Summary
The purpose of the present article was to evaluate the previously unreported vascular alterations in Leber's Hereditary Optic Neuropathy (LHON) 3460 mitochondrial DNA (mtDNA) mutation. Among the three primary mtDNA mutations, namely 11778, 14484, and 3460, LHON 3460 is the most rare and historically recognized as having the poorest visual prognosis. Optical coherence tomography angiography (OCTA) is a novel imaging modaility providing high-resolution microcirculation maps and enhancing visualization of the optic disc and peripapillary capillary beds. We herein exploit the advantages of OCTA, for the first time, to assess the optic nerve head and peripapillary microvasculature changes in an affected patient and compare these vascular changes with an asymptomatic carrier for LHON 3460, serving as a control. Vascular changes in LHON 11778 and 14484 have classically shown microvasculature attenuation localized specifically to the temporal peripapillary quadrant. In the present case, however, OCTA in LHON 3460, the most severe of the three mutational subtypes, illustrated significant vascular attenuation involving the nasal peripapillary region in addition to the temporal peripapillary microvascular changes classically seen in LHON. Our findings suggest that vascular measures may serve useful for objectively assessing mitochondrial disease. Further OCTA studies involving the nasal peripapillary region may be warranted to further understand vascular pathogenesis in LHON.
Keywords: Leber's hereditary optic neuropathy, optical coherence tomography angiography, peripapillary microvasculature, vascular biomarkers
1. Introduction
Vascular pathology in Leber's hereditary optic neuropathy (LHON) has been previously shown (1-3). These studies have looked at LHON across all mutational subtypes as a whole without assessing the severity of vascular attenuation attributed to the individual subtypes of LHON mitochondrial DNA (mtDNA) mutations. Among the three primary mtDNA mutations, namely 11778, 14484, and 3460, LHON 3460 is the most rare and historically recognized as having the poorest visual prognosis (4). Individual reports of vascular changes in LHON 11778 and 14484 have been shown (5-7), however, an isolated vascular evaluation of the LHON 3460 primary mutation variant is lacking. Fundus photographs or fluorescein angiography have historically been used to describe oculovascular alterations in LHON (8-12). Optical coherence tomography angiography (OCTA), a novel imaging modality, can be used to noninvasively evaluate the peripapillary retinal and vascular circulations without the need for dye injection (13). Our laboratory has recently shown that assessing vascular changes in mitochondrial optic neuropathies using OCTA may offer several advantages including supplementing our understanding of LHON pathophysiology (2,3). In contrast to 11778 and 14484, there are currently no clinical trials for the 3460 mtDNA mutation, the most severe of the three primary LHON mutations (4). Therefore, further evaluation of vascular parameters in mitochondrial optic neuropathies may be warranted to serve as an objective measure of disease progression, and monitoring the efficacy of purported therapies. Given the severe nature of the 3460 mtDNA mutation, we hypothesized that vascular attenuation would similarly be more severe in LHON 3460 expanding beyond the classically observed temporal peripapillary region. We hereby report the optic nerve head (ONH) and peripapillary microvasculature changes associated with LHON 3460 using an advanced imaging modality known as OCTA.
2. Case Report
A 22-year-old male with LHON, genetically confirmed for the 3460 mtDNA mutation, presented to the Doheny Eye Center in September 2018 with a 2-month history of vision loss beginning with the left eye, followed by vision loss in the right eye one month later. Family history was significant for bilateral vision loss in the mother's cousin at age 30. The patient endorsed alcohol consumption amounting to two to three bottles of beer per day. The patient also had a one-year history of exposure to kitchen smoke, having an occupation as a restaurant server. The patient began Idebenone 250 mg PO TID and was scheduled for a 6-month follow up.
