Abstract
Objective:
Racial discrimination (RD) is hypothesized to dysregulate the production of stress reactive hormones among African Americans. Psychological processes that may mediate the association between RD and such dysregulation (e.g., cortisol/DHEA ratio) are not well articulated. Organizational religious involvement (ORI) has been discussed as a psychological protective factor within the context of RD, but our understanding of ORI as a physiological protective factor remains limited. We evaluated whether RD was directly and indirectly (through depressive symptoms) associated with an imbalance of cortisol and DHEA hormones, and whether ORI buffered these direct and/or indirect pathways.
Design:
Data were drawn from the Flint Adolescent Study, an ongoing interview study of youth that began in 1994. Participants were 188 African American emerging adults (47.3% Female, ages 20–22). We used mediation and moderated-mediation analyses, as outlined by Hayes (2012), to evaluate the study aims.
Results:
We found that depressive symptoms mediated the association between RD and the cortisol/DHEA ratio. We also found that depressive symptoms mediated the association between RD and the cortisol/DHEA ratio for individuals reporting low and moderate levels of ORI, but not at high levels.
Conclusions:
Our findings support the socio-psychobiological model of racism and health (Chae et al., 2011) and suggest that the psychological toll of RD can confer physiological consequences. Moreover, ORI may disrupt pathways from RD to cortisol/DHEA ratio by buffering the psychological toll of RD.
Keywords: racial discrimination, neuroendocrine response, religion, resilience
Epidemiological research has firmly established that African Americans bear a disproportionate share of health problems (see Lewis et al., 2015). To understand the causes of racial disparities in health, scholars have pointed to racial discrimination (RD) as a psychosocial stressor that contributes to poor health among African Americans (see Williams & Mohammed, 2013). Guided by Chae and colleagues’ (2011) socio-psychobiological conceptual model of racism and health, we posit that one mechanism through which RD may lead to poor health is through dysregulation of stress-reactive biological systems (e.g., the hypothalamic-pituitary-adrenal (HPA) axis; indexed by the glucocorticoid cortisol; Adam et al., 2015; Bergus & Sarnyai, 2015; Lee et al., 2017). Disrupted stress biology is proposed to underlie many health conditions (Cohen, Janicki-Deverts, & Miller, 2007), and scholars have reported that RD can disrupt the production of hormones such as cortisol and dehydroepiandrosterone (DHEA) that are involved in the stress process (Lucas et al., 2017; Ratner, Halim, & Amodio, 2013). To our knowledge, no study, has examined whether RD can contribute to an imbalance of multiple stress reactive hormones. Yet, imbalances in the cortisol/DHEA ratio have been associated with various mental (Sollberger & Ehlert, 2016) and physical health problems (Guder et al., 2007), suggesting that this type of dysregulated stress response might be one pathogenic pathway by which RD deteriorates health.
Despite these interesting findings, research on RD and stress reactive hormones is limited in two important ways. First, although research consistently reveals that chronic RD generates vulnerability for mental health problems (e.g., Hurd et al., 2014), and that mental health problems contribute to the imbalance of the cortisol/DHEA ratio (Sollberger & Ehlert, 2016), these associations have not been examined in the same study. Furthermore, evidence of the association between RD and biological stress regulation (i.e., cortisol, DHEA) has been mixed, with several researchers reporting no direct effect (Fuller-Rowell et al., 2012; Lee et al., 2017). We posit that one reason for the inconsistency in prior work is that the association between RD and dysregulation of biological stress indicators like the cortisol/DHEA ratio may become established through indirect psychological pathways, such as depressive symptoms. To better understand the risk and resilience processes, we propose to test this model in the current study.
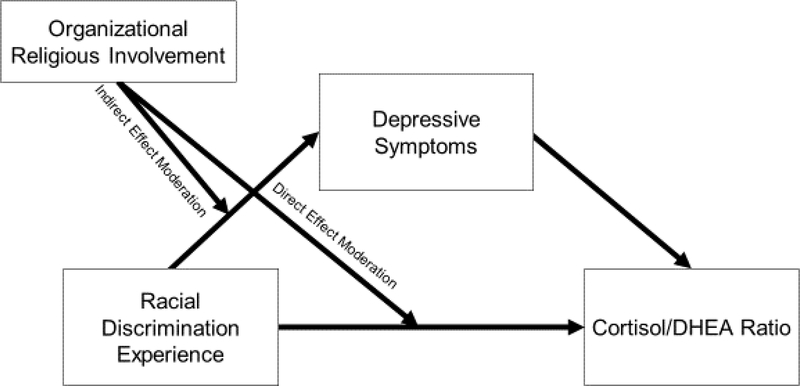
The second limitation pertains to the limited number of studies that examine protective factors in pathways that link RD to physiological stress responses. Organizational religious involvement (hereafter we use ORI) has been identified as a key protective factor that can moderate the adverse psychological effects of RD among African Americans (e.g., Hope, Assari, Cole-Lewis, & Caldwell, 2017). Researchers have mostly theorized mechanisms by which ORI might offer physiological protection from RD (Assari et al., 2015). Scholars, for instance, posit that ORI may moderate the physiological consequences of RD by mitigating the psychological consequences of RD (Assari, 2013; Assari, 2014; Assari et al., 2015), but studies have not examined both processes in the same model. Thus, we do not know whether ORI reduces the physiological toll of RD directly or by moderating the psychological influence of RD (see Figure 1 for conceptual model).
Figure 1.
Conceptual model bridging racial discrimination to the cortisol/DHEA ratio, with organizational religious involvement as protective factor
To address these gaps in the literature, our study entailed two aims. First, we investigated whether RD was directly and indirectly (through depressive symptoms) associated with an imbalance in the cortisol/DHEA ratio. Second, we explored whether ORI moderated the direct and indirect pathway from RD to the cortisol/DHEA ratio (see Figure 1).
Racial Discrimination and the HPA axis
RD, defined as “differential treatment on the basis of race that disadvantages a racial group” (National Research Council, 2004, p. 4), is a ubiquitous stressor among African Americans. With over 90% of African American emerging adults reporting multiple RD experiences in a given year (e.g., race-related microaggressions), researchers have consistently found that RD exacts psychological and physiological tolls (Lewis et al., 2015). Chae and colleagues (2011) have proposed a socio-psychobiological framework of racial health disparities, in which RD can set in motion psychological, behavioral, and biological vulnerabilities that, in combination, can accelerate health deterioration. Among African Americans, RD exposure has been linked with poorer mental health outcomes (Hurd, Varner, Caldwell, & Zimmerman, 2014), higher allostatic load (Geronimus, 2006), shorter telomere length (Lee, Kim, & Neblett, 2016), dysregulation of the HPA axis (Lee et al., 2017), and poorer health behaviors (Jackson, Knight, & Rafferty, 2010). RD, to this end, may contribute substantially to racial disparities in rates of disease morbidity, comorbidity, severity, and mortality (Williams & Mohammed, 2013).
