Summary
Nipah virus, an enveloped ribonucleic acid virus, has been a major cause of encephalitis out-breaks with high mortality, primarily in the Indo-Bangladesh regions. Except for the first outbreak in Malaysia-Singapore, which was related to contact with pigs and the outbreak in Philippines associated with horse slaughter, most other outbreaks have affected the Indo- Bangladesh regions. The Indo-Bangladesh outbreaks were associated with consumption of raw date palm sap contaminated by fruit bats and had a very high secondary attack rate. The patient usually presents with fever, encephalitis and/or respiratory involvement with or without thrombocytopenia, leukopenia and transaminitis. Diagnosis can be confirmed by isolation and nucleic acid amplification in the acute phase or antibody detection during the convalescent phase. Treatment is mostly limited to supportive care and syndromic management of acute encephalitis syndrome. Ribavirin, m102.4 monoclonal antibody and favipiravir are the only anti-virals with some activity against Nipah virus. Standard precautions, hand hygiene and personal protective equipments are the cornerstone of comprehensive infection prevention and control strategy. With the recent outbreaks affecting newer geographical areas, there is a need for physicians to be aware of this disease and keep abreast of its current detection and management strategies.
Keywords: Henipavirus, encephalitis, India, Bangladesh
1. Introduction
The name 'Nipah' comes from a Malaysian village, where the first outbreak was reported in 1998-1999 (1,2). The outbreak of Nipah virus (NiV) disease in Malaysia involved more than 250 cases of febrile encephalitis in farm and abattoir workers. This outbreak caused widespread panic and considerable socio-economic disruption. Although, no further outbreaks were reported from Malaysia the virus has been responsible for outbreaks in other parts of the world, mainly in Bangladesh and India (2). Recent outbreak of Nipah virus in Kerala in May 2018 brought this emerging-re-emerging virus into the spotlight again.
The high mortality rate, broad species tropism, multiple plausible modes of transmission, risk of person-person transmission and documented cases of health care workers being affected during outbreaks has left the medical community perplexed. While a lot remains to be deciphered about the virus and many efforts to unravel its mysteries are ongoing, this article has tried to review and synthesize the available information about this virus and its clinical aspects. The clinical information presented in this article can be used as a guiding tool for physicians in an outbreak setting.
2. The virus
Nipah virus is an enveloped paramyxovirus with negative-stranded polarity and a non-segmented RNA genome consisting of helical nucleocapsids. NiV has subtle differences in its makeup when compared to a typical paramyxovirus. It has reticular cytoplasmic inclusions close to the endoplasmic reticulum unlike other paramyxoviruses. Also, NiV is on average larger than typical paramyxoviruses. There are only minor ultrastructural differences between Hendra virus (HeV) and NiV and they have significant cross reactivity on serological tests, and are therefore grouped as Henipavirus (3). Within NiV, two different strains have been identified, the Malaysian (MY) and the Bangladesh (BD) strains. The two strains are approximately 92% identical on sequencing but appear to be significantly different in their pathogenicity and transmissibility (4-7).
3. Epidemiology
The first outbreak of Nipah virus in Malaysia- Singapore (1998-1999) was initially thought to be Japanese encephalitis (JE), however on further investigation, it was later identified as Nipah virus (Figure 1) (3,8,9). The second outbreak of this disease was in a geographically non-contiguous location, in the Meherpur district of Bangladesh and Siliguri city of West Bengal, India in 2001 (Figures 1 and 2). The Indo-Bangladesh outbreaks were significantly different from the previous outbreak in Malaysia in terms of modes of transmission, clinical features and case fatality rates. Human to human transmission and nosocomial infections (via droplets and/or fomites) were a prominent feature in this outbreak. Also, the secondary attack rates were higher and the disease was more severe and rapidly progressive compared to the Malaysian outbreak. Apart from the neurological manifestations, acute respiratory distress syndrome (ARDS) and respiratory failure with multi organ dysfunction syndrome (MODS) were probably the major reason for higher mortality. Since then, there has been an outbreak in Bangladesh almost every year and a total of 17 outbreaks have been reported until 2015. There was another small outbreak in 2007 in the Nadia district of West Bengal, India (Figures 1 and 2). In the outbreak in the Philippines in March-May, 2014, both horses and humans were affected with fruit bats being the possible source of infection. Interestingly, this outbreak was significantly associated with horse slaughter and horse meat consumption (Figures 1 and 2) (10). The Indian outbreak of 2018 affected primarily the Kozhikode district and nearby area of Kerala (Figures 1 and 2) (11).
