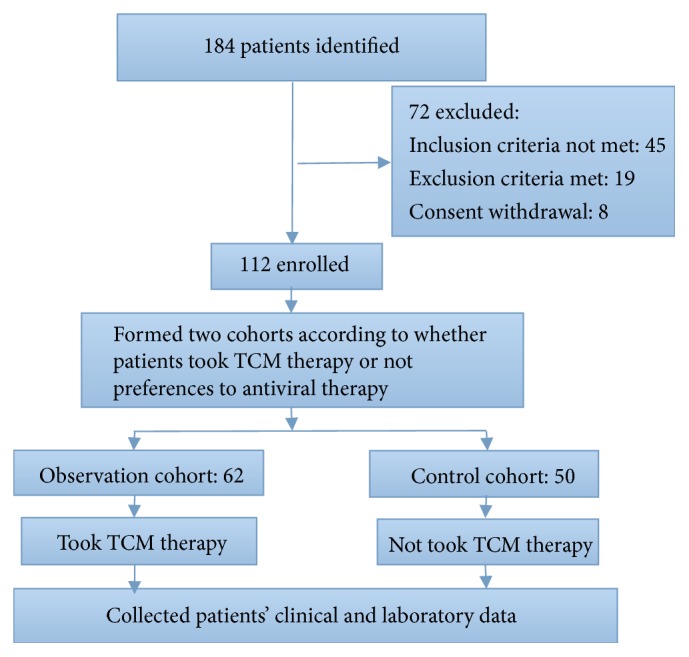
The research flowchart.
3.2. Baseline Characteristics
No significant differences were found in gender, age, serum ALT, AST, TBIL, ALB, PTA, HBV-DNA levels, HBeAg (±), CTP, and MELD scores (p > 0.05). And there were no differences in complications between the two groups before treatment (p > 0.05). The baseline characteristics of patients are shown in Table 1.
Table 1.
Baseline characteristics.
| Characteristics | Observation group | Control group |
t/χ2 | P-value |
|---|---|---|---|---|
| Age (years) | 41.7 ± 9.9 | 42.5 ± 10.2 | 1.341 | 0.381 |
| Males (%) | 43(69.4) | 35(70.0) | 0.005 | 0.941 |
| ALT (U/L) | 438.5 ± 85.8 | 456.9 ± 89.6 | 0.594 | 0.618 |
| AST (U/L) | 401.7 ± 78.5 | 410.7 ± 86.3 | 0.641 | 0.431 |
| TBIL (umol /L) | 356.3 ± 80.5 | 347.6 ± 78.1 | 0.684 | 0.513 |
| PTA (%) | 17.1 ± 2.4 | 16.7 ±3.2 | 0.298 | 0.701 |
| ALB (g/L) | 26.1 ± 6.4 | 26.7 ±6.0 | 0.562 | 0.487 |
| Creatinine (umol/L) | 72.3 ± 15.1 | 69.6 ± 12.9 | 1.270 | 0.301 |
| Sodium (mmol/L) | 130.9 ± 38.3 | 129.1 ± 40.5 | 1.615 | 0.231 |
| CTP points | 12.2 ± 1.7 | 13.0 ± 2.3 | 1.304 | 0.209 |
| MELD points | 28.9 ± 5.1 | 26.7 ± 4.9 | 0.732 | 0.809 |
| HBeAg-positive (%) | 21 (33.9) | 18 (36.0) | 0.055 | 0.814 |
| HBV-DNA (log10 copies/mL) |
6.4 ± 1.3 | 6.7 ± 1.8 | 0.781 | 0.512 |
| Ascites (%) | 50 (80.6) | 37 (74.0) | 0.705 | 0.401 |
| Hepatic encephalopathy(II-IV) (%) | 22 (35.5) | 17 (34.0) | 0.027 | 0.870 |
| Severe infection (%) | 16 (25.8) | 13 (26.0) | 0.001 | 0.981 |
| Hemorrhage of upper gastrointestinal tract (%) | 21 (33.9) | 18 (36) | 0.055 | 0.814 |
| Hepatorenal syndrome (%) | 6 (9.7) | 5 (10.0) | 0.003 | 0.955 |
All values are expressed as mean ± SD or number (%).
