Abstract
Because an intervention’s clinical benefit depends on who receives it, a key to improving the efficiency of lung cancer screening with low-dose computed tomography (LDCT) is to incentivize its use among the current or former smokers who are most likely to benefit from it. Despite its clinical advantages and cost-effectiveness, only 3.9 percent of the eligible population underwent LDCT screening in 2015. Using individual lung cancer mortality risk, we developed a policy simulation model to explore the potential impact of implementing risk-targeted incentive programs, compared to either implementing untargeted incentive programs or doing nothing. We found that compared to the status quo, an untargeted incentive program that increased overall LDCT screening from 3,900 (baseline) to 10,000 per 100,000 eligible people would save 12,300 life-years and accrue a net monetary benefit (NMB) of $771 million over a lifetime horizon. Increasing screening by the same amount but targeting higher-risk people would yield an additional 2,470–6,600 life-years and an additional $210–$560 million NMB, depending on the extent of the risk targeting. Risk-targeted incentive programs could include provider-level bonuses, health plan premium subsidies, and smoking cessation programs to maximize their impact. As clinical medicine becomes more personalized, targeting and incentivizing higher-risk people will help enhance population health and economic efficiency.
Background
As knowledge and acceptance of the differential benefits of medical interventions across population subgroups continue to grow, it is essential to incorporate individual risk information into the design of incentive programs.
Conventional incentive programs aim to encourage the use of high-value services (or discourage the use of low-value ones) among all enrollees. Empirical studies have shown that these programs, such as pay-for-performance or value-based insurance design,1–4 increase the use of targeted services, albeit modestly.5–10 In contrast, risk-targeted incentive programs seek to selectively incentivize people who can benefit the most. The potential impact of these programs has not been explored. Online appendix exhibit A schematically illustrates how different types of policies incorporate individualized risk information and incentives.11
Using lung cancer screening as an example, this article aims to evaluate the potential impact of implementing hypothetical risk-targeted incentive programs to promote the use of low-dose computed tomography (LDCT) screening among higher-risk people. LDCT screening is well suited for use in an exploration of risk-targeted incentive programs. Clinically, it has been shown to reduce lung cancer mortality by 20 percent, compared to chest x-ray screening.12 Although the LDCT-eligible population is relatively large, only 3.9 percent (262,700 of 6.8 million people) underwent LDCT screening in 2015.13 The low uptake of LDCT screening indicates a need for policy levers to promote its use.14 Also, using a rich data set from the National Lung Screening Trial enabled us to characterize how LDCT screening and baseline risk interact to influence benefits.
Although the US Preventive Services Task Force defines the LDCT-eligible population as current or former heavy smokers ages 55–80,15 multiple risk-prediction models have shown that LDCT screening confers the greatest benefit (that is, avoided lung cancer mortality) on those at the highest risk of lung cancer mortality within the eligible population.16–18
Examples from other clinical areas and programs suggest that risk-targeted incentive programs for LDCT screening might include provider-level financial bonuses, health plan premium subsidies, and smoking cessation or counseling programs.19–21 Targeting incentives or altering the magnitude of those incentives for different plan members (for example, providing greater insurance premium supports for higher-risk people) could promote LDCT screening selectively among higher-risk people.
Our previous risk-stratified cost-effectiveness analysis showed that risk targeting could improve LDCT screening efficiency (compared to chest x-ray screening).22 Building upon our prior analysis, we developed a health policy simulation model to explore the potential impact of implementing risk-targeted incentive programs, compared to either implementing untargeted incentive programs (for example, conventional pay-for-performance) or doing nothing (status quo).
Study Data And Methods
Study Design
Despite having limited empirical data, we used the best available information to project population-level health and economic gains corresponding to different LDCT uptake scenarios across risk-stratified subgroups. The previous literature has described a general analytic framework for policy-oriented economic evaluation and the value of implementation approach, which examines whether it is worth expending resources to promote participation in cost-effective programs.23,24
To estimate the benefits of risk-targeted strategies, we compared two hypothetical incentive programs: an untargeted incentive program that aimed to increase LDCT uptake uniformly across all members of the eligible population and a risk-targeted incentive program that aimed to increase LDCT uptake preferentially among higher-risk people.
