Abstract
Direct-acting antiviral medications (DAAs) have revolutionized care for hepatitis C positive (HCV+) liver (LT) and kidney (KT) transplant recipients. SRTR registry data were integrated with national pharmaceutical claims (2007–2016) to identify HCV treatments before 1/2014 (pre-DAA) and after (post-DAA), stratified by donor (D) and recipient (R) serostatus and payer. Pre-DAA, 18% of HCV+ LT recipients were treated within 3 years and without differences by donor serostatus or payer. Post-DAA, only 6% of D-/R+ recipients, 19.8% of D+/R+ recipients with public insurance, and 11.3% with private insurance were treated within 3 years (P<0.0001). Liver transplant recipients treated for HCV pre-DAA experienced higher rates of graft loss (adjusted hazard ratio [aHR] 1.341.852.10, P<.0001) and death (aHR 1.471.681.91, p<0.0001). Post-DAA, HCV treatment was not associated with death (aHR 0.340.671.32, p=0.25) or graft failure (aHR 0.320.641.26, p=0.20) in D+R+ LT recipients. Treatment increased in D+R+ KT recipients (5.5% pre-DAA vs. 12.9% post-DAA), but did not differ by payer status. DAAs reduced the risk of death after D+/R+ KT by 57% (0.190.430.95, p=0.04) and graft loss by 46% (0.270.541.07, p=0.08). HCV treatment with DAAs appears to improve HCV+ LT and KT outcomes; however, access to these medications appears limited in both LT and KT recipients.
INTRODUCTION
Direct-acting antivirals (DAAs) have dramatically altered the care of patients with chronic hepatitis C virus (HCV) infection.1–6 For the last two decades, chronic HCV infection was the leading indication for liver transplant (LT) in the United States. Treatment of HCV infection prior to 2014 consisted primarily of interferon- and ribavirin-based treatment regimens, which had limited efficacy, were associated with debilitating side effects, and, generally, were contraindicated in patients with decompensated cirrhosis or, in the case of ribavirin, advanced chronic kidney disease (CKD).7,8 DAAs reduce morbidity and result in sustained virological response 12 weeks after completing treatment (SVR12) rates greater than 94% for most genotypes in both compensated and decompensated patients.4,9,10 DAAs have also been safely used in the post LT setting to prevent recurrent inflammation and fibrosis, which was universal in the absence of effective pretransplant treatment. Historically, recurrence of HCV in the liver graft resulted in cirrhosis in 20%−30% of LT recipients and fibrosing cholestatic hepatitis C in 2%−9% within the first 12 months.11,12 DAAs have been shown to achieve SVR12 after LT in multiple clinical trials, although the impact of DAAs on longer-term liver and kidney transplant outcomes has not been reported.12
Among patients with end-stage renal disease (ESRD), HCV prevalence is 5 times greater than in the general population.13,14 Historically, HCV-infected ESRD patients on dialysis were 60% more likely to die than their non-infected counterparts. Prior to DAAs, interferon-based regimens had low levels of efficacy and high rates of intolerability in this population and were generally contraindicated in HCV-infected kidney transplant (KT) recipients owing to unacceptable rates of rejection and allograft dysfunction. Because most HCV-infected KT recipients in the pre-DAA era were therefore either untreated or intolerant to pre-transplant interferon/ribavirin, these patients experienced higher rates of graft loss and death than non-infected patients.15 In contrast, DAAs are both safe and well tolerated in patients with advanced chronic kidney disease (CKD), with SVR12 rates from 95–98%.16,17 DAA treatment in KT recipients successfully eradicates the virus without negatively affecting graft function in clinical series.18,19
While DAA treatment is revolutionary, access to it has been hampered nationally by its high cost.20–22 Initial regimens resulted in total health care expenditures exceeding $100,000 USD (United States Dollars) per treatment course. As more DAAs have entered the market and competition has increased, costs have diminished, but remain in excess of $25,000 per course, depending on specific agent. Consequently, major private payers developed preauthorization processes that initially restricted use to patients with defined clinical conditions such as demonstrated hepatic fibrosis, cirrhosis, or advanced kidney disease, despite evidence suggesting clinical benefits even in patients without advanced liver disease.23 While long-term economic analyses suggest that DAAs are cost effective, few are cost saving despite reducing the need for transplant.24–26 Furthermore, the cost savings are accrued far in the future when many patients have changed health insurance, diminishing enthusiasm for broader treatment of patients without qualifying conditions.
While clinical trial experience with DAAs in the posttransplant LT and KT populations have demonstrated high SVR rates, no large-scale, population-based assessment of access to DAA treatment, effects on longer-term transplant outcomes, or increases in the cost of treatment has been conducted. Using a unique data set linking pharmacy claims data and transplant registry outcomes, we developed a national cohort of HCV-positive transplant recipients with sufficient power to assess DAA use in LT and KT recipients before and after introduction of DAAs, characteristics associated with posttransplant HCV treatment, and the independent impact of these medications on patient and graft outcomes.
METHODS
Data sources
We conducted a retrospective cohort study using linked health care databases in the US to ascertain patient characteristics, pharmacy fill records, and outcome events for LT and KT recipients. This study used transplant data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR system includes data on all donors, waitlisted candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services, provides oversight of the activities of the OPTN and SRTR contractors. Baseline demographic information ascertained for LT and KT recipients from OPTN included age, sex, and race as reported by the transplant centers.