3.3. 12-Week Survival Rate
There were 39 (62.9%) cases survived in the observation group and 18 (36.0%) patients survived in the control group after 12-week treatment. The mean survival time of the patients in the observation group was 66.7 d, while the mean survival time was 45.5 d in the control group. Compared to the control group, the cumulative survival rate was significantly improved in the observation group (P = 0.001). The results are shown in Figure 2.
Figure 2.
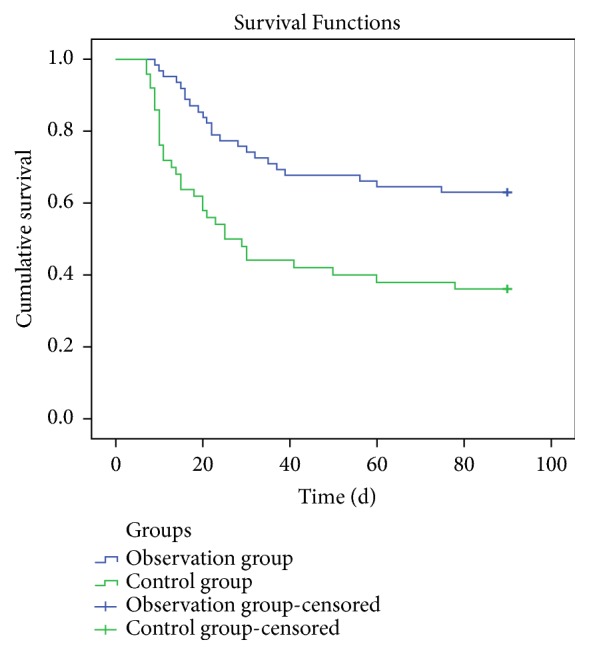
Survival curve of patients in the two groups after 12-week treatment.
3.4. Biochemical Characteristics
As for the ALT and AST levels between the two groups, there were statistical differences at week 4 (P < 0.05), but no significant difference at weeks 8 and 12 (all P > 0.05). As time went on, the TBIL levels were decreased in both groups, while the ALB and PTA were increased in both groups. But the effects were greater in the observation group at 4, 8, and 12 weeks, respectively (P<0.05). The results are shown in Tables 2–4.
Table 2.
Baseline characteristics at 4 weeks.
| Characteristics | Observation group | Control group |
t | P-value |
|---|---|---|---|---|
| ALT (U/L) | 214.2 ± 71.7 | 273.2 ± 80.5 | 2.936 | 0.018★ |
| AST (U/L) | 215.9 ± 77.2 | 269.4 ± 76.2 | 2.538 | 0.040★ |
| TBIL (umol /L) | 261.7 ± 62.3 | 301.9 ± 72.5 | 3.732 | 0.001★ |
| PTA (%) | 34.3 ± 8.3 | 27.9 ±9.8 | 2.151 | 0.032★ |
| ALB (g/L) | 29.5 ± 6.8 | 27.0 ±6.5 | 2.653 | 0.048★ |
| CTP points | 8.9 ± 2.1 | 10.1 ± 2.8 | 0.988 | 0.056 |
| MELD points | 22.5 ± 5.9 | 24.1 ± 4.9 | 0.547 | 0.094 |
All values are expressed as mean ± SD. ★Statistical significant difference between groups.
Table 3.
