Abstract
Cannabis use disorder (CUD) is the most common illicit substance use disorder and individuals with CUD have high rates of comorbid anxiety disorders. Comorbidity between CUD and anxiety disorders is of public health relevance given that although motivation enhancement therapy (MET) combined with cognitive-behavioral therapy (CBT) is an efficacious intervention for CUD, outcomes are worse for patients with elevated anxiety. The current study tested the acceptability and efficacy of the integration of a transdiagnostic anxiety CBT (i.e., treatment of patients with any anxiety disorder) with MET-CBT (integrated cannabis and anxiety reduction treatment, or ICART) for CUD compared to MET-CBT alone. Treatment-seeking cannabis users (56.4% male, Mage = 23.2, 63.3% non-Hispanic White) with CUD and at least one comorbid anxiety disorder were randomly assigned to ICART (n = 27) or MET-CBT (n = 28). Patients in the ICART condition attended significantly more treatment sessions than those in the MET-CBT condition. Patients in the ICART condition were more likely to be abstinent post-treatment than those in MET-CBT. Further, treatment produced decreases in cannabis use and related problems. Notably, therapy type did not moderate the impact of treatment on frequency of use and related problems.Together, these data suggest that ICART may be at least as efficacious as a gold-standard psychosocial CUD treatment, MET-CBT, for a difficult-to-treat subpopulation of cannabis users.
Keywords: cannabis, marijuana, anxiety disorders, dual diagnosis, integrated treatment
Cannabis is the most commonly used drug with approximately 24 million Americans endorsing past year use (Center for Behavioral Health Statistics and Quality, 2017). Notably, rates of use, including daily use, continue to rise (Center for Behavioral Health Statistics and Quality, 2017). Corresponding to the increase in cannabis use rates, rates of cannabis use disorder (CUD) are also increasing. To illustrate, CUD rates have nearly doubled since 2002 (Hasin et al., 2015) and rates of CUD are greater than opioid and methamphetamine use disorders combined (Center for Behavioral Health Statistics and Quality, 2017).
Quitting cannabis is often very difficult for regular users (Moore & Budney, 2003) and situations involving negative affect are among the most difficult situations in which to abstain during cessation attempts (Buckner, Zvolensky, & Ecker, 2013). This seems especially true for individuals with elevated anxiety, given that anxiety is related to greater cannabis problems and greater perceived barriers to quitting (e.g,. Buckner & Carroll, 2010; Zvolensky et al., 2018). In fact, over 40% of individuals with CUD have a comorbid anxiety disorder (Teesson et al., 2012). The high rates of anxiety disorders among patients with CUD is concerning given that motivation enhancement therapy combined with cognitive-behavioral therapy (MET-CBT; Steinberg et al., 2005) is an efficacious intervention for CUD (e.g., Marijuana Treatment Project Research Group, 2004), yet patients with elevated anxiety report more cannabis use and related problems following treatment (Buckner & Carroll, 2010). Importantly, decreases in anxiety during CUD treatment are related to better outcomes (Buckner & Carroll, 2010), indicating a need to target anxiety during CUD treatment.
The high rates of comorbid anxiety and substance use disorders (SUD) and the poorer outcomes for these patients have led to explicit calls for the development of personalized treatments for dually diagnosed patients (National Insitute of Drug Abuse, 2013) that treat anxiety and SUD in an integrated fashion that addresses the reciprocal nature of these disorders (Stewart & Conrod, 2008). Given that a variety of anxiety disorders (e.g., social anxiety, generalized anxiety, panic) are related to cannabis use and CUD (e.g., Buckner, Heimberg, Ecker, & Vinci, 2013; Marmorstein, White, Loeber, & Stouthamer-Loeber, 2010; Zvolensky et al., 2006), it may be beneficial to incorporate a transdiagnostic anxiety treatment into CUD treatment for these patients. Transdiagnostic anxiety treatments are designed to treat underlying processes common to all anxiety disorders to facilitate the use of similar techniques to alleviate symptoms regardless of type of particular anxiety disorder. False Safety behavior Elimination Treatment (FSET; Schmidt, Buckner, Pusser, Woolaway-Bickel, & Preston, 2012) is one such transdiagnostic CBT that addresses several anxiety disorders simultaneously by addressing False Safety Behaviors (FSB), or behaviors that help one avoid or alleviate false threats (i.e., phobic stimuli). FSBs are common across anxiety conditions because they often temporarily alleviate anxiety (e.g., avoiding a phobic stimulus); however, repeated use of FSBs can contribute to the maintenance of anxiety disorders (Salkovskis, Clark, & Hackmann, 1991). Thus, FSET involves the identification and elimination of FSBs and has been found to decrease anxiety and depression and improve quality of life (Schmidt et al., 2012).
FSB are common among cannabis users, with the majority of cannabis users, regardless of level of trait anxiety, endorsing FSB use (Buckner, Zvolensky, Businelle, & Gallagher, 2017). Further, FSB use is robustly related to more frequent cannabis use (Buckner et al., 2017). Notably, although there is indirect evidence that anxiety is related to using cannabis as a FSB (anxiety is associated with more coping motivated cannabis use or use to manage negative affectivity; e.g., Buckner, Bonn-Miller, Zvolensky, & Schmidt, 2007; Buckner, Heimberg, Matthews, & Silgado, 2012; Zvolensky et al., 2009), anxiety severity is also related to greater use of non-cannabis FSB (e.g., avoiding anxiety provoking situations) and use of such FSB is related to more cannabis problems among anxious users (Buckner et al., 2017). Thus, FSET may be especially appropriate for integration with CUD treatment to treat anxiety-CUD comorbidity given that these emerging data indicate that (1) FSB use is common among cannabis users, (2) use of FSB, even non-cannabis FSB, is related to more cannabis use and related problems, and (3) anxious users are especially vulnerable to FSB use, which at least partially accounts for the relation between anxiety severity and cannabis problems. Given that anxious cannabis users may rely on cannabis and other FSB to manage negative affectivity, to the exclusion of more adaptive coping strategies, FSB use may reinforce maladaptive beliefs that phobic stimuli are to be avoided, thereby increasing anxiety (and reliance on cannabis to manage anxiety) in the long-term.