A strong conceptual and empirical case exists for examining the HPA axis as a biological pathway by which RD deteriorates health among African Americans. RD experiences during adolescence and emerging adulthood have been linked to dampened awakening response, diurnal rhythm, and circulating levels of cortisol among African Americans (Adam et al., 2015; Bergus & Sarnyai, 2015; Lee et al., 2017). Although acute stressors have been associated with increases in circulating cortisol levels, scholars have pointed to the attenuation hypothesis (see Susman, 2006) to explain why cortisol levels are attenuated within the context of RD (Lee et al., 2017). The attenuation hypothesis contends that the HPA axis downregulates cortisol production following chronic stress to limit the body’s exposure to cortisol (Susman, 2006). Since RD experiences are chronic among African Americans (Lee et al., 2017), it comes as no surprise that scholars have largely documented blunted cortisol production in the context of RD exposure.
RD-induced alterations to cortisol production may be better understood when considered in conjunction with DHEA. DHEA, a hormone that is synchronously released with cortisol from the adrenal cortex, is known to antagonize the adverse physiological effects of cortisol (Kaminska, Harris, Gijsbers, & Dubrovsky, 2000). Scholars have traditionally theorized that elevated cortisol and attenuated DHEA production (i.e., higher cortisol/DHEA ratio) increases the body’s exposure to unopposed cortisol, thereby increasing risk for physical health problems such as acute stroke (Blum et al., 2013). While higher cortisol/DHEA ratios have generally been observed among individuals experiencing chronic stress (e.g., Moriguchi Jeckel et al., 2010) and major depressive disorder (Sollberger & Ehlert, 2016), studies also found lower cortisol/DHEA ratios to be associated with diagnoses of depression and post-traumatic stress disorder (Gill, Vythilingam, & Page, 2008; Yehuda et al., 2006). Drawing from the attenuation hypothesis (Susman, 2006), Karmin and Kertes (2017) argued that the HPA axis may downregulate cortisol production in the context of prolonged stress but upregulate DHEA to better meet the physiological demands of the chronic stressor(s). As RD is a pervasive and prevalent stressor in the lives of African Americans, it may dysregulate the HPA axis by creating an imbalance in circulating levels of cortisol and DHEA that over time can contribute to poorer health.
Depressive Symptoms as a Mediating Pathway
RD invokes poorer psychological well-being and physiological health among African Americans (Berger & Sarnyai, 2015). Chae and colleagues (2011) suggested that RD can generate psychological risks, which can, in turn, promote biological vulnerabilities (e.g., dysregulation of the HPA axis). To date, a few researchers have elucidated psychological factors that mediate the effect of RD on biological outcomes such as telomere length (Chae et al., 2015; Lee et al., 2017) and cortisol concentration (Lee et al., 2017). For example, Lee and colleagues (2017) found that RD indirectly, but not directly, influenced cortisol concentrations through anxiety symptoms. Psychological reactions to RD, to this end, may influence the regulation of biological systems engaged in the stress response such as the HPA axis (Berger & Sarnyai, 2015).
Embracing this line of thinking, two separate bodies of research point to the role of depressive symptoms specifically as a mediator of the link between RD and the cortisol/DHEA ratio. First, cross-sectional and longitudinal studies have documented a positive association between RD and depressive symptoms among African Americans (Hurd et al., 2014). Second, depressive symptoms have been associated with an imbalance in the cortisol/DHEA ratio (Sollberger & Ehlert, 2016). Considered within Chae’s socio-psychobiological model of racism and health (2011), these studies suggest that depressive symptoms may mediate the association between RD and an imbalance of the cortisol/DHEA ratio.
Organizational Religious Involvement as a Protective Factor
Although psychological pathways from RD to physiological outcomes have been documented (Lee et al., 2017), no study, to date, has examined the role of protective factors within these mechanisms. organizational religious involvement (ORI), defined as “…public, institutional forms of religious involvement [e.g., service attendance],” has been consistently documented as a psychological protective factor for African Americans contending with racism-related stress (Mattis & Watson, 2009 for review). Guided by the risk and resilience model (Begun, 1993), ORI (e.g.,service attendance) has been found to mitigate the psychological consequences of RD among African Americans (Bierman, 2006; Ellison, Musick, & Henderson, 2008; Ellison et al., 2017; Hope et al., 2017; Lee, Neblett, & Jackson, 2015). Ellison and colleagues (2017), for instance, found that service attendance buffered the adverse effects of RD on mental health outcomes and life satisfaction. Bierman (2006) similarly found that religious service attendance was psychologically protective for African Americans, but not White Americans. Moreover, religious social support offered by fellow congregants reduced the influence of RD on psychiatric disorders, suggesting that ORI may promote resilience by extending religious social support.
Scholars have proposed multiple mechanisms through which ORI offers psychological protection within the context of RD. Most notably, researchers have identified service attendance and organized religious activities (e.g., choir, volunteerism) as psychologically salutary for African Americans by increasing opportunities for social support (Hope et al., 2017; Mattis & Watson, 2009). Given the centrality of religion in the lives of African Americans, predominantly African Americans faith-based communities are fertile ground for receiving social and emotional support within the context of RD (Hope et al., 2017). For example, African Americans who access predominantly Black religious communities may be uniquely positioned to receive empathy and emotional validation from leaders and fellow co-religionists (Mattis & Watons, 2009). In addition to emotional support, one might surmise that religious social support promotes social assets such as mentorship (Cook, 2000) and mental health counseling from a religious leader (Krause, 2003) that underlies the psychologically protective role of ORI in the context of RD. Mentorship, for example, has been associated with less mental health problems and risk behaviors (e.g., substance use, sexual risk behaviors) over time among Black youth (Hurd & Zimmerman, 2010). Relational support structures in predominantly Black faith-based communities, to this end, may buffer the mental health consequences of RD (Hope et al., 2017).
ORI may also increase exposure to religious teachings that emphasize liberationist theology (Mattis et al., 2003) and activities that promote volunteerism and civic engagement (Hope & Spencer), which, in turn, promotes psychological well-being within the context of RD. For instance, exposure to liberationist theology may reinforce beliefs that RD experiences are not without meaning and that the divine is able to exact social, political, and economic justice to members of oppressed groups (Mattis et al., 2003). This belief, in turn, may cultivate greater optimism and diminish the mental health consequences of RD (Lee et al., 2015). Hope and Spencer (2017) have also noted that African Americans are more likely to spend time in volunteer and civic engagement activities, which may promote positive mental health outcomes in the context of RD. Thus, ORI may both enhance mental health among African Americans burdened by RD and offset the psychological pathways linking RD to its physiological toll.
Although considerable evidence points to ORI as a psychologically protective factor in the presence of RD, research examining ORI as a physiologically protective factor is limited. In a few studies, ORI has been associated with better physiological functioning in the context of stress (Hill, Bradshaw, & Burdette, 2016; Hill, Rote, Ellison, & Burdette, 2014). For example, Hill and colleagues (2016) noted that religious involvement may increase social resources, promote health behaviors, improve psychological functioning, and decrease stress exposure to mitigate allostatic load – such as, neuroendocrine functioning – within the context of stress (e.g, RD stress). Researchers have not, however, tested whether ORI reduces the physiological consequences of RD. Thus, it is important to test whether ORI shields against physiological imbalance by first reducing the psychological toll of RD (i.e., indirect effect moderation). It is also plausible, although limited in evidence (Cooper et al., 2013), that ORI may directly mitigate the physiological effect of RD (i.e., direct effect moderation; see Figure 1).
Current Study
To advance our understanding of risk and resilience in the context of RD, our first aim was to investigate whether depressive symptoms mediated the association between RD and the cortisol/DHEA ratio. We hypothesized that RD exposure would increase depressive symptoms, which, in turn, would associate with imbalances between cortisol and DHEA. Second, in light of past studies on ORI as a protective factor (e.g., Hope et al., 2017), we tested whether ORI directly or indirectly moderated the above mediational pathway (see Figure 1).