Figure 1.
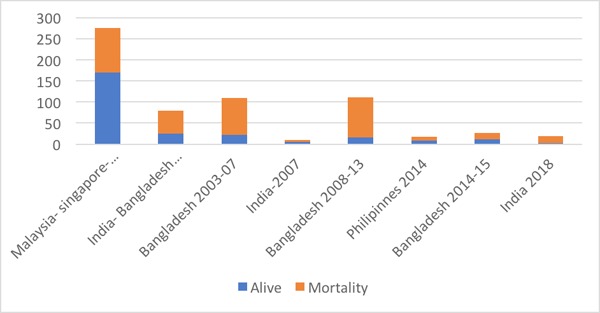
Timeline of Nipah Virus outbreaks across the world with total number of cases reported in each outbreak.
Figure 2.
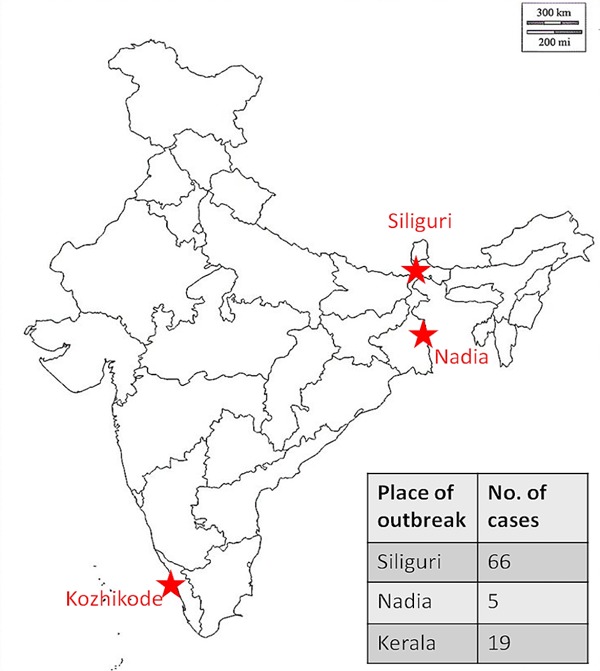
Nipah virus outbreaks in India.
The Malaysian outbreak was primarily due to close contact with affected pigs. No human to human transmission was reported (8,12,13). The Indo- Bangladesh outbreaks, including the recent Kerala outbreak, was remarkable in various aspects, mode of transmission being one of them. A significant association of NiV disease and consumption of raw date palm sap contaminated by fruit bats was found by Luby et al. (14) Another study by Rahman et al. also concluded with similar results (15). In date palm sap the virus remains stable for at least 7 days at 22°C and is extremely tolerant to a wide range of pH (from 3 to 11) (16). Human to human and nosocomial transmission was also documented in the Indo-Bangladesh outbreaks. In the Siliguri outbreak of 2001, a single patient admitted to a private hospital infected 23 hospital staff and 8 visitors (17). Poor adherence to standard precautions was probably the major reason for this. Also, the difference in strains (BD vs. MY) contributed to the difference in the transmission rates. A study by Clayton et al. showed that ferrets infected with the BD strain had higher RNA levels in the blood as well as increased shedding of the virus in oral secretions, possibly explaining the higher secondary attack rates as well as more severe infection in the Indo-Bangladesh outbreak. It is noteworthy that viral shedding was seen even in the incubation period (18). The major research findings in different studies are tabulated in Table 1.
Table 1. Summary of major research findings.
