Abstract
Background
Biomarkers may facilitate clinical decisions in order to guide antimicrobial treatment and prediction of prognosis in community-acquired pneumonia (CAP). We measured serum C-reactive protein, procalcitonin (PCT) and calprotectin levels, and plasma pentraxin 3 (PTX3) and presepsin levels, along with whole-blood white cell counts, at three time-points, and examined their association with microbial aetiology and adverse clinical outcomes in CAP.
Methods
Blood samples were obtained at hospital admission, clinical stabilisation and 6-week follow-up from 267 hospitalised adults with CAP. Adverse short-term outcome was defined as intensive care unit admission and 30-day mortality. Long-term outcome was evaluated as 5-year all-cause mortality.
Results
Peak levels of all biomarkers were seen at hospital admission. Increased admission levels of C-reactive protein, PCT and calprotectin were associated with bacterial aetiology of CAP, while increased admission levels of PCT, PTX3 and presepsin were associated with adverse short-term outcome. In univariate and multivariate regression models, white blood cells and calprotectin at 6-week follow-up were predictors of 5-year all-cause mortality.
Conclusions
Calprotectin emerges as both a potential early marker of bacterial aetiology and a predictor for 5-year all-cause mortality in CAP, whereas PCT, PTX3 and presepsin may predict short-term outcome.
Short abstract
In 267 adults with community-acquired pneumonia, systemic calprotectin emerges as an early marker of bacterial aetiology and a predictor of 5-year mortality, whereas systemic procalcitonin, pentraxin 3 and presepsin are predictors of short-term outcome http://ow.ly/dz6S30nAFvn
Introduction
Community-acquired pneumonia (CAP) remains a frequent infectious condition, responsible for considerable short- and long-term morbidity and mortality [1]. Despite preventive measures, the burden of this disease is expected to increase in the coming years [2]. For optimal management and use of healthcare resources in CAP, early identification of the causative agent(s), recognition of disease severity and prediction of unfavourable outcome is of major importance. Biomarkers, applied in synergy with clinical assessment and CAP-specific severity scores, may provide additional information on disease severity and on the distinction between bacterial and viral aetiology. At present, however, biomarkers that discriminate viral infections from bacterial and mixed viral–bacterial causes of CAP are not precise enough to allow pathogen-specific therapy [3], nor have biomarkers provided a definite advantage over CAP-specific severity scores for predicting poor short- and long-term prognosis [4, 5]. Thus, new noninvasive diagnostic and prognostic tools could benefit the assessment and management of CAP, to better guide therapeutic options, avoid antibiotic overuse and improve clinical short-term outcomes [6]. Biomarkers could also identify patients at risk of poor long-term outcomes, defining a subgroup of CAP survivors that should be followed more carefully.
White blood cell (WBC) count and the short pentraxin C-reactive protein (CRP) are widely available inflammatory biomarkers of low to intermediate value for prediction of microbial patterns and disease severity in CAP [7–9]. The calcitonin precursor procalcitonin (PCT) is assumed to be a superior marker of bacterial aetiology and outcome in CAP compared with the former two [4, 7, 10], in addition to being useful for guiding the duration of antibiotic treatment in CAP [11]. In comparison, soluble CD14 (sCD14) subtype, known more simply as presepsin, is a molecule suggested to be more specific to systemic infections than WBCs, CRP and PCT, potentially serving as a predictor of severe disease and short-term mortality in CAP [12, 13]. More recently, the neutrophil-associated biomarker calprotectin and the long pentraxin 3 (PTX3), reflecting local and not systemic inflammation, have emerged as promising acute-phase markers of systemic infections [14, 15], but the utility of these markers in CAP is less clear [16, 17].
We have previously reported a high diagnostic microbial yield in a well-defined cohort of 267 hospitalised adult patients with CAP [18]. In this study, our objective was to quantify levels of WBCs, CRP and PCT, as established inflammatory biomarkers, along with calprotectin, PTX3 and presepsin, at three study time-points, and examine the association between these biomarkers and microbial aetiology, and short- and long-term outcome in CAP.
Materials and methods
Study population and design
The study was performed in an acute-care, 270-bed general hospital in Drammen, Vestre Viken Hospital Trust, in south-eastern Norway between January 1, 2008, and January 31, 2011. A total of 267 patients aged ≥18 years admitted with suspected pneumonia to the Dept of Internal Medicine were consecutively recruited. Within the first 48 h of hospital admission, patients were screened for eligibility by determining the presence of CAP criteria, defined by 1) a new pulmonary infiltrate on chest radiography, 2) rectal temperature >38.0°C and 3) at least one of the following symptoms or signs: cough (productive or nonproductive), dyspnoea, respiratory chest pain, crackles or reduced respiratory sounds. Patients were excluded from the study if they had been hospitalised within the past 2 weeks or if the chest radiograph uncovered noninfectious findings. Immunocompromised patients (i.e. primary or acquired immunodeficiency, active malignancy, and patients using immunosuppressive drugs, as defined by Holter et al. [19]) were not excluded from the study in order to reflect the total population being referred to this local hospital. The inclusion process is presented in figure S1. Patients were invited to an outpatient follow-up ∼6 weeks after hospital discharge, i.e. during the convalescent phase of CAP. All patients provided written informed consent. The study was approved by the Regional Committee for Medical and Health Research Ethics in South-Eastern Norway (ref. number S-06266a) and a waiver of consent was obtained from the committee to link patient data to death certificates (2012/467 A).
