Summary
Trisomy 9 including mosaic and partial trisomy is less frequently seen chromosomal abnormality in live born children. The pure or partial trisomy 9 frequently been reported in prenatal diagnosis and product of conception. However few studies reported partial trisomy 9 in live born children. In addition data on genotype and phenotype correlation of partial trisomy is not well understood except few case reports. Here we report a case of partial trisomy 9 and monosomy 14 with a 46,XY,der(9)t(9;14)(q22.1;q11.2)pat,-14 karyotype in a 5-year old dysmorphic child. The proband was confirmed as trisomic for 9pter->9q22.1 and monosomic for 14pter->q11.2 due to paternal t(9;14)(q22.1;q11.2) balanced translocation using a combination of conventional and molecular cytogenetic (fluorescence in situ hybridization, array-comparative genomic hybridization) techniques. The clinical features similar to pure trisomy 9 is due to duplication of the large region of chromosome 9. However, the present report of partial trisomy 9 and monosomy 14 is a novel case report and showing comparatively longer survival which have not been previously reported in the literature. The parent of the proband was counseled for the future pregnancies.
Keywords: Partial trisomy 9, partial monosomy 14, unbalanced translocation, developmental delay, dysmorphic features
1. Introduction
Trisomy 9 is one of the rare chromosomal abnormalities associated with complex phenotype involving multiple malformations of limbs, cardiac, renal and central nervous systems (1). The clinical severity in trisomy 9 is not precisely correlated with the extent of trisomic material (2). Prenatal growth retardation, postnatal mental retardation and early mortality are known to be associated with trisomy 9 (3). The trisomy 9 can classify as pure trisomy and partial trisomy 9 (4,5). The pure trisomy 9 can occurs due to different type of adjacent-2 segregation mechanism as well as non-disjunction of chromosomes in one of the parent during gametogenesis (6). The partial trisomy includes somatic mosaicism and translocation. The phenotype associated with it relies on the number of cellular lines that are trisomic and the additional chromosome material translocated to different chromosomes (7,8). However, majority of the pure and partial trisomy 9 ends in spontaneous abortion (3,8).
The published literature of pure or partial trisomy 9 provides limited information mainly related to prenatal diagnosis, electively terminated pregnancies or autopsy cases and not on long time survivors of partial trisomy 9 (5,9,10). The developmental complications in partial trisomy 9 have been reported in few case studies (11). So far 150 cases of pure and partial trisomy 9 have been reported in the database of National organization for rare disorders (NORD) (12,13). However there is dearth of information regarding developmental status of pure or partial trisomy 9 cases in the literature.
Here, we report a novel case of partial trisomy 9 and monosomy 14 in a five year old child with dysmorphic features.
2. Case Report
A 5-year old male child, the first born of a healthy non-consanguineous couple was referred to our clinic for chromosomal analysis due to delayed milestones and dysmorphic features. He had been born of full term normal delivery with moderately low birth weight. Mother's age was 33 years with no previous bad obstetric history and the father was 35 years old.
On clinical examination, the child had facial dysmorphic features like prominent forehead with mild macrocephaly, low hair line, slight downward slanting of the eyes with epicanthic folds, hypertelorism, low set ears, large pinnae, long face, bulbous nose, thin upper lip, long philtrum, high arched palate and webbed neck. The proband also had skeletal and limb anomalies including kyphoscoliosis rocker bottom feet, short middle interphalangeal elevated foot, clinodactyly of fifth finger and pilonidal sinus. Speech development was severely retarded and the gross and fine motor development was poor. The Social Quotient (SQ) was 80. The Magnetic resonance imaging (MRI) and Computer tomography (CT) scan of brain revealed generalized cerebral atrophy, dilated ventricles, and arachnoid cyst. The 2-Dimentional echocardiogram (2D-ECHO) reported tiny patent ductus arteriosus (PDA) with normal left ventricular output. Ultra sonography (USG) of abdomen revealed small size of both kidneys. Brainstem Evoked Response Audiometric (BERA) test revealed bilateral moderate to severe hearing loss. Ophthalmic examination showed telecanthus.
The study was carried out with the consent of one of the parent.