| Author and year of publication (ref.) | Major finding related to epidemiology and transmission |
Major finding related to clinical features and Diagnosis |
Major finding related to therapeutic options |
|---|---|---|---|
| CDC, 1999 (8,9) | First outbreak report of Nipah. Transmission to pig abattoir workers. | Serology is cross reactive to Hendra. | -- |
| Reynes et al, 2005 (4); Wacharapluesadee et al. 2005 (5) | Presence of NiV in bats. | -- | -- |
| Mounts et al, 2001 (13) | No human to human and nosocomial transmission for the Malaysian strain of NiV causing Malaysia-Singapore outbreaks. | -- | -- |
| Goh et al, 2000 (19) | -- | Predominantly neurological symptoms and no significant r espi r a tory symp toms in Malaysian strain of NiV. | -- |
| Chadha et al, 2006 (17); Arunkumar et al, 2018 (53) | Strong evidence of human to human transmission for the Bangladesh strain of NiV causing Bangladeshi and Indian outbreaks. | S i g n i f i c a n t r e s p i r a t o r y involvement leading to ARDS in Bangladesh strain of NiV. | -- |
| Luby et al, 2006 (14); Rahaman et al, 2012 (15) | Date palm s ap consumpt ion contaminated by bat excreta and saliva is a significant risk factor for transmission. | -- | -- |
| Chong et al, 2001 (33) | -- | Serology is cross reactive to Hendra. | Ribavirin can substantially reduce mor tal i ty (36%) without any significant adverse reactions. |
| Wright et al, 2005 (29); Aljofan et al, 2009 (30) | -- | -- | In vitro activity of ribavirin on halting the replication of NiV in cell cultures. |
| Bossart et al, 2009 (38); Bossart et al, 2011 (39) | -- | -- | Role of monoclonal antibody m102.4 in preventing transmission and halting disease progression among animal model of ferrets and African green monkey. |
| Dawes et al, 2018 (40) | -- | Respiratory involvement and higher mortality in Bangladesh strain, compared to Malaysia strain. | Promising role of favipiravir in protecting NiV infected animals (hamster model). |
4. Clinical features
Clinical Features of Nipah typically includes fever with encephalitis and or respiratory involvement (Figure 3) (17,19-23). Asymptomatic infection was reported in 8% of patients with laboratory-confirmed cases in Malaysia. No such data of asymptomatic NiV infection is available from Bangladesh and Indian outbreaks. However, cases with mild and nonspecific features were identified. Fever, headache, dizziness, myalgia, vomiting and loose stools have been documented as non-specific prodromal symptoms in various outbreaks of Nipah. The Malaysian outbreak documented that 55 percent of patients had a reduced level of consciousness and prominent brain-stem dysfunction (10). Distinctive clinical signs included segmental myoclonus (32%), areflexia, hypotonia, hypertension, and tachycardia and thus suggests the involvement of the brain stem and upper cervical spinal cord. Brain stem dysfunction and neurological clinical signs include abnormal doll's eye reflex, reflexes, vasomotor changes, and myoclonic jerks. Cerebellar dysfunction was seen in eight patients in the Malaysian outbreak. In Siliguri outbreak (2001) in India, fever followed by altered sensorium (97%) developing over the next 3 to 4 days was the presenting complaint. 34 percent of the cases had convulsions (20). Similarly, in Bangladesh outbreaks, fever with altered sensorium was the most common presentation. A case series of four outbreaks from Bangladesh shows altered mental status (90%), headache (73%), severe weakness (67%) and seizures (23%) as common neurological manifestation (17). Nipah encephalitis may present with relapse or residual deficits in survivors. Relapse of encephalitis has been demonstrated with a time range of months to years after recovery from acute infection. Psychiatric and neurological complications (depression, personality changes, deficits in attention, verbal, and/ or visual memory) after recovery are also well known. Goh et al. reports from their Malaysian outbreak experience that 15 percent of their patients (14 patients) had residual deficits out of which five remained in a vegetative state (19). Respiratory involvement is well documented in severe cases. ARDS (50% to 66% cases) was documented in Malaysian outbreak. Siliguri outbreak witnessed 54 percent cases had associated respiratory symptoms particularly in the later stage of illness.
Figure 3.
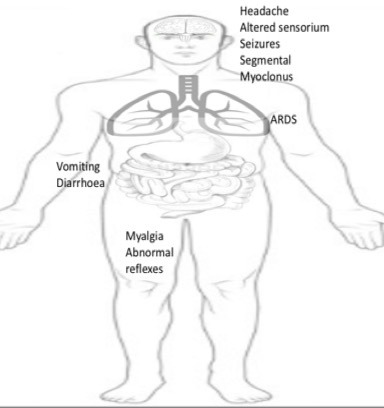
Clinical features of Nipah Virus disease.
5. Diagnosis
Common hematologic abnormalities in NiV infection include thrombocytopenia (30%) and leukopenia (11%) (Table 2). Elevated liver enzymes have been seen in 40% of patients, and hyponatraemia is sometimes found. Hemoglobin, renal indices and electrolytes other than sodium are usually normal. Lymphocytic pleocytosis with raised proteins similar to any other viral meningitis may be seen in cerebrospinal fluid.