Data collection and definitions
Baseline data collection and definitions have been described elsewhere [18, 19]. In short, demographic, clinical and laboratory data were collected within 48 h of admission, mean±sd time from hospital admission to study inclusion was 0.6±0.5 days and 260 of 267 (97%) patients were included within 24 h. The microbial aetiology of CAP was established by use of a comprehensive array of microbiological tests (i.e. bacterial cultures, serology, urinary antigen assays and PCR). In the present study, clinical stabilisation was evaluated daily during the first 12 days of hospitalisation, with CRP and WBCs measured every second day, according to the following criteria (1 point for each criterion): 1) unchanged antibiotic treatment the last 2 days, 2) improvement of general condition, 3) morning rectal temperature <38.0°C and 4) >25% decrease in CRP or WBC levels. Clinical stabilisation was defined as a score of ≥3 points.
Blood sampling
Blood samples were obtained at hospital admission, clinical stabilisation and at the 6-week follow-up, with serum and plasma samples drawn into pyrogen-free vacutainer tubes. Tubes for plasma samples contained EDTA as an anticoagulant. Serum or plasma was separated from whole blood within 60 min by refrigerated centrifugation at 2000g for 12 min and stored in several aliquots at −80°C.
Biomarker analysis
Serum CRP was measured by turbidimetry (Abbott Architect ci16200; Abbott Diagnostics, Abbott Park, IL, USA), while full-blood WBCs with automated differential count were counted on a CELL-DYN 4000 haematology analyser (Abbott Diagnostics) and an Advia 120 Hematology System (Siemens Healthineers, Erlangen, Germany). Serum PCT was measured using a chemiluminescent assay (Advia Centaur BRAHMS PCT; Siemens Healthineers). Serum calprotectin was analysed with an ELISA (Calpro AS, Oslo, Norway) on an automated ELISA instrument (Dynex DS2 Automated ELISA System; Dynex Technologies Inc., Chantilly, VA, USA). Both plasma PTX3 and presepsin levels were analysed by commercially available sandwich ELISAs (R&D Systems, Minneapolis, MN, USA, and MyBioSource Inc., San Diego, CA, USA, respectively).
Outcome measures
Based on the microbiological results, four aetiological groups were analysed: 1) bacterial, 2) viral, 3) viral–bacterial and 4) unknown. Disease severity was evaluated by the validated CURB-65 severity score (confusion, urea >7 mmol·L−1, respiratory rate ≥30 breaths·min−1, blood pressure <90 mmHg (systolic) or ≤60 mmHg (diastolic), age ≥65 years); patients with a CURB-65 score of ≤2 were classified into low-risk and ≥3 into high-risk groups [20]. Short-term outcome was defined as a composite endpoint of intensive care unit (ICU) admission and 30-day mortality. Long-term outcome was evaluated as 5-year all-cause mortality.
Statistical analysis
Categorical variables are presented as n (%). Continuous variables are presented as mean±sd for normally distributed data or median (interquartile range) for skewed data. Differences in biomarker levels between aetiologies and time points were analysed with Kruskal–Wallis and Friedman tests for multiple-group comparisons, and Mann–Whitney and Wilcoxon signed-rank tests for two-group comparisons. Correlation analysis between continuous variables was performed with Spearman's rank-order correlation. Univariate and multivariate logistic regression analysis examined the association between biomarkers and bacterial aetiology or short-term outcome. The diagnostic accuracy of biomarkers for predicting bacterial aetiology or short-term outcome was calculated by area under the curve (AUC). In dichotomous analysis, bacterial aetiology was defined as pure bacterial or mixed viral–bacterial CAP. Cut-off values were determined by use of the Youden index. Survival from 30 days until death or the end of the follow-up period was described for all survivors using Kaplan–Meier plots. Survival distributions were compared with the log-rank test. Univariate and multivariate Cox regression analysis examined the association between biomarkers and long-term outcome. Continuous variables were log-transformed before inclusion in regression analysis if skewed. A two-sided p-value <0.05 was considered to be significant. Statistical analyses were performed using STATA version 15.0 for Windows (Stata Corp LP, College Station, TX, USA) and SPSS version 25.0 for Windows (IBM Corp, Armonk, NY, USA).
Results
Baseline characteristics
Baseline characteristics of the study cohort have previously been reported [18] and are briefly summarised in table S1. Median age was 66 (52–78) years and 172 (64%) patients had at least one comorbid condition. Microbial aetiology was established in 167 (63%) patients; 73 (28%) patients had a bacterial infection, 41 (15%) a viral infection and 51 (19%) a viral–bacterial infection, while 100 (37%) had CAP of unknown aetiology (table 1).
TABLE 1.