The primary aim of the current study was to test the utility of MET-CBT with integrated cannabis and anxiety reduction treatment (ICART), that integrates FSET for anxiety disorders with MET-CBT for CUD to simultaneously treat CUD and anxiety disorders (see Table 1). First, we examined treatment acceptability by testing whether participants in the ICART condition would be less likely to attend treatment after learning that treatment includes fading of false safety behaviors, given that anxious cannabis users tend to engage in more FSB (Buckner et al., 2017) and other avoidance strategies (Buckner, Heimberg, & Schmidt, 2011; Buckner, Zvolensky, Farris, & Hogan, 2014). Second, we tested whether the integration of FSET into MET-CBT would dilute the effects of MET-CBT by testing whether both treatments resulted in abstinence from cannabis as well as decreases in cannabis use frequency and related problems. Given its focus on reducing FSB, we also tested whether ICART would produce greater reductions in anxiety than MET-CBT.
Table 1.
Treatment Session Topics
| Session # | MET-CBT for CUD | ICART for CUD and Anxiety |
|---|---|---|
| 1 | • Psychoeducation: CUD • Personalized Feedback Report • Barriers to Quitting |
• Psychoeducation: anxiety disorders • Psychoeducation: CUD • Psychoeducation: reciprocal relation between anxiety & cannabis • Reasons for Quitting • Personalized Feedback Report • Change Plan • Barriers to Quitting |
| 2 | • Change Plan • Supporters • Reasons for Quitting • New Cannabis Coping Strategies |
• Psychoeducation: anxiety • Psychoeducation: emotional processing model of anxiety & how it relates to cannabis • New Cannabis Coping Strategies |
| 3 | • Coping with other life problems | • Psychoeducation: false safety behaviors & strategies to eliminate them |
| 4 | • Functional analysis • Increase pleasant activities • Relaxation Training • Coping with boredom |
• Cannabis & other substance use as false safety behaviors • Function analysis • Cannabis coping strategies |
| 5 | • Coping with Cravings • Urge Surfing |
•Antiphobic attitude • Coping with cannabis cravings • Urge surfing |
| 6 | • Managing thoughts about cannabis use | • Other false safety behaviors (checking, reassurance seeking, companions, avoidance of bodily sensations) • Managing thoughts related to cannabis use |
| 7 | • Problem-solving | • Avoidance as a false safety behavior • Managing Negative Moods & Depression as triggers for cannabis • Increasing pleasant activities |
| 8 | • Cannabis refusal skills | • Other false safety behaviors (cognitive avoidance, idiosyncratic behaviors) • Seemingly irrelevant decisions |
| 9 | • Elective Topic (planning for emergencies & coping with a lapse; seemingly irrelevant decisions; managing negative moods; assertiveness) | • Problem-solving |
| 10 | • Cannabis refusal skills | |
| • Assertiveness | ||
| 11 | • Planning for emergencies & coping with a lapse | |
| 12 | • Prevention of relapse of anxiety disorders and CUD |
Note. MET-CBT = motivation enhancement therapy integrated with cognitive behavioral therapy (nine sessions). ICART = integrated cannabis and anxiety reduction treatment (12 sessions). In both treatments, motivational interviewing techniques were used to explore and resolve ambivalence and increase motivation to change cannabis use throughout treatment as clinically appropriate.
Methods
Participants and Procedures
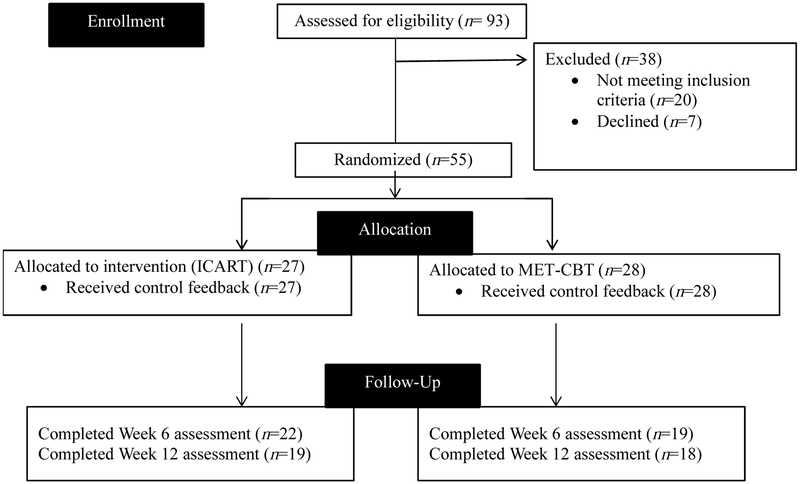
Participants (N = 55) were recruited from the community (via flyers, newspaper ads, online advertisements) to participate in a randomized controlled trial examining the efficacy of two psychosocial interventions for CUD (clinicaltrials.gov #NCT01875796). Inclusion criteria included being 18-65 years of age, current cannabis use (confirmed via urine sample using a 50 ng/ml positive cutoff, which detects cannabis use up to 78 hours post-ingestion), meeting DSM-5 criteria for both CUD and an anxiety disorder and having used cannabis in the past week to manage anxiety. Of the 93 participants who completed a baseline screening appointment, 31 were deemed ineligible. Exclusion criteria and the number of individuals excluded for each reason were: unable/unwilling to commit to 12 sessions and assessments due to scheduling/availability (n = 8), no longer interested in CUD treatment (n = 7), psychiatric disorder that precluded participation (e.g., possible psychotic disorder; n = 4), current participation in other anxiety or substance use treatment (n = 4), CUD not primary substance use disorder (n = 2), cannabis not substance of choice for anxiety management (n = 2), did not meet DSM-5 criteria for CUD (n = 1) or anxiety disorder (n = 2), and severe active suicidal ideation (n = 1). Four participants did not return attempts to contact to a baseline appointment. Three others dropped out prior to their first treatment session. Hence, 55 attended at least one study treatment session (see Figure 1). All participants met DSM-5 criteria for CUD. Comorbid diagnoses included social anxiety disorder (67.3%), generalized anxiety disorder (25.5%), alcohol use disorder (25.5%), major depressive disorder (21.8%), panic disorder (18.2%), pervasive depressive disorder (9.1%), other specified anxiety disorder (9.1%), adjustment disorder (9.1%), post-traumatic stress disorder (7.3%), specific phobia (5.5%), agoraphobia (5.5%), and other specified trauma disorder (3.6%)1. The majority (70.9%) met DSM criteria for more than one comorbid disorder, with 41.8% meeting for two, 21.8% meeting for three, 5.5% meeting for four, and 1.8% meeting for five comorbid disorders. Descriptive information by condition is presented in Table 2.