Method
Participants
The sample for this study included 188 African Americans from an ongoing longitudinal study of school-dropout and alcohol/substance use (Zimmerman & Schmeelk-Cone, 2003). The original sample consisted of 850 ninth graders enrolled in one of four public high schools in a large, Midwestern city. To be eligible for the original study, participants needed to have an 8th grade GPA of 3.0 or lower and no diagnosis of an emotional disorder or a developmental disability. Participants in the present study were assessed at the sixth wave (2001; Mage = 20.98, SDage = 0.64) and were included if they had complete cortisol and DHEA data (see Table 1). Pregnant participants and participants with a salivary blood protein contamination level greater than or equal to 3 mg/dL were excluded (n = 10).
Table 1.
Descriptive Statistics and Intercorrelations Between Study Variables
| 1 | 2 | 3 | 4 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | % or M(SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sex | -- | 52.7% Male | |||||||||||||||||||
| 2. Age | −.14ᵀ | -- | 20.98 (0.67) | ||||||||||||||||||
| 3. Educational Attainment | .23** | −.33** | -- | 4.78 (2.10) | |||||||||||||||||
| 4. Racial Discrimination (Wave 5) | −.07 | −.01 | −.04 | -- | 0.85 (0.81) | ||||||||||||||||
| 4. Racial Discrimination (Wave 6) | −.15* | .03 | −.08 | .40** | -- | 0.90 (0.91) | |||||||||||||||
| 5. Depressive Symptoms | .16** | −.06 | −.05 | .18* | .31** | -- | 0.68 (0.69) | ||||||||||||||
| 6. Alcohol Use | −.14ᵀ | −.07 | .01 | .19* | .20** | .20** | -- | 4.78 (3.78) | |||||||||||||
| 7. Cigarette Use | −.16ᵀ | .05 | −.25** | .04 | .06 | .02 | .40** | -- | 5.80 (2.82) | ||||||||||||
| 8. Exercise | −.19** | −.01 | −.05 | −.07 | .07 | −.04 | .04 | .07 | -- | 2.55 (1.27) | |||||||||||
| 9. Fruit Consumption | .00 | −.04 | .08 | −.05 | .06 | −.16* | −.14ᵀ | −.12 | .21** | -- | 2.70 (1.20) | ||||||||||
| 10. Fat Food Consumption | −.02 | −.02 | −.04 | .11 | .13ᵀ | .03 | .15* | .05 | .08 | −.08 | -- | 3.84 (1.15) | |||||||||
| 11. BMI | .19* | −.02 | .04 | −.02 | −.12 | −.06 | −.08 | −.18* | −.04 | .18* | −.07 | -- | 26.59 (6.97) | ||||||||
| 12. Perceived Stress | .04 | .06 | −.16* | .18* | .32** | .56* | .14ᵀ | .16* | .02 | −.10 | .06 | −.04 | -- | 2.43 (0.59) | |||||||
| 13. Doctor Visits | .12 | .08 | −.02 | .08 | .11 | .07 | −.08 | −.05 | .19* | .17* | .06 | .02 | .03 | -- | 1.76 (2.02) | ||||||
| 14. Chronic Illness | −.01 | −.02 | .08 | −.02 | −.05 | .09 | .03 | −.03 | .17* | .15* | −.03 | .16* | .03 | .15** | -- | 0.27 (0.54) | |||||
| 15. Organizational Religious Inv. | .03 | .06 | .13ᵀ | −.01 | .04 | −.14* | −.31** | −.22** | .14ᵀ | .29** | −.01 | .13ᵀ | .11 | .09 | .04 | -- | .09 (0.92) | ||||
| 16. Saliva Collection Start Time | −.04 | .05 | −.01 | .06 | −.02 | −.15* | .01 | .06 | −.11 | −.24** | .06 | −.10 | −.07 | −.06 | .02 | −.04 | -- | 1:54PM (2.97 hrs) | |||
| 17. Cortisol Concentration (ug/dl) | −.16* | .01 | .05 | .02 | −.07 | .03 | .00 | .13ᵀ | −.06 | −.03 | .06 | .01 | .16* | −.13ᵀ | −.09 | .04 | −.22** | -- | 0.27 (0.23) | ||
| 18. DHEA Concentration (pg/ml) | −.32** | .06 | −.01 | .11 | .10 | .02 | .05 | .14* | −.01 | .04 | .06 | −.04 | .06 | −.06 | .04 | −.04 | .01 | .41** | -- | 127.75 (107.67) | |
| 19. Log(Cortisol/DHEA ratio) | .22** | −.08 | .03 | .00 | −.11 | −.02 | −.04 | −.03 | .05 | .01 | −.07 | −.07 | .07 | .02 | −.05 | .09 | −.16* | .25** | −.57** | -- | −0.74 (0.29) |
Note.
p < .10.
p < .05.
p < .01.
BMI = Body Mass Index.
Procedure
Our study was approved by the University of Michigan Institutional Review Board. Participants completed face-to-face interviews for approximately 60 minutes by trained interviewers in the participants’ home or in the community setting (e.g., public library). Following the interview, participants were self-administered a paper-and-pencil questionnaire that included questions about demographics, RD experiences, and health behaviors. Additional methodological details can be found elsewhere (Zimmerman & Schmeelk-Cone, 2003).
Measures
Cortisol and DHEA.
Saliva samples were collected at three designated time-points during the wave 6 interviews. Saliva was only collected from participants who reported not eating, drinking, or using tobacco in the hour prior to study onset. Following consent procedures, participants were instructed to rinse their mouth with water. For each saliva collection, participants pooled saliva in their mouth for one minute and then expectorated through a straw into a cryotube. Participants were interviewed between 7:10 AM and 4:35 PM (Mtime = 1:43 PM, SD = 2 hours, 59 minutes) and saliva samples were collected approximately 10-, 32-, and 62-minutes following consent. Immediately following collection, the saliva samples were placed on ice and refrigerated, then transported to a −80 degree Fahrenheit freezer for storage.
Cortisol and DHEA were determined by high sensitivity salivary cortisol and salivary DHEA enzyme immunoassays (Salimetrics, State College, PA). Saliva samples were thawed and centrifuged at 3,000 rpm for 15 minutes prior to assay, and the assays followed standard enzyme immunoassay procedures (see Granger, Cicchetti, Rogosch, Hibel, & Flores, 2007; Klimes-Dougan et al., 2001). Saliva samples were assayed for cortisol in duplicate and averaged for analyses. The lower limit of sensitivity of this cortisol assay is .007 ug/DL (Zimmerman & Schmeelk-Cone, 2003). Intra- and inter-assay coefficients of variability were below 8% and 7%, respectively. Saliva samples were assayed for DHEA in singlet and the test uses 50 μl of saliva and has a minimum detection limit of 10 pg/ml. Raw cortisol (ug/dl) and DHEA (pg/ml) concentrations assayed from sample 1 were used to calculate the cortisol/DHEA ratio. A natural log transformation was performed on the cortisol/DHEA ratio to account for positive skew.
Racial discrimination (RD).