Best-corrected visual acuities (BCVA) were 20/200 in the right eye (OD) and 20/400 in the left eye (OS). Fundus examination revealed temporal pallor, peripapillary telangiectasias, and pseudo-edema in both eyes (OU) (Figures 1A and 1E). Pupils were equal and reactive to light with no afferent pupillary defects. The patient exhibited dyschromatopsia on Ishihara plate testing, scoring 5/14 OD and 2/14 OS. Humphrey visual field (HVF) testing showed mild, bilateral cecocentral scotomas (Figures 1B and 1F). Structural OCT displayed bilateral retinal nerve fiber layer (RNFL) pseudoedema (Figures 1C and 1G) and retinal ganglion cell complex (RGCC) thinning (Figures 1D and 1H). OCTA images of the ONH and peripapillary superficial vasculature were obtained using Spectral Domain-OCT (Cirrus HD-OCT, software V.6.0; Carl Zeiss Meditec, Inc., Dublin, CA, USA; the scan size of the optic nerve head was 6 × 6 mm). The same set of images were acquired for the patient's 38-year-old mother, sharing the mtDNA 3460 mutation, though asymptomatic with no significant past medical history, thus serving as a control and compared (Figure 2). Close inspection of the superficial vascular networks demonstrated visible attenuation of the temporal microvascular networks of the ONH and peripapillary area (yellow circles) along with peripapillary telangiectatic blood vessels and vascular tortuosity (yellow arrows) greater for the patient's right (Figure 2B) and left eyes (Figure 2C) relative to the asymptomatic mother (Figure 2A). Intriguingly, however, vascular attenuation was also seen in the nasal peripapillary region as well (orange circles). OCT cross-sections (Figures 2D and 2F) overlaying retinal flow showed significant perfusion defects in the temporal and nasal peripapillary nerve fiber layer in the affected patient (Figures 2B and 2C: red arrows) relative to the asymptomatic mother, where perfusion was preserved in these correspoinding areas (Figure 2D: green arrows).
Figure 1.
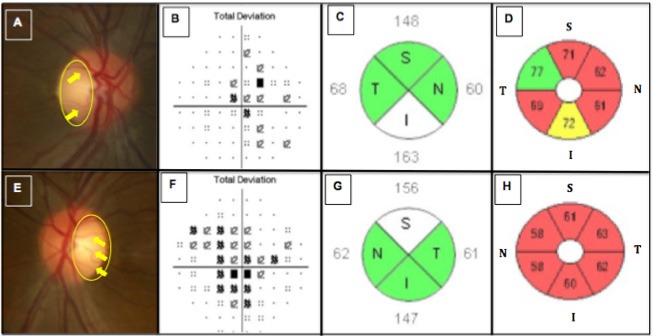
Shows images of the right and left eyes in the LHON patient. Disc photographs (A, E) show temporal pallor (yellow circle) and peripapillary telangiectatic blood vessels along with vascular tortuosity (yellow arrows) for the right (A) and left eyes (E), respectively. Humphrey visual field testing revealed cecocentral scotomomas more severe in the left (F) than in the right eye (B), having mean deviations (MD) of -3.82 and -2.75, respectively. Spectral-Domain optical coherence tomography (SD-OCT) imaging of the peripapillary nerve fiber layer (pRNFL) (C,G) showed relative axonal swelling, most pronounced in the inferior and superior quadrants of the right (C) and left (G) eyes, respectively. SD-OCT imaging of the macular retinal ganglion cell and inner plexiform layers (RGC-IPL) (D,H) revealed diffuse ganglion cell atrophy, more severe in the primarily involved left eye (H) than the secondarily involved right eye (D).
Figure 2.