Figure 1.
Flow of participants through the trial.
Table 2.
Descriptive Information by Condition
| MET-CBT | ICART | ||||||
|---|---|---|---|---|---|---|---|
| Variable | M or % | SD | M or % | SD | F | p | d |
| Age | 23.2 | 7.5 | 23.1 | 7.4 | 0.00 | .959 | .01 |
| Sex (% male) | 64.3 | 48.1 | 1.46 | .228 | .16 | ||
| Race | 1.09 | .780 | .14 | ||||
| Non-Hispanic White | 64.3 | 63.0 | |||||
| Non-Hispanic African American | 21.4 | 25.9 | |||||
| Hispanic White | 10.7 | 11.1 | |||||
| Multiracial | 3.6 | 0.0 | |||||
| Highest Educational Level | 1.18 | .757 | .15 | ||||
| High School | 10.7 | 18.5 | |||||
| Some College | 67.9 | 59.3 | |||||
| Technical Degree | 3.6 | 7.4 | |||||
| Bachelor’s degree | 17.9 | 14.8 | |||||
| Employment | 0.67 | .880 | .11 | ||||
| Full-time | 17.9 | 25.9 | |||||
| Part-time | 50.0 | 44.4 | |||||
| Age of first cannabis use | 15.1 | 3.2 | 17.0 | 3.3 | 3.71 | .061 | .58 |
| # of years of cannabis use | 7.8 | 8.8 | 5.7 | 6.3 | 0.85 | .360 | .28 |
| Primary cannabis use method | |||||||
| Blunts | 70.8 | 68.2 | 0.04 | .845 | .03 | ||
| Joints | 70.8 | 50.0 | 2.09 | .148 | .15 | ||
| # serious cannabis quit attempts | 5.8 | 20.1 | 1.5 | 1.0 | 1.02 | .318 | .30 |
| Drug treatment history | 7.1 | 7.7 | 0.01 | .939 | .01 | ||
| Anxiety treatment history | 32.1 | 53.8 | 2.60 | .107 | .22 | ||
| # Comorbid diagnoses | 1.9 | 0.9 | 2.3 | 1.0 | 2.86 | .096 | .46 |
Treatments
Both treatments were manualized and delivered as individual weekly sessions (Table 1). Participants in each condition were permitted 12 weeks to complete their allocated number of treatment sessions. Therapists were five advanced graduate students in clinical psychology. Clinicians completed didactics on CBT for anxiety and for substance use disorders, had prior experience conducting CBT for anxiety and/or substance use, and completed a two-day didactic training workshop on FSET led by FSET developer, Dr. Norman Schmidt. All treatment sessions were video-recorded for review during weekly supervision with the first author, a licensed clinical psychologist, for fidelity to the treatment manual. Upon completion of Week 12 assessments, those in the MET-CBT were offered ICART at no cost.
Motivation Enhancement Therapy/Cognitive Behavior Therapy for Cannabis Use Disorders (MET-CBT).
The Substance Abuse and Mental Health Services Administration’s (SAMHSA) manual for MET-CBT was used (Steinberg et al., 2005). The protocol consists of nine sessions, with the first session consisting of motivational enhancement strategies, goal setting, and treatment planning.Other sessions included functional analysis, coping with cravings/urges, cognitive restructuring, seemingly irrelevant decisions, managing negative moods, and planning for emergencies/coping with a lapse.
Integrated Cannabis and Anxiety Reduction Treatment.
ICART is a 12-session CBT protocol that integrates FSET, a transdiagnostic CBT for anxiety disorders (Schmidt et al., 2012) with MET-CBT for CUD as described above. Techniques are taught in an integrated fashion to teach patients to manage symptoms of anxiety and cannabis simultaneously (for more detail, see Buckner et al., 2016; Buckner, Zvolensky, Schmidt, et al., 2014). Psychoeducation includes discussion of the relation between anxiety and cannabis use, as well as psychoeducation about false safety behaviors. Patients are taught to reduce avoidance of phobic threats through the reduction of false safety behaviors that maintain anxiety conditions (Salkovskis et al., 1991). Patients are taught to identify when cannabis is used as a false safety behavior, as well as to identify other types of false safety behaviors. They are then encouraged to eliminate false safety behavior use, while being taught skills to manage cravings and other high-risk cannabis use situations.
Measures
Anxiety disorder diagnoses were determined using the Anxiety Disorders Interview Schedule for DSM-5 (ADIS-5; Brown & Barlow, 2014), a structured diagnostic interview designed to provide detailed coverage of anxiety and related disorders. The ADIS-IV has been shown to be a reliable and valid measure of DSM-IV (American Psychiatric Association, 2000) anxiety and mood disorders (Brown, DiNardo, Lehman, & Campbell, 2001). Cannabis use disorders were assessed using the Structured Clinical Interview for DSM-IV-TR Axis I disorders, Research Version, Non-patient Edition (SCID; First, Spitzer, Gibbon, & Williams, 2002), with additional questions added to assess DSM-5 criteria (American Psychiatric Association, 2013). The SCID was used to assess substance use disorders given that the psychometrics of substance use disorder section of the ADIS has not been examined, whereas the SCID substance use disorder section has very good psychometric properties, including good inter-rater reliability (e.g., Buckner, Schmidt, Bobadilla, & Taylor, 2006). To test inter-rater reliability in the current study, the clinical interviews were conducted at screening and again at baseline by two independent doctoral students in clinical psychology (the baseline rater was blind to prior ratings). Baseline ratings were conducted on average 12.7 (SD = 11.7) days after screening. All participants met criteria for CUD at both the screening and baseline appointments to be eligible for the study. Intraclass Correlation Coefficient of two ratings for anxiety disorder diagnosis was excellent (ICC = .82, 95% CI: .70-.90).