Microbial findings in 267 hospitalised patients with community-acquired pneumonia
| Bacterial pathogens | Patients with positive findings | Viral pathogens | Patients with positive findings |
| Streptococcus pneumoniae | 81 (30%) | Influenza viruses§ | 40 (15%) |
| Bordetella pertussis | 15 (6%) | Rhinovirus | 32 (12%) |
| Haemophilus influenzae | 14 (5%) | Parainfluenza virusesƒ | 8 (3%) |
| Mycoplasma pneumoniae | 10 (4%) | Respiratory syncytial virus | 7 (3%) |
| Chlamydophila pneumoniae | 7 (3%) | Metapneumovirus | 7 (3%) |
| Legionella pneumophila | 7 (3%) | Enterovirus | 5 (2%) |
| Enterobacteriaceae# | 6 (2%) | Adenovirus | 1 (0.4%) |
| Moraxella catarrhalis | 5 (2%) | ||
| Miscellaneous¶ | 3 (1%) | ||
| Haemophilus parainfluenzae | 2 (1%) | ||
| Total+ | 126 (47%) | Total+ | 92 (34%) |
#: Escherichia coli, Pseudomonas aeruginosa or Enterobacter spp.; ¶: group A Streptococcus, Prevotella spp. or Dialister pneumosintes; +: number of patients does not sum to number of pathogens because some patients had multiple pathogens detected; §: influenza A and B viruses; ƒ: parainfluenza virus types 1–3.
Biomarker levels at study time points
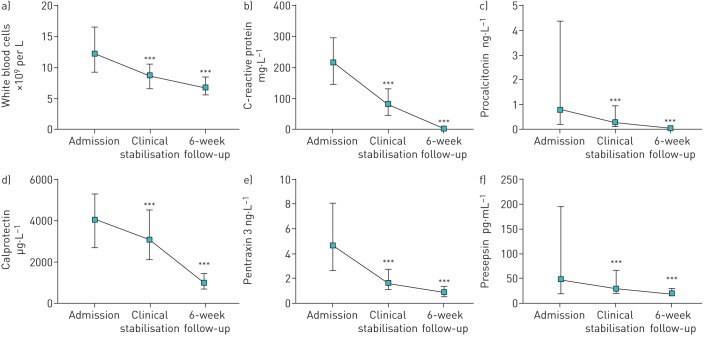
Peak levels of all examined biomarkers were seen at hospital admission, with significantly lower levels seen at clinical stabilisation during hospitalisation (p<0.001 for all) and at the 6-week follow-up (p<0.001 for all) (figure 1). Neutrophil granulocytes correlated strongly with WBCs at all three time points (correlation coefficient r=0.982, r=0.953 and r=0.937 at time points 1–3, respectively; p<0.001 for all).
FIGURE 1.
Biomarker levels at hospital admission, clinical stabilisation and 6-week follow-up in 267 hospitalised patients with community-acquired pneumonia. a) White blood cells; b) C-reactive protein; c) procalcitonin; d) calprotectin; e) pentraxin 3; f) presepsin. *: p<0.05; **: p<0.01; ***: p<0.001. Two-group comparison performed with Wilcoxon signed-rank test.
Biomarker levels at hospital admission in relation to microbial aetiology
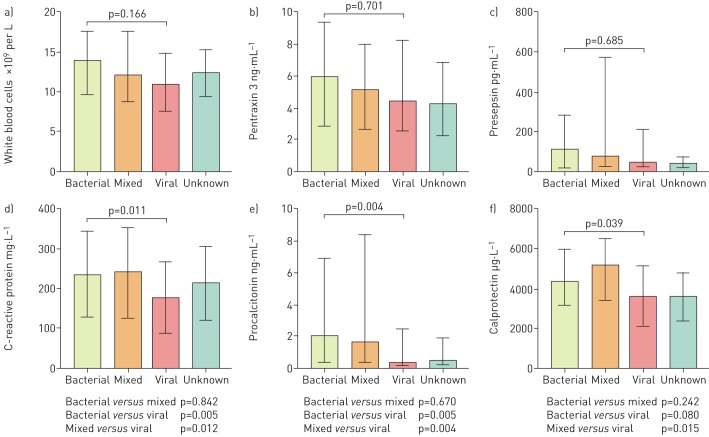
The distributions of the biomarkers WBC, PTX3 and presepsin were similar at hospital admission in relation to microbial aetiology (figure 2a–c). For CRP and PCT, however, admission levels were significantly higher in bacterial and viral–bacterial CAP compared with viral CAP (figure 2d and e), while calprotectin levels were higher in viral–bacterial CAP compared with viral CAP (figure 2f).
FIGURE 2.
Biomarker levels at hospital admission stratified by microbial aetiology in 267 hospitalised patients with community-acquired pneumonia. Data are presented as medians with interquartile ranges. Multiple group comparison performed with Kruskal–Wallis test and two-group comparison performed with Mann–Whitney test. Patients with unknown aetiology were excluded from statistical analysis. a) White blood cells; b) pentraxin 3; c) presepsin; d) C-reactive protein; e) procalcitonin; f) calprotectin.