Baseline characteristics at 8 weeks.
| Characteristics | Observation group | Control group |
t | P-value |
|---|---|---|---|---|
| ALT (U/L) | 56.7 ± 14.6 | 60.4 ± 16.4 | 0.849 | 0.115 |
| AST (U/L) | 70.5 ± 20.8 | 74.3 ± 22.9 | 0.641 | 0.189 |
| TBIL (umol /L) | 127.3 ± 39.8 | 196.3 ± 70.1 | 2.942 | 0.001★ |
| PTA (%) | 50.2 ± 10.8 | 38.3 ±9.6 | 1.843 | 0.015★ |
| ALB (g/L) | 33.6 ± 7.9 | 28.6 ±6.7 | 2.093 | 0.032★ |
| CTP points | 6.8 ± 2.3 | 8.9 ± 3.2 | 1.085 | 0.037★ |
| MELD points | 14.6 ± 3.7 | 18.5 ± 4.1 | 1.867 | 0.026★ |
All values are expressed as mean ± SD. ★Statistical significant difference between groups.
Table 4.
Baseline characteristics at 12 weeks.
| Characteristics | Observation group | Control group | t | P-value |
|---|---|---|---|---|
| ALT (U/L) | 47.3 ± 13.8 | 51.1 ± 14.8 | 0.913 | 0.203 |
| AST (U/L) | 51.4 ± 15.6 | 57.8 ± 16.7 | 0.863 | 0.392 |
| TBIL (umol /L) | 58.5 ± 25.3 | 102.5 ± 39.6 | 2.649 | 0.001★ |
| PTA (%) | 62.5 ± 11.7 | 46.7 ±10.9 | 2.537 | 0.012★ |
| ALB (g/L) | 38.5 ± 8.2 | 29.9 ±6.8 | 2.363 | 0.021★ |
| CTP points | 5.3 ± 2.5 | 8.2 ± 4.1 | 2.043 | 0.025★ |
| MELD points | 10.5 ± 3.2 | 14.7 ± 4.5 | 1.992 | 0.032★ |
All values are expressed as mean ± SD. ★Statistical significant difference between groups.
3.5. CTP and MELD Scores
The CTP and MELD scores in both groups were decreased after treatment. But the CTP and MELD scores at 8-week and 12-week were lower in the observation than in the control group (P < 0.05). The results are shown in Tables 2–4.
3.6. Safety
No patient developed severe drug-induced adverse events.
4. Discussion
Chronic liver disease is a serious health problem. Among it, chronic HBV infection is the most difficult one worldwide [5]. China has always maintained a high morbidity of chronic HBV infection. And in China, approximately 90% of ACLF is induced by chronic HBV infection [23]. HBV-ACLF is a devastating syndrome with extremely high mortality [24]. Multiple organ failure is the main cause of death in 77% of patients with HBV-ACLF [5]. As all we know, the prognosis of HBV-ACLF depends on the capability of liver cell regeneration, the degree of hepatic damage, and the prevention of multisystem organ failure. Liver transplantation [16] has been the only definitive therapy to salvage these patients; however, it is not readily available and feasible in many parts of the world, and the artificial liver support system cannot fully meet the needs [16]. Therefore, it is time to identify a novel strategy to cure HBV-ACLF.
In China, traditional Chinese medicine (TCM) has been used to cure diseases successfully for centuries [25, 26]. In our previous study [14], we used MSND to treat acute liver failure in rat induced by D-galactosamine. Our results indicated that MSND could improve liver function, reduce necrosis in the liver tissues, and remarkably prolong the survival time. It meant that MSND could gain more time for the regeneration of liver cells. So MSND might be a useful means to save these patients. And in this retrospective cohort, our results indicated that MSND could reduce CTP and MELD scores at weeks 8 and week 12 in patients with HBV-ACLF (P<0.05). Meanwhile, MSND could improve the biochemical parameters of patients with HBV-ACLF. The results were mainly reflected by the decreasing of ALT and AST at week 4 (P<0.05), the decreasing of IBIL at 4, 8, and 12 weeks, respectively (P<0.05), and the increasing of ALB at 4, 8, and 12 weeks, respectively (P<0.05). But there were no significant differences (P>0.05) at week 8 and week 12 for ALT and AST levels between the two groups. The possible reason might be that after massive necrosis of hepatocytes in patients with hepatitis B virus related acute-on-chronic liver failure, transaminase has maintained a high level for quite a long time, and transaminase would be progressively exhausted; finally, ALT and AST would decrease or even be normal. So there were no significant differences at week 8 and week 12. At the same time, MSND could significantly increase PTA at 4, 8, 12 weeks, respectively (P<0.05). Thus, MSND could prolong the survival times of the hepatitis B virus related acute-on-chronic liver failure patients finally. Therefore, it could be concluded that MSND was a useful therapeutic agent for HBV-ACLF.