The Structured Interview Guide for the Hamilton Anxiety Scale (SIGH-A; Hamilton, 1959) was used to assess anxiety symptom severity. This measure was developed to assess anxiety in clinical populations and it demonstrates high inter-rater and test-retest reliability (Shear et al., 2001). The scale also demonstrated good internal consistency in the current study at baseline (α = .842). The SIGH-A was administered at screening and again at baseline by two independent doctoral students in clinical psychology; intraclass Correlation Coefficient was excellent (ICC = .82, 95% CI: .67-.90).
The clinician-administered Timeline Follow Back (TLFB; Sobell & Sobell, 1992) was used to assess quantity of cannabis use during the 30 days prior to the baseline appointment. Participants reported the number of “joints” (i.e., cannabis cigarettes) used per day. If participants used methods other than joints (e.g., bong, bowl), they were asked to estimate the number of joints that what they used would have equaled. The TLFB is a reliable and valid self-report measure of cannabis use (O'Farrell, Fals-Stewart, & Murphy, 2003; Robinson, Sobell, Sobell, & Leo, 2014).
The Marijuana Problems Scale (MPS; Stephens, Roffman, & Curtin, 2000) assessed the severity of 19 cannabis-related problems experienced in the past 90 days from 0 (no problem) to 2 (serious problem). Items were summed. The measure achieved good internal consistency in prior work (e.g,. Ecker & Buckner, 2014; Lozano, Stephens, & Roffman, 2006) and in the current sample (α = .87).
Procedure
Participants provided informed consent prior to participation and the study protocol was approved by the university’s Institutional Review Board. After completion of the screening appointment, participants were randomly assigned to receive either Integrated Cannabis and Anxiety Reduction Treatment (ICART) or motivational enhancement/cognitive behavior therapy (MET-CBT) for CUD via urn randomization to control for age, sex, cannabis use frequency, primary anxiety diagnosis, and CUD severity. Participants in the ICART condition completed 12 therapy sessions, whereas participants in the MET-CBT control condition completed 9. Participants were assessed at baseline (Week 0), week 6, and week 12. Each assessment included a clinical interview (to assess psychiatric symptoms, HAM-A, and TLFB), urine analysis, and self-report measures (e.g., MPS). In cases in which randomized participants did not attend a treatment or assessment appointment, these participants were contacted and invited to attend all remaining treatment sessions and assessment appointments through the follow-up period. Participants were permitted to make-up missed treatment sessions during the first 12 weeks of the study. If participants missed an appointment, a research assistant attempted to contract the participant every two days until they contacted the participant. No participant was excluded for failure to attend an appointment. Participants were compensated $25 for completion of each assessment (regardless of results of urine analysis).
Data Analytic Strategy
Comparisons between treatment conditions on baseline characteristics were performed using a one-way analysis of variance (ANOVA) for continuous measures and chi-square tests for dichotomous ones (Table 2). The differences between conditions on treatment acceptability, study attrition, and cannabis abstinence were also examined using chi-square tests. Missing urine specimens were considered positive for cannabis per recommendations concerning research on substance use (Nathan & Lansky, 1978). Bayesian modeling (e.g., Berry et al., 2006; Goodman, 2005; Spiegelhalter, Myles, Jones, & Abrams, 1999; Wijeysundera, Austin, Hux, Beattie, & Laupacis, 2009) conducted using R (R Core Team, 2013) was used to estimate the interaction of time (pre-treatment, mid-treatment, and post-treatment assessments) and treatment condition on cannabis use (joints), cannabis problems, and anxiety. Bayesian approaches allow for the estimation of the probability of a non-zero effect, which can be informative for treatment development. This effect is the alternative hypothesis in traditional hypothesis testing. By using the observed data and incorporating prior information about potential parameter values (i.e., priors), a posterior distribution is generated and allows for examination of the probability of obtaining a certain parameter (e.g., a non-zero effect). This contrasts with traditional “frequentist” methods (e.g., ANOVA) which can only inform the likelihood (i.e., p < .05) that the observed effect could occur if the null hypothesis were true. Additionally, these Bayesian methods are robust with small sample sizes (Irony & Simon, 2006; Lilford, Thornton, & Braunholtz, 1995; Temple, 2005).
Specifically, Bayesian, multilevel models evaluated three outcomes (i.e. anxiety symptoms, cannabis problems, and safety behaviors) as a function of time, treatment and the interaction of time and treatment. Adequate fit of response distributions was assessed by a qualitative evaluation of posterior distributions and the Widely Applicable Information Criterion (WAIC) fit index (Vehtari, Gelman, & Gabry, 2017). Bayesian credible interval (i.e., CBI) is the interval that contains the parameter of interest with 95% probability given the observed data. The Student-t with identity link function provided the best appropriate fit to the anxiety symptoms, whereas a skew normal distribution with identity link function provided a more appropriate fit for the cannabis problems and use frequency data because it adequately accommodates non-zero skewness. Specification of diffuse, neutral prior distributions used ~Normal [μ = 0, σ2 = 1×103 for coefficients and ~Folded- t [df = 3, μ = 0, σ2 = 100] for the standard deviation of level one and two variance terms. The priors reflect the absence of credible evidence for the effect size of this emerging treatment. We considered a Bayesian probability of 80% or greater that an effect exists to merit further consideration (Johnson, 2013).