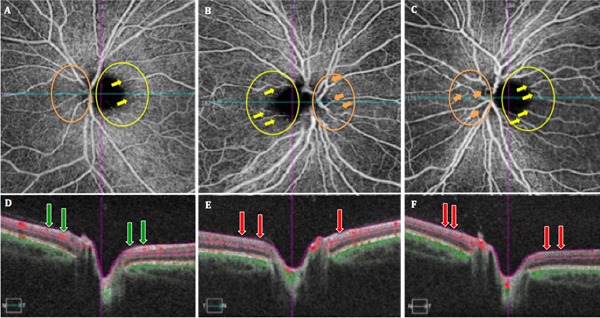
Shows optical coherence tomography angiography (OCTA) images (A-C) of the superficial vascular networks for the peripapillary nerve fiber layer (pRNFL) in the asymptomatic LHON mtDNA3460 carrier (patient's mother) (A), and affected LHON mtDNA 3460 patient right (B) and left (C) eyes. OCT cross-sections (D-F) show overlaying retinal flow (red) on OCT reflectance (gray scale).
3. Discussion
OCTA provides high-resolution, noninvasive visualization of the ONH and peripapillary microvasculature (13,14). We hereby characterize the vascular alterations associated with LHON 3460, the most severe of the three primary mtDNA mutations, as seen in an affected patient and compared with an asymptomatic carrier, serving as a control. Peripapillary vascular alterations, classically observed on funduscopy, have been referred to as hallmarks of LHON (9-11,15). OCTA of our affected LHON 3460 patient similarly exhibitted these hallmark features of circumpapillary microangiopathy, dilated tortuous vasculature, and significant capillary dropout in the temporal region, corresponding with the small axons of the papillomacular bundle (PMB) (15,16). Similar vascular changes have been reported in LHON 11778 and 14484 (15-17). In contrast to 11778 and 14484 mutations, OCTA in our affected 3460 mtDNA patient illustrated significant vascular attenuation involving the nasal peripapillary region when compared with the asymptomatic carrier, the temporal and nasal peripapillary microvasculature remained intact. In addition, nasal perfusion defects were more severe for the subacutely affected left eye (Figures 2C and 2F) compared to the acutely affected right eye (Figures 2B and 2E). Our laboratory recently investigated the vascular parameters in late disease stages in patients with chronic LHON (2). In the current case, however, we report the presence of nasal peripapillary vascular pathology even in the early stages of disease. These nasal peripapillary defects have not been observed in previously reported cases of in LHON 11778 and 14484 (5,7), which are classically referred to as less severe subtypes of LHON and exhibit vascular attenuation solely in the temporal peripapillary region. Further, OCTA of the asymptomatic LHON 3460 carrier revealed circumpapillary microangiopathy. This is consistent with previous studies and further confirms the subclinical vascular changes historically observed funduscopically in asymptomatic carrers (11,17).
Our findings demonstrate that the poor disease course characteristic of LHON 3460 is similarly reflected in the severity of vascular pathology. In our case of LHON 3460, vascular attenuation extended beyond the temporal peripapillary region and also involved the nasal peripapillary area. This supports the preferential involvement of the small axons comprising the PMB. However, the present study also illustrates that in cases of severe mitchondrial dysfunction such as in LHON 3460, surrounding peripapillary areas may also be secondarily involved. In conclusion, vascular changes may serve as useful objective measures of disease. To date, clinical trials are solely in place for LHON 11778, the most common and least severe mutational subytype (4,15). Additional OCTA studies assessing the severity of vascular changes attributed to LHON mutational subtypes individually may be warranted to further understand LHON pathophysiology and navigate future directions for potential treatments of severe disease.
References
- 1. Balducci N, Cascavilla ML, Ciardella A, La Morgia C, Triolo G, Parisi V, Bandello F, Sadun AA, Carelli V, Barboni P. Peripapillary vessel density changes in Leber's hereditary optic neuropathy: A new biomarker. Clin Exp Ophthalmol. 2018; 46:1055-1062. [DOI] [PubMed] [Google Scholar]
- 2. Borrelli E, Balasubramanian S, Triolo G, Barboni P, Sadda SR, Sadun AA. Topographic macular microvascular changes and correlation with visual loss in chronic Leber hereditary optic neuropathy. Am J Ophthalmol. 2018; 192:217-228. [DOI] [PubMed] [Google Scholar]
- 3. Ghasemi Falavarjani K, Tian JJ, Akil H, Garcia GA, Sadda SR, Sadun AA. Swept-source optical coherence tomography angiography of the optic disk in optic neuropathy. Retina. 2016; 36 Suppl 1:S168-S177. [DOI] [PubMed] [Google Scholar]
- 4. Yu-Wai-Man P, Chinnery PF. Leber Hereditaroy Optic Neuropathy. GeneRev. 2016. https://www.ncbi.nlm.nih.gov/books/NBK1174/ (accessed November 10 2018).