In our study, the formula MSND consists of Aconitum carmichaelii, Zingiber officinale, Glycyrrhiza uralensis, Panax ginseng, Prunus mume, etc. The formula has warming and nourishing yang-qi effect in deficiency of vital energy and eliminating cold-dampness. Previous studies [13, 27–32] showed that the Chinese medicines used in this study had widespread biological activities, including protecting the damaged hepatocytes. And our previous [14] study had explored the possible mechanisms of the effects of MSND on protecting liver function. Our results revealed that MSND could decrease the expressions of the HMGB1, TLR4, and NF-κB mRNAs, downregulate the expression of Caspase-3 mRNA to reduce apoptosis in hepatocytes, and increase the PCNA-positive rate [14].
In addition, the trial had limitations. A retrospective study is conducted by looking backwards at clinical events that have occurred. The trial cannot follow the randomization principle. The selection bias is the main disadvantage of the retrospective study. The randomized, controlled trials (RCT) have the highest grade evidence, but HBV-ACLF is such a serious disease, it was not ethical to conduct an RCT. And a prospective study needs larger size and takes too long to be conducted. Therefore, a retrospective cohort study was conducted by us. At the same time, in order to improve the validity of this study, we set an inclusion and exclusion criteria, and so on.
5. Conclusion
This trial showed that modified sini decoction could protect the liver function and improve the survival rates of patients with hepatitis B virus related acute-on-chronic liver failure. This study might provide an effective and safe option for patients with HBV-ACLF.
Acknowledgments
All cases were obtained from the Hospital of Chengdu University of Traditional Chinese Medicine. The authors are particularly grateful for the excellent professional assistance from their colleagues in the Department of Infectious Diseases, Hospital of Chengdu University of Traditional Chinese Medicine.
Abbreviations
- ALT:
Alanine aminotransferase
- AST:
Aspartate aminotransferase
- TBIL:
Total bilirubin
- PTA:
Prothrombin activity
- ALB:
Albumin
- CTP:
Child-Turcotte Pugh
- MELD:
Model for endstage liver disease
- ACLF:
Acute-on-chronic liver failure
- HBV:
Hepatitis B virus
- TCM:
Traditional Chinese medicine.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Yang F. J., Peng L., Liu Y. Y., Gao Z. L., Han T., Huang J. R. Research advances in diagnosis and treatment of liver failure in 2016. Zhonghua Gan Zang Bing Za Zhi. 2017;25(2):94–99. doi: 10.3760/cma.j.issn.1007-3418.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G. Acute-on-chronic liver failure: an old entity in search of clarity. Hepatol Commun. 2018;2(12):1421–1424. doi: 10.1002/hep4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Q., Ding W., Jiang L., et al. Comparative transcriptome analysis of peripheral blood mononuclear cells in hepatitis B-relatedacute-on-chronic liver failure. Scientific Reports. 2016;6 doi: 10.1038/srep20759.20759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z. H., Wu C. C., Chen X. W., Li X., Li J., Lu M. J. Genetic variation of hepatitis B virus and its significance for pathogenesis. World Journal of Gastroenterology. 2016;22(1):126–144. doi: 10.3748/wjg.v22.i1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Hu X.-Y., Zhong S., et al. Entecavir vs lamivudine therapy for naïve patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. World Journal of Gastroenterology. 2014;20(16):4745–4752. doi: 10.3748/wjg.v20.i16.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W. J., Lai R. T., Lu J., et al. Correlation between circulating miR-122 and prognosis of chronic HBV-related liver failure. Journal of Digestive Diseases. 2016;17(5):334–339. doi: 10.1111/1751-2980.12348. [DOI] [PubMed] [Google Scholar]