Results
Treatment Acceptability and Retention
Treatment acceptability was assessed two ways. First, we tested the number of participants who attended more than one therapy session (per Budney, Higgins, Radonovich, & Novy, 2000). Using this metric, acceptability did not significantly differ between conditions: ICART = 88.9%, MET-CBT = 92.9%, χ2 (1, N = 55) = 0.26, p = .609. Next, given that treatment conditions differed in number of sessions, we tested whether conditions differed on the number of participants who completed nine treatment sessions (i.e., the number of sessions in the MET-CBT condition). Participants in ICART (59.3%) were more likely to complete nine sessions than those in the MET-CBT condition (25%), χ2 (1, N = 55) = 6.63, p = .010, Cramer’s V = .35. On average, participants in the ICART condition (M = 8.26, SD = 4.01) attended significantly more treatment sessions than those in the MET-CBT condition (M = 6.00, SD = 3.04), F (1, 534) = 5.57, p = .022, d = .65. There was no difference between ICART and MET-CBT on completion of the Week 12 assessment, χ2 (1, N = 55) = 0.23, p = .631, Cramer’s V = .07.
Baseline Differences and Correlations among Study Variables
Conditions did not significantly differ on any baseline variable (Table 2). Correlations among study variables are presented in Table 3. Given the age range of our sample, we tested whether age was related to study variables; it was not. At each timepoint, anxiety severity was significantly associated with more severe cannabis-related problems. Means and standard deviations for outcome variables at all three timepoints (Weeks 0, 6, 12) by condition are presented in Table 4. Conditions did not differ on baseline levels of outcome variables except that the ICART condition reported greater baseline anxiety, F (1, 54) = 7.36, p = .009, d = .75.
Table 3.
Bivariate correlations among study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | ||||||||||
| 2. # joints (baseline) | .03 | |||||||||
| 3. # joints (week 6) | .00 | .54** | ||||||||
| 4. # joints (week 12) | −.22 | .69** | .69** | |||||||
| 5. Cannabis problems (baseline) | −.16 | .08 | −.02 | .02 | ||||||
| 6. Cannabis problems (week 6) | .08 | .22 | .36* | −.08 | .16 | |||||
| 7. Cannabis problems (week 12) | .17 | .18 | .33* | .19 | .26 | .51** | ||||
| 8. Anxiety (week 0) | −.10 | .29* | .17 | .04 | .28* | .22 | .08 | |||
| 9. Anxiety (week 6) | −.15 | .16 | .27 | .04 | .18 | .39* | .22 | .63** | ||
| 10. Anxiety (week 12) | −.19 | .22 | .46** | .15 | .14 | .14 | .34* | .57** | .70** |
p < .05
p < .01
Table 4.
Means and Standard Deviations for Outcome Variables at Each Timepoint by Condition
| Variable | Assessment point |
MET-CBT | ICART | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| # past-month joints | Baseline | 67.94 | 65.49 | 60.47 | 37.16 |
| Week 6 | 25.28 | 30.25 | 28.24 | 25.85 | |
| Week 12 | 22.89 | 43.56 | 11.12 | 13.76 | |
| Cannabis problem severity | Baseline | 10.67 | 6.15 | 15.18 | 6.74 |
| Week 6 | 6.44 | 5.48 | 9.35 | 5.30 | |
| Week 12 | 5.11 | 6.61 | 5.47 | 4.91 | |
| Anxiety severity | Baseline | 17.83 | 7.20 | 23.71 | 7.76 |
| Week 6 | 13.28 | 7.82 | 17.47 | 7.11 | |
| Week 12 | 10.61 | 6.91 | 12.24 | 7.21 | |
Impact of Treatment
Cannabis Use.
First, we tested whether patients in the ICART condition were more likely to test negative for cannabis at any point during treatment (Week 6 or Week 12) and found that although 11.1% of patients in the ICART condition tested negative for cannabis use via urinalysis during treatment compared to 3.6% of patients in MET-CBT, this difference was not statistically significant, χ2 = 1.16, p = .282, Cramer’s V = .15. Regarding abstinence rates at Week 12 specifically, 12% of patients in the ICART condition tested negative for cannabis use via urinalysis at Week 12, compared to 0% of patients in the MET-CBT condition, χ2 = 3.56, p = .059, Cramer’s V = .26.
Regarding self-reported cannabis use (# of past-month joints), there was not a reliable interaction (probability of an interaction = 73.7%) between treatment condition and time (β = −0.55, 95% CI = [−2.33, 1.24]). Self-reported cannabis use reliably decreased over time in the MET-CBT condition (probability of a non-zero slope = 99.6%; β= −2.19, 95% CBI = [−4.08, −0.52). Additionally, cannabis use reliably decreased over time in the ICART condition (probability of a non-zero slope > 99.9%; β= −2.41, 95% CBI = [−3.56, −1.33]). Thus, both treatments appeared to reduce cannabis use to a similar degree.
Cannabis-Related Problems.
There was not a reliable interaction (probability of an interaction = 28.2%) between treatment condition and time (β = 0.10, 95% CI = [−0.24, 0.42]) on cannabis problems. Cannabis problems reliably decreased over time in the MET-CBT condition (probability of a non-zero slope > 99.9%; β = −0.48, 95% CBI = [−0.72, −0.24]). Additionally, cannabis problems reliably decreased over time in the ICART condition (probability of a non-zero slope > 99.9%; β = −0.43, 95% CBI = [−0.73, −0.16]). Thus, both treatments appeared to reduce cannabis problems to a similar degree.
Anxiety.
There was a reliable interaction (probability of an interaction = 97.0%) between treatment condition and time (β = −0.34, 95% CI = [−0.67, −0.01]) on anxiety scores. Anxiety reliably decreased over time in the control group (probability of a non-zero slope = 99.0%; β = −0.59, 95% CBI = [−0.84, −0.35]). Additionally, anxiety reliably decreased over time in the active group (probability of a non-zero slope > 99.9%; β = −0.93, 95% CBI = [−1.17, −0.69]). Thus, although both treatments reduced anxiety reliably, the active condition reduced to a greater degree relative to control.