Pharmacy fill data were assembled by linking SRTR records for LT and KT recipients with billing claims from a large US pharmaceutical claims data (PCD) warehouse that collects prescription drug fill records including self-paid fills and those reimbursed by private and public payers. PCD comprises National Council for Prescription Drug Program format prescription claims aggregated from multiple sources including claims warehouses, retail pharmacies, and prescription benefit managers for approximately 60% of US retail pharmacy transactions. Individual claim records include the pharmacy fill date with the national drug code identifying agent and dosage. After Institutional Review Board and HRSA approvals, PCD records were linked with SRTR records for transplant recipients. We applied a deterministic de-identification strategy wherein patient identifiers (last name, first name, date of birth, sex, and ZIP code of residence) were transformed before delivery to the Saint Louis University researchers with Health Information Portability and Accountability Act and Health Information Technology for Economic and Clinical Health (HITECH) -certified encryption technology from PCD. The patient de-identification software employs multiple encryption algorithms in succession to guarantee that the resulting “token” containing encrypted patient identifiers can never be decrypted. However, the algorithm yields the same results for a given set of data elements, such that linkages by unique anonymous tokens are possible.27
Sample and clinical characteristics
We identified adult LT and KT recipients (age ≥18 years) with SRTR records of transplants between 2007 and 2016 and available pharmaceutical fill records for up to 36 months posttransplant. Recipient clinical and demographic characteristics, characteristics of the donated organ, and other transplant factors including ischemic time and sharing, were defined by the OPTN Transplant Candidate and Recipient Registration forms (Table 1). Patients were identified has being HCV+ based on HCV serostatus at the time of transplant as noted on the Transplant Recipient Registration (TRR) form. As patients who were HCV antibody positive but nucleic acid testing (NAT) negative may be classified as positive on the TRR, we further identified patients who were given an HCV antibody or NAT-positive donor organ as evidence of an active viremic state (as use of HCV+ organs in NAT-negative recipients remains uncommon). Patient insurance coverage was dichotomized as public (Medicaid, Medicare, self-pay) vs. private (all others). Patient and graft outcomes were determined from SRTR registry data.
Table 1.
HCV Positive Liver and Kidney Transplant Recipient Treatment Patterns
| Liver Pre-DAA | HCV D+/R+ | HCV D-/R+ | HCV D-/R- | HCV D+/R- |
|---|---|---|---|---|
| Total subjects | 1,132 | 14,640 | 20,864 | 0 |
| PCD eligibility at Tx | 926 | 11,348 | 15,902 | 0 |
| 1 year PCD eligibility post-Tx | 985 | 12,488 | 18,433 | 0 |
| HCV Rx: 3 months post-Tx | 3 | 98 | 3 | 0 |
| HCV Rx: 1 year post-Tx | 59 | 820 | 29 | 0 |
| HCV Rx: post-Tx | 335 | 3,907 | 153 | 0 |
| Listing to Tx in days (Mean) | 303 | 282 | 225 | NA |
|
Liver Post-DAA | ||||
| Total subjects | 1,166 | 6,254 | 14,320 | 133 |
| PCD eligibility at transplant | 890 | 4,908 | 10,716 | 85 |
| 1 year PCD eligibility post-Tx | 483 | 3,183 | 6,071 | 47 |
| HCV Rx: 3 months post-Tx | 47 | 176 | 6 | 1 |
| HCV Rx: 1 year post-Tx | 155 | 614 | 26 | 5 |
| HCV Rx: post-Tx | 181 | 793 | 31 | 11 |
| Listing to Tx in days (Mean) | 328 | 336 | 241 | 315 |
| Kidney Pre-DAA | HCV D+/R+ | HCV D-/R+ | HCV D-/R- | HCV D+/R- |
| Total subjects | 1,377 | 3,173 | 95,715 | 232 |
| PCD eligibility at transplant | 1,066 | 2,480 | 75,378 | 184 |
| 1 year PCD eligibility post-Tx | 1,256 | 2,925 | 89,704 | 212 |
| HCV Rx: 3 months post-Tx | 0 | 2 | 4 | 0 |
| HCV Rx: 1 year post-Tx | 11 | 9 | 6 | 1 |
| HCV Rx: post-Tx | 215 | 298 | 77 | 12 |
| Listing to Tx in days (Mean) | 395 | 826 | 708 | 411 |
| Kidney Post-DAA | ||||
| Total subjects | 1,083 | 1,608 | 54,412 | 273 |
| PCD eligibility at transplant | 852 | 1,247 | 42,892 | 157 |
| 1 year PCD eligibility post-Tx | 482 | 756 | 26,383 | 82 |
| HCV Rx: 3 months post-Tx | 19 | 19 | 2 | 1 |
| HCV Rx: 1 year post-Tx | 144 | 82 | 13 | 11 |
| HCV Rx: post-Tx | 181 | 119 | 25 | 11 |
| Listing to Tx in days (Mean) | 396 | 871 | 749 | 384 |
Abbreviations: D/R; donor/recipient hepatitis status; DAA, direct acting antiviral; HCV, hepatitis C virus; Rx, treatment for HCV; PCD, pharmaceutical claims data; Tx, transplant.
HCV Medication
Using pharmacy fill records, we identified claims for approved HCV medications and combinations. In the pre-DAA era, defined as before January 2014, HCV treatment was defined as pegylated interferon, interferon, and ribavirin. DAA-era HCV treatments, with or without ribavirin, are shown in Table S1.
Analyses
Demographic characteristics:
Donor and recipient characteristics were drawn from the SRTR data. Pre- and post-DAA-era differences were assessed using Student t-test and chi-squared analyses as appropriate.
Propensity to receive HCV treatment:
Kaplan-Meier survival analysis was performed to identify the proportion of patients receiving HCV treatment by era and primary payer. Multivariate regression analyses were separately performed for LT and KT recipients to assess factors correlated with HCV treatment before and after introduction of DAAs. Donor and recipient characteristics, including primary payer, were included as independent variables.
Cost of treatment analysis:
The direct cost of HCV treatment was calculated using pharmacy claims for LT and KT recipients before and after introduction of DAAs.
Survival analysis:
Posttransplant survival was assessed in HCV+ patients who did and did not receive HCV treatment in the pre- and post-DAA eras. Multivariate Cox proportional hazard models were constructed with HCV treatment as a time-varying covariate. Models were separately constructed for patients noted to be HCV+ on the TRR and for HCV+ patients who received HCV+ donor organs. Donor and recipient characteristics were included as covariates in the model.
Statistical significance:
For all models, P<0.05 was used to determine statistical significance. Analyses were conducted using SAS version 9.4, Cary, NC.