3. Cytogenetics
Peripheral blood cultures were set up at 37°C for 72 hours according to standard procedure (14). The cultures were stimulated with phytohaemagglutinin (PHA) arrested with colchicine (50 ug/mL) and treated with hypotonic solution (KCL- 0.56 g/100 mL). The cells were fixed in carnoy's solution (Methanol: Glacial acetic acid; 3:1). The chromosomal preparations obtained were subjected to GTG banding (15). At least 30 metaphases were scored and karyotype (approximately 400 band resolution) according to International System of Chromosome Nomenclature 2016 (ISCN 2016) (6). Applied Spectral Imaging software system (Inc. Carlsbad, USA) interfaced with Nikon 90i microscope (Japan) was used for analysis.
The chromosomal analysis of child revealed 46,XY,+der(9)t(9;14)(q22.1;q11.2)pat,-14 karyotype (Figure 1A). The karyotype of mother was normal while the father had balanced translocation 46,XY,t(9;14) (q22.1;q11.2) (Figure 1B). Fluorescence in situ hybridization (FISH) was carried out using centromere specific and whole chromosome painting probe (Kreatech, Leica biosystems, Germany) of chromosome 9 (Cat. No. KBI30009G) and 14 (Cat. No. KBI30014R). FISH results revealed partial trisomy 9 and monosomy 14 in proband (Figures 1C and 1D). The chromosome breakpoints further confirmed by array-CGH (CytoPrime microarray system with Chromosome Analysis Suite software designed by affymetrix). Array-CGH revealed arr[GRCh38] 9p24.3q22.1(203861_87860633)x3 pat (Figures 2A and 2B). Array-CGH analysis shows a single copy gain of chromosome 9 at band 9p24.3- >q22.1 which spans 87.657 Mbps genomic material consisting of 432 genes. The array-CGH result suggested partial trisomy 9.
Figure 1.
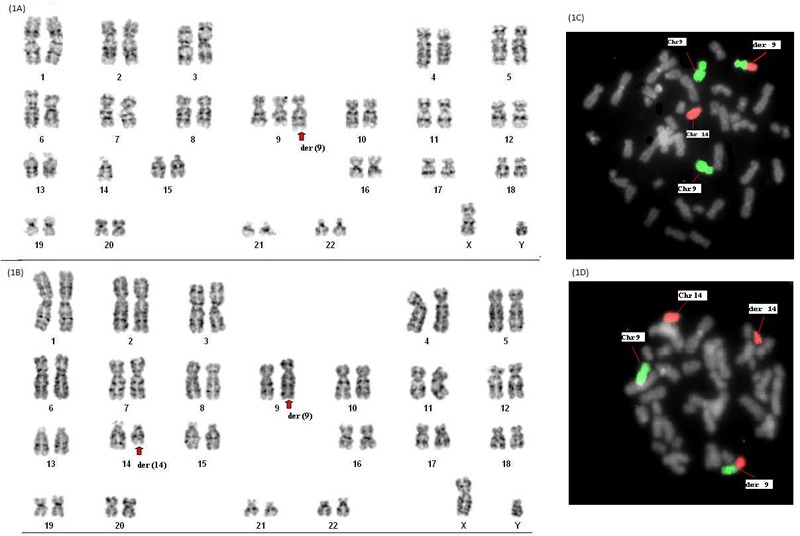
Karyotype and FISH images of Proband and father of the affected child. (A), GTG banded karyotype of proband showing 46,XY,+der(9)t(9;14)(q22.1;q11.2)pat,-14; (B), Balanced translocation showing 46,XY,t(9;14)(q22.1;q11.2) in father; (C), Whole chromosome painting (FISH) shows partial trisomy 9 [Green (9) and red (14)] in proband; (D), FISH image showing balanced translocation in father.
Figure 2.