Discussion
Data provide preliminary support for the utility of ICART, a psychosocial treatment that integrates the transdiagnostic CBT, FSET, for anxiety disorders (Riccardi, Korte, & Schmidt, 2017; Schmidt et al., 2012) with MET-CBT for CUD (Steinberg et al., 2002) in the treatment of CUD with comorbid anxiety disorders. Specifically, ICART seemed to be an acceptable treatment given that participants in the ICART condition were not more likely to drop out after session one, despite learning that treatment would entail reducing their reliance on FSB and other avoidance strategies. This is especially important given that anxious cannabis users use more FSB (Buckner et al., 2017) including avoidance (Buckner et al., 2011; Buckner, Zvolensky, Farris, et al., 2014), which could have placed them at risk for dropping out of treatment rather than learn to face anxiety-providing stimuli without FSB. Further supporting the utility of ICART, ICART was associated with greater abstinence from cannabis than MET-CBT, a gold standard psychosocial treatment for CUD. Further ICART produced decreases in cannabis use and related problems, and treatment condition did not moderate observed reductions in these cannabis outcomes, indicating that the addition of FSET to MET-CBT did not dilute the impact of MET-CBT on cannabis outcomes. These data are especially promising when considered in light of our finding that participants in the ICART condition evinced greater anxiety at baseline than those in the MET-CBT condition, given that anxiety is associated with worse CUD treatment outcomes (Bonn-Miller & Moos, 2009; Buckner & Carroll, 2010).
Providing patients with skills to manage their cannabis cravings and high-risk use situations while simultaneously teaching them skills to manage their anxiety is a unique strategy of this treatment and it shows great promise. The ICART manual was developed such that techniques from FSET (Riccardi et al., 2017; Schmidt et al., 2012) work in concert with those from MET-CBT (Steinberg et al., 2002) to increase motivation to change problematic cannabis-related behaviors while also targeting the patient’s anxiety. ICART produced significant decreases in anxiety, which may result in better long-term cannabis-related outcomes given that negative affect predicts lapse among cannabis users undergoing quit attempts (Buckner, Zvolensky, et al., 2013) and that anxiety severity was associated with more cannabis problems at each timepoint in the current study.
It is noteworthy that ICART is 12 sessions as opposed to the 9 sessions of MET-CBT (Steinberg et al., 2005). This slightly longer course of treatment was necessary to permit the incorporation of FSET materials into the MET-CBT manual and is consistent with the notion that comorbidity can result in a longer course of treatment. Given that patients with elevated anxiety have poorer MET-CBT outcomes after 9 sessions (Buckner & Carroll, 2010), this longer course of treatment may be necessary for patients to learn and use specific anxiety-management skills, which led to greater decreases in anxiety in the ICART condition. Importantly, the longer course of treatment did not result in less treatment adherence, as participants in the ICART condition attended more treatment sessions than those in MET-CBT.
Notably, low rates of abstinence were obtained in both conditions. In fact, no MET-CBT participant tested negative for THC at Week 12 and only 12% of ICART participants tested negative. This finding is consistent with results from other outpatient samples undergoing MET-CBT for CUD who were recruited regardless of level of anxiety. To illustrate, only approximately 10% of participants tested abstinent after completing 14 sessions of MET-CBT (Budney et al., 2000). In fact, abstinence, if achieved, is rarely maintained following CUD treatment, whereas reductions in use and use-related problems tend to be more appropriate indices of treatment success (c.f., Marijuana Treatment Project Research Group, 2004). This may be especially true for patients with CUD and comorbid anxiety given that anxiety is related to greater post-MET-CBT cannabis use and related problems (Buckner & Carroll, 2010). Thus, our finding that ICART was associated with more treatment sessions attended and greater likelihood of cannabis abstinence is indicative of greater clinically significant improvement in regard to CUD treatment outcome research (Carroll et al., 2006).
The current study must be considered in light of limitations that can inform future directions in research on treatment of CUD. First, the sample for this pilot study was relatively small as was the follow-up period and replication with larger samples over longer follow-up periods will be an important next step. Second, although the sample was relatively diverse in terms of sex and race (56.4% male, 63.3% non-Hispanic White), replication with larger samples will facilitate examination of whether outcomes vary as a function of demographic characteristics. Third, given that ICART involved more sessions than MET-CBT, additional work is necessary to control for therapy time to isolate the impact of ICART skills on outcomes and to test the hypothesis that access to additional treatment sessions may have impacted outcomes. Fourth, although conditions did not differ on likelihood of complete the Week 12 assessment, ICART participants were offered weekly treatment sessions between Weeks 9-12, whereas MET-CBT participants that completed treatment during the first nine weeks of the study did not. Future work is necessary to determine whether access to treatment during those weeks impacted Week 12 assessment results.
In sum, although additional research is warranted to test longer term outcomes associated with ICART for comorbid CUD and anxiety disorders, these pilot data are promising. The excellent response demonstrated by these patients suggests that integrating FSET (Riccardi et al., 2017; Schmidt et al., 2012) with MET-CBT for CUD (Steinberg et al., 2002) may be useful in helping anxious patients achieve their cannabis use goals while simultaneously learning to manage their chronically elevated anxiety. Given that nearly over 40% of individuals with CUD have a comorbid anxiety disorder (Teesson et al., 2012) combined with data that these patients have more severe pathology and poorer outcomes (Bonn-Miller & Moos, 2009; Buckner & Carroll, 2010), treatments that simultaneously treat anxiety and cannabis in an integrated manner has the potential to benefit a great many patients.
Highlights.
Integrated cannabis and anxiety reduction treatment (ICART) was pilot tested
ICART demonstrated evidence of acceptability
ICART resulted in greater cannabis abstinence than MET-CBT
Both ICART and MET-CBT reduced cannabis use and related problems
Acknowledgements
This work was supported by the National Institute of Drug Abuse (R34DA031937), awarded to Dr. Julia Buckner. The authors declare no conflict of interest. We thank Kathleen Carroll, PhD, for her consultation on study design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Percentages add up to greater than 100% as participants met for more than one.