The participants’ perceived RD was measured using the 18-item Daily Life Experience scale (DLE; Harrell, 1997) at waves 5 and 6. The DLE assesses the frequency of racial microaggressions and overt discriminatory encounters experienced during the past 12 months on a 6-point Likert-type scale ranging from 0 (never happened to me) to 5 (once a week or more). Items were averaged, with higher scores reflecting higher levels of RD experience (αwave 5 = .96; αwave 6 = .95). Prior research has demonstrated the validity and reliability of the DLE among African American young adults (e.g., Lee et al., 2017). Sample items include “because of your race, how often were you observed or followed while in public places” or “because of your race, how often were you laughed at, made fun of, or taunted?”
Depressive Symptoms.
The participants’ depressive symptoms were measured using 6 items from the Brief Symptom Inventory (BSI; Derogatis & Spencer, 1982). The BSI assesses the frequency of depressive symptoms (e.g., feeling lonely, feeling worthless) experienced during the past week. Response options ranged from 0 (not at all) to 4 (extremely). Items were averaged, with higher scores indicating higher levels of depressive symptoms (α = .85).
Organized Religious Involvement (ORI).
ORI was measured using two items that assessed the frequency of service attendance and whether the participant was involved in other religious activities (e.g., choir; bible study). The frequency of service attendance was scaled from 0 (never) and 7 (more than once a week), and participation in other religious activities ranged from 0 to 3 activities. Standard scores for service attendance and other religious activities were calculated for each participant to account for differences in the Likert scales. Standard scores for service attendance and other religious activities was then averaged to approximate ORI.
Covariates.
Participants self-reported their sex at Wave 1 (i.e., 0 = male, 1 = female). The socioeconomic status (SES) of participants was estimated using their level of educational attainment (i.e., no high school degree, high school degree/GED, 1–4 years of college) at wave 7. The participants also disclosed their age at wave 6. We also assessed the participant’s alcohol and cigarette use in the past 30 days. Participant’s responses ranged on an 8-point Likert type scale of 0 (none) to 7 (40+ times) for alcohol use and cigarette use, separately. Participants also self-reported various health behaviors on a Likert-type scale of 0 (Almost Never) to 4 (Everyday), including the frequency of exercise and consumption of fatty foods and fruits/vegetables in the past week. The participants’ body-mass-index was calculated by using their self-reported weight and height at wave 6. Lifetime prevalence of chronic illnesses (i.e., asthma, chronic bronchitis/emphysema, diabetes, high blood pressure/hypertension; coded as “yes” or “no” if ever diagnosed) was assessed at wave 6, and the number of doctor visits in the past year due to an illness. Lastly, interview time was included as a control variable to account for the diurnal decline in cortisol.
Analytic Approach
All study analyses were implemented in SPSS version 24 (SPSS, Inc., Chicago, IL, USA). Preliminary data analyses entailed descriptive statistics and inter-correlations between study variables. To assess depressive symptoms as a mediator in the association between RD and the cortisol/DHEA ratio (Aim 1), we used Hayes’ (2013) PROCESS macro (i.e., model 4; see Hayes, 2013). The PROCESS macros use ordinary least squares estimation to compute model parameters for various path models (e.g., moderated-mediation). Bias-corrected bootstrapped confidence intervals (i.e., 5,000 bootstrapped samples) were used to estimate the indirect effect.
To assess the second aim of the study, ORI was included as a moderator to the mediation model in Aim 1. We implemented a moderated-mediation model using PROCESS macro (i.e., model 8; see Hayes, 2013). A mean-centered product between discrimination and ORI suggested a moderated-mediation effect. If the mean-centered product predicted depressive symptoms (i.e., indirect effect moderation), we probed the indirect effect of our original mediation model at the mean, and ±1 standard deviation at the mean, of the focal moderator. If the mean-centered product between RD and ORI predicted the cortisol/DHEA ratio (i.e., direct effect moderation), we probed the effect of RD and the cortisol/DHEA ratio at the mean, and ±1 standard deviation at the mean, of ORI. Non-significant mean-centered products were removed from the model, and a simpler moderated-mediation model was estimated (i.e., model 7; see Hayes, 2013). In all models, we controlled for sex, age, educational attainment, study onset time, tobacco use, alcohol use, exercise frequency, fruit consumption, fatty food consumption, chronic illness, doctor visits, BMI, perceived stress, and RD encounters from one year ago. The PROCESS macro requires complete data and implements listwise deletion to handle missing data (i.e., 21.3% (n = 40) had missing data). Lastly, we assessed if the cortisol/DHEA ratio mediated the association between RD and depressive symptoms to rule out an alternative mechanism linking RD, depressive symptoms, and the cortisol/DHEA ratio.
Results
Descriptive statistics and inter-correlations between the study variables can be found in Table 1. RD was associated with more depressive symptoms (r = .20), whereas ORI was associated with fewer depressive symptoms (r = −.15). The cortisol/DHEA ratio was not correlated with RD or depressive symptoms.
Aim 1: Mediation Model from Racial discrimination to Cortisol/DHEA ratio
As shown in Table 2, an increase in RD was associated with more depressive symptoms (b = .155), while depressive symptoms predicted a lower cortisol/DHEA ratio (b = −.114). Furthermore, although the direct effect of RD on the cortisol/DHEA ratio was not significantly different from zero, RD had a significant indirect effect on the cortisol/DHEA ratio through depressive symptoms (b = −.018; 95% CI = −.047, −.004).
Table 2.
Direct and Indirect Effects from Racial Discrimination to the Cortisol/DHEA Ratio
| Outcome: Depressive Symptoms | b | s.e. | p | Biased Corrected 95% C.I. |
|---|---|---|---|---|
| Racial Discrimination (Wave 6) | .155 | .061 | .013 | .034, .276 |
| Racial Discrimination (Wave 5) | −.001 | .065 | .923 | −.131, .128 |
| Sex | .268 | .106 | .013 | .058, .478 |
| Age | −.059 | .077 | .443 | −.211, .093 |
| Educational Attainment | .014 | .026 | .577 | −.036, .065 |
| Alcohol Use | .026 | .015 | .083 | −.003, .054 |
| Cigarette Use | −.015 | .019 | .441 | −.054, .024 |
| Exercise | −.019 | .041 | .629 | −.099, .060 |
| Fruit Consumption | −.077 | .045 | .085 | −.165, .011 |
| Fatty Food Consumption | −.011 | .044 | .805 | −.097, .075 |
| BMI | −.006 | .008 | .431 | −.022, .009 |
| Perceived Stress | .515 | .089 | < .001 | .339, .692 |
| Doctor Visits | .022 | .025 | .374 | −.027, .072 |
| Chronic Illness | .126 | .091 | .167 | −.053, .304 |
| Organizational RI | −.061 | .059 | .299 | −.177, .055 |
| Saliva Collection Start Time | −.669 | .43 | .130 | −1.537, .199 |
| Constant | .812 | 1.731 | .639 | −2.617, 4.241 |
| Outcome: Cortisol/DHEA Ratio | b | s.e. | p | 95% C.I. |
| Depressive Symptoms | −.114 | .049 | .022 | −.211, −.017 |
| Racial Discrimination (Wave 6) | −.028 | .033 | .405 | −.094, .038 |
| Racial Discrimination (Wave 5) | .010 | .035 | .776 | −.059, .078 |
| Sex | .176 | .058 | .004 | .062, .291 |
| Age | −.046 | .041 | .260 | −.127, .035 |
| Educational Attainment | −.002 | .014 | .892 | −.029, .025 |
| Alcohol Use | .005 | .008 | .571 | −.011, .020 |
| Cigarette Use | .003 | .010 | .747 | −.017, .024 |
| Exercise | .021 | .022 | .341 | −.022, .063 |
| Fruit Consumption | −.010 | .024 | .667 | −.058, .037 |
| Fatty Food Consumption | −.020 | .023 | .389 | −.066, .026 |
| BMI | −.006 | .004 | .169 | −.014, .003 |
| Perceived Stress | .134 | .054 | .014 | .028, .240 |
| Doctor Visits | .015 | .013 | .255 | −.011, .042 |
| Chronic Illness | −.019 | .048 | .698 | −.114, .077 |
| Saliva Collection Start Time | −.416 | .235 | .079 | −.880, .049 |
| Organizational RI | .022 | .031 | .474 | −.039, .084 |
| Constant | .134 | .919 | .885 | −1.686, 1.953 |
| Indirect Effect | −.018 | .009 | -- | −.047, −.003 |
Note. BMI = Body Mass Index. C.I. = Confidence Interval.
