Chronic kidney disease (CKD) is common and appears to be progressive in sickle cell disease (SCD) and is also associated with increased mortality in this condition (Ataga, et al 2014). Albuminuria is a risk factor for worsening kidney function (Ataga, et al 2014). Albuminuria and changes in glomerular filtration rate occur well after substantial structural and functional tissue damage have ensued (Guasch, et al 1996). Available biomarkers of CKD fail to provide information on underlying causal biochemical pathways, limiting the discovery of novel therapeutic or preventive measures. Metabolite profiling and the bioinformatics tools to study them may aid in the discovery of novel associations with disease markers that uncover pathophysiological pathways in SCD. This exploratory study evaluated plasma metabolomics profiles in SCD patients with and without albuminuria.
SCD patients with normal albuminuria (urine albumin-creatinine ratio [UACR] < 30 mg/g) and moderately or severely increased albuminuria (UACR ≥ 30 mg/g) were recruited from an adult sickle cell clinic. The study design and patient eligibility criteria have been previously described (Ataga, et al 2016). The study was approved by the Institutional Review Board. All subjects gave written informed consent.
Plasma samples (150 μl) were prepared by adding 0.9% saline solution (100 μl) containing 2.5 mM formate (chemical shift indicator). Proton nuclear magnetic resonance (1H NMR) spectra of plasma samples were acquired on a Bruker Avance III 950 MHz NMR spectrometer using a Carr-Purcell-Meiboom-Gill pulse sequence and 128 scans. NMR peaks were library-matched to metabolites and the relative concentration of metabolites was determined using Chenomx NMR Suite 8.1 Professional software (Chenomx, Edmonton, Alberta, Canada) (Appendix S1). Orthogonal projections to latent structures discriminant analysis (OPLS-DA) was performed using SIMCA software (Sartorius Stedim Biotech, Umea, Sweden). Variable influence on projection (VIP) plots were inspected and metabolites with a VIP ≥ 1.0 and a jack-knife confidence interval that did not include zero were considered important in differentiating the groups. Statistical analyses using the metabolite relative concentrations were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC). Hypothesis testing (two-sided) was conducted using the Wilcoxon Rank Sum test for continuous variables and Fisher’s Exact Test for categorical variables. Fold-changes were calculated as the ratio of the median concentrations. There was no adjustment for multiple testing. Due to the small sample size, p-values < 0.1 were considered to be important associations.
Plasma samples from 23 subjects with HbSS were analysed. One subject, with normal albuminuria and marked hyperbilirubinaemia, was removed from the analyses following concerns that his hyperbilirubinaemia might have falsely affected the spectral patterns. The median age of the remaining 22 subjects was 43.5 years, 12 were female, and 6 subjects had normal albuminuria (Table I). There were differences between the albuminuria groups for systolic blood pressure (p=0.04), diastolic blood pressure (p=0.08) and fetal haemoglobin (p=0.095). The albuminuria groups were distinguished by OPLS-DA (Figure 1) and the VIP plot is shown in Figure S1. Hypothesis testing found that higher levels of betaine and proline were associated with albuminuria (p=0.04 and p=0.07, respectively) (Table SI). Higher dimethylamine (DMA) level was also associated with albuminuria (p=0.098), with a more than 200-fold change. OPLS-DA identified six metabolites important in differentiating subjects with albuminuria: betaine (VIP=1.79), proline (VIP=1.69), DMA (VIP=1.54), glutamate (VIP=1.30), leucine (VIP=1.06) and lysine (VIP=1.02).
Table I:
Baseline Demographics and Laboratory Data
| Characteristic | Normal Albuminuria (n = 6) | Albuminuria (n = 16)+ | p-value** |
|---|---|---|---|
| Age, years | 41.0 (13.0) | 43.5 (17.5) | 0.49 |
| Female, n (%) | 5 (83.3%) | 7 (43.8%) | 0.16 |
| Weight, kg | 63.9 (34.4) | 68.3 (17.2) | 0.97 |
| Height, cm | 166.3 (11.8) | 169.3 (9.8) | 1.0 |
| Systolic blood pressure, mm Hg | 107.5 (24.0) | 132.0 (31.0) | 0.04 |
| Diastolic blood pressure, mm Hg | 57.0 (14.0) | 66.5 (21.5) | 0.08 |
| Hydroxycarbamide use, yes, n (%) | 5 (83.3%) | 13 (81.3%) | 1.0 |
| Renin-angiotensin-aldosterone system blocking agent use, yes, n (%) | 0 (0%) | 4 (25%) | 0.53 |
| White blood cell count, x 109/l | 6.9 (2.2) | 7.6 (5.6) | 0.47 |
| Haemoglobin, g/l | 91 (16) | 88 (15) | 0.91 |
| Platelet count, x 109/l | 335.5 (173.0) | 312.0 (140.5) | 0.41 |
| Reticulocyte count percentage | 5.2 (7.0) | 8.0 (5.7) | 0.27 |
| Fetal haemoglobin percentage | 19.3 (10.5) | 11.6 (9.8) | 0.095 |
| Creatinine, μmol/l | 53.0 (17.7) | 61.9 (44.2) | 0.13 |
| *Estimated glomerular filtrate rate, ml/min/1.73m2 | 124.0 (11.7) | 120.2 (70.4) | 0.45 |
| Lactate dehydrogenase, u/l | 660.0 (395.0) | 907.0 (568.0) | 0.13 |
| Total bilirubin, μmol/l | 22.2 (44.5) | 44.5 (29.1) | 0.19 |
All values are given as median (interquartile range unless otherwise specified.