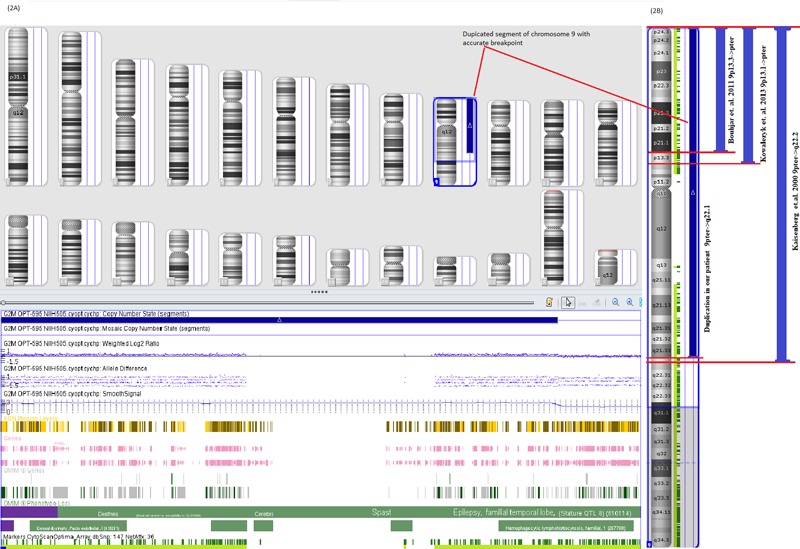
Array-CGH findings. (A), Array-CGH image showing duplication of chromosome 9, region 9pter->9q22.1 (Proband); (B), Array-CGH image showing duplicated region of chromosome 9 in our case and its comparison with previous studies.
4. Discussion
Genomic imbalances due to chromosomal abnormalities are major cause of multiple congenital anomalies including dysmorphic features and developmental delay. The chromosomal abnormalities including balanced translocations, inversions and extra marker chromosomes passed through the parents cause unbalanced genomic changes in the fetus or children resulting in the abnormal phenotype (16).
Trisomy 9 is a fairly uncommon aneuploidy accounting for only 2.7% of all trisomy and mainly results in early miscarriages (17). Pure trisomy 9 presents a wide variety of congenital anomalies affecting most of the vital organs and limb extremities. However, the severity is highly variable from case to case (18,19). Partial trisomy 9 due to balanced translocation between chromosome 9 and other chromosomes of parents have been reported in literature (20,21). In our case, chromosome analysis using GTG banding revealed der(9) chromosome and the karyotype was 46,XY,+der(9)t(9;14)(q22.1;q11.2)pat,-14. The array- CGH detected the accurate break point to be 9p24.3- >q22.1 which could not be confirmed by conventional method. Total 432 genes are present in this breakpoint region and out of which 222 genes are found to be associated with Mendelian inheritance (22). It is evident that the der(9) chromosome came from father due to the presence of balanced translocation 46,XY,t(9;14) (q22.1;q11.2). The adjacent-2 segregation taking place during the gametogenesis in one of the parent may be the reason for the unbalanced genotype (6). Molecular cytogenetic analysis of proband using FISH revealed that the der(9) was indeed derived from the father and the child was trisomic for chromosome region 9pter- >9q22.1 and monosomic for 14pter->14q11.2 region. Hence, the karyotype of proband was confirmed to be 46,XY,+der(9)t(9;14)(q22.1;q11.2)pat,-14.
In literature review we found that nine such cases with slightly similar breakpoint 9pter->9q22.2 have been reported (Table 1). However, the other partial trisomy related to region 9q12->9q32 also reported in the literature. The National organization for rare disorders (NORD) and Tracking Rare Incidence Syndromes (TRIS) project are the important database for rare diseases like trisomy 9. According to their database 150 cases of trisomy 9 has been reported so far. Of them only few cases had longer survival more than 3-4 years (12,13). According to published literature majority of trisomy 9 cases involving breakpoint region 9pter->9q22.2 manifest clinical features such as webbed neck, rocker bottom feet, slanting eyes, growth retardation and delayed milestones (23). Similar clinical abnormalities were seen in this case, additionally classical anomalies like CNS involvement (cerebral atrophy, dilated ventricles), and cardio vascular involvement (PDA) and renal impairment (hypoplastic kidneys) were also present. The presence of these anomalies makes the proband a classical case of partial trisomy 9 in terms of clinical diagnosis and management. Hence reporting of such cases is essential to characterize the disorder as well as to increase the awareness and knowledge in the medical community.
Table 1. Correlation of clinical features and chromosome breakpoints in patients with 9p duplication.