Approval:
This project was reviewed and approved by the Institutional Review Board of Saint Louis University.
RESULTS
Between 2007 and 2016, there were 58,509 LTs were performed; pharmacy claims data for at least 1 year after transplant were available for 41,690 (71%) of these (Table 1). Among patients with claims, 15,671 (38%) were HCV donor negative and recipient positive (D-/R+), 1,468 (3.5%) were D+/R+, and 47 (0.1%) were recorded as D+/R-. In the same period, 157,873 KTs were performed; pharmacy claims data were available for 121,800 (71%). In this population, 3,681 (3.0%) were D-/R+, 1,738 (1.4%) were D+/R+, and 294 (0.2%) were D+/R-.
Use of HCV medications:
Overall, 12.9% of patients undergoing LT received HCV medications within 3 years after transplant. Among serologic subgroups, treatment prevalence varied from 0.75% of HCV D-/R- patients to 35.2% of D+/R+ patients (Figure 1A). In the pre-DAA era, 4.0% of HCV D+/R+ and 5.5% of D-/R+ received HCV treatment within 1 year. Post-DAA, 13.5% of D+/R+ patients and 4.4% of D-/R+ patients received treatment within 1 year. By 3 years, 17.0% D+/R+ and 17.1% D-/R+ patients received treatment in the pre-DAA era. Post-DAA, 15.3% of D+/R+ and 5.5% of D-/R+ recipients had received treatment at the end of follow-up (2.3 years and 2.6 years, respectively). Clinical factors associated with use of HCV treatments pre-DAA include male sex (adjusted hazard ratio [aHR] 0.830.911.00), diabetes (aHR 0.800.890.98), hypertension (aHR 1.031.131.25), higher model for end-stage liver disease (MELD) score (15–30, aHR 1.011.101.20; >30, aHR 1.001.181.38 vs MELD<15), and black race (aHR 0.720.810.90). Post-DAA, donor HCV+ status (aHR 1.661.952.29), higher MELD score (15–30, aHR 1.231.421.65; >30, aHR 1.431.812.28), and black race (aHR 0.650.790.96) were associated with the likelihood of HCV treatment (Table S2A).
Figure 1:
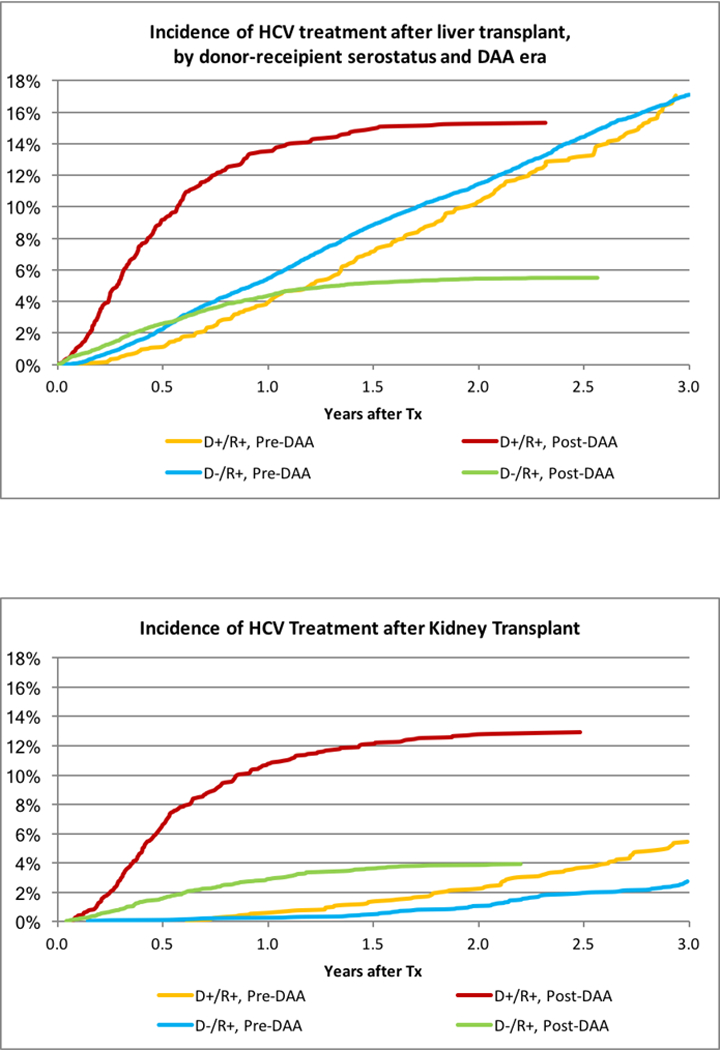
(A) Incidence of HCV treatment after liver transplant, by donor-recipient serostatus and DAA era. (B) Incidence of HCV treatment after kidney transplant.
The impact of payer on LT recipient access differed by DAA era. Pre-DAA, HCV+ LT recipients with private insurance were equally likely to be treated with HCV medications (Figure 2A). After adjustment for donor and recipient characteristics, HCV+ recipients with private insurance were somewhat more likely to be treated (aHR 1.011.101.19). However, in the post-DAA era, both D+/R+ and D-/R+ recipients with private insurance were significantly less likely to receive HCV treatment (Figure 2B). Nearly 20% of D+R+ recipients with public insurance, compared with 11% of recipients with private insurance (P<0.0001), received treatment. After adjustment for other donor and recipient characteristics, D+/R+ recipients with private insurance were 45% less likely than publicly insured recipients to receive HCV treatment (aHR 0.430.550.71, p<.0001). Among D-/R+ recipients, 6.2% with public insurance received DAA treatment, compared with 5.0% with private insurance (aHR 0.740.840.96, p=0.01).