Includes subjects with moderately increased and severely increased albuminuria. N=15 for foetal haemoglobin percentage, lactate dehydrogenase, and total bilirubin.
Glomerular filtration rate estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation
Wilcoxon Rank Sum Test for continuous variables; Fisher’s Exact Test for categorical variables.
Normal albuminuria – urine albumin-creatinine ratio (UACR) < 30 mg/g
Albuminuria – UACR ≥ 30 mg/g
Figure 1.
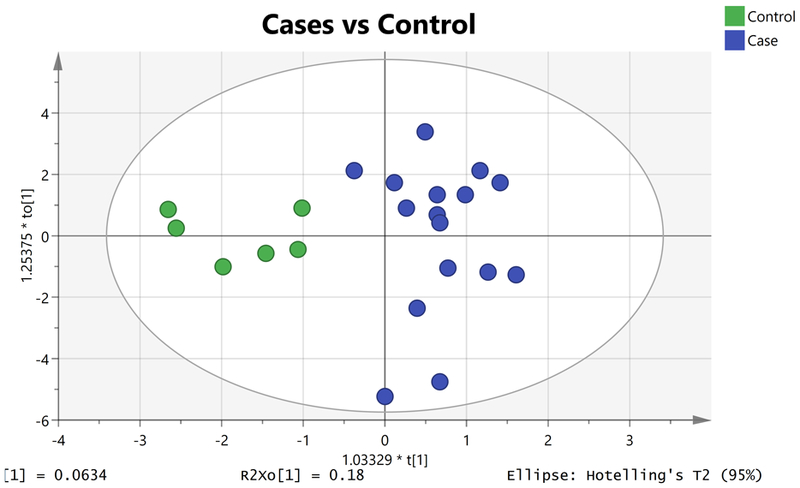
Orthogonal projections to latent structures discriminant analysis (OPLS-DA) Scores Plot showing separation of albuminuria (blue circles, right) from normal albuminuria (green circles, left) [R2X = 0.4; R2Y= 0.8; Q2=−0.1].
Urine levels of both DMA and proline are associated with proteinuria in patients with primary focal segmental glomerulosclerosis (Kalantari, et al 2016), a common pathological finding in SCD glomerulopathy (Ataga, et al 2014). DMA, a product of asymmetric dimethylarginine (ADMA) metabolism, plays a role in nitric oxide (NO) modulation. The NO synthesis pathway is proposed to be associated with chronic renal failure (Vallance, et al 1992). A significant portion of ADMA, an endogenous inhibitor of NO synthases, is metabolized by dimethylarginine dimethylaminohydrolase to DMA and citrulline. The observed association of DMA with albuminuria suggests that its substrate, ADMA, may also be associated with albuminuria. Plasma ADMA is significantly higher in SCD subjects compared to controls, and is associated with haemolysis markers, levels of soluble adhesion molecules (Schnog, et al 2005), elevated tricuspid regurgitant jet velocity and increased mortality. The association of DMA with albuminuria supports the hypothesis that endothelial dysfunction contributes to the pathophysiology of SCD-related glomerulopathy (Ataga, et al 2016).
Proline, a non-essential amino acid synthesized from L-glutamate or arginine, was also associated with albuminuria. Proline build-up could be due to increased synthesis or decreased breakdown by the enzymes proline oxidase or proline dehydrogenase (PRODH), respectively. As the level of lactate, an inhibitor of PRODH, is elevated in SCD patients compared to control subjects following a shift towards anaerobic metabolism (Petto, et al 2011), high lactate levels may contribute to the development of albuminuria in SCD via increased proline levels.