- 5. De Rojas JO, Rasool N, Chen RWS, Horowitz J, Odel JG. Optical coherence tomography angiography in Leber hereditary optic neuropathy. Neurology. 2016; 2016; 87; 2065-2066. [DOI] [PubMed] [Google Scholar]
- 6. Gaier ED, Gittinger JW, Cestari DM, Miller JB. Peripapillary capillary dilation in Leber hereditary optic neuropathy revealed by optical coherence tomographic angiography. JAMA Ophthalmol. 2016; 134:1332-1334. [DOI] [PubMed] [Google Scholar]
- 7. Matsuzaki M, Hirami Y, Uyama H, Kurimoto Y. Optical coherence tomography angiography changes in radial peripapillary capillaries in Leber hereditary optic neuropathy. Am J Ophthalmol Case Rep. 2018; 9:51-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nikoskelainen EK, Huoponen K, Juvonen K, Lamminen T, Nummelin K, Savontaus ML. Ophthalmologic findings in Leber hereditary optic neuropathy, with special reference to mtDNA mutations. Ophthalmology. 1996; 103:504-514. [DOI] [PubMed] [Google Scholar]
- 9. N ikos k e l a inen E, Hoyt WF, Numme l in K. Ophthalmoscopic findings in Leber's hereditary optic neuropathy. I. Fundus findings in asymptomatic family members. Arch Ophthalmol. 1982; 100:1597-1602. [DOI] [PubMed] [Google Scholar]
- 10. N ikos k e l a inen E, Hoyt WF, Numme l in K. Ophthalmoscopic findings in Leber's hereditary optic neuropathy: II. The fundus findings in the affected family members. Arch Ophthalmol. 1983; 101:1059-1068. [DOI] [PubMed] [Google Scholar]
- 11. Nikoskelainen E, Nummelin K, Hoyt WF, Schatz H. Fundus findings in Leber's hereditary optic neuroretinopathy III. Fluorescein angiographic studies. Arch Ophthalmol. 1984; 102:981-989. [DOI] [PubMed] [Google Scholar]
- 12. Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012; 20:4710-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang X, Jia Y, Spain R, Potsaid B, Liu JJ, Baumann B, Hornegger J, Fujimoto JG, Wu Q, Huang D. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol. 2014; 98:1368-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akil H, Falavarjani KG, Sadda SR, Sadun AA. Optical coherence tomography angiography of the optic disc; an overview. J Ophthalmic Vis Res. 2017; 12:98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadun AA, La Morgia C, Carelli V. Mitochondrial optic neuropathies: Our travels from bench to bedside and back again. Clin Exp Ophthalmol. 2013; 41:702-712. [DOI] [PubMed] [Google Scholar]
- 16. Pan BX, Ross-Cisneros FN, Carelli V, Rue KS, Salomao SR, Moraes-Filho MN, Moraes MN, Berezovsky A, Belfort R Jr, Sadun AA. Mathematically modeling the involvement of axons in Leber's hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2012; 53:7608-7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sadun AA, Salomao SR, Berezovsky A, Sadun F, DeNegri AM, Quiros PA, Chicani F, Ventura D, Barboni P, Sherman J, Sutter E, Belfort R Jr, Carelli V. Subclinical carriers and conversion in leber's hereditary optic neuropathy: A prospective psychosocial study. Trans Am Ophthalmol Soc. 2006; 104:51-61. [PMC free article] [PubMed] [Google Scholar]