In logistic regression analysis of continuous variables, increased admission levels of CRP (OR 1.90, 95% CI 1.16–3.12; p=0.011), PCT (OR 1.44, 95% CI 1.15–1.81; p=0.002) and calprotectin (OR 2.15, 95% CI 1.05–4.45; p=0.036) were significantly associated with bacterial aetiology of CAP (table 2). The diagnostic accuracy for discriminating bacterial infections from viral infections of CAP was, however, of moderate value for these three markers (table 2). The optimal cut-off value for discriminating bacterial infections from viral infections for CRP was 176 mg·L−1 (sensitivity 72%, specificity 51%), for PCT was 0.45 ng·mL−1 (sensitivity 70%, specificity 65%) and for calprotectin was 3476 µg·L−1 (sensitivity 73%, specificity 50%).
TABLE 2.
Logistic regression analysis and diagnostic accuracy of biomarker levels at hospital admission for prediction of bacterial aetiology in 267 hospitalised patients with community-acquired pneumonia
| Biomarker | Univariate analysis | Area under the curve | ||
| OR (95% CI) | p-value | AUC (95% CI) | p-value | |
| CRP | 1.90 (1.16–3.12) | 0.011 | 0.66 (0.56–0.75) | 0.003 |
| PCT | 1.44 (1.15–1.81) | 0.002 | 0.67 (0.58–0.77) | 0.001 |
| Calprotectin | 2.15 (1.05–4.41) | 0.036 | 0.63 (0.52–0.73) | 0.022 |
CRP: C-reactive protein; PCT: procalcitonin.
When comparing biomarkers at admission between encapsulated bacteria (e.g. Streptococcus pneumoniae or Haemophilus influenzae) and atypical bacterial infections (e.g. Mycoplasma pneumoniae, Bordetella pertussis, Chlamydophila pneumoniae or Legionella pneumophila), patients with CAP caused by encapsulated bacteria had significantly higher levels of PCT (p=0.049) and WBCs (p=0.003). No significant differences in biomarker levels were seen for CRP, calprotectin, PTX3 or presepsin (p=0.379, p=0.449, p=0.281 and p=0.063, respectively).
Biomarker levels at hospital admission in relation to short-term outcome
A total of 51 (19%) patients were admitted to the ICU (n=48, 18%) and/or died within 30 days of admission (n=10, 4%). Compared with the remaining study cohort, patients with an adverse short-term outcome had higher admission levels of PCT, PTX3 and presepsin, but not of CRP, WBCs or calprotectin (table S2). In line with this finding, high admission levels of PCT, PTX3 and presepsin were all associated with an adverse short-term outcome in univariate logistic regression analysis and in analyses adjusted for the CURB-65 severity score (table 3).
TABLE 3.
Logistic regression analysis of biomarker levels at hospital admission and associations to adverse short-term outcome in 267 hospitalised patients with community-acquired pneumonia
| Biomarker | Univariate analysis | Multivariate analysis# | ||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| PCT | 1.40 (1.16–1.69) | 0.001 | 1.29 (1.06–1.58) | 0.012 |
| PTX3 | 2.46 (1.49–4.06) | <0.001 | 1.88 (1.11–3.19) | 0.018 |
| Presepsin | 1.40 (1.12–1.74) | 0.003 | 1.32 (1.04–1.67) | 0.022 |
PCT: procalcitonin; PTX3: pentraxin 3. #: adjusted for the CURB-65 severity score (confusion, urea >7 mmol·L−1, respiratory rate ≥30 breaths·min−1, blood pressure <90 mmHg (systolic) or ≤60 mmHg (diastolic), age ≥65 years).
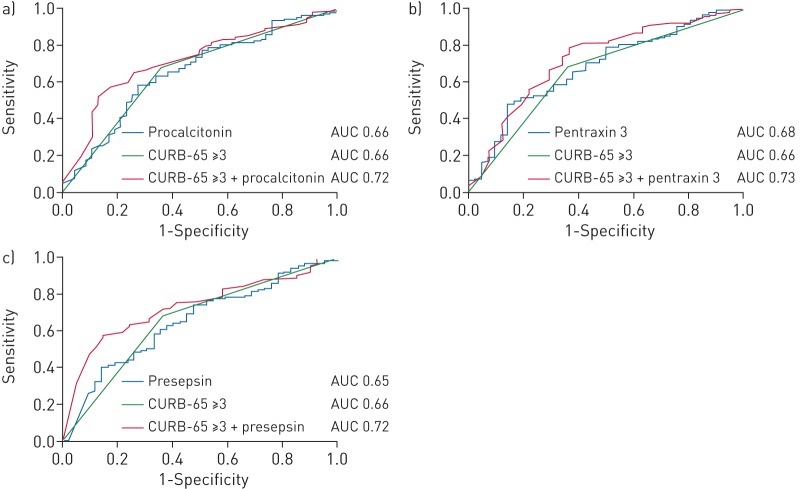
However, admission levels of PCT (AUC 0.66, 95% CI 0.57–0.75; p=0.001), PTX3 (AUC 0.68, 95% CI 0.60–0.77; p<0.001) and presepsin (AUC 0.65, 95% CI 0.56–0.74; p=0.002) provided only moderate value of discrimination between patients with an adverse and nonadverse short-term outcome (figure 3). The optimal cut-off value for discriminating between patients with an adverse and nonadverse short-term outcome for PCT was 0.91 ng·mL−1 (sensitivity 72%, specificity 58%), for PTX3 was 4.22 ng·mL−1 (sensitivity 83%, specificity 50%) and for presepsin was 64.5 pg·mL−1 (sensitivity 64%, specificity 61%).