Plasma level of betaine (N,N,N-trimethylglycine), an important methyl donor and osmolyte, was associated with albuminuria. Betaine, the major methyl donor for the enzyme betaine homocysteine methyltransferase (BHMT), catalyses the re-synthesis of methionine from homocysteine. Mild elevation in plasma homocysteine is documented in SCD (Dhar, et al 2004). Although plasma homocysteine correlates with serum creatinine in SCD (Dhar, et al 2004), it is uncertain whether an increased homocysteine level contributes to renal dysfunction or accumulates with renal insufficiency. Urinary excretion of glycine betaine is elevated in kidney disease and in diabetes, but the elevation in diabetes is not correlated with microalbuminuria or plasma creatinine (Lever, et al 1994). Confirmatory studies are required to determine whether betaine could serve as an early indicator of SCD glomerulopathy.
The significance of the relationship between changes in glutamate, lysine and leucine, and albuminuria in SCD is uncertain. However, alterations in glutamate are reported to predict the development of albuminuria in individuals with hypertension, while L-lysine is a potent inhibitor of renal tubule protein reabsorption (Gonzalez-Calero, et al 2016).
We report, for the first time, metabolomic pathways associated with albuminuria in SCD. The associations of DMA and proline with albuminuria further highlight our finding of an association of albuminuria with impaired flow-mediated dilation of the brachial artery (Ataga, et al 2016). We also found associations of glutamate, lysine and leucine with albuminuria. This study is limited by the small sample size and absence of a validation cohort. Our future goal is to conduct an adequately powered study to identify metabolites that could identify patients at increased risk of worsening CKD, and facilitate the development of novel therapies for SCD glomerulopathy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants R01HL111659 (LE, DRA, KIA), and the NIH Eastern Regional Comprehensive Metabolomics Resource Core (ERCMRC), funded by the NIH Common Fund under grant U24 DK097193 (Sumner, PI).
REFERENCES
- Ataga KI, Derebail VK & Archer DR (2014) The glomerulopathy of sickle cell disease. American journal of hematology, 89, 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataga KI, Derebail VK, Caughey M, Elsherif L, Shen JH, Jones SK, Maitra P, Pollock DM, Cai J, Archer DR & Hinderliter AL (2016) Albuminuria Is Associated with Endothelial Dysfunction and Elevated Plasma Endothelin-1 in Sickle Cell Anemia. PLoS ONE, 11, e0162652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar M, Bellevue R, Brar S & Carmel R (2004) Mild hyperhomocysteinemia in adult patients with sickle cell disease: a common finding unrelated to folate and cobalamin status. American journal of hematology, 76, 114–120. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Calero L, Martin-Lorenzo M, Martinez PJ, Baldan-Martin M, Ruiz-Hurtado G, Segura J, de la Cuesta F, Barderas MG, Ruilope LM, Vivanco F & Alvarez-Llamas G (2016) Hypertensive patients exhibit an altered metabolism. A specific metabolite signature in urine is able to predict albuminuria progression. Transl Res, 178, 25–37 e27. [DOI] [PubMed] [Google Scholar]
- Guasch A, Cua M & Mitch WE (1996) Early detection and the course of glomerular injury in patients with sickle cell anemia. Kidney international, 49, 786–791. [DOI] [PubMed] [Google Scholar]
- Kalantari S, Nafar M, Samavat S, Parvin M, Nobakht MGBF & Barzi F (2016) 1 H NMR-based metabolomics exploring urinary biomarkers correlated with proteinuria in focal segmental glomerulosclerosis: a pilot study. Magn Reson Chem, 54, 821–826. [DOI] [PubMed] [Google Scholar]
- Lever M, Sizeland PC, Bason LM, Hayman CM, Robson RA & Chambers ST (1994) Abnormal glycine betaine content of the blood and urine of diabetic and renal patients. Clin Chim Acta, 230, 69–79. [DOI] [PubMed] [Google Scholar]
- Petto J, de Jesus JB, Vasques LM, Pinheiro RL, Oliveira AM, Spinola KA & Silva Wdos S (2011) Resting blood lactate in individuals with sickle cell disease. Rev Bras Hematol Hemoter, 33, 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnog JB, Teerlink T, van der Dijs FP, Duits AJ, Muskiet FA & Group CS (2005) Plasma levels of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, are elevated in sickle cell disease. Annals of hematology, 84, 282–286. [DOI] [PubMed] [Google Scholar]
- Vallance P, Leone A, Calver A, Collier J & Moncada S (1992) Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet, 339, 572–575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