Aim 2: Moderated-Mediation Models with Organizational Religious Involvement
To assess whether ORI moderated the direct and indirect effect from RD to the cortisol/DHEA ratio, we estimated a moderated-mediation model (i.e., conditional process model; see Figure 1). ORI moderated the influence of RD on depressive symptoms (b = −.094), but not the direct effect of RD on the cortisol/DHEA ratio. As a result, we estimated an indirect effect moderation model without direct effect moderation (Table 3). In particular, RD had an indirect effect on the cortisol/DHEA ratio through depressive symptoms at low (b = −.029; 95% CI = −.079, −.006) and moderate levels of ORI (b = −.019; 95% CI = −.051, −.005), but not at a high level.
Table 3.
Organizational Religious Involvement as a Protective Factor (Indirect Effect Moderation)
| Depressive Symptoms (Mediator) | b | s.e. | p | Bias Corrected 95% C.I. |
|---|---|---|---|---|
| Racial Discrimination (Wave 6) | .172 | .061 | .005 | .052, .292 |
| Racial Discrimination (Wave 5) | −.013 | .064 | .846 | −.139, .114 |
| Sex | .273 | .105 | .011 | .065, .481 |
| Age | −.046 | .076 | .543 | −.196, .104 |
| Educational Attainment | .022 | .026 | .401 | −.029, .072 |
| Alcohol Use | .021 | .014 | .157 | −.008, .049 |
| Cigarette Use | −.013 | .019 | .504 | −.051, .025 |
| Exercise | −.018 | .039 | .652 | −.097, .061 |
| Fruit Consumption | −.084 | .044 | .058 | −.171, .003 |
| Fatty Food Consumption | −.011 | .043 | .799 | −.095, .074 |
| BMI | −.007 | .008 | .373 | −.022, .008 |
| Perceived Stress | .519 | .087 | < .001 | .345, .692 |
| Doctor Visits | .022 | .025 | .374 | −.027, .071 |
| Chronic Illness | .102 | .089 | .252 | −.074, .279 |
| Organizational Religious Involvement | −.021 | .021 | .320 | −.062, .020 |
| Organizational Religious Involvement X Racial Discrimination | −.048 | .020 | .018 | −.087, −.009 |
| Saliva Collection Start Time | −.676 | .430 | .119 | −1.528, .177 |
| Constant | .698 | 1.718 | .685 | −2.704, 4.101 |
| Cortisol/DHEA Ratio (Outcome) | b | s.e. | p | Bias Corrected 95% C.I. |
| Depressive Symptoms | −.117 | .049 | .018 | −.214, −.021 |
| Racial Discrimination (Wave 6) | −.026 | .033 | .435 | −.092, .040 |
| Racial Discrimination (Wave 5) | .010 | .034 | .766 | −.058, .079 |
| Sex | .173 | .058 | .003 | .059, .287 |
| Age | −.043 | .040 | .293 | −.123, .037 |
| Educational Attainment | −.004 | .013 | .974 | −.027, .026 |
| Alcohol Use | .003 | .008 | .672 | −.012, .018 |
| Cigarette Use | .003 | .010 | .807 | −.018, .023 |
| Exercise | .023 | .021 | .275 | −.019, .065 |
| Fruit Consumption | −.007 | .024 | .746 | −.054, .039 |
| Fatty Food Consumption | −.018 | .023 | .443 | −.063, .028 |
| BMI | −.006 | .004 | .192 | −.014, .003 |
| Perceived Stress | .135 | .053 | .013 | .029, .241 |
| Doctor Visits | .016 | .013 | .242 | −.011, .042 |
| Chronic Illness | −.022 | .048 | .650 | −.117, .073 |
| Saliva Collection Start Time | −.422 | .234 | .074 | −.886, .041 |
| Constant | .017 | .911 | .985 | −1.787, 1.822 |
| Indirect Effect: −1 SD ORI | −.035 | .018 | -- | −.070, −.003 |
| Indirect Effect: Mean of ORI | −.020 | .011 | -- | −.042, −.001 |
| Indirect Effect: +1 SD ORI | −.006 | .008 | -- | −.022, .013 |
Note. ORI = Organizaitonal Religious Involvement. BMI = Body Mass Index.
Sensitivity Analysis
We assessed whether cortisol/DHEA ratio mediated the association between RD and depressive symptoms. The indirect effect of RD on depressive symptom through the cortisol/DHEA ratio was not significant (b = .018; 95% CI = −.002, .063). Further, indirect and direct effect moderation were not observed in the moderated-mediation model (see Table 4).
Table 4.
Sensitivity Analysis
| Indirect Effect | b | s.e. | Bias Corrected 95% CI |
|---|---|---|---|
| Racial Discrimination → Cortisol/DHEA → Depressive Symptoms |
.006 | .011 | −.013, .031 |
| Conditional Indirect Effects | |||
| −1 SD ORI | .019 | .023 | −.013, .080 |
| Mean of ORI | .008 | .012 | −.011, .039 |
| +1 SD ORI | −.003 | .013 | −.035, .017 |
Note. C.I. = Confidence Interval.
Discussion
RD is a salient stressor that can increase susceptibility to poorer psychological well-being and physiological health among African Americans (see Lewis et al., 2015). Moreover, Chae and colleagues’ (2011) socio-psychobiological model suggests that RD may indirectly exact physiologic tolls by generating greater psychological risk (e.g., depressive symptoms; Chae et al., 2011). Our findings are consistent with this model, suggesting that depressive symptoms mediate the association between RD and the imbalance of the cortisol/DHEA ratio. Specifically, in line with prior research, we found that RD was associated with higher levels of depressive symptoms (e.g., Hurd et al., 2014), and that symptoms of depression were associated with lower cortisol/DHEA ratios (e.g., Gill et al., 2008). Importantly, a direct effect of RD on the ratio of cortisol/DHEA was not observed. This may suggest that the mental health consequences of RD may precede the imbalance of stress reactive hormones. Alternatively, RD encounters may shape acute cortisol reactivity, but not regulatory (i.e., basal) levels of cortisol as assessed in our study (Korous, Causadias, & Casper, 2017). Together, our findings indicate that RD may invoke dysregulations in the neuroendocrine system by deteriorating mental health.