Figure 2:
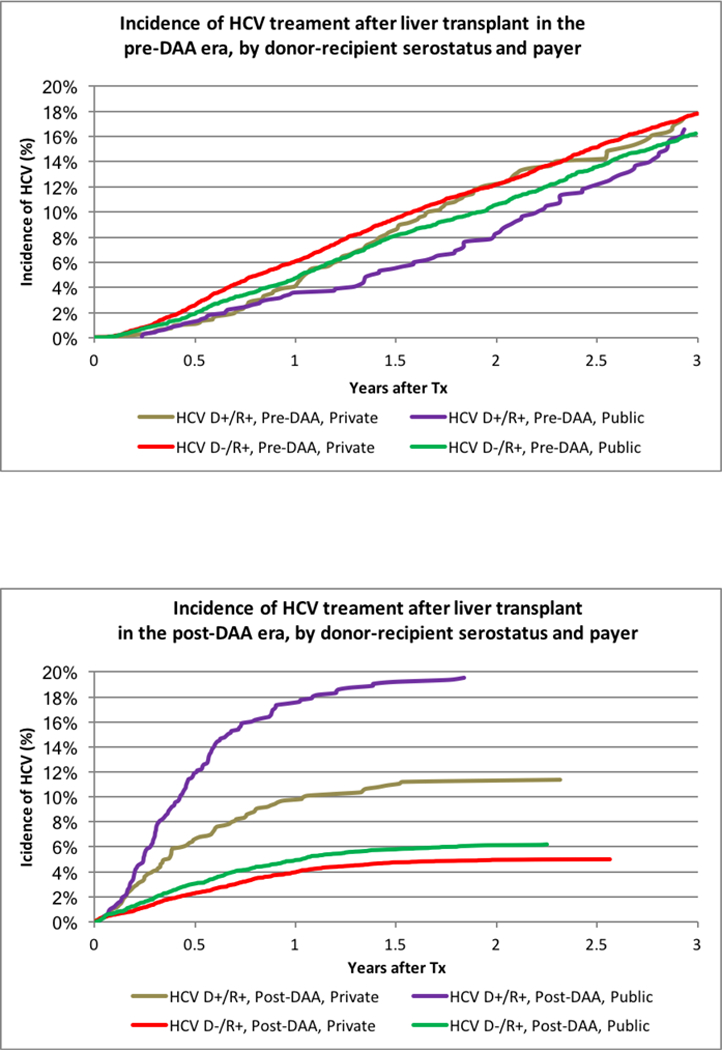
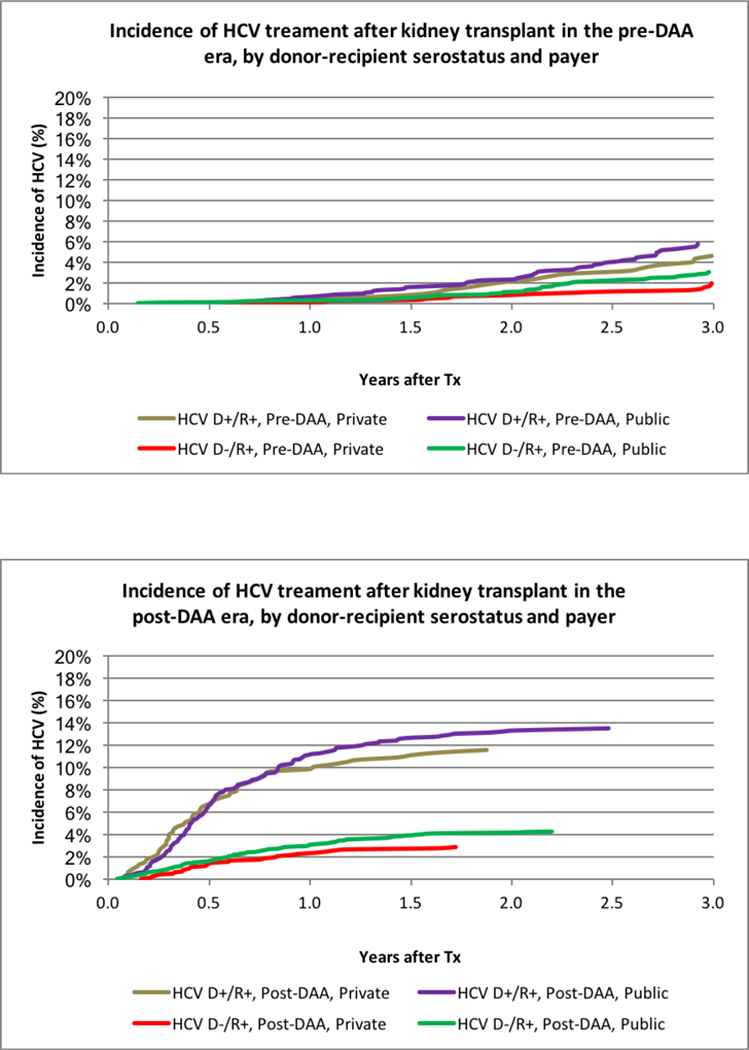
(A) Incidence of HCV treatment after liver transplant in the pre-DAA era, by donor-recipient serostatus and payer. (B) Incidence of HCV treatment after liver transplant in the post-DAA era, by donor-recipient serostatus and payer. (C) Incidence of HCV treatment after kidney transplant in the pre-DAA era, by donor-recipient serostatus and payer. (D) Incidence of HCV treatment after kidney transplant in the post-DAA era, by donor-recipient serostatus and payer.
As expected, HCV treatment was substantially less common in KT recipients; overall, 1.4% received HCV treatments within 3 years of transplant. Utilization was highest, at 22.8%, for D+R+ recipients; 11.3% of D-/R+ recipients received treatment (Figure 1B). Pre-DAA, 2.8% of D-/R+ and 5.5% of D+R+ recipients received treatment with HCV medications within 3 years. Post-DAA, 3.9% of D-/R+ and 12.9% of D+/R+ recipients received treatment (P<.0001). Differences by payer in the post-DAA era were modest. Among D+R+ recipients, 13.5% with public insurance received treatment compared with 11.5% with private insurance (p=0.35). Among D-/R+ recipients, 4.3% with public insurance received treatment, compared with 2.9% with private insurance (p=0.05). Adjustment for donor and recipient characteristics, there were HCV treatment in R+ KT patients was more likely with D+ (Pre-DAA: aHR 1.432.02 2.84 vs. Post DAA aHR: 2.312.93 3.71). In the post-DAA era, other patients whose race with not black or white and older patients were more likely to be treated. (Table S2b).
