Summary
Extramammary Paget's disease (EMPD) is a rare skin malignant tumor. The prognosis of EMPD with distant metastasis is poor, however an effective therapy has not yet established. Recently, EpCAM (epithelial cell adhesion molecule, CD326) has attracted attention as both prognostic marker and therapeutic target in several cancers. Besides, EpCAM is an important surface marker of circulating tumor cell (CTC) in the collection of CTC. Thus, the purpose of our study was to examine the expression levels of EpCAM and evaluate the correlation between its intensity of EpCAM and the clinical characteristics of EMPD. The expression of EpCAM in EMPD was examined using immunohistochemistry. Skin samples were obtained from 32 patients with EMPD. We found that almost all EMPD tissues (90.6%, 29/32) were positive for EpCAM. Furthermore, the staining intensity of EpCAM protein negatively correlated with the presence of distant metastasis. Overexpression of EpCAM in EMPD cells suggests that EpCAM may be a novel therapeutic target and the research of CTC may be newly developed in EMPD. Based on these findings, EpCAM may be a meaningful molecule in EMPD.
Keywords: Extramammary Paget's disease, epithelial cell adhesion molecule, CD326, distant metastasis, immunohistochemistry, circulating tumor cell
1. Introduction
Extramammary Paget's disease (EMPD) is a rare skin malignant tumor that shows erythematous plaques and erosions in pubic or axillary lesions. Although the prognosis of EMPD with distant metastasis is poor, an effective therapy has not yet established. Thus, there is need to investigate molecular targets for EMPD.
Recently, EpCAM (epithelial cell adhesion molecule, CD326) has attracted attention as both prognostic marker and therapeutic target in several cancers (1). EpCAM is involved in not only cell adhesion but also cellular signaling, cell migration, proliferation and differentiation (2). EpCAM is overexpressed in many malignant tumors, e.g. prostatic, ovarian, urothelial and breast carcinoma (2). The anti-EpCAM antibody (catumaxomab) has been authorized for treatment of malignant ascites in cancer patients (3). On the other hand, EpCAM has been reported to be a tumor suppressive protein in certain types of cancers such as oral, rectal and endometrial carcinoma (4-6) although the molecular mechanism of the tumor suppressive function of EpCAM in several cancers has not been found yet (7). Moreover, EpCAM is an important surface marker of circulating tumor cell (CTC) (8), and the CellSearch system (CTC detector of circulating EpCAM+CD45- cells) is approved by FDA (9).
Thus, the purpose of our study was to examine the expression levels of EpCAM and evaluate the correlation between its intensity of EpCAM and the clinical characteristics of EMPD.
2. Materials and Methods
2.1. Patients
Skin samples were obtained from 32 patients with EMPD. All patients with EMPD were diagnosed by clinical and histopathological findings. These findings were comprehensively analyzed by more than five dermatologists. Paraffin-embedded sections were collected from patients undergoing operation or mapping biopsy in Kumamoto University Hospital between 2011 and 2018. All patients underwent imaging tests for the presence or absence of metastasis. Most of EMPD patients were treated with definitive operation or radiotherapy although EMPD patients with unresectable and metastasis received chemotherapy or palliative radiotherapy. Institutional review board approval and written informed consent were obtained according to the Declaration of Helsinki.
2.2. Immunohistochemical staining
Paraffin-embedded sections were mounted on glass slides, then dewaxed in Clear Plus (FALMA, Japan) and rehydrated in graded alcohols. Immunohistochemistry (IHC) antigen activation was performed by incubation with antigen retrieval solution (pH9, Nichirei, Japan) for 10 min at 121℃. The sections were then incubated overnight with the antibody against EpCAM (Dako, CA, USA, 1:50) at 4℃. The immunoreactivity was visualized with DAB. We used the following grading system: - negative staining, + for slight staining, ++ for strong staining, as described previously (10).
2.3. Statistical analysis
Correlations were assessed according to Fisher's correlation coefficient and its statistical analyses were performed using GraphPad Prism version 7 (MDF, Tokyo, Japan). A p value less than 0.05 was considered significant.
3. Results and Discussion
First, we investigated EpCAM protein expression in EMPD using immunohistochemical staining. Immunohistochemistry of EpCAM revealed that EMPD cells had more intense staining than the normal keratinocytes cells in same tissue samples (Figure 1). Almost all EMPD tissues (90.6%, 29/32) were positive for EpCAM.