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders, text revision (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Berry DA, Inoue L, Shen Y, Venier J, Cohen D, Bondy M, … Munsell MF (2006). Chapter 6: Modeling the Impact of Treatment and Screening on U.S. Breast Cancer Mortality: A Bayesian Approach. JNCI Monographs, 2006(36), 30–36. doi: 10.1093/jncimonographs/lgj006 [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, & Moos RH (2009). Marijuana discontinuation, anxiety symptoms, and relapse to marijuana. Addictive Behaviors, 34(9), 782–785. doi: 10.1016/j.addbeh.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Brown TA, & Barlow DH (2014). Anxiety Disorders Interview Schedule for DSM-5 (ADIS-5) - Adult Version: Oxford. [Google Scholar]
- Brown TA, DiNardo PA, Lehman CL, & Campbell LA (2001). Reliability of DSM-IV anxiety and mood disorders: Implications for the classification of emotional disorders. Journal of Abnormal Psychology, 110(1), 49–58. doi: 10.1037/0021-843X.110.1.49 [DOI] [PubMed] [Google Scholar]
- Buckner JD, Bonn-Miller MO, Zvolensky MJ, & Schmidt NB (2007). Marijuana use motives and social anxiety among marijuana-using young adults. Addictive Behaviors, 32(10), 2238–2252. doi: 10.1016/j.addbeh.2007.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, & Carroll KM (2010). Effect of anxiety on treatment presentation and outcome: Results from the Marijuana Treatment Project. Psychiatry Research, 178(3), 493–500. doi: 10.1016/j.psychres.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Ecker AH, Beighley JS, Zvolensky MJ, Schmidt NB, Shah SM, & Carroll KM (2016). Integrated cognitive behavioral therapy for comorbid cannabis use and anxiety disorders. Clinical Case Studies, 15(1), 68–83. doi: 10.1177/1534650115590857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Heimberg RG, Ecker AH, & Vinci C (2013). A biopsychosocial model of social anxiety and substance use. Depression and Anxiety, 30(3), 276–284. doi: 10.1002/da.22032 [DOI] [PubMed] [Google Scholar]
- Buckner JD, Heimberg RG, Matthews RA, & Silgado J (2012). Marijuana-related problems and social anxiety: The role of marijuana behaviors in social situations. Psychology of Addictive Behaviors, 26(1), 151–156. doi: 10.1037/a0025822 [DOI] [PubMed] [Google Scholar]
- Buckner JD, Heimberg RG, & Schmidt NB (2011). Social anxiety and marijuana-related problems: The role of social avoidance. Addictive Behaviors, 36(1-2), 129–132. doi: 10.1016/j.addbeh.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB, Bobadilla L, & Taylor J (2006). Social anxiety and problematic cannabis use: Evaluating the moderating role of stress reactivity and perceived coping. Behaviour Research and Therapy, 44(7), 1007–1015. doi: 10.1016/j.brat.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ, Businelle MS, & Gallagher MW (2017). Direct and indirect effects of false safety behaviors on cannabis use and related problems. The American Journal on Addictions. doi: 10.1111/ajad.12659 [DOI] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ, & Ecker AH (2013). Cannabis use during a voluntary quit attempt: An analysis from ecological momentary assessment. Drug and Alcohol Dependence, 132(3), 610–616. doi: 10.1016/j.drugalcdep.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ, Farris SG, & Hogan J (2014). Social anxiety and coping motives for cannabis use: The impact of experiential avoidance. Psychology of Addictive Behaviors, 28(2), 568–574. doi: 10.1037/a0034545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ, Schmidt NB, Carroll KM, Schatschneider C, & Crapanzano K (2014). Integrated cognitive behavioral therapy for cannabis use and anxiety disorders: rationale and development. Addictive Behaviors, 39(3), 495–496. doi: 10.1016/j.addbeh.2013.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, & Novy PL (2000). Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology, 68(6), 1051–1061. doi: 10.1037//0022-006X.68.6.1051 [DOI] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, … Rounsaville BJ (2006). The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. Journal of Consulting and Clinical Psychology, 74(5), 955–966. doi: 10.1037/0022-006X.74.5.955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2017). 2016 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; Retrieved from https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.pdf. [Google Scholar]
- Ecker AH, & Buckner JD (2014). Cannabis use behaviors and social anxiety: The roles of perceived descriptive and injunctive peer norms. Journal of Studies on Alcohol and Drugs, 75(74). [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Goodman SN (2005). Introduction to Bayesian methods I: measuring the strength of evidence. Clinical Trials, 2(4), 282–290. doi: 10.1191/1740774505cn098oa [DOI] [PubMed] [Google Scholar]
- Hamilton M (1959). The assessment of anxiety states by rating. The British Journal of Medical Psychology, 32(1), 50–55. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, … Grant BF (2015). Prevalence of Marijuana Use Disorders in the United States Between 2001-2002 and 2012-2013. JAMA Psychiatry, 72(12), 1235–1242. doi: 10.1001/jamapsychiatry.2015.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irony TZ, & Simon R (2006). Applications of Bayesian Methods to Medical Device Trials In Becker KM & Whyte JJ (Eds.), Clinical Evaluation of Medical Devices: Principles and Case Studies (pp. 99–116). Totowa, NJ: Humana Press. [Google Scholar]
- Johnson VE (2013). Revised standards for statistical evidence. Proceedings of the National Academy of Sciences of the United States of America, 110(48), 19313–19317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilford RJ, Thornton JG, & Braunholtz D (1995). Clinical trials and rare diseases: a way out of a conundrum. BMJ : British Medical Journal, 311(7020), 1621–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano BE, Stephens RS, & Roffman RA (2006). Abstinence and moderate use goals in the treatment of marijuana dependence. Addiction, 101(11), 1589–1597. doi: 10.1111/j.1360-0443.2006.01609.x [DOI] [PubMed] [Google Scholar]