FIGURE 3.
Receiver operating characteristic curves for biomarkers and CURB-65 severity score (confusion, urea >7 mmol·L−1, respiratory rate ≥30 breaths·min−1, blood pressure <90 mmHg (systolic) or ≤60 mmHg (diastolic), age ≥65 years) at hospital admission for prediction of an adverse short-term outcome. a) Procalcitonin; b) pentraxin 3; c) presepsin. AUC: area under the curve.
Biomarker levels combined with the CURB-65 severity score in relation to short-term outcome
A CURB-65 severity score ≥3 was significantly associated with an adverse short-term outcome (OR 3.83, 95% CI 2.00–7.32; p<0.001) and provided moderate value of discrimination (AUC 0.66, 95% CI 0.58–0.75; p<0.001) for short-term outcome prediction (figure 3). The combination of a CURB-65 score ≥3 with admission levels of PCT (AUC 0.72, 95% CI 0.64–0.79; p<0.001), PTX3 (AUC 0.73, 95% CI 0.64–0.82; p<0.001) and presepsin (AUC 0.73, 95% CI 0.65–0.81; p<0.001) improved the diagnostic accuracy for discriminating between patients with an adverse and nonadverse short-term outcome, although only significantly for presepsin (p-value for difference: p=0.077, p=0.097 and p=0.042, respectively) (figure 3).
Biomarker levels at hospital admission in relation to long-term outcome
Of 257 short-term survivors of CAP, 67 (26%) died within 5 years post-discharge. Cumulative 5-year survival rate was 74% (95% CI 68–79%). In univariate Cox regression analysis, increased admission levels of PTX3 were associated with 5-year all-cause mortality (hazard ratio (HR) 1.63, 95% CI 1.13–2.35; p=0.010). However, when adjusting for age and clinically relevant comorbid conditions (heart failure, active malignant disease, chronic obstructive pulmonary disease and renal disease), PTX3 levels were no longer significantly associated with 5-year all-cause mortality (HR 1.47, 95% CI 0.99–2.18; p=0.052).
Biomarker levels at 6-week follow-up in relation to long-term outcome
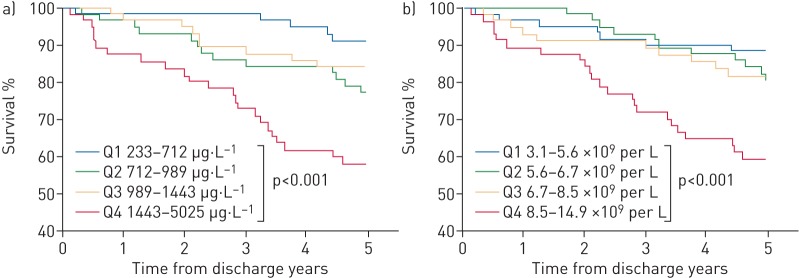
Increased levels of WBCs, CRP, PCT, calprotectin and PTX3 in samples obtained at the 6-week follow-up were associated with 5-year all-cause mortality in univariate Cox regression analysis (table 4). In multivariate Cox regression analysis with adjustment for age and clinically relevant comorbid conditions, only calprotectin and WBCs remained significantly associated with 5-year all-cause mortality (table 4 and figure 4).
TABLE 4.
Cox regression analysis of biomarker levels at the 6-week follow-up and associations to 5-year all-cause mortality in 267 hospitalised patients with community-acquired pneumonia
| Biomarker | Univariate analysis | Multivariate analysis# | ||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| WBCs | 4.74 (1.89–11.87) | 0.001 | 2.81 (1.06–7.44) | 0.037 |
| CRP | 1.67 (1.34–2.08) | <0.001 | 1.29 (0.99–1.67) | 0.055 |
| PCT | 2.82 (1.71–4.63) | 0.001 | 1.73 (0.97–3.09) | 0.063 |
| Calprotectin | 2.56 (1.62–4.04) | <0.001 | 2.18 (1.27–3.74) | 0.005 |
| PTX3 | 2.31 (1.49–3.59) | <0.001 | 1.26 (0.83–1.92) | 0.282 |
HR: hazard ratio; WBC: white blood cell; CRP: C-reactive protein; PCT: procalcitonin; PTX3: pentraxin 3. #: adjusted for age, heart failure, active malignant disease, chronic obstructive pulmonary disease and renal disease.
FIGURE 4.
Kaplan–Meier plots of biomarkers at 6-week follow-up and associations with 5-year all-cause mortality, stratified by quartiles of a) calprotectin (p<0.001 by the log-rank test) and b) white blood cell count (p<0.001). Q: quartile.