Figure 1.
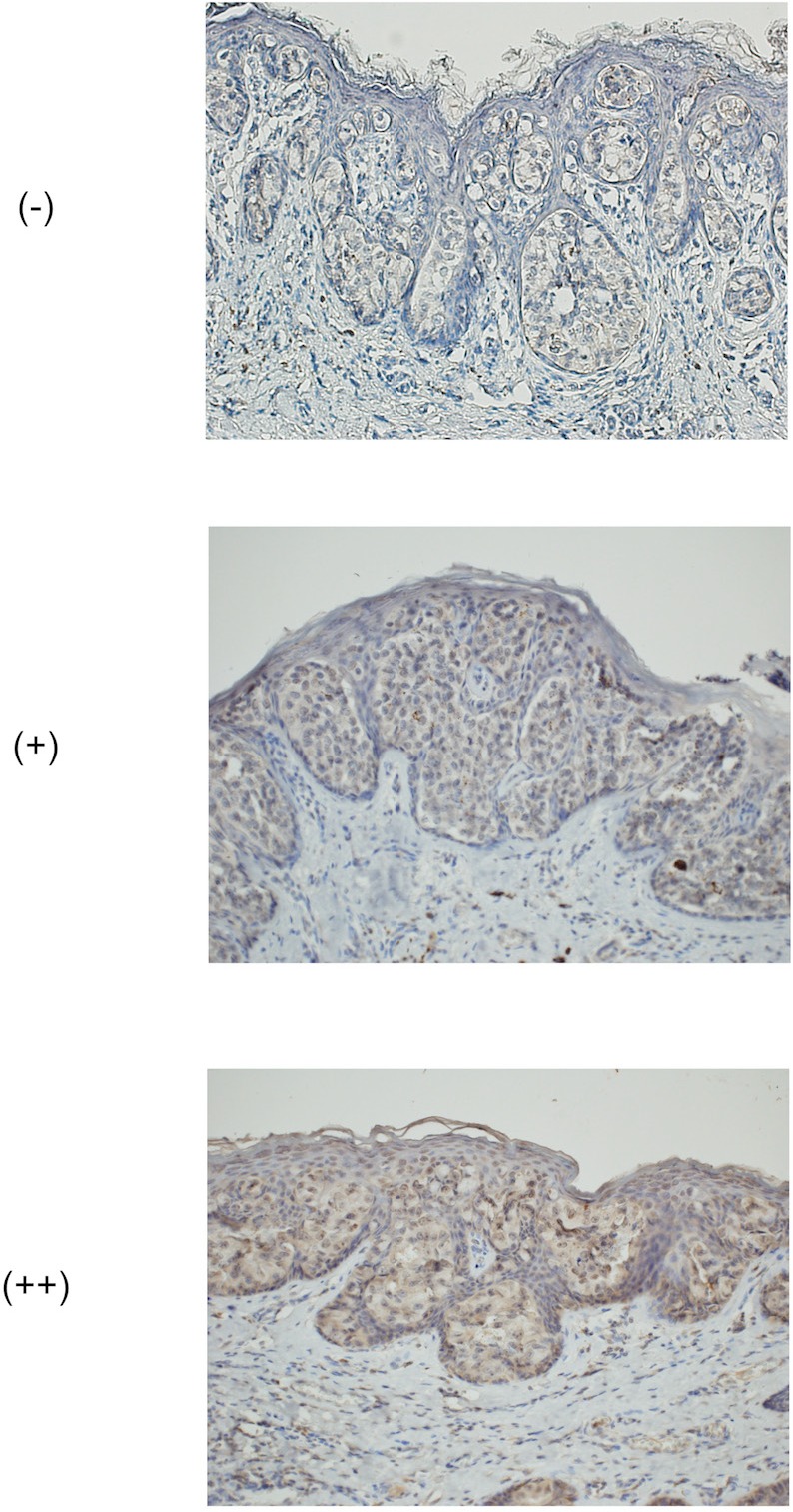
Representative images of semi-quantitative scoring using the immunohistochemical staining (EpCAM). ([-], [+], [++]) (original magnification ×200). -: negative staining, +: slight staining, ++: strong staining.