Table 2. Laboratory and Radiological diagnosis of Nipah Virus disease.
|
|
|
|
|
|
|
|
NiV is a biosafety level (BSL) 4 agent, however, BSL 2 laboratory facilities are sufficient for routine diagnosis if the virus is inactivated during specimen collection and isolation is not attempted. Laboratory diagnosis of a patient with a clinical history of NiV can be made during the acute and convalescent phases of the disease by using a combination of tests. Samples should be transported at 4°C and processed as early as possible. During the early stage of illness - virus isolation and reverse transcriptase polymerase chain reaction assay (RT PCR) from throat and nasal swabs, cerebrospinal fluid (CSF), urine, and blood is recommended (24). During the convalescent phase, antibody detection by enzyme linked immunosorbent assay (ELISA-IgG and IgM) from serum or CSF may be used.
Advanced diffusion weighted (DW) magnetic resonance imaging (MRI) of the brain can give useful radiological evidence of Nipah encephalitis. Lim et al. suggested that MRI pattern may be useful in differentiating Nipah from its closely differential related Japanese encephalitis/other encephalitis in most cases (25). It can also help to diagnose exposed individuals, particularly at the height of an epidemic even before serologic confirmation is available (19). This finding derived from Malaysian experience during Nipah outbreak needs validation from other occurrences. More experience is required for its validation but this can be a potentially crucial finding to diagnose Nipah encephalitis and decide treatment and post exposure prophylaxis. MRI in acute Nipah encephalitis shows multifocal discrete lesions probably due to areas of micro-infarction. These discrete high-signal-intensity lesions usually measure about 2-7 mm and are disseminated throughout the brain, mainly in the subcortical and deep white matter of the cerebral hemispheres. Mass effect or edema is not usually seen. In relapse or late onset Nipah encephalitis, MRI characteristically shows multiple areas of patchy and confluent cortical involvement (26,27).
6. Differential diagnosis
Nipah is an important differential diagnosis in patients with fever and encephalitis and/or ARDS in context of relevant epidemiology with an ongoing outbreak in the area or relevant travel history to affected areas. But any fever or encephalitis may mimic disease and differentials need to be seen in correct perspective. The following differentials should be considered in patients with suspected Nipah infection: a) Japanese encephalitis (JE), b) Measles, c) Rabies, d) Dengue encephalitis, e) Cerebral malaria, f) Scrub typhus, g) Leptospirosis, h) Herpes encephalitis and i) Bacterial meningitis (Table 3).
Table 3. Differential diagnosis of Nipah Virus disease.
| Differential diagnoses | Differentiating features |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7. Treatment and post exposure prophylaxis
Treatment of NiV disease is mostly limited to supportive care and syndromic management of acute encephalitis syndrome (28). Under the current circumstances, specific pharmacological options should not be treated as alternatives to infection control measures. More evidence needs to be generated for considering post-exposure prophylaxis in individuals who were in close contact with confirmed Nipah cases. However, three pharmacological options have been explored for the possible treatment and post-exposure prophylaxis of NiV infection: Ribavirin, m102.4 monoclonal antibody and Favipiravir.
7.1. Ribavirin
In vitro studies and animal studies have shown conflicting results in the efficacy of ribavirin against NiV and Hendra, with some studies showing effective inhibition of viral replication in cell lines (29,30), whereas some studies in animal models showed that ribavirin treatment only delayed but did not prevent death after Nipah or Hendra virus infection (31,32). Only one in-vivo human study by Chong et al. has evaluated the role of ribavirin during the Malaysia outbreak of NiV, between 1998 and 1999 (33). In this study, patients who were managed prior to the availability of ribavirin during the outbreak or who refused ribavirin were taken as controls, and all patients who were still in the acute phase of the illness were offered ribavirin, either oral or intravenous, based on availability. A total of 140 patients treated with ribavirin were retrospectively compared with 52 control NiV patients who did not receive ribavirin due to unavailability and 2 patients who didn't consent There was no significant difference in the incidence of adverse reactions in the treatment group as compared to controls. The study showed 45 deaths in the treated group (32%) and 29 in the controls (54%), with a total of 36% reduction in mortality. However, as treatment allocation was not randomized, it is possible that treated patients had better clinical outcomes because they received better general medical care than the untreated patients.