It is important to mention that our results are inconsistent with some other work that has documented greater cortisol and lower DHEA concentrations (i.e., a higher cortisol/DHEA ratio) among individuals with major depressive disorder and elevated depressive symptoms (see Sollberger & Ehlert, 2016). Two separate lines of research can inform our understanding of why depressive symptoms, in some cases, predict a lower cortisol and higher DHEA (i.e., a lower cortisol/DHEA ratio). First, scholars have found that depressive symptoms are associated with a blunted cortisol response to a stressor (Burke, Fernald, Gertler, & Adler, 2005), as well as a dampened cortisol awakening response and diurnal rhythm (Doane et al., 2013; Stetler & Miller, 2005). Second, researchers have found higher DHEA concentrations among patients diagnosed with major depressive disorder (Assies et al., 2004), as well as healthy individuals reporting higher levels of depressive symptoms (Morrison, Ten Have, Freeman, Sammel, & Grisso, 2001). Together, these findings may begin to explain why depressive symptoms, in some cases, may reduce cortisol levels, while elevating DHEA.
The persistence of mixed results between symptoms of psychopathology and the cortisol/DHEA ratio hints that an imbalance between stress-reactive hormones, regardless of its direction, reflects higher allostatic load (Shirtcliff, Peres, Dismukes, Lee, & Phan, 2014). While some researchers have reported positive associations between psychopathology and cortisol/DHEA ratio (e.g., Markopoulou et al., 2009; Young et al., 2002), others have documented a negative association (e.g., Gill et al., 2008; Shirotsuki et al., 2009; Yehuda, 2006). Furthermore, low cortisol/DHEA ratios have been associated with poorer health outcomes such as pro-inflammatory immune response (Gill et al., 2008). Our study, to this end, is one of the first to suggest that, at the very least, the psychological consequences of RD may contribute to the imbalance of cortisol and DHEA concentrations.
With regard to ORI, we found that ORI buffered the indirect effect of RD on the cortisol/DHEA ratio. Specifically, ORI mitigated the influence of RD on depressive symptoms, which, in turn, offset the indirect influence of RD on the cortisol/DHEA ratio. Furthermore, we did not find evidence of a direct effect between RD and the cortisol/DHEA ratio across different levels of ORI. Our results, when considered in tandem, suggest that ORI counteracts the influence of RD on biological stress regulation by offsetting the psychological consequences of RD. Various mechanisms may underlie the protective influence of ORI in the context of RD. Religious social support (Hope et al., 2016), religious teachings that emphasize liberation and hope (Mattis et al., 2003), and civic engagement opportunities (Hope & Spencer, 2017) may be important mechanisms by which ORI can buffer the psychological consequences of RD. In light of our findings, future investigations should examine the psychologically salutary assets and resources afforded by ORI to ameliorate the physiologically deleterious effects of RD.
Although our study advances our understanding of how RD, depressive symptoms, and ORI work in concert in relation to biological stress response, several limitations suggest a cautious interpretation of our results. First, while we were able to detect direct and indirect effects in our sample, it is possible that our standard errors are biased due to the limited sample size (MacKinnon et al., 2007). Thus, it is important to validate our findings in a larger sample of African Americans. Second, due to documented instabilities in the level of cortisol across days (e.g., Doane et al., 2015), our one day assessment of cortisol may not generalize to the participant’s global experience. Accordingly, psychological pathways from RD to the cortisol/DHEA ratio after taking into account day-to-day variability in cortisol level should be examined. Third, it is possible that our limited measure of ORI broadly captures religiosity domains, which, in turn, would upwardly bias the protective effects of ORI. It is, therefore, important to measure religious involvement using a multidimensional measure to more accurately assess the unique contribution of ORI on psychophysiological outcomes. Fourth, religious affiliation was not assessed in the original study and it is possible that our assessment of ORI as a protective factor may not generalize to a non-Christian sample. Given the documented differences in ORI across religious groups (Pearce & Denton, 2011), it is important to investigate how ORI promotes resilience within various religious groups. A final potential limitation concerns the measurement of RD as a unidimensional construct. Our measure was limited to the frequency of racial hassles. Future studies can advance our understanding of RD and stress reactive hormones by examining other dimensions of RD such as institutional forms (e.g., racial profiling by law enforcement, residential segregation; Williams & Mohammed, 2013).
Notwithstanding these limitations, our results contribute to the growing body of evidence that indicates the socio-psychobiological pathways by which RD “gets under the skin” (Chae et al., 2011). By identifying depressive symptoms as a mediator of the association between RD and the imbalance of cortisol and DHEA, our findings suggest that that the psychological and physiological consequences of RD may work together to accelerate the deterioration of health. In particular, an individual’s psychological response to RD may generate susceptibility to greater physiological risk and poorer health. Our findings also illustrate that the mental health benefits of ORI may ultimately benefit the physical health of African Americans in the context of racism-related stress. Our study represents a preliminary step towards determining how ORI can shield African Americans from the adverse physiological effects of RD. Future investigations should mechanistically examine how ORI and different types of religious involvement offer physiological protection to African Americans contending with RD. Mechanisms such as gratitude (Krause, 2006), forgiveness (Krause & Ellison, 2003), and religious coping (Pargament, 1997) are plausible pathways by which ORI might offer protection to African Americans within the context of RD. By testing these mechanisms, interventionists, researchers, and clinicians will be able to identify approaches to address racism-related stress within faith-based communities.
Acknowledgments
This research was supported by a grant from NIDA (R01-DA-07484–09). The first author (D.B.L.) was supported by a grant from the NICHD (T32 HD 79350–2). The second author (M.K.P.) was supported by a grant from the NICHD (T32 HD007109–36).
Contributor Information
Melissa K. Peckins, Email: mpeckins@umich.edu.
Alison L. Miller, Email: alimill@umich.edu.
Meredith O. Hope, Email: mohope@umich.edu.
Enrique W. Neblett, Email: eneblett@unc.edu.
Shervin Assari, Email: assari@umich.edu.
Jaime Muñoz-Velázquez, Email: jaimemv@umich.edu.
Marc A. Zimmerman, Email: marcz@umich.edu.