Impact of HCV treatment on patient and graft survival:
LT outcomes among all HCV+ recipients have improved in the post-DAA era (Figure 3A). Among all HCV+ LT recipients, after adjustment for donor and recipient characteristics, HCV requiring treatment pre-DAA was associated with a significantly higher risk of posttransplant mortality (aHR 1.471.681.91, p<0.0001) and all cause graft failure (aHR 1.641.852.10, p<.0001) (Figure 4A). In contrast, there was not increased risk of death or graft failure post-DAA (aHR for death: 0.740.941.19, p=0.61; aHR for graft failure: 0.740.941.19, p=0.62) (Figure 4; Table S3A). In D-/R+ recipients, HCV treatment pre-DAA was not associated with an increased risk of death (p=0.96) or graft failure (p=0.40). Among D+/R+ post-DAA, the reduction in the risk of death (aHR 0.340.671.32, p=0.25) and graft failure (aHR 0.320.641.26, p=0.20) within three years of transplant was not statistically significant. (Table S3B).
Figure 3:
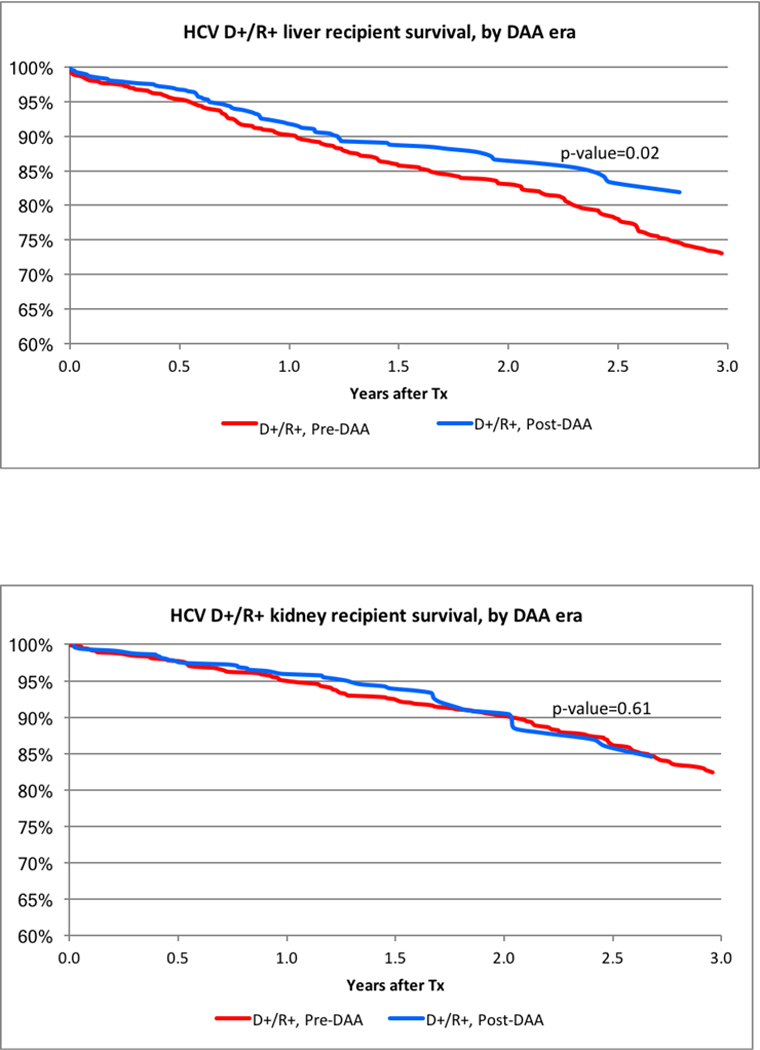
(A) HCV D+/R+ liver recipient survival, by DAA era. (B) HCV D+/R+ kidney recipient survival, by DAA era.
Figure 4:
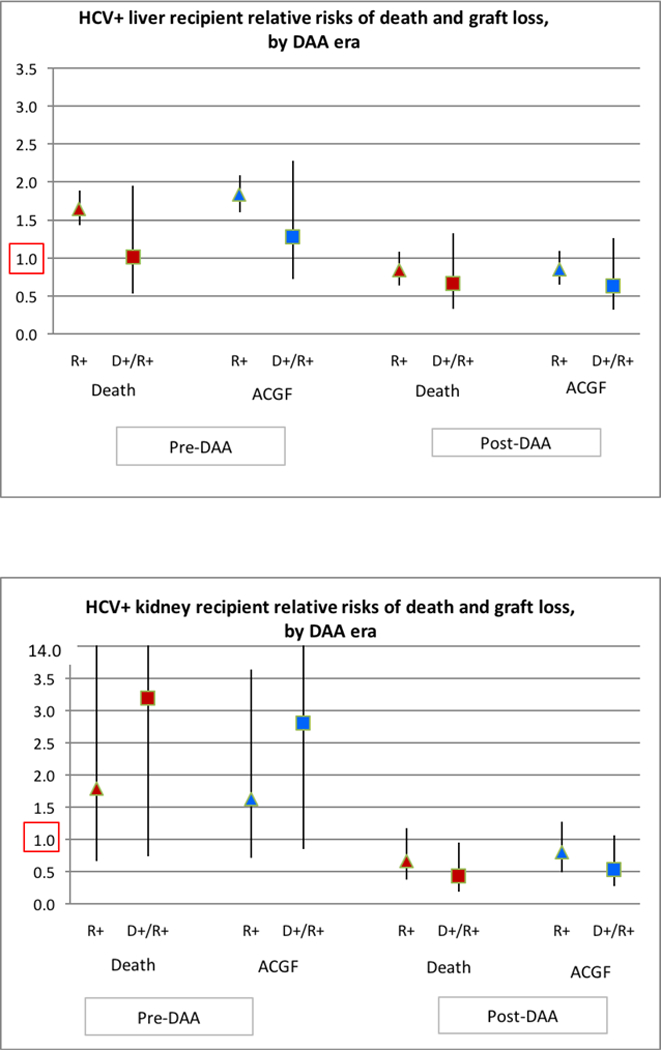
(A) HCV+ liver recipient relative risks of death and graft loss, by DAA era. (B) HCV+kidney recipient relative risks of death and graft loss, by DAA era.