The dosage of ribavirin in Nipah virus has not been defined but treatment can be initiated in the lines of that suggested by WHO for Lassa fever with a loading dose of 30 mg/kg for children and 2,000 mg/kg for adults, followed by 10 days of therapy (4 g in divided doses for first four days and 2 g in divided doses for next six days). Oral bioavailability of ribavirin is reported to be between 32.6% and 52%, with evidence of first-pass metabolism. Ribavirin is not bound to plasma proteins (34). Ribavirin was found to cross the blood-brain barrier following oral administration with a mean CSF/plasma ratio of 0.7 (35). The serious adverse drug reactions with ribavirin are: Neutropenia (8% to 40%), anemia (11% to 35%; children & adolescents: 11%), lymphocytopenia (12% to 14%) and suicidal ideations (36). Most of the side-effects with ribavirin have been noticed with long term administration. Ribavirin has been found to be teratogenic in animal studies on rodents and rabbits, but no human teratogenic studies are available. Due to the long terminal half-life of elimination of the drug, the minimum interval following treatment with ribavirin before pregnancy can be safely initiated is estimated to be 7 months (36).
The Infectious Diseases Society of America has recommended in 2008 the use of ribavirin in cases of NiV infections (37). Owing to the positive in-vivo and in-vitro results and a considerable safety profile for short term courses, a strong case can be made favoring short course high dose ribavirin for therapy. However, a controlled trial is lacking to resolve the status, for which a pre-approval should be taken from the appropriate authority, so that the trial may be immediately started with the onset of a future outbreak.
7.2. Monoclonal Antibody m102.4
The experimental monoclonal antibody, m102.4, which targets the ephrin-B2 and ephrin-B3 receptor binding domain of the Henipavirus G envelope glycoprotein is a potent cross-reactive neutralizing antibody in vitro. It was effective in protecting ferrets from lethal NiV challenge (38). In May 2010, in Queensland, Australia, m102.4 was offered as a trial on compassionate grounds to a mother and daughter who were exposed to Hendra virus from their infected horse. Both of them did not develop Hendra virus infection, although it is still not known whether treatment was effective or whether the patients did not get infected. In an animal study comprised of 14 African green monkey (AGM) subjects, m102.4 prevented infection and death after injection of a lethal dose of NiV in 12 AGM subjects. Both the control AGM subjects contracted severe infection and developed encephalitis as well as ARDS (39).
7.3. Favipiravir
The viral RNA-dependent RNA polymerase inhibitor favipiravir was developed by Toyama Chemical Company as an antiviral for use against influenza. In a Syrian hamster model for Nipah virus infection, favipiravir was successfully used in lethally challenged hamsters (40).
8. Prognosis
Case fatality rates ranges from 40% to 100%. Poor prognostic factors from the Malaysian outbreak included old age, more severe brain-stem involvement presenting as a reduced level of consciousness, vomiting, abnormal doll's-eye reflex, abnormal pupils, hypertension, and tachycardia during the course of the illness (19).
9. Disease prevention
The morbidity and mortality of healthcare workers involved in care of patients with NiV is a major concern (13,17,41-44). Although, there is still a lot of confusion about transmission and spread of this paramyxovirus, there is a need to lay guidelines for protection of the healthcare workforce based on present evidence and resources available. A leaf can be drawn from the successful containment of Ebola and severe acute respiratory syndrome (SARS) outbreaks, which affected health care workers (HCWs) (45). Standard precautions, hand hygiene and personal protective equipment (PPE) remain as pillars of comprehensive infection prevention and control strategy (46,47).
All hospitals should adhere to standard infection control precautions for all patient-care activities and aerosol-generating procedures. In case of NiV infection in health-care settings, additional measures, such as droplet, contact and airborne precautions should be applied. Droplet precautions rely on isolation (one-patient isolation rooms or cohorting [i.e., grouping patients infected with the same infectious agents together to confine their care to one area and prevent contact with susceptible patients]) and keeping the patient with an existing roommate. A patient that meets the criteria for a suspect Nipah case should immediately be isolated and infection control precautions instituted. In general, hospitals in at-risk areas need to be prepared for the management of Nipah cases via hospital screening, admission procedures and triage, and the management of visitor access and movement should be in place to minimize potential exposure. Standard precautions should be applied while handling patients, handling the deceased, handling the specimens, cleaning and waste disposal.
Hand hygiene: Handwashing with soap and water or alcohol-based hand rub before and after patient contact. Evidence from Bangladesh suggested that Nipah virus can survive on surfaces and be a potential source of spread of infection to caregivers (44). Lack of hand hygiene practices and scarcity of water in the setting was possibly responsible for HCWs being affected in the outbreak (44). The importance of hand hygiene cannot be over emphasized and it remains the crucial cornerstone for preventing spread of infection (44,46,48).
Wearing of PPE when performing an aerosol generating procedure or a patient examination. Highest level of protection (Level B/A OSHA) is recommended for Nipah. Infections in HCWs with SARS or Ebola during the respective outbreaks were very commonly attributed to improper PPE removal or doffing (49-52).