References
- Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, … Eccles JS (2015). Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: A 20-year prospective study. Psychoneuroendocrinology, 62, 279–291. doi: 10.1016/j.psyneuen.2015.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assari S (2013). Race and ethnicity, religion involvement, church-based social support and subjective health in United States: a case of moderated mediation. International Journal of Preventive Medicine, 4(2), 208–217. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3604855/ [PMC free article] [PubMed] [Google Scholar]
- Assari S (2014). Chronic medical conditions and Major Depressive Disorderer: Differential role of positive religious coping among African Americans, Caribbean Blacks and non-hispanic whites. International Journal of Preventive Medicine, 5(4), 405–413. [PMC free article] [PubMed] [Google Scholar]
- Assari S, Moghani Lankarani M, Malekahmadi MR, Caldwell CH, & Zimmerman M (2015). Baseline religion involvement predicts subsequent salivary cortisol levels among male but not female Black youth. International Journal of Endocrinology and Metabolism, 13(4), 1–8. doi: 10.5812/ijem.31790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assies J, Visser I, Nicolson NA, Eggelte TA, Wekking EM, Huyser J, … Schene AH (2004). Elevated salivary dehydroepiandrosterone-sulfate but normal cortisol levels in medicated depressed patients: Preliminary findings. Psychiatry Research, 128(2), 117–122. doi: 10.1016/j.psychres.2004.05.016 [DOI] [PubMed] [Google Scholar]
- Begun AL (1993). Human behavior and the social environment: The vulnerability, risk, and resilience model. Journal of Social Work Education, 29(1), 26–35. doi: 10.1080/10437797.1993.10778796 [DOI] [Google Scholar]
- Berger M, & Sarnyai Z (2015). “More than skin deep”: Stress neurobiology and mental health consequences of racial discrimination. Stress, 18(1), 1–10. doi: 10.3109/10253890.2014.989204 [DOI] [PubMed] [Google Scholar]
- Blum CA, Mueller C, Schuetz P, Fluri F, Trummler M, Mueller B, … Christ-Crain M (2013). Prognostic value of dehydroepiandrosterone-sulfate and other parameters of adrenal function in acute ischemic stroke. PLoS ONE, 8(5), 1–9. Ddoi: 10.1371/journal.pone.0063224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Fernald LC, Gertler PJ, & Adler NE (2005). Depressive symptoms are associated with blunted cortisol stress responses in very low-income women. Psychosomatic Medicine, 67(2), 211–216. doi: 10.1097/01.psy.0000156939.89050.28 [DOI] [PubMed] [Google Scholar]
- Chae DH, Epel ES, Nuru-Jeter AM, Lincoln KD, Taylor RJ, Lin J, ... & Thomas SB (2016). Discrimination, mental health, and leukocyte telomere length among African American men. Psychoneuroendocrinology, 63, 10–16. doi: 10.1016/j.psyneuen.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Nuru-Jeter AM, Lincoln KD, & Francis DD (2011). Conceptualizing racial disparities in health: Advancement of a socio-psychobiological approach. Du Bois Review, 8(1), 63–77. doi: 10.1017/S1742058X11000166 [DOI] [Google Scholar]
- Cohen S, Janicki-Deverts D, & Miller GE (2007). Psychological stress and disease. JAMA, 298(14), 1685–1687. doi: 10.1001/jama.298.14.1685 [DOI] [PubMed] [Google Scholar]
- Cooper SM, Brown C, Metzger I, Clinton Y, & Guthrie B (2013). Racial discrimination and African American adolescents’ adjustment: Gender variation in family and community social support, promotive and protective factors. Journal of Child and Family Studies, 22(1), 15–29. doi: 10.1007/s10826-012-9608-y [DOI] [Google Scholar]
- Derogatis LR, & Spencer PM (1982). The Brief Symptoms Inventory procedures manual-I. Baltimore, MD: Johns Hopkins University School of Medicine, Clinical Psychometric Research Unit. [Google Scholar]
- Doane LD, Chen FR, Sladek MR, Van Lenten SA, & Granger D A. (2015). Latent trait cortisol ( LTC ) levels : Reliability, validity, and stability. Psychoneuroendocrinology, 55, 21–35. doi: 10.1016/j.psyneuen.2015.01.017 [DOI] [PubMed] [Google Scholar]
- Doane LD, Mineka S, Zinbarg RE, Craske M, Griffith JW, & Adam EK (2013). Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Development and Psychopathology, 25(3), 629–642. doi: 10.1017/S0954579413000060 [DOI] [PubMed] [Google Scholar]
- Ellison CG, DeAngelis RT, & Güven M (2017). Does religious involvement mitigate the effects of major discrimination on the mental health of African Americans? Findings from the Nashville Stress and Health Study. Religions, 8(9), 195. doi: 10.3390/rel8090195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Doan SN, & Eccles JS (2012). Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendocrinology, 37(1), 107–118. doi: 10.1016/j.psyneuen.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, & Bound J (2006). “ Weathering “ and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health, 96(5), 826–833. doi: 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J, Vythilingam M, & Page GG (2008). Low cortisol, high DHEA, and high levels of stimulated TNF-α, and IL-6 in women with PTSD. Journal of Traumatic Stress, 21(6), 530–539. doi: 10.1002/jts.20372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Cicchetti D, Rogosch FA, Hibel LC, Teisl M, & Flores E (2007). Blood contamination in children’s saliva: Prevalence, stability, and impact on the measurement of salivary cortisol, testosterone, and dehydroepiandrosterone. Psychoneuroendocrinology, 32, 724–733. doi: 10.1016/j.psyneuen.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Güder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, … Störk S (2007). Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation, 115(13), 1754–1761. doi: 10.1161/CIRCULATIONAHA.106.653964 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Vazquez DM (2001). Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology, 13(3), 515–538. doi: 10.1017/S0954579401003066 [DOI] [PubMed] [Google Scholar]
- Harrell SP, Merchant MA, & Young SA (1997). Psychometric properties of the racism and life experiences scales (RaLES). Unpublished Manuscript. [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press, New York, NY. [Google Scholar]
- Hayes AF (2012). PROCESS SPSS Macro. [Computer Software and Manual]. Retrieved from http://www.afhayes.com/public/process.pdf
- Hill TD, Bradshaw M, & Burdette AM (2016). Health and biological functioning. In Yamane D (Ed.), Handbook of religious and society (pp. 11–28). Dordrecht, the Netherlands: Springer. [Google Scholar]
- Hill TD, Rote SM, Ellison CG, & Burdette AM (2014). Religious attendance and biological functioning: A multiple specification approach. Journal of Aging and Health, 26(5), 766–785. doi: 10.1177/0898264314529333 [DOI] [PubMed] [Google Scholar]
- Hope EC, & Spencer MB (2017). Civic engagement as an adaptive coping response to conditions of inequality: An application of Phenomenological Variant of Ecological Systems Theory (PVEST). In Cabrera NJ & Leyendecker B (Eds.), Handbook on Positive Development of Minority Children and Youth (pp. 421–435). Cham, Switzerland: Springer International Publishing. doi: 10.1007/978-3-319-43645-6 [DOI] [Google Scholar]
- Hope MO, Assari S, Cole-Lewis YC, & Caldwell CH (2017). Religious social support, discrimination, and psychiatric disorders among Black adolescents. Race and Social Problems, 9(2), 102–114. doi: 10.1007/s12552-016-9192-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd NM, Varner FA, Caldwell CH, & Zimmerman MA (2014). Does perceived racial discrimination predict changes in psychological distress and substance use over time? An examination among Black emerging adults. Developmental Psychology, 50(7), 1910–1918. doi: 10.1037/a0036438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JS, Knight KM, & Rafferty JA (2010). Race and unhealthy behaviors: Chronic stress, the HPA Axis, and physical and mental health disparities over the life course. American Journal of Public Health, 100(5), 933–939. doi: 10.2105/AJPH.2008.143446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin HS, & Kertes DA (2017). Cortisol and DHEA in development and psychopathology. Hormones and Behavior, 89, 69–85. doi: 10.1016/j.yhbeh.2016.11.018 [DOI] [PubMed] [Google Scholar]