Discussion
First, in this cohort of 267 hospitalised patients with CAP, calprotectin was an independent marker of bacterial aetiology, and provided a diagnostic accuracy for discriminating bacterial from viral infections comparable to the established inflammatory biomarkers CRP and PCT, thus suggesting it primarily reflects bacterially mediated inflammation. Secondly, the novel inflammatory biomarkers PTX3 and presepsin were, in addition to PCT, but not CRP, associated with an adverse short-term outcome in CAP, also after adjustment for the CURB-65 severity score. Thirdly, admission levels of the biomarkers examined were not useful for long-term outcome prediction but importantly, levels of calprotectin along with WBCs in the convalescent phase of CAP, at the 6-week follow up, were independent predictors of 5-year all-cause mortality.
A considerable overlap of CRP levels in bacterial, mixed viral–bacterial and viral CAP was found in the present study, in line with observations from clinical practice and other similar studies [3, 7, 21]. Nevertheless, CRP was an independent marker of bacterial aetiology with a moderate discriminatory value, and results for CRP were comparable to PCT. The calcitonin precursor PCT is believed to rise rapidly in the systemic circulation, within 2–3 h, in response to infectious bacterial stimuli and may reach levels 100 000-fold above normal in sepsis, while viral stimuli do not induce the same PCT response [3, 7]. Thus, in most analyses of PCT in lower respiratory tract infections such as CAP, this inflammatory marker has offered a moderately good ability to differentiate bacterial from viral aetiology [22, 23], a finding which also was confirmed in our study. However, our data suggest that PCT may not be superior to CRP for discriminating between bacterial and viral aetiology in CAP.
Calprotectin is a complex of S100A8 and S100A9, two damage-associated molecular pattern molecules, which are primarily released from activated or necrotic neutrophils, including via neutrophil extracellular traps (NETs), although monocytes and macrophages also are cellular sources to circulating calprotectin levels [24]. The calprotectin complex functions as an innate immune mediator in systemic infections like pneumonia and sepsis, exerting its effects via activation of pattern recognition molecules, and by recruiting neutrophils and other immune cells to the site of inflammation [25, 26]. Previously, elevated levels of calprotectin have been reported in both serum and bronchoalveolar lavage fluid from patients with CAP caused by S. pneumoniae [16]. In our study, calprotectin levels had a dynamic temporal profile and were associated with bacterial aetiology, with highest levels in patients with mixed viral–bacterial aetiology. Importantly, however, none of the examined biomarkers could reliably separate bacterial from viral infections in CAP and determine if antibiotic treatment could be safely avoided.
The inflammatory markers PCT, PTX3 and presepsin were all associated with an adverse short-term outcome, defined as ICU admission or 30-day mortality, and discriminated between adverse and nonadverse short-term outcomes with moderate accuracy. By combining a CURB-65 severity score ≥3 with biomarker levels, the prognostic accuracy improved significantly for presepsin only. In accordance with our results, admission levels of PCT have, in multiple previous studies, been associated with short-term mortality, mostly providing moderate to high prognostic value [4, 27]. Presepsin is an inflammatory biomarker derived from the sCD14 protein [28]. Compared with PCT and other existing biomarkers, presepsin levels are suggested to rise earlier in systemic infections and to be more infection specific [12, 28]. In the few studies performed in CAP, presepsin has been found to be higher in patients with a severe course, serving as an independent predictor of severe CAP and 28-day mortality [13, 29]. Our study supports the previous findings on presepsin as a potential novel biomarker predicting short-term outcome. Similarly, PTX3, which, like CRP, is part of the pentraxin family of pattern recognition molecules, predicted short-term outcome in our cohort. In contrast to CRP, PTX3 is produced at the site of infection by numerous cell types, including neutrophils and monocytes, upon stimulation by inflammatory signals (e.g. cytokines or microbial moieties) [30]. In sepsis, PTX3 is believed to act as an acute-phase protein, reach peak levels within 6–8 h and be a predictor of short-term mortality [30, 31]; and in CAP, PTX3 has been reported to correlate with disease severity [17]. The results from our study support the hypothesis that PTX3 may be a prognostic marker in CAP, showing, for the first time, an association between plasma PTX3 levels and adverse short-term outcome in CAP.
It has become increasingly clear that CAP survivors have a higher risk of long-term mortality after hospitalisation in comparison to the general population [32]. The reason for this higher risk of death post-pneumonia is insufficiently understood, although it may in part be attributed to an increase in major cardiovascular events [19, 33]. Previously, admission levels of cardiovascular disease (CVD)-associated biomarkers (troponin T, N-terminal pro-B-type natriuretic peptide and trimethylamine-N-oxide), but not typical inflammatory markers like WBCs, CRP and PCT, have been associated with poor long-term prognosis in CAP [5, 34–36]. In comparison, elevated hospital discharge levels of interleukin-6, as an indication of persisting inflammation, were found to be associated with 1-year mortality in the Genetic and Inflammatory Markers of Sepsis study [37]. Admission levels of inflammatory biomarkers were not independent predictors of long-term mortality in our cohort. However, in samples from the convalescent phase ∼6 weeks after discharge, both calprotectin and WBC levels were associated with 5-year all-cause mortality in analyses adjusted for age and relevant comorbidities.