10. Conclusion
Nipah virus outbreak should be suspected in relevant epidemiological settings (e.g. history of travel or residence in known geographical areas with Nipah transmission or contact with pigs or bats) in clusters of patients presenting with acute encephalitis with or without ARDS, high secondary attack rate and very high mortality. These patients should be managed with appropriate infection control measures. Until the time when newer drugs are developed for its effective treatment, the role of drugs like ribavirin has to be clearly established with the help of properly designed trials. Effective control measures in the community to prevent its transmission from animals (bats/pigs) to humans in disease prone areas have to be instituted. In the battle of virus vs. man, hopefully the latter will turn out to be victorious in the long run.
References
- 1. Chua KB. Nipah virus outbreak in Malaysia. J Clin Virol. 2003; 26:265-275. [DOI] [PubMed] [Google Scholar]
- 2. Kulkarni DD, Tosh C, Venkatesh G, Senthil Kumar D. Nipah virus infection: Current scenario. Indian J Virol. 2013; 24:398-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ksiazek TG, Rota PA, Rollin PE. A review of Nipah and Hendra viruses with an historical aside. Virus Res. 2011; 162:173-183. [DOI] [PubMed] [Google Scholar]
- 4. Reynes JM, Counor D, Ong S, Faure C, Seng V, Molia S, Walston J, Georges-Courbot MC, Deubel V, Sarthou JL. Nipah virus in Lyle's flying foxes, Cambodia. Emerg Infect Dis. 2005; 11:1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wacharapluesadee S, Lumlertdacha B, Boongird K, Wanghongsa S, Chanhome L, Rollin P, Stockton P, Rupprecht CE, Ksiazek TG, Hemachudha T. Bat Nipah virus, Thailand. Emerg Infect Dis. 2005; 11:1949-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daszak P, Plowright R, Epstein JH, Pulliam J, Abdul Rahman S, Field HE, Smith CS, Olival KJ, Luby S, Halpin K. The emergence of Nipah and Hendra virus: pathogen dynamics across a wildlife-livestock-human continuum. Disease Ecology: Community structure and pathogen dynamics. 2006; 2006; 186-201. [Google Scholar]
- 7. Hayman DT, Suu-Ire R, Breed AC, McEachern JA, Wang L, Wood JL, Cunningham AA. Evidence of henipavirus infection in West African fruit bats. PloS One. 2008; 3:e2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention (CDC) Outbreak of Hendra-like virus--Malaysia and Singapore, 1998-1999. MMWR Morb Mortal Wkly Rep. 1999; 48:265-269. [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention (CDC) Update: Outbreak of Nipah virus ‒ Malaysia and Singapore, 1999. MMWR Morb Mortal Wkly Rep. 1999; 48:335-337. [PubMed] [Google Scholar]
- 10. Ching PK, de Los Reyes VC, Sucaldito MN, et al. Outbreak of henipavirus infection, Philippines, 2014. Emerg Infect Dis. 2015; 21:328-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ajith Kumar AK, Anoop Kumar AS. Deadly Nipah outbreak in Kerala: Lessons learned for the future. Indian J Crit Care Med. 2018; 22:475-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parashar UD, Sunn LM, Ong F, et al. Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998-1999 outbreak of severe encephalitis in Malaysia. J Infect Dis. 2000; 181:1755-1759. [DOI] [PubMed] [Google Scholar]
- 13. Mounts AW, Kaur H, Parashar UD, Ksiazek TG, Cannon D, Arokiasamy JT, Anderson LJ, Lye MS; Nipah Virus Nosocomial Study Group. A cohort study of health care workers to assess nosocomial transmissibility of Nipah virus, Malaysia, 1999. J Infect Dis. 2001; 183:810-813. [DOI] [PubMed] [Google Scholar]
- 14. Luby SP, Rahman M, Hossain MJ, Blum LS, Husain MM, Gurley E, Khan R, Ahmed BN, Rahman S, Nahar N, Kenah E, Comer JA, Ksiazek TG. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis. 2006; 12:1888-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rahman MA, Hossain MJ, Sultana S, et al. Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector Borne Zoonotic Dis. 2012; 12:65-72. [DOI] [PubMed] [Google Scholar]