Among HCV+ KT recipients, there was no significant improvement in unadjusted patient survival post-DAA compared with pre-DAA (Figure 3B). In the adjusted analysis, pre-DAA HCV treatment was associated with a non-statistically significant increased risk of death (aHR0.67 1.804.87, p=0.25) and graft failure (aHR 0.711.613.62, p=0.24) (Figure 4B; Table S4A). There was a non-significant protective effect of treatment for KT recipients post-DAA for mortality (aHR 0.370.661.18, p=0.16) and graft failure (aHR 0.490.791.27, p=0.33) (Table S3; Table S4A). Among D+R+ KT recipients, DAA treatment was associated with non-significant reduction in graft failure (aHR 0.270.54 1.07, P=0.08) and a statistically significant lower risk of death after KT (0.190.430.95, p=0.04). (Table S4B)
Economic analysis:
The cost of HCV treatment increased dramatically for LT and KT recipients in the DAA era. The mean direct cost of HCV treatment for D+R+ recipients after LT increased from $9,772 USD pre-DAA to $120,096 USD in 2014–2017 (p<.0001. For KT, cost of treatment increased from $4,489 USD to $106,747 USD (p<0.0001).
DISCUSSION
Introduction of DAAs has markedly improved care for LT and KT recipients with chronic HCV infection. Among LT recipients, our data support findings from smaller clinical studies that suggest that DAAs in the posttransplant setting improves posttransplant outcomes. In the pre-DAA era, HCV reinfection after LT was universal and treatment was generally reserved only for recipients with early aggressive recurrence resulting in fibrosing cholestatic hepatitis C or other complications. Treatment was often ineffective, resulting in accelerated graft loss and death, as confirmed in this analysis. DAAs, by contrast, are well tolerated and recommended for all actively infected patients.7,9,28 Based on national data and early follow-up, there is a consistent pattern of improved outcomes in LT recipients with HCV treated with DAAs. In KT recipients, treatment of HCV is less common than in LT recipients. However, a similar protective effect is noted in D+/R+ KT recipients, who have reduced mortality and, likely, graft loss if they receive posttransplant DAAs. Access to these expensive medications however appears limited, as less than 20% of D+/R+ LT and KT recipients receive them, and DAA treatment rates appear to be even lower among privately insured patients.
The availability of effective posttransplant HCV treatment allows LT and KT recipients to time treatment to achieve the greatest benefit.24,29 Pretransplant patients who are pre-cirrhotic or who have well-compensated disease may benefit from early HCV treatment with stabilization or regression of chronic liver disease, potentially avoiding LT entirely. Ahmed et al. recently reported a Markov analysis comparing delayed or immediate HCV treatment among patient waiting for LT.30 The benefit of HCV treatment varied according to clinical condition. Among patients with decompensated liver disease, pre-LT treatment was associated with improved survival (9.3 vs. 8.7 quality-adjusted life-years) but higher costs ($304,800 USD vs. $283,789 USD). Results were sensitive to MELD score at evaluation, such that pre-LT treatment was of less benefit to patients with greater decompensation. Decompensated patients may be further disadvantaged by pre-LT treatment, as the availability of HCV-infected donor organs has historically been significantly greater, allowing earlier transplant. Among patients with stable liver disease who undergo transplant due to hepatocellular carcinoma, immediate HCV treatment was associated with a gain of 11.5 quality-adjusted life-years vs. 10.4 for delayed treatment; however, health care expenditures were increased by $82,000 USD per patient. Our data suggest that HCV treatment after transplant is no longer associated with a decrement in graft or patient survival, and may in fact be protective. In light of the organ shortage, reserving treatment of patients with decompensated cirrhosis until after LT may be clinically and economically beneficial, provided they have access to DAAs after transplant.
Dialysis patients infected with HCV are frequently encouraged to seek DAA treatment before transplant despite demonstrated efficacy of DAA in the posttransplant setting.13,31 In recipients with available, compatible live donors, this strategy can be justified as it allows viral clearance prior to transplant, thereby avoiding potential post-transplant drug-drug interactions and mitigating risk of any early HCV-related complications, for example new onset diabetes. However, patients who are waiting for deceased donor organs may delay HCV treatment until after transplant to allow greater access to HCV+ donor organs, which is associated with a marked reduction in expected waiting times.32 Importantly, these data are the first to suggest improved patient and allograft survival among HCV+ KT patients who are treated with DAAs early post-transplant, yet less than 15% of patients who receive a HCV D+ organ received timely DAA treatment.