- Marijuana Treatment Project Research Group. (2004). Brief treatments for cannabis dependence: Findings from a randomized multisite trial. Journal of Consulting and Clinical Psychology, 72(3), 455–466. doi: 10.1037/0022-006X.72.3.455 [DOI] [PubMed] [Google Scholar]
- Marmorstein NR, White HR, Loeber R, & Stouthamer-Loeber M (2010). Anxiety as a predictor of age at first use of substances and progression to substance use problems among boys. Journal of Abnormal Child Psychology, 38(2), 211–224. doi: 10.1007/s10802-009-9360-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, & Budney AJ (2003). Relapse in outpatient treatment for marijuana dependence. Journal of Substance Abuse Treatment, 25(2), 85–89. doi: 10.1016/S0740-5472(03)00083-7 [DOI] [PubMed] [Google Scholar]
- Nathan PE, & Lansky D (1978). Common methodological problems in research on the addictions. Journal of Consulting and Clinical Psychology, 46(4), 713–726. doi: 10.1037/0022-006X.46.4.713 [DOI] [PubMed] [Google Scholar]
- National Insitute of Drug Abuse. (2013). Strategic Plan. Retrieved from http://www.drugabuse.gov/about-nida/organization/divisions/division-basic-neuroscience-behavioral-research-dbnbr/strategic-plan [Google Scholar]
- O'Farrell TJ, Fals-Stewart W, & Murphy M (2003). Concurrent validity of a brief self-report drug use frequency measure. Addictive Behaviors, 28(2), 327–337. doi: 10.1016/S0306-4603(01)00226-X [DOI] [PubMed] [Google Scholar]
- R Core Team. (2013). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Riccardi CJ, Korte KJ, & Schmidt NB (2017). False Safety Behavior Elimination Therapy: A randomized study of a brief individual transdiagnostic treatment for anxiety disorders. Journal of Anxiety Disorders, 46, 35–45. doi: 10.1016/j.janxdis.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, & Leo GI (2014). Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychology of Addictive Behaviors, 28(1), 154–162. doi: 10.1037/a0030992 [DOI] [PubMed] [Google Scholar]
- Salkovskis PM, Clark DM, & Hackmann A (1991). Treatment of panic attacks using cognitive therapy without exposure or breathing retraining. Behaviour Research and Therapy, 29(2), 161–166. doi: 10.1016/0005-7967(91)90044-4 [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Buckner JD, Pusser AT, Woolaway-Bickel K, & Preston JL (2012). Randomized controlled trial of False Safety Behavior Elimination Therapy (F-SET): A unified cognitive behavioral treatment for anxiety psychopathology. Behavior Therapy, 43(3), 518–532. doi: 10.1016/j.beth.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, … Frank DM (2001). Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A). Depression and Anxiety, 13(4), 166–178. [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back: A technique for assessing self-reported alcohol consumption In Litten RZ & Allen JP (Eds.), Measuring alcohol consumption: Psychosocial and biochemical methods. (pp. 41–72). Totowa, NJ US: Humana Press. [Google Scholar]
- Spiegelhalter DJ, Myles JP, Jones DR, & Abrams KR (1999). An introduction to bayesian methods in health technology assessment. BMJ, 319(7208), 508–512. doi: 10.1136/bmj.319.7208.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg KL, Roffman RA, Carroll KM, Kabela E, Kadden R, Miller M, & Duresky D (2002). Tailoring cannabis dependence treatment for a diverse population. Addiction, 97, 135–142. doi: 10.1046/j.1360-0443.97.s01.5.x [DOI] [PubMed] [Google Scholar]
- Steinberg KL, Roffman RA, Carroll KM, McRee B, Babor TF, Miller M, … Stephens R (2005). Brief Counseling for Marijuana Dependence: A Manual for Treating Adults. DHHS Publication No. (SMA) 05-4022. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Stephens RS, Roffman RA, & Curtin L (2000). Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology, 68(5), 898–908. doi: 10.1037/0022-006X.68.5.898 [DOI] [PubMed] [Google Scholar]
- Stewart SH, & Conrod PJ (Eds.). (2008). Anxiety and substance use disorders: The vicious cycle of comorbidity. New York: Springer. [Google Scholar]
- Teesson M, Slade T, Swift W, Mills K, Memedovic S, Mewton L, … Hall W (2012). Prevalence, correlates and comorbidity of DSM-IV Cannabis use and Cannabis use disorders in Australia. Australian and New Zealand Journal of Psychiatry, 46(12), 1182–1192. doi: 10.1177/0004867412460591 [DOI] [PubMed] [Google Scholar]
- Temple R (2005). How FDA currently makes decisions on clinical studies. Clinical Trials, 2(4), 276–281. doi: 10.1191/1740774505cn097oa [DOI] [PubMed] [Google Scholar]
- Vehtari A, Gelman A, & Gabry J (2017). Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Statistics and Computing, 27(5), 1413–1432. [Google Scholar]
- Wijeysundera DN, Austin PC, Hux JE, Beattie WS, & Laupacis A (2009). Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. Journal of Clinical Epidemiology, 62(1), 13–21.e15. doi: 10.1016/j.jclinepi.2008.07.006 [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bernstein A, Sachs-Ericsson N, Schmidt NB, Buckner JD, & Bonn-Miller MO (2006). Lifetime associations between cannabis, use, abuse, and dependence and panic attacks in a representative sample. Journal of Psychiatric Research, 40(6), 477–486. doi: 10.1016/j.jpsychires.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Marshall EC, Johnson K, Hogan J, Bernstein A, & Bonn-Miller MO (2009). Relations between anxiety sensitivity, distress tolerance, and fear reactivity to bodily sensations to coping and conformity marijuana use motives among young adult marijuana users. Experimental and Clinical Psychopharmacology, 17(1), 31–42. doi: 10.1037/a0014961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Rogers AH, Manning K, Hogan JBD, Paulus DJ, Buckner JD, … Schmidt NB (2018). Anxiety sensitivity and cannabis use problems, perceived barriers for quitting, and fear of quitting. Psychiatry Research, 263, 115–120. doi: 10.1016/j.psychres.2018.03.006 [DOI] [PubMed] [Google Scholar]