- 16. Fogarty R, Halpin K, Hyatt AD, Daszak P, Mungall BA. Henipavirus susceptibility to environmental variables. Virus Res. 2008; 132:140-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chadha MS, Comer JA, Lowe L, Rota PA, Rollin PE, Bellini WJ, Ksiazek TG, Mishra A. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis. 2006; 12:235-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clayton BA, Middleton D, Bergfeld J, Haining J, Arkinstall R, Wang L, Marsh GA. Transmission routes for Nipah virus from Malaysia and Bangladesh. Emerg Infect Dis. 2012; 18:1983-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goh KJ, Tan CT, Chew NK, Tan PS, Kamarulzaman A, Sarji SA, Wong KT, Abdullah BJ, Chua KB, Lam SK. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N Engl J Med. 2000; 342:1229-1235. [DOI] [PubMed] [Google Scholar]
- 20. Harit AK, Ichhpujani RL, Gupta S, Gill KS, Lal S, Ganguly NK, Agarwal SP. Nipah/Hendra virus outbreak in Siliguri, West Bengal, India in 2001. Indian J Med Res. 2006; 123:553-560. [PubMed] [Google Scholar]
- 21. Hossain MJ, Gurley ES, Montgomery JM, et al. Clinical presentation of Nipah virus infection in Bangladesh. Clin Infect Dis. 2008; 46:977-984. [DOI] [PubMed] [Google Scholar]
- 22. Clayton BA. Nipah virus: Transmission of a zoonotic paramyxovirus. Curr Opin Virol. 2017; 22:97-104. [DOI] [PubMed] [Google Scholar]
- 23. Paton NI, Leo YS, Zaki SR, Auchus AP, Lee KE, Ling AE, Chew SK, Ang B, Rollin PE, Umapathi T, Sng I, Lee CC, Lim E, Ksiazek TG. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet. 1999; 354:1253-1256. [DOI] [PubMed] [Google Scholar]
- 24. Daniels P, Ksiazek T, Eaton BT. Laboratory diagnosis of Nipah and Hendra virus infections. Microbes Infect. 2001; 3:289-295. [DOI] [PubMed] [Google Scholar]
- 25. Lim CT. MR imaging in Nipah virus infection. Neurology Asia. 2009; 14:49-52. [Google Scholar]
- 26. Sarji SA, Abdullah BJ, Goh KJ, Tan CT, Wong KT. MR imaging features of Nipah encephalitis. AJR Am J Roentgenol. 2000; 175:437-442. [DOI] [PubMed] [Google Scholar]
- 27. Lee KE, Umapathi T, Tan CB, Tjia HT, Chua TS, Oh HM, Fock KM, Kurup A, Das A, Tan AK, Lee WL. The neurological manifestations of Nipah virus encephalitis, a novel paramyxovirus. Ann Neurol. 1999; 46:428-432. [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention. Treatment. Nipah Virus (NiV). https://www.cdc.gov/vhf/nipah/treatment/index.
- 29. Wright PJ, Crameri G, Eaton BT. RNA synthesis during infection by Hendra virus: an examination by quantitative real-time PCR of RNA accumulation, the effect of ribavirin and the attenuation of transcription. Arch Virol. 2005; 150:521-532. [DOI] [PubMed] [Google Scholar]
- 30. Aljofan M, Saubern S, Meyer AG, Marsh G, Meers J, Mungall BA. Characteristics of Nipah virus and Hendra virus replication in different cell lines and their suitability for antiviral screening. Virus Res. 2009; 142:92-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Georges-Courbot MC, Contamin H, Faure C, Loth P, Baize S, Leyssen P, Neyts J, Deubel V. Poly (I)-poly (C12U) but not ribavirin prevents death in a hamster model of Nipah virus infection. Antimicrob Agents Chemother. 2006; 50:1768-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freiberg AN, Worthy MN, Lee B, Holbrook MR. Combined chloroquine and ribavirin treatment does not prevent death in a hamster model of Nipah and Hendra virus infection. J Gen Virol. 2010; 91:765-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chong HT, Kamarulzaman A, Tan CT, Goh KJ, Thayaparan T, Kunjapan SR, Chew NK, Chua KB, Lam SK. Treatment of acute Nipah encephalitis with ribavirin. Ann Neurol. 2001; 49:810-813. [DOI] [PubMed] [Google Scholar]
- 34. Laskin OL, Longstreth JA, Hart CC, Scavuzzo D, Kalman CM, Connor JD, Roberts RB. Ribavirin disposition in high risk patients for acquired immunodeficiency syndrome. Clin Pharmacol Ther. 1987; 41:546-555. [DOI] [PubMed] [Google Scholar]
- 35. Connor E, Morrison S, Lane J, Oleske J, Sonke RL, Connor J. Safety, tolerance, and pharmacokinetics of sys temic r ibavi r in in chi ldren with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1993; 37:532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grayson ML, Cosgrove SE, Crowe S, Hope W, McCarthy JS, Mills J, Mouton JW, Paterson DL. Kucers' The use of antibiotics: A clinical review of antibacterial, antifungal, antiparasitic, and antiviral drugs, -Three volume set. CRC Press, FL, USA. 2017. [Google Scholar]