Next, to analyze the relationship between the intensity of EpCAM protein expression levels and clinical findings of patients with EMPD, we classified semi-quantitative scoring of immunoreactivity ([-], [+], [++]) (Figure 1 and Table 1). The expression of EpCAM in EMPD patients with distant metastasis was significantly decreased compared to that in patients without distant metastasis (p = 0.036), although it did not correlate any other clinical findings (age: p = 0.365, sex: p = 0.087, the degree of invasiveness: p = 0.826, the presence of lymph node metastasis: p = 0.132) (Table 2).
Table 1. Expression of EpCAM protein and clinical manifestations in the patients with extramammary Paget's disease.
| Case | Age | Sex | The degree of invasiveness | Lymph node metastasis | Distant metastasis | EpCAM |
|---|---|---|---|---|---|---|
| 1 | 85 | Male | invasive | + | - | ++ |
| 2 | 78 | Male | in situ | - | - | ++ |
| 3 | 77 | Male | in situ | - | - | + |
| 4 | 76 | Female | in situ | - | - | - |
| 5 | 81 | Male | in situ | - | - | ++ |
| 6 | 66 | Male | invasive | - | - | + |
| 7 | 76 | Female | in situ | - | - | + |
| 8 | 68 | Male | invasive | + | + | - |
| 9 | 71 | Female | in situ | - | - | ++ |
| 10 | 72 | Male | invasive | + | - | + |
| 11 | 69 | Male | in situ | - | - | + |
| 12 | 69 | Male | in situ | - | - | + |
| 13 | 71 | Female | in situ | - | - | + |
| 14 | 78 | Male | in situ | + | + | - |
| 15 | 87 | Female | in situ | - | - | ++ |
| 16 | 63 | Female | in situ | - | - | ++ |
| 17 | 82 | Male | in situ | - | - | + |
| 18 | 67 | Female | in situ | - | - | + |
| 19 | 78 | Female | in situ | - | - | ++ |
| 20 | 76 | Male | in situ | - | - | + |
| 21 | 78 | Male | in situ | - | - | + |
| 22 | 74 | Male | invasive | + | + | + |
| 23 | 64 | Female | in situ | - | - | ++ |
| 24 | 54 | Male | in situ | - | - | + |
| 25 | 64 | Male | in situ | - | - | + |
| 26 | 65 | Female | in situ | - | - | ++ |
| 27 | 60 | Male | in situ | - | - | + |
| 28 | 76 | Female | in situ | - | - | + |
| 29 | 90 | Female | in situ | - | - | + |
| 30 | 59 | Female | in situ | - | - | + |
| 31 | 83 | Male | invasive | + | - | + |
| 32 | 94 | Female | invasive | - | - | ++ |
Table 2. Correlation between the semiquantitative evaluation of the EpCAM protein and patient characteristics .
| Items | EpCAM |
p values | ||
| - | + | ++ | ||
|---|---|---|---|---|
| Age (from 54 to 94) | 0.365 | |||
| Sex | 0.087 | |||
| male (n = 18) | 2 | 13 | 3 | |
| female (n = 14) | 1 | 6 | 7 | |
| The degree of invasiveness | 0.826 | |||
| invasive (n = 6) | 1 | 3 | 2 | |
| microinvasion (n = 1) | 0 | 1 | 0 | |
| in situ (n = 25) | 2 | 15 | 8 | |
| Lymph node metastasis | 0.132 | |||
| - (n = 26) | 1 | 16 | 9 | |
| + (n = 6) | 2 | 3 | 1 | |
| Distant metastasis | 0.036 | |||
| - (n = 29) | 1 | 18 | 10 | |
| + (n = 3) | 2 | 1 | 0 | |
In this study, we presented two novel findings: first, we found that almost all EMPD tissues were positive for EpCAM. Furthermore, the staining intensity of EpCAM protein negatively correlated with the presence of distant metastasis.
EpCAM is overexpressed in several cancers such as adenocarcinomas of colon, stomach, pancreas and lung (11-13). As with these cancers, our results showed that almost all EMPD tissues were positive for EpCAM. It suggests that EpCAM may be a novel therapeutic target in EMPD.