| Authors [Year] (ref.) | Region/loci duplicated | Reported clinical anomalies |
|---|---|---|
| Our Patient | 9p24.3->q22.1 | Macrocephaly, slight downward slanting of the eyes with epicanthic folds, hypertelorism, low set ears, large pinnae, long face, bulbous nose, thin upper lip, long philtrum, high arched palate and webbed neck, kyphoscoliosis, rocker bottom feet, short middle interphalangeal, clinodactyly of the fifth finger, pilonidalsinus, cerebral atrophy, dilated ventricles and arachnoid cyst, congenital heart disease, small size kidneys |
| Fryns J P et al. [1979] (29) | 9p | Retardate psychomotor development, hypertelorism, antimagoloid slant, globular nose, protruding ears, dilated ventricles |
| Wilson GN et al. [1985] (2) | 9pter->q22 | Microcephaly , prominent nasal root, bulbous nose, and down-turned comers of the mouth |
| Smart RD et al.[1988] (30) | 9pter->q22.1 | Enlarged ventricles, facial dysmorphism |
| Chih C P et al. [1999] (31) | 9pter->q22 | Enlarged cysternamegna with bilateral ventriculomegaly |
| Von Kaisenberg CS et al. [2000] (32) | 9pter->q22.2 | Dandy Walker malformation* and cerebella vermis hypoplasia in fetus |
| Bouhjar IB et al. [2011] (27) | 9p13.3->pter | Typical dysmorphic features but not mental retardation |
| Lyons JM et al. [2013] (33) | Partial 9p and partial monosomy Yq | Neuro developmental delay, growth delay, dysmorphic features, small genitalia |
| Kowalezyk M. et al. [2013] (28) | 9p13.1->pter duplication with 9 p deletion | Craniofacial anomalies, Dandy Walker malformation*, delayed development, mental retardation |
| Brambila-Tapia AJ et al. [2014] (21) | Pure trisomy 9p13.1 | Psychomotor delay, short stature, open anterior fontanelle, dysplastic ears, facial dysmorphism, Long and broad first toes, CNS and skeletal alterations |
Bolding represents features in common with our patient. *Partial anomalies present in our case.
Moreover, partial monosomy 14pter->14q11.2 was also detected through conventional cytogenetics in the proband. Few cases of partial monosomy 14q11.2 have been reported in the literature (Table 2) and most of the anomalies overlap with trisomy 9 features (24,25). However we have not found any gain or loss in array- CGH results, showing involvement of heterochromatic region in the translocation. The reported studies shows recurrent 100 kb micro deletions in the chromosomal region 14q11.2 involving CHD1 gene are associated with autism and macrocephaly. Other study also suggested that the deletion of 114 kb in the region of 14q11.2 involving genes SUPTT16H, CHD8, RAB2B which are strongly associated with autism and facial dysmorphism (26). Hence cytogenetic analysis of the children with dysmorphic features is essential in view of autism spectrum disorder and its further management.
Table 2. Comparative clinical features and chromosome break points in patients with monosomy 14.
| Authors [Year] (ref.) | Region/loci duplicated | Reported clinical anomalies |
|---|---|---|
| Our Patient | 14pter-> 14q11.2 | Macrocephaly, slight downward slanting of the eyes with epicanthic folds, hypertelorism, low set ears, large pinnae, long face, bulbous nose, thin upper lip, long philtrum, high arched palate and webbed neck, kyphoscoliosis, rocker bottom feet, short middle interphalangeal, clinodactyly of the fifth finger, pilonidalsinus, cerebral atrophy, dilated ventricles and arachnoid cyst, congenital heart disease, small size kidneys |
| Short E M et al. [1972] (25) | (14q-)+ deletion | Delayed development, Intellectual disability |
| Petek E et al. [2003] (24) | 14q interstitial deletion | Small fontanelle, sloping forehead, micropthalmia, malformed pinnae |
| Prontera P et al. [2014] (26) | 14q11.2 deletion | Macrocephaly, facial dysmorphism with Autism |
Bolding represents features in common with our patient.
The cytogenetic screening of future pregnancies is important as there is a high risk of giving birth to chromosomally abnormal child, due to the presence of balanced translocation in the father which is likely to undergo abnormal meiotic segregation. Over the last decade recent advancement in molecular cytogenetic has proved to be an important tool for identification of complex chromosomal abnormalities including minor losses and gains with accurate breakpoint (27). The Copy number Variations (CNV's) are found to be associated with several disease conditions (28).
In conclusion, the present case of partial trisomy 9 and monosomy 14 is novel findings and showing comparatively longer survival which has not been previously reported in the literature. Further, the combination of conventional and advance molecular cytogenetic tools have proven to be essential in accurate identification of breakpoint in affected children. The present case reports will help in appropriate genetic counseling and prevention of genetic disease through prenatal diagnosis.
Acknowledgements
The study was carried out with Institutional core grant.