The observation that calprotectin and WBCs, which correlated strongly with neutrophils and are the main source of systemic calprotectin [24], both were predictors of long-term prognosis suggests an impact of WBCs and neutrophil activity on the continued inflammatory process beyond the acute phase of CAP. CVD is the most common cause of long-term mortality in CAP survivors [19, 38]. Based on the role of neutrophils in the pathogenesis of CVD [39, 40], one might speculate that persistent activation of these cells could contribute to poor long-term prognosis in the CAP population. However, this will have to be further investigated in forthcoming studies. Nonetheless, our findings suggest that patients with persistent elevation of calprotectin along with WBCs should be followed more carefully, especially for the development of CVD.
Limitations and strengths
The measure short-term outcome was defined as a composite endpoint of ICU admission and 30-day mortality, and a majority of patients were categorised with the softer ICU survivor outcome parameter, which may have affected our results. Strengths of our study include the number of patients with an established microbiological diagnosis (63%), the long follow-up period of 5 years and the coverage of data on long-term mortality, as only one patient was lost to follow-up, resulting in 5-year all-cause mortality data on 99.6% of the population.
Conclusion
Calprotectin may represent a new marker of both bacterial aetiology and 5-year all-cause mortality, while PTX3 and presepsin are potential novel predictors of short-term outcome in CAP. The elevated calprotectin and WBC levels in the convalescent phase may reflect unfavourable persistent inflammation associated with the increased risk of long-term mortality.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00014-2019.Supplement (241.5KB, pdf)
Acknowledgements
We gratefully thank Kåre Bø, Thomas Skrede, Anita Johansen and Britt Hiaasen (Dept of Internal Medicine, Drammen Hospital, Vestre Viken Hospital Trust, Drammen, Norway) for collection of patient data; Ola Bjørang, Helvi H. Samdal and Carina Thilesen (Dept of Medical Microbiology, Vestre Viken Hospital Trust, Drammen, Norway) for excellent laboratory assistance; and Nihal Perera (Oslo Center of Biostatistics and Epidemiology, Research Support Services, Oslo University Hospital, Oslo, Norway) who contributed to the design of the database.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: W.W. Siljan has nothing to disclose.
Conflict of interest: J.C. Holter has nothing to disclose.
Conflict of interest: A.E. Michelsen has nothing to disclose.
Conflict of interest: S.H. Nymo has nothing to disclose.
Conflict of interest: T. Lauritzen has nothing to disclose.
Conflict of interest: K. Oppen has nothing to disclose.
Conflict of interest: E. Husebye has nothing to disclose.
Conflict of interest: T. Ueland has nothing to disclose.
Conflict of interest: T.E. Mollnes has nothing to disclose.
Conflict of interest: P. Aukrust has nothing to disclose.
Conflict of interest: L. Heggelund has nothing to disclose.
Support statement: This work was supported by Vestre Viken Hospital Trust, Norway. The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Wunderink RG, Waterer GW. Community-acquired pneumonia. N Engl J Med 2014; 370: 1863. [DOI] [PubMed] [Google Scholar]
- 2.Ewig S, Torres A. Community-acquired pneumonia as an emergency: time for an aggressive intervention to lower mortality. Eur Respir J 2011; 38: 253–260. [DOI] [PubMed] [Google Scholar]
- 3.Kruger S, Welte T. Biomarkers in community-acquired pneumonia. Expert Rev Respir Med 2012; 6: 203–214. [DOI] [PubMed] [Google Scholar]
- 4.Viasus D, Del Rio-Pertuz G, Simonetti AF, et al. Biomarkers for predicting short-term mortality in community-acquired pneumonia: a systematic review and meta-analysis. J Infect 2016; 72: 273–282. [DOI] [PubMed] [Google Scholar]
- 5.Alan M, Grolimund E, Kutz A, et al. Clinical risk scores and blood biomarkers as predictors of long-term outcome in patients with community-acquired pneumonia: a 6-year prospective follow-up study. J Intern Med 2015; 278: 174–184. [DOI] [PubMed] [Google Scholar]
- 6.Christ-Crain M, Muller B. Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J 2007; 30: 556–573. [DOI] [PubMed] [Google Scholar]
- 7.Esposito S, Di Gangi M, Cardinale F, et al. Sensitivity and specificity of soluble triggering receptor expressed on myeloid cells-1, midregional proatrial natriuretic peptide and midregional proadrenomedullin for distinguishing etiology and to assess severity in community-acquired pneumonia. PLoS One 2016; 11: e0163262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalmers JD, Singanayagam A, Hill AT. C-reactive protein is an independent predictor of severity in community-acquired pneumonia. Am J Med 2008; 121: 219–225. [DOI] [PubMed] [Google Scholar]
- 9.Muller B, Harbarth S, Stolz D, et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis 2007; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruger S, Ewig S, Marre R, et al. Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. Eur Respir J 2008; 31: 349–355. [DOI] [PubMed] [Google Scholar]
- 11.Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18: 95–107. [DOI] [PubMed] [Google Scholar]
- 12.Shozushima T, Takahashi G, Matsumoto N, et al. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother 2011; 17: 764–769. [DOI] [PubMed] [Google Scholar]