- 37. Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, Hartman BJ, Kaplan SL, Scheld WM, Whitley RJ; Infectious Diseases Societyof America. The management of encephalitis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008; 47:303-327. [DOI] [PubMed] [Google Scholar]
- 38. Bossart KN, Zhu Z, Middleton D, Klippel J, Crameri G, Bingham J, McEachern JA, Green D, Hancock TJ, Chan YP, Hickey AC, Dimitrov DS, Wang LF, Broder CC. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 2009; 5:e1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bossart KN, Geisbert TW, Feldmann H, Zhu Z, Feldmann F, Geisbert JB, Yan L, Feng YR, Brining D, Scott D, Wang Y, Dimitrov AS, Callison J, Chan YP, Hickey AC, Dimitrov DS, Broder CC, Rockx B. A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge. Sci Transl Med. 2011; 3:105ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dawes BE, Kalveram B, Ikegami T, Juelich T, Smith JK, Zhang L, Park A, Lee B, Komeno T, Furuta Y, Freiberg AN. Favipiravir (T-705) protects against Nipah virus infection in the hamster model. Sci Rep. 2018; 8:7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gurley ES, Montgomery JM, Hossain MJ, Islam MR, Molla MA, Shamsuzzaman SM, Akram K, Zaman K, Asgari N, Comer JA, Azad AK, Rollin PE, Ksiazek TG, Breiman RF. Risk of nosocomial transmission of Nipah virus in a Bangladesh hospital. Infect Control Hosp Epidemiol. 2007; 28:740-742. [DOI] [PubMed] [Google Scholar]
- 42. Stone R. Epidemiology. Breaking the Chain in Bangladesh. Science. 2011; 331:1128-1131. [DOI] [PubMed] [Google Scholar]
- 43. Luby SP, Gurley ES, Hossain MJ. Transmission of human infection with Nipah Virus. Clin Infect Dis. 2009; 49:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gurley ES, Montgomery JM, Hossain MJ, et al. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg Infect Dis. 2007; 13:1031-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Personal protective equipment for use in a filovirus disease outbreak: Rapid advice guideline. Geneva: World Health Organization; 2016. http://www.ncbi.nlm.nih.gov/books/NBK401170/ (accessed on August 17, 2018). [PubMed]
- 46. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health Care Infection Control Practices Advisory Committee. 2007 guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007; 35:S65-S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garner JS. Guideline for isolation precautions in hospitals. The Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1996; 17:53-80. [DOI] [PubMed] [Google Scholar]
- 48. Boyce JM, Pittet D; Healthcare Infection Control Practices Advisory Committee; HICPAC/SHEA/APIC/ IDSA Hand Hygiene Task Force. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep. 2002; 51:1-45, quiz CE1-4. [PubMed] [Google Scholar]
- 49. Weber DJ, Rutala WA, Schaffner W. Lessons learned: Protection of healthcare workers from infectious disease risks. Crit Care Med. 2010; 38:S306-S314. [DOI] [PubMed] [Google Scholar]
- 50. Centers for Disease Control and Prevention (CDC). Cluster of severe acute respiratory syndrome cases among protected health-care workers--Toronto, Canada, April 2003. MMWR Morb Mortal Wkly Rep. 2003; 52:433-436. [PubMed] [Google Scholar]
- 51. Zamora JE, Murdoch J, Simchison B, Day AG. Contamination: A comparison of 2 personal protective systems. CMAJ. 2006; 175:249-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kreuels B, Addo MM, Schmiedel S. Severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2015; 372:1377. [DOI] [PubMed] [Google Scholar]
- 53. Arunkumar G, Chandni R, Chandni R, Mourya DT, Singh SK, Sadanandan R, Sudan P, Bhargava B; NIPAH: Nipah Investigators People And Health. Outbreak Investigation of Nipah Virus Disease in Kerala, India, 2018. J Infect Dis. 2018; 10.1093/infdis/jiy612 [DOI] [PubMed] [Google Scholar]