References
- 1. Feingold M, Atkins L. A case of trisomy 9. J Med Genet. 1973; 10:184-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson GN, Raj A, Baker D. The phenotypic and cytogenetic spectrum of partial trisomy 9. Am J Med Genet. 1985; 20:277-282. [DOI] [PubMed] [Google Scholar]
- 3. Yeo L, Waldron R, Lashley S, Day-Salvatore D, Vintzileos AM. Prenatal sonographic findings associated with nonmosaic trisomy 9 and literature review. J Ultrasound Med. 2003; 22:425-430. [DOI] [PubMed] [Google Scholar]
- 4. Sepulveda W, Wimalasundera RC, Taylor MJ, Blunt S, Be C, De La Fuente S. Prenatal ultrasound findings in complete trisomy 9. Ultrasound Obstet Gynecol. 2003; 22:479-483. [DOI] [PubMed] [Google Scholar]
- 5. Chitayat D, Hodgkinson K, Luke A, Winsor E, Rose T, Kalousek D. Prenatal diagnosis and fetopathological findings in five fetuses with trisomy 9. Am J Med Genet. 1995; 56:247-251. [DOI] [PubMed] [Google Scholar]
- 6. McGowan-Jordan J, Simons A, Schmid M, (eds.). An international system for human cytogenomic nomenclature. Reprint of Cytogenetic and Genome Res, S Karger, Basel, New York, 2016, 149:1-2. [Google Scholar]
- 7. Saura R, Traore W, Taine L, Wen ZQ, Roux D, Maugey-Laulom B, Ruffie M, Vergnaud A, Horovitz J. Prenatal diagnosis of trisomy 9. Six cases and a review of the literature.. [DOI] [PubMed] [Google Scholar]
- 8. Zen PR, Rosa RF, Rosa RC, Graziadio C, Paskulin GA. New report of two patients with mosaic trisomy 9 presenting unusual features and longer survival. Sao Paulo Med J. 2011; 129:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Francke U, Benirschke K, Jones OW. Prenatal diagnosis of trisomy 9. Humangenetik. 1975; 29:243-250. [DOI] [PubMed] [Google Scholar]
- 10. Schwartz S, Ashai S, Meijboom EJ, Schwartz MF, Sun CC, Cohen MM. Prenatal detection of trisomy 9 mosaicism. Prenat Diagn. 1989; 9:549-554. [DOI] [PubMed] [Google Scholar]
- 11. Ben Slama S, Ouertani I, Dimassi K, Bacha D, Lahmar A, Mzabi S. Complete trisomy 9 with unusual phenotype association. Tunis Med. 2016; 94:895. [PubMed] [Google Scholar]
- 12. National organization for rare disorders (NORD). Rare Disease Database. https://rarediseases.org/rare-diseases/chromosome-9-trisomy-9p-multiple-variants (accessed December 5, 2018).
- 13. Bruns D. Presenting physical characteristics, medical conditions, and developmental status of long-term survivors with trisomy 9 mosaicism. Am J Med Genet A. 2011; 155:1033-1039. [DOI] [PubMed] [Google Scholar]
- 14. Moorhead PS, Nowell PC, Mellman WJ, Battips DM, Hungerford DA. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960; 20:613-616. [DOI] [PubMed] [Google Scholar]
- 15. Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971; 2:971-972. [DOI] [PubMed] [Google Scholar]
- 16. Liu HY, Huang J, Li T, Wu D, Wang HD, Wang Y, Wang T, Guo LJ, Guo QN, Huang FF, Wang RL, Wang YT. Clinical and molecular cytogenetic analyses of four patients with imbalanced translocations. Mol Cytogenet. 2016; 9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruns DA, Campbell E. Twenty-Five additional cases of trisomy 9 mosaic: Birth information, medical conditions and developmental status. Am J Med Genet A. 2015; 167A:997-1007. [DOI] [PubMed] [Google Scholar]
- 18. López-Félix J, Flores-Gallegos L, Garduño-Zarazúa L, Leis-Márquez T1, Juárez-García L1, Meléndez- Hernández R, Castelazo-Morales E, Mayén-Molina D. Partial trisomy 9: Prenatal diagnosis and recurrence within same family. Clin Case Rep. 2017; 5:986-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leube B, Majewski F, Drechsler M, Royer-Pokora B. Unbalanced cryptic translocation der (14)t(9;14) (q34.3;q32.33) identified by subtelomeric FISH. Clin Dysmorphol. 2003; 12:261-265. [DOI] [PubMed] [Google Scholar]