- 13.Liu B, Yin Q, Chen YX, et al. Role of Presepsin (sCD14-ST) and the CURB65 scoring system in predicting severity and outcome of community-acquired pneumonia in an emergency department. Respir Med 2014; 108: 1204–1213. [DOI] [PubMed] [Google Scholar]
- 14.Gao S, Yang Y, Fu Y, et al. Diagnostic and prognostic value of myeloid-related protein complex 8/14 for sepsis. Am J Emerg Med 2015; 33: 1278–1282. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Qu X, Liu F, et al. Pentraxin 3 as a prognostic biomarker in patients with systemic inflammation or infection. Mediators Inflamm 2014; 2014: 421429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Achouiti A, Vogl T, Endeman H, et al. Myeloid-related protein-8/14 facilitates bacterial growth during pneumococcal pneumonia. Thorax 2014; 69: 1034–1042. [DOI] [PubMed] [Google Scholar]
- 17.Kao SJ, Yang HW, Tsao SM, et al. Plasma long pentraxin 3 (PTX3) concentration is a novel marker of disease activity in patients with community-acquired pneumonia. Clin Chem Lab Med 2013; 51: 907–913. [DOI] [PubMed] [Google Scholar]
- 18.Holter JC, Muller F, Bjorang O, et al. Etiology of community-acquired pneumonia and diagnostic yields of microbiological methods: a 3-year prospective study in Norway. BMC Infect Dis 2015; 15: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holter JC, Ueland T, Jenum PA, et al. Risk factors for long-term mortality after hospitalization for community-acquired pneumonia: a 5-year prospective follow-up study. PLoS One 2016; 11: e0148741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bello S, Minchole E, Fandos S, et al. Inflammatory response in mixed viral-bacterial community-acquired pneumonia. BMC Pulm Med 2014; 14: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Self WH, Balk RA, Grijalva CG, et al. Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia. Clin Infect Dis 2017; 65: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruger S, Ewig S, Papassotiriou J, et al. Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with CAP: results from the German competence network CAPNETZ. Respir Res 2009; 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiopu A, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm 2013; 2013: 828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Zoelen MA, Vogl T, Foell D, et al. Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. Am J Respir Crit Care Med 2009; 180: 1098–1106. [DOI] [PubMed] [Google Scholar]
- 26.Achouiti A, Vogl T, Van der Meer AJ, et al. Myeloid-related protein-14 deficiency promotes inflammation in staphylococcal pneumonia. Eur Respir J 2015; 46: 464–473. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez JF, Sibila O, Restrepo MI. Predicting ICU admission in community-acquired pneumonia: clinical scores and biomarkers. Expert Rev Clin Pharmacol 2012; 5: 445–458. [DOI] [PubMed] [Google Scholar]
- 28.Masson S, Caironi P, Spanuth E, et al. Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: data from the Albumin Italian Outcome Sepsis trial. Crit Care 2014; 18: R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klouche K, Cristol JP, Devin J, et al. Diagnostic and prognostic value of soluble CD14 subtype (Presepsin) for sepsis and community-acquired pneumonia in ICU patients. Ann Intensive Care 2016; 6: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daigo K, Inforzato A, Barajon I, et al. Pentraxins in the activation and regulation of innate immunity. Immunol Rev 2016; 274: 202–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauri T, Bellani G, Patroniti N, et al. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med 2010; 36: 621–629. [DOI] [PubMed] [Google Scholar]
- 32.Eurich DT, Marrie TJ, Minhas-Sandhu JK, et al. Ten-year mortality after community-acquired pneumonia. A prospective cohort. Am J Respir Crit Care Med 2015; 192: 597–604. [DOI] [PubMed] [Google Scholar]
- 33.Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015; 313: 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vestjens SMT, Spoorenberg SMC, Rijkers GT, et al. High-sensitivity cardiac troponin T predicts mortality after hospitalization for community-acquired pneumonia. Respirology 2017; 22: 1000–1006. [DOI] [PubMed] [Google Scholar]
- 35.Ottiger M, Nickler M, Steuer C, et al. Trimethylamine-N-oxide (TMAO) predicts fatal outcomes in community-acquired pneumonia patients without evident coronary artery disease. Eur J Intern Med 2016; 36: 67–73. [DOI] [PubMed] [Google Scholar]
- 36.Chang CL, Mills GD, Karalus NC, et al. Biomarkers of cardiac dysfunction and mortality from community-acquired pneumonia in adults. PLoS One 2013; 8: e62612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yende S, D'Angelo G, Kellum JA, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med 2008; 177: 1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eurich DT, Marrie TJ, Minhas-Sandhu JK, et al. Risk of heart failure after community acquired pneumonia: prospective controlled study with 10 years of follow-up. BMJ 2017; 356: j413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guasti L, Dentali F, Castiglioni L, et al. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb Haemost 2011; 106: 591–599. [DOI] [PubMed] [Google Scholar]
- 40.Langseth MS, Opstad TB, Bratseth V, et al. Markers of neutrophil extracellular traps are associated with adverse clinical outcome in stable coronary artery disease. Eur J Prev Cardiol 2018; 25: 762–769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00014-2019.Supplement (241.5KB, pdf)