- Kaminska M, Harris J, Gijsbers K, & Dubrovsky B (2000). Dehydroepiandrosterone sulfate (DHEAS) counteracts decremental effects of corticosterone on dentate gyrus LTP. implications for depression. Brain Research Bulletin, 52(3), 229–234. doi: 10.1016/S0361-9230(00)00251-3 [DOI] [PubMed] [Google Scholar]
- Korous KM, Causadias JM, & Casper DM (2017). Racial discrimination and cortisol output: A meta-analysis. Social Science & Medicine, 193, 90–100. doi:j.socscimed.2017.09.042 [DOI] [PubMed] [Google Scholar]
- Klimes–Dougan B, Hastings PD, Granger DA, Usher BA, & Zahn-Waxler C (2001). Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology, 13(3), 695–719. doi: 10.1017/S0954579401003157 [DOI] [PubMed] [Google Scholar]
- Krause N (2006). Gratitude toward God, stress, and health in late life. Research on Aging, 28(2), 163–183. doi: 10.1177/0164027505284048 [DOI] [Google Scholar]
- Krause N, & Ellison CG (2003). Forgiveness by God, forgiveness of others, and psychological well–being in late life. Journal for the scientific study of religion, 42(1), 77–93. doi: 10.1111/1468-5906.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DB, Kim ES, & Neblett EW (2017). The link between discrimination and telomere length in African American adults. Health Psychology, 36(5), 458–467. doi: 10.1037/hea0000450 [DOI] [PubMed] [Google Scholar]
- Lee DB, & Neblett EW (2017). Religious development in African American adolescents: Growth patterns that offer protection. Child Development, 1–15. doi: 10.1111/cdev.12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DB, Neblett EW, & Jackson V (2015). The role of optimism and religious involvement in the association between race-related stress and anxiety symptomatology. Journal of Black Psychology, 41(3), 221–246. doi: 10.1177/0095798414522297 [DOI] [Google Scholar]
- Lee DB, Peckins MK, Heinze JE, Miller AL, Assari S, & Zimmerman MA (2017). Psychological pathways from racial discrimination to cortisol in African American males and females. Journal of Behavioral Medicine. doi: 10.1007/s10865-017-9887-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin JS, Taylor RJ, & Chatters LM (1995). A multidimensional measure of religious involvment for African Americans. The Sociological Quarterly, 36(1), 157–173. Retrieved from http://www.jstor.org/stable/4121282 [Google Scholar]
- Lewis TT, Cogburn CD, & Williams DR (2015). Self-reported experiences of discrimination and health: Scientific advances, ongoing controversies, and emerging issues. Annual Review of Clinical Psychology, 11(1), 407–440. doi: 10.1146/annurev-clinpsy-032814-112728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Wegner R, Pierce J, Lumley MA, Laurent HK, & Granger DA (2017). Perceived discrimination, racial identity, and multisystem stress response to social evaluative threat among African American men and women. Psychosomatic Medicine, 79(3), 293–305. doi: 10.1097/PSY.0000000000000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, & Fritz MS (2007). Mediation analysis. Annual Review Psychology, 58, 593–614. doi: 10.1146/annurev.psych.58.110405.085542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markopoulou K, Papadopoulos A, Juruena MF, Poon L, Pariante CM, & Cleare AJ (2009). The ratio of cortisol/DHEA in treatment resistant depression. Psychoneuroendocrinology, 34(1), 19–26. doi: 10.1016/j.psyneuen.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Mattis JS, Fontenot DL, & Hatcher-Kay CA (2003). Religiosity, racism, and dispositional optimism among African Americans. Personality and Individual Differences, 34(6), 1025–1038. doi: 10.1016/S0191-8869(02)00087-9 [DOI] [Google Scholar]
- Mattis JS, & Watson CR (2009). Religion and spirituality. In Neville HA, Tynes BM, & Utsey SO (Eds.), Handbook of African American Psychology (pp. 91–102). Thousand Oaks, CA: SAGE Publications. [Google Scholar]
- Moriguchi Jeckel CM, Lopes RP, Berleze MC, Luz C, Feix L, Argimon IIDL, … Bauer ME (2010). Neuroendocrine and immunological correlates of chronic stress in “strictly healthy” populations. NeuroImmunoModulation, 17(1), 9–18. doi: 10.1159/000243080 [DOI] [PubMed] [Google Scholar]
- Morrison MF, Ten Have T, Freeman EW, Sammel MD, & Grisso JA (2001). DHEA-S levels and depressive symptoms in a cohort of African American and Caucasian women in the late reproductive years. Biological Psychiatry, 50(1), 705–711. doi: 10.1016/S0006-3223(01)01169-6 [DOI] [PubMed] [Google Scholar]
- National Research Council. (2004). Measuring racial discrimination. Panel on Methods for Assessing Discrimination. (Blank RM, Dabady M, & Citro CF, Eds.). Committee on National Statistics, Division of Behavioral and Social Sciences and Education. Washington, DC: The National Academies Press. doi: 10.17226/10887 [DOI] [Google Scholar]
- Pargament KI: The Psychology of Religion and Coping. New York, Guilford, 1997. [Google Scholar]
- Pearce L, & Denton ML (2011). A faith of their own: Stability and change in the religiosity of America’s adolescents. Oxford University Press. [Google Scholar]
- Ratner KG, Halim ML, & Amodio DM (2013). Perceived stigmatization, ingroup pride, and immune and endocrine activity: Evidence from a community sample of Black and Latina women. Social Psychological and Personality Science, 4(1), 82–91. doi: 10.1177/1948550612443715 [DOI] [Google Scholar]
- Shirotsuki K, Izawa S, Sugaya N, Yamada KC, Ogawa N, Ouchi Y, … Nomura S (2009). Salivary cortisol and DHEA reactivity to psychosocial stress in socially anxious males. International Journal of Psychophysiology, 72(2), 198–203. doi: 10.1016/j.ijpsycho.2008.12.010 [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Peres JC, Dismukes AR, Lee Y, & Phan JM (2014). Hormones: Commentary: Riding the Physiological Roller Coaster: Adaptive Significance of Cortisol Stress Reactivity to Social Contexts. Journal of Personality Disorders, 28(1), 40–51. doi: 10.1521/pedi.2014.28.1.40 [DOI] [PubMed] [Google Scholar]
- Sollberger S, & Ehlert U (2016). How to use and interpret hormone ratios. Psychoneuroendocrinology, 63, 385–397. doi: 10.1016/j.psyneuen.2015.09.031 [DOI] [PubMed] [Google Scholar]
- Stetler C, & Miller GE (2005). Blunted cortisol response to awakening in mild to moderate depression: Regulatory influences of sleep patterns and social contacts. Journal of Abnormal Psychology, 114(4), 697–705. doi: 10.1037/0021-843X.114.4.697 [DOI] [PubMed] [Google Scholar]
- Susman EJ (2006). Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews, 30(3), 376–389. doi: 10.1016/j.neubiorev.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Williams DR, & Mohammed SA (2013). Racism and health I: Pathways and scientific evidence. American Behavioral Scientist, 57(8), 1152–1173. doi: 10.1177/0002764213487340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Brand SR, Golier JA, & Yang R-K (2006). Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatrica Scandinavica, 114(3), 187–193. doi: 10.1111/j.1600-0447.2006.00801.x [DOI] [PubMed] [Google Scholar]
- Young AH, Gallagher P, & Porter RJ (2002). Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. American Journal of Psychiatry, 159(7), 1237–1239. doi: 10.1176/appi.ajp.159.7.1237 [DOI] [PubMed] [Google Scholar]
- Zimmerman MA, & Schmeelk-Cone KH (2003). A longitudinal analysis of adolescent substance use and school motivation among African American youth. Journal of Research on Adolescence, 13(2), 185–210. doi: 10.1111/1532-7795.1302003 [DOI] [Google Scholar]