Overexpression of EpCAM was associated with an advanced stage of the disease and linked to worse overall survival in certain tumor types (2). On the other hand, EpCAM could be a tumor suppressive protein in certain types of cancers (7). In gastric cancer, EpCAM appeared to be associated with a more favorable prognosis (14). Besides, the loss of EpCAM in rectal cancer was associated with the reduction of cell-cell adhesion and the augmentation of migration function (15). Our results showed that the EpCAM expression negatively correlated with the presence of distant metastasis. Taken together, these findings suggest that the reduced expression of EpCAM may enhance the metastatic ability in EMPD.
Moreover, EpCAM is an important surface marker of CTC (8). Overexpression of EpCAM in EMPD cells may facilitate the research of CTC in EMPD. Based on these findings, EpCAM may be a meaningful molecule in EMPD although further investigations are needed.
Acknowledgements
This study was supported in part by a grant for scientific research from the Japanese Ministry of Education, Science, Sports and Culture and by project research from the Japanese Ministry of Health, Labour and Welfare.
References
- 1. Schnell U, Cirulli V, Giepmans BN. EpCAM: Structure and function in health and disease. Biochim Biophys Acta. 2013; 1828:1989-2001. [DOI] [PubMed] [Google Scholar]
- 2. Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treat Rev. 2012; 38:68-75. [DOI] [PubMed] [Google Scholar]
- 3. Linke R, Klein A, Seimetz D. Catumaxomab: Clinical development and future directions. MAbs. 2010; 2:129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hwang EY, Yu CH, Cheng SJ, Chang JY, Chen HM, Chiang CP. Decreased expression of Ep-CAM protein is significantly associated with the progression and prognosis of oral squamous cell carcinomas in Taiwan. J Oral Pathol Med. 2009; 38:87-93. [DOI] [PubMed] [Google Scholar]
- 5. Gosens MJ, van Kempen LC, van de Velde CJ, van Krieken JH, Nagtegaal ID. Loss of membranous Ep- CAM in budding colorectal carcinoma cells. Mod Pathol. 2007; 20:221-232. [DOI] [PubMed] [Google Scholar]
- 6. Wen KC, Sung PL, Chou YT, Pan CM, Wang PH, Lee OK, Wu CW. The role of EpCAM in tumor progression and the clinical prognosis of endometrial carcinoma. Gynecol Oncol. 2018; 148:383-392. [DOI] [PubMed] [Google Scholar]
- 7. Huang L, Yang Y, Yang F, Liu S, Zhu Z, Lei Z, Guo J. Functions of EpCAM in physiological processes and diseases (Review). Int J Mol Med. 2018; 42:1771-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Y, Wu S, Bai F. Molecular characterization of circulating tumor cells-from bench to bedside. Semin Cell Dev Biol. 2018; 75:88-97. [DOI] [PubMed] [Google Scholar]
- 9. Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019; 20:71-88. [DOI] [PubMed] [Google Scholar]
- 10. Yamada-Kanazawa S, Kajihara I, Fukushima S, Jinnin M, Masuzawa M, Masuzawa M, Amoh Y, Hoshina D, Abe R, Ihn H. Inhibition of heat shock protein 90 exerts an antitumour effect in angiosarcoma: Involvement of the vascular endothelial growth factor signalling pathway. Br J Dermatol. 2017; 177:456-469. [DOI] [PubMed] [Google Scholar]
- 11. Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004; 35:122-128. [DOI] [PubMed] [Google Scholar]
- 12. Rao CG, Chianese D, Doyle GV, Miller MC, Russell T, Sanders RA Jr, Terstappen LW. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int J Oncol. 2005; 27:49-57. [PubMed] [Google Scholar]
- 13. Brezicka T. Expression of epithelial-cell adhesion molecule (Ep-CAM) in small cell lung cancer as defined by monoclonal antibodies 17-1A and BerEP4. Acta Oncol. 2005; 44:723-727. [DOI] [PubMed] [Google Scholar]
- 14. Warneke VS, Behrens HM, Haag J, Krüger S, Simon E, Mathiak M, Ebert MP, Röcken C. Members of the EpCAM signalling pathway are expressed in gastric cancer tissue and are correlated with patient prognosis. Br J Cancer. 2013; 109:2217-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gosens MJ, van Kempen LC, van de Velde CJ, van Krieken JH, Nagtegaal ID. Loss of membranous Ep- CAM in budding colorectal carcinoma cells. Mod Pathol. 2007; 20:221-232. [DOI] [PubMed] [Google Scholar]