- 7.Nian X., Xu Z., Liu Y., Chen J., Li X., Xu D. Association between hepatitis B virus basal core promoter/precore region mutations and the risk of hepatitis B-related acute-on-chronic liver failurein the Chinese population: an updated meta-analysis. Hepatology International. 2016;10(4):606–615. doi: 10.1007/s12072-016-9716-7. [DOI] [PubMed] [Google Scholar]
- 8.Song E., Fabian J., Boshoff P. E., et al. Adult liver transplantation in Johannesburg, South Africa (2004-2016): blancing good outcomes, constrained resources and limited donors. South African Medical Journal. 2018;108(11):929–936. doi: 10.7196/SAMJ.2018.v108i11.13286. [DOI] [PubMed] [Google Scholar]
- 9.Yuan D., Liu F., Wei Y. G., et al. Adult-to-adult living donor liver transplantation for acute liver failure in China. World Journal of Gastroenterology. 2012;18(48):7234–7241. doi: 10.3748/wjg.v18.i48.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu X. Y., Zhang Y., Chen G., Zhong S., Fan X. J. A prospective cohort study on the influence of high doses of herbs for clearing heat and resolving stasis on survival rates in patients with hepatitis B-related acute-on-chronic liverfailure. Zhong Xi Yi Jie He Xue Bao. 2012;10(2):176–185. doi: 10.3736/jcim20120208. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J., Ma X., Shi M., et al. Serum metabolomics analysis reveals that obvious cardioprotective effects of low dose Sini decoction against isoproterenol-induced myocardial injury in rats. Phytomedicine. 2017;31:18–31. doi: 10.1016/j.phymed.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Wang L. C., Lei B. J., Li L., Zhao J. B. Pathological study of the therapeutic effect of wen-yang herbs on experimental liver cirrhosis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004;35(4):532–535. [PubMed] [Google Scholar]
- 13.Wang L.-C., Zhao L.-S., Tang H., Liu L., Liu C., Lei B.-J. Influence of wen-yang herbs on hemodynamics in liver fibrotic rats. Chinese Journal of Hepatology. 2005;13(6):421–424. [PubMed] [Google Scholar]
- 14.Luo J., Zhang Y., Hu X., et al. The effects of modified sini decoction on liver injury and regeneration in acute liver failure induced by d-galactosamine in rats. Journal of Ethnopharmacology. 2015;161:53–59. doi: 10.1016/j.jep.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Fan Z., EnQiang C., Yao D. L., et al. Neutrophil-lymphocyte ratio predicts short term mortality in patients with hepatitis B virus-related acute-on-chronic liver failure treated with an artificial liver support system. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0175332.e0175332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong Y., Guo Y., Liu X., et al. Serum glycopatterns as novel potential biomarkers for diagnosis of acute-on-chronic hepatitis B liver failure. Scientific Reports. 2017;7 doi: 10.1038/srep45957.45957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liver Failure and Artificial Liver Group. Guideline for diagnosis and treatment of liver failure. Zhonghua Gan Zang Bing Za Zhi. 2019;27(1):18–26. doi: 10.3760/cma.j.issn.1007-3418.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Jiang S. W., Wang P., Xiang X. G., et al. Serum soluble ST2 is a promising prognostic biomarker in HBV-related acute-on-chronic liver failure. Hepatobiliary & Pancreatic Diseases International. 2017;16(2):181–188. doi: 10.1016/s1499-3872(16)60185-6. [DOI] [PubMed] [Google Scholar]
- 19.Professional Committee of Hepatology. China Association of Traditional Chinese Medicine Criteria of differentiation of symptoms and sings of traditional Chinese medicine for viral hepatitis (trial implementation) Zhong Yi Za Zhi. 1992;5:39–40. [Google Scholar]