The cost of HCV therapy with DAAs increased markedly compared with interferon and ribavirin. The cost of care is further increased for HCV patients with co-existing organ dysfunction. Patients with chronic kidney disease or ESRD who require HCV treatment incur four-fold higher per member per month costs than HCV patients without ESRD ($5481 USD vs. $1922 USD p<0.001). Despite these high costs, DAA treatment in the majority of patients has been found to be generally cost effective, with some regimens characterized as cost saving in the non-transplant population.33,34 While early treatment regimens, such as those identified in this data set, were very expensive, competition from newly released agents has dramatically reduced the cost of treatment in an effort to increase market share. Treatment costs have also been reduced after shorter durations of care and have been demonstrated to be equally effective. A recent meta-analysis assessing the cost effectiveness of pretransplant treatment revealed that 71% of analyses found second-generation DAAs to be cost saving and 22% cost effective, while only 7% were not cost effective. Further savings may be expected through reduced graft loss and need for retransplantation (LT) and HCV related kidney disease. Because use of kidneys from HCV-infected donors can markedly reduce wait times for transplant for HCV-infected kidney candidates, the cost-saving realized by a shorter dialysis burden in these patients must be accounted for as well, especially in regions associated with lengthy waiting times.35 It is therefore unclear why access to treatment with DAAs should be limited for transplant recipients, who are even more likely to benefit from treatment than dialysis patients.24,36
This analysis has several key limitations. First, we lack information regarding viral load and genotype in both the pre- and posttransplant setting. Therefore, it is impossible to determine definitively which patients were actively infected at the time of transplant. This is particularly important in the current era, as HCV seropositivity in the absence of a positive NAT is consistent with virological cure, either related to prior administration of efficacious therapy or spontaneous clearance. This may account for the lower rate of utilization of HCV treatment in the post-DAA era among D-/R+ recipients. Second, the price of HCV medications has fallen since 2016 as new medications have been developed and marketed. This competition has resulted in somewhat lower costs for treatment regimens than reported in this analysis. Therefore, the reduced treatment access for privately insured patients that we found may have improved, as insurance companies start to develop new policies relating to DAAs. Third, despite our assembling the largest cohort reported of treatment in HCV+ recipients, the number of presumptively viremic patients (D+/R+) is still limited in this national study. This may limit inferences about the impact of treatment and comparisons of treatments. Additional data with longer periods of follow up may provide important insight into the benefits of these treatments. Finally, among KT patients, the severity of HCV-related liver disease, an important outcome determinant, was unknown. However, in this large-scale analysis, it is unlikely to have differed between eras.
In conclusion, HCV treatment patterns have changed with the introduction of highly effective DAAs. Fewer HCV D-/R+ LT patients are treated in the posttransplant setting, as many may have been treated prior to transplant, while rates have increased for D+/R+ patients. In contrast to the reduced survival observed in LT patients treated in the pre-DAA era, HCV treatment with DAAs was not associated with poor outcomes in D+/R+ HCV+ LT recipients. Future studies capturing larger samples may demonstrate improved survival following DAA treatment. In KT patients, HCV treatment remains rare, but is more common in the DAA era and appears to improve outcomes in HCV+ KT recipients. Finally, DAAs were associated with a 10-fold increase the cost of HCV treatment after LT and KT compared with medications in the pre-DAA era. These data suggest that patients with private insurance have reduced access to DAAs, which may result in higher rates of graft failure and death in patients who receive HCV+ donor organs or remain viremic at the time of transplant. Further study is needed to determine the optimal time and treatment strategy for transplant candidates and recipients infected with HCV.
Supplementary Material
ACKNOWLEDGMENTS
This work was conducted under the auspices of the Minneapolis Medical Research Foundation (MMRF), contractor for the Scientific Registry of Transplant Recipients (SRTR), as a deliverable under contract no. HHSH250201000018C (US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a US Government-sponsored work, there are no restrictions on its use. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. The authors thank SRTR colleague Nan Booth, MSW, MPH, ELS, for manuscript editing.
This work was supported by grants from the Saint Louis University Liver Center and the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK102981.
Abbreviations:
- aHR
adjusted hazard ratio
- aOR
adjusted odds ratio
- BMI
body mass index
- CI
confidence interval
- DAA
Direct-acting antiviral
- eGFR
estimated glomerular filtration rate
- ESLD
end-stage liver disease
- ESRD
end-stage renal disease
- HCV
hepatitis C virus
- HRSA
Health Resources and Services Administration
- KT
kidney transplant
- LT
liver transplant
- MELD
model for end-stage liver disease
- NAT
nucleic acid testing
- OPTN
Organ Procurement and Transplantation Network
- PCD
pharmaceutical claims data warehouse
- SRTR
Scientific Registry of Transplant Recipients
- SVR
sustained viral response
- TRR
transplant recipient registration
- US
United States
- USD
United States dollars
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Institution at which work was performed: Saint Louis University, St. Louis, MO, USA
REFERENCES
- 1.Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149(3):649–659. [DOI] [PubMed] [Google Scholar]
- 2.Alqahtani SA, Afdhal N, Zeuzem S, et al. Safety and tolerability of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic hepatitis C virus genotype 1 infection: Analysis of phase III ION trials. Hepatology. 2015;62(1):25–30. [DOI] [PubMed] [Google Scholar]
- 3.Reddy KR, Bourliere M, Sulkowski M, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: An integrated safety and efficacy analysis. Hepatology. 2015;62(1):79–86. [DOI] [PubMed] [Google Scholar]
- 4.Curry MP, O’Leary JG, Bzowej N, et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. The New England journal of medicine. 2015;373(27):2618–2628. [DOI] [PubMed] [Google Scholar]
- 5.Cheung MCM, Walker AJ, Hudson BE, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. Journal of hepatology. 2016;65(4):741–747. [DOI] [PubMed] [Google Scholar]