- 20. Deng L, Peng Y, Liu J, Wen J, Xia Y, Liang D, Wu L. Brief Report. Adult patient presenting an interstitial (9) (q21.32q31.1) direct duplication resulting from the malsegregation of a paternal balanced insertional translocation. Birth Defects Res A Clin Mol Teratol. 2014; 100:294-299. [DOI] [PubMed] [Google Scholar]
- 21. Brambila-Tapia AJ, Neira VA, Vásquez-Velásquez AI, Jimenez-Arredondo RE, Chávez-González EL, Picos- Cárdenas VJ, Fletes-Rayas AL, Figuera LE. Pure 9p trisomy derived from a terminal balanced unreciprocal translocation. Genet Couns. 2014; 25:289-297. [PubMed] [Google Scholar]
- 22. Hamosh A, Scott AF, Amberger J, Valle D, McKusick VA. Online Mendelian Inheritance in Man (OMIM). Hum Mutat. 2000; 15:57-61. [DOI] [PubMed] [Google Scholar]
- 23. Smith DW. Recognizable p a t t e r n s o f human malformation. Major Probl Clin Pediatr. 1976; 7:1-497. [PubMed] [Google Scholar]
- 24. Petek E, Plecko-Startinig B, Windpassinger C, Egger H, Wagner K, Kroisel PM. Molecular characterization of a 3.5 Mb interstitial 14q deletion in a child with several phenotypic anomalies. J Med Genet. 2003; 40:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Short EM, Solitare GB, Breg WR. A case of partial 14 trisomy 47,XY,(14q-)+ and translocation t(9p+;14q-) in mother and brother. J Med Genet. 1972; 9:367-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prontera P, Ottaviani V, Toccaceli D, Rogaia D, Ardisia C, Romani R, Stangoni G, Pierini A, Donti E. Recurrent~100 Kb microdeletion in the chromosomal region 14q11.2, involving CHD8 gene, is associated with autism and macrocepahly. Am J Med Genet A. 2014; 164A:3137-3141. [DOI] [PubMed] [Google Scholar]
- 27. Bouhjar IB, Hannachi H, Zerelli SM, Labalme A, Gmidène A, Soyah N, Missaoui S, Sanlaville D, Elghezal H, Saad A. Array-CGH study of partial trisomy 9p without mental retardation. Am J Med Genet A. 2011; 155A:1735-1739. [DOI] [PubMed] [Google Scholar]
- 28. Kowalczyk M, Tomaszewska A, Podbioł-Palenta A, Constantinou M, Wawrzkiewicz-Witkowska A, Kowalski J, Kałużewski B, Zajaczek S, Srebniak MI. Another rare case of a child with de novo terminal 9p deletion and co-existing interstitial 9p duplication: Clinical findings and molecular cytogenetic study by array-CGH. Cytogenet Genome Res. 2013; 139:9-16. [DOI] [PubMed] [Google Scholar]
- 29. Fryns JP, Casaer P, Van den Berghe H. Partial duplication of the short arm of chromosome 9 (p13 leads to p22) in a child with typical 9p trisomy phenotype. Hum Genet. 1979; 46:231-235. [DOI] [PubMed] [Google Scholar]
- 30. Smart RD, Viljoen DL, Fraser B. Partial trisomy 9 - further delineation of the phenotype. Am J Med Genet. 1988; 31:947-951. [DOI] [PubMed] [Google Scholar]
- 31. Chen CP, Shih JC. Prenatal diagnosis of bilateral ventriculomegaly and an enlarged cisterna magna in a fetus with partial trisomy 9 and partial trisomy 21. Prenat Diagn. 1999; 19:1175-1176. [DOI] [PubMed] [Google Scholar]
- 32. von Kaisenberg CS, Caliebe A, Krams M, Hackelöer BJ, Jonat W. Absence of 9q22-9qter in trisomy 9 does not prevent a Dandy‐Walker phenotype. Am J Med Genet. 2000; 95:425-428. [PubMed] [Google Scholar]
- 33. Lyons MJ, Fuller JD, Montoya Mdel C, DuPont BR, Holden KR. Unbalanced translocation involving partial trisomy 9p and partial monosomy yq with neurodevelopmental delays. J Child Neurol. 2013; 28:524-526. [DOI] [PubMed] [Google Scholar]