- 20.Lin B.-L., Chen J.-F., Qiu W.-H., et al. Allogeneic bone marrow–derived mesenchymal stromal cells for hepatitis B virus–related acute-on-chronic liver failure: a randomized controlled trial. Hepatology. 2017;66(1):209–219. doi: 10.1002/hep.29189. [DOI] [PubMed] [Google Scholar]
- 21.Piotrowski D., Saczewska-Piotrowska A., Jaroszewicz J., Boron-Kaczmarska A. Predictive power of Model for End-Stage Liver Disease and Child-Turcotte-Pugh score for mortality in cirrhotic patients. Clinical and Experimental Hepatology. 2018;4(4):240–246. doi: 10.5114/ceh.2018.80125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machicao V. I. Model for end-stage liver disease–sodium score: the evolution in the prioritization of liver transplantation. Clinics in Liver Disease. 2017;21(2):275–287. doi: 10.1016/j.cld.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Huang C., Yu K. K., Zheng J. M., Li N. Steroid treatment in patients with acute-on-chronic liver failure precipitated by hepatitis B: a 10-year cohort study in a university hospital in East China. Journal of Digestive Diseases. 2019;20(1):38–44. doi: 10.1111/1751-2980.12691. [DOI] [PubMed] [Google Scholar]
- 24.Lu J., Lin L., Ye C., et al. Serum NGAL is superior to cystatin C in predicting the prognosis of acute-on-chronic liver failure. Annals of Hepatology. 2019;18(1):155–164. doi: 10.5604/01.3001.0012.7907. [DOI] [PubMed] [Google Scholar]
- 25.Xu F., Cui W., Kong Q., Tang Z., Dong J. A real-world evidence study for distribution of traditional chinese medicine syndrome and its elements on respiratory disease. Evidence-Based Complementary and Alternative Medicine. 2018;2018:11. doi: 10.1155/2018/8305892.8305892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W., Chen H. Y., Yang Y. H., et al. An investigation of the prescription patterns of chinese herbal products for chronic glomerulonephritis patients: a hospital-based cross-sectional study. Evidence-Based Complementary and Alternative Medicine. 2018;2018:11. doi: 10.1155/2018/5080764.5080764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park G., Kim K. M., Choi S., Oh D. S. Aconitumcarmichaelii protects against acetaminophen-induced hepatotoxicity via B-cell lymphoma-2 protein-mediated inhibition of mitochondrial dysfunction. Environmental Toxicology and Pharmacology. 2016;42:218–225. doi: 10.1016/j.etap.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Chen J., Li W.-C., Gu X.-L. Optimized extraction, preliminary characterization, and in vitro antioxidant activity of polysaccharides from Glycyrrhiza uralensis fisch. Medical Science Monitor. 2017;23:1783–1791. doi: 10.12659/MSM.900471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park H. S., Cho J. H., Kim K. W., Chung W. S., Song M. Y. Effects of Panax ginseng on obesity in animal models: a systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine. 2018;2018:16. doi: 10.1155/2018/2719794.2719794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saba E., Jeong D., Irfan M., et al. Anti-inflammatory activity of Rg3-enriched korean red ginseng extract in murine model of sepsis. Evidence-Based Complementary and Alternative Medicine. 2018;2018:11. doi: 10.1155/2018/6874692.6874692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan A., Pan J. H., Cho S., Lee S., Kim Y. J., Park Y. H. Investigation of the hepatoprotective effect of Prunus mume Sieb. et Zucc extract in a mouse model of alcoholic liver injury through high-resolution metabolomics. Journal of Medicinal Food. 2017;20(8):734–743. doi: 10.1089/jmf.2016.3874. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y., Zhao J., Han Q., et al. The effect and mechanism of chinese herbal formula sini tang in heart failure after myocardial infarction in rats. Evidence-Based Complementary and Alternative Medicine. 2018;2018:7. doi: 10.1155/2018/5629342.5629342 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