- 6.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of Sustained Virologic Response with Direct-Acting Antiviral Treatment on Mortality in Patients with Advanced Liver Disease. Hepatology. 2017. [DOI] [PubMed] [Google Scholar]
- 7.Spengler U Direct antiviral agents (DAAs) - A new age in the treatment of hepatitis C virus infection. Pharmacol Ther. 2018;183:118–126. [DOI] [PubMed] [Google Scholar]
- 8.Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57(5):1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mucke MM, Mucke VT, Lange CM, Zeuzem S. Managing hepatitis C in patients with the complications of cirrhosis. Liver international : official journal of the International Association for the Study of the Liver. 2018;38 Suppl 1:14–20. [DOI] [PubMed] [Google Scholar]
- 10.Mucke MM, Mucke VT, Lange CM, Zeuzem S. Special populations: treating hepatitis C in patients with decompensated cirrhosis and/or advanced renal impairment. Liver international : official journal of the International Association for the Study of the Liver. 2017;37 Suppl 1:19–25. [DOI] [PubMed] [Google Scholar]
- 11.Coilly A, Roche B, Duclos-Vallee JC, Samuel D. Optimum timing of treatment for hepatitis C infection relative to liver transplantation. Lancet Gastroenterol Hepatol. 2016;1(2):165–172. [DOI] [PubMed] [Google Scholar]
- 12.Felmlee DJ, Coilly A, Chung RT, Samuel D, Baumert TF. New perspectives for preventing hepatitis C virus liver graft infection. Lancet Infect Dis. 2016;16(6):735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadoul M, Martin P. Hepatitis C Treatment in Chronic Kidney Disease Patients: The Kidney Disease Improving Global Outcomes Perspective. Blood Purif. 2017;43(1–3):206–209. [DOI] [PubMed] [Google Scholar]
- 14.Patel PR, Thompson ND, Kallen AJ, Arduino MJ. Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;56(2):371–378. [DOI] [PubMed] [Google Scholar]
- 15.Mendizabal M, Reddy KR. Chronic hepatitis C and chronic kidney disease: Advances, limitations and unchartered territories. Journal of viral hepatitis. 2017;24(6):442–453. [DOI] [PubMed] [Google Scholar]
- 16.Bruchfeld A, Roth D, Martin P, et al. Elbasvir plus grazoprevir in patients with hepatitis C virus infection and stage 4–5 chronic kidney disease: clinical, virological, and health-related quality-of-life outcomes from a phase 3, multicentre, randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2017;2(8):585–594. [DOI] [PubMed] [Google Scholar]
- 17.Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naive chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Alimentary pharmacology & therapeutics. 2018;47(2):259–267. [DOI] [PubMed] [Google Scholar]
- 18.Sawinski D, Bloom RD. Novel Hepatitis C Treatment and the Impact on Kidney Transplantation. Transplantation. 2015;99(12):2458–2466. [DOI] [PubMed] [Google Scholar]
- 19.Colombo M, Aghemo A, Liu H, et al. Treatment With Ledipasvir-Sofosbuvir for 12 or 24 Weeks in Kidney Transplant Recipients With Chronic Hepatitis C Virus Genotype 1 or 4 Infection: A Randomized Trial. Annals of internal medicine. 2017;166(2):109–117. [DOI] [PubMed] [Google Scholar]
- 20.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid Reimbursement of Sofosbuvir for the Treatment of Hepatitis C Virus Infection in the United States. Annals of internal medicine. 2015;163(3):215–223. [DOI] [PubMed] [Google Scholar]
- 21.Campbell CA, Canary L, Smith N, Teshale E, Ryerson AB, Ward JW. State HCV Incidence and Policies Related to HCV Preventive and Treatment Services for Persons Who Inject Drugs - United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2017;66(18):465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabiri M, Chhatwal J, Donohue JM, et al. Long-term disease and economic outcomes of prior authorization criteria for Hepatitis C treatment in Pennsylvania Medicaid. Healthc (Amst). 2017;5(3):105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith-Palmer J, Cerri K, Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefits. BMC Infect Dis. 2015;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samur S, Kues B, Ayer T, et al. Cost Effectiveness of Pre- vs Post-Liver Transplant Hepatitis C Treatment With Direct-Acting Antivirals. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018;16(1):115–122 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chhatwal J, Samur S, Bethea ED, et al. Transplanting HCV-positive livers into HCV-negative patients with preemptive antiviral treatment: A modeling study. Hepatology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bethea ED, Chen Q, Hur C, Chung RT, Chhatwal J. Should we treat acute hepatitis C? A decision and cost-effectiveness analysis. Hepatology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axelrod DA, Naik AS, Schnitzler MA, et al. National Variation in Use of Immunosuppression for Kidney Transplantation: A Call for Evidence-Based Regimen Selection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(8):2453–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadiparthi C, Cholankeril G, Perumpail BJ, et al. Use of direct-acting antiviral agents in hepatitis C virus-infected liver transplant candidates. World journal of gastroenterology : WJG. 2018;24(3):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cholankeril G, Joseph-Talreja M, Perumpail BJ, et al. Timing of Hepatitis C Virus Treatment in Liver Transplant Candidates in the Era of Direct-acting Antiviral Agents. J Clin Transl Hepatol. 2017;5(4):363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed A, Gonzalez SA, Cholankeril G, et al. Treatment of patients waitlisted for liver transplant with all-oral direct-acting antivirals is a cost-effective treatment strategy in the United States. Hepatology. 2017;66(1):46–56. [DOI] [PubMed] [Google Scholar]
- 31.Jadoul M, Martin P. Should all dialysis patients with hepatitis C be treated? If so, before or after kidney transplantation? Seminars in dialysis. 2017;30(5):395–397. [DOI] [PubMed] [Google Scholar]
- 32.Shelton BA, Sawinski D, Mehta S, Reed RD, MacLennan PA, Locke JE. Kidney transplantation and waitlist mortality rates among candidates registered as willing to accept a hepatitis C infected kidney. Transplant infectious disease : an official journal of the Transplantation Society. 2017. [DOI] [PubMed] [Google Scholar]
- 33.He T, Lopez-Olivo MA, Hur C, Chhatwal J. Systematic review: cost-effectiveness of direct-acting antivirals for treatment of hepatitis C genotypes 2–6. Alimentary pharmacology & therapeutics. 2017;46(8):711–721. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal R, Chen Q, Goel A, et al. Cost-effectiveness of hepatitis C treatment using generic direct-acting antivirals available in India. PloS one. 2017;12(5):e0176503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawinski D, Wyatt CM, Locke JE. Expanding the use of hepatitis C-viremic kidney donors. Kidney international. 2017;92(5):1031–1033. [DOI] [PubMed] [Google Scholar]
- 36.Belli LS, Duvoux C, Berenguer M, et al. ELITA consensus statements on the use of DAAs in liver transplant candidates and recipients. Journal of hepatology. 2017;67(3):585–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