Risk Stratification Among The National Lung Screening Trial Participants
Using individual-level data from 53,086 participants in the National Lung Screening Trial, we applied a published multivariable model that predicts lung cancer mortality risk based on eight variables: age, sex, race, family history, body mass index, smoking exposure, years since smoking cessation, and history of emphysema.18 Based on individual lung cancer mortality risk, we categorized all trial participants into five equal-size risk groups (people with the lowest, second-lowest, middle, second-highest, and highest risk). Appendix exhibit B describes the characteristics of trial participants across these five risk groups.11
Short- And Long-Term Outcomes Of Low-Dose Computed Tomography Screening
Considering the importance of the time horizon in economic evaluations,25 we first compared outcomes for LDCT and chest x-ray screening for each of the five risk groups over both a short-term (seven-year) and a long-term (lifetime) time horizon. The short-term horizon corresponded to the follow-up duration for the National Lung Screening Trial, as reflected in the data. For long-term outcomes, we extrapolated the trial data beyond seven years, using a multistate risk model that projected the likelihood over a lifetime of being in each of three health states: having no cancer, living with lung cancer, and death. We used this model to project accrued life-years and health care costs. Costs reflected initial screening, follow-up diagnosis and treatment, and age-specific background medical costs. We discounted both future life-years and costs at 3 percent annually. Our previous publication provides additional technical details.22
Based on projected life-years lived and health care costs incurred with either LDCT or chest x-ray screening for each of the National Lung Screening Trial participants, we calculated added life-years gained and costs incurred from LDCT screening, compared to chest x-ray screening (that is, the incremental benefits and costs of using LDCT instead of chest x-ray), for different LDCT uptake scenarios for each risk group.
To provide a single summary measure of policy value, we also calculated the net monetary benefit (NMB)—that is, the monetary value of life-years gained minus incurred health care costs—for LDCT, compared to chest x-ray, screening. Although the value of a life-year can depend on context and methodology, we assigned it a value of $100,000 based on the literature.26–29
Low-Dose Computed Tomography Uptake Under Different Policy Scenarios
Based on the 2015 National Health Interview Survey data and US Preventive Services Task Force eligibility criteria, 6.82 million US adults were eligible for annual LDCT screening. Exhibit 1 shows our assumptions for LDCT screening uptake under different policy scenarios.
Exhibit 1:
Assumed uptake of low-dose computed tomography (LDCT) screening under different policy scenarios
| Untargeted incentive programs that increase uptake per 100,000 to: | Incentive programs that increase uptake to 5,000 per 100,000, by degree of risk targeting | Incentive programs that increase uptake to 10,000 per 100,000, by degree of risk targeting | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 5,000 | 10,000 | Conservative | Moderate | Aggressive | Conservative | Moderate | Aggressive | |
| LDCT screening rates (per 100,000) | 3,900 | 5,000 | 10,000 | 5,000 | 5,000 | 5,000 | 10,000 | 10,000 | 10,000 |
| Number of people receiving LDCT screening, by risk group | |||||||||
| All | 266,000 | 341,000 | 682,000 | 341,000 | 341,000 | 341,000 | 682,000 | 682,000 | 682,000 |
| Lowest | 53,200 | 68,200 | 136,400 | 34,100 | 17,000 | 0 | 68,200 | 34,100 | 0 |
| Second lowest | 53,200 | 68,200 | 136,400 | 51,200 | 34,100 | 17,000 | 102,300 | 68,200 | 34,100 |
| Middle | 53,200 | 68,200 | 136,400 | 68,200 | 68,200 | 51,200 | 136,400 | 136,400 | 102,300 |
| Second highest | 53,200 | 68,200 | 136,400 | 85,200 | 102,300 | 102,300 | 170,500 | 204,600 | 204,600 |
| Highest | 53,200 | 68,200 | 136,400 | 102,300 | 119,400 | 170,500 | 204,600 | 238,700 | 341,000 |
SOURCE Authors’ analysis. NOTES In 2015, 6.82 million US adults were eligible for annual LDCT screening, and the current baseline estimate is that 3,900 out of every 100,000 eligible adults receive the screening (as explained in the text).
Status Quo:
Assuming that in the absence of incentive programs (that is, at baseline) 3,900 per 100,000 adults who were eligible (according to the US Preventive Services Task Force criteria) received LDCT screening, we estimated that 266,000 US adults would undergo LDCT screening annually (3.9 percent of 6.82 million eligible people).
Scenario 1: Untargeted Incentive Programs:
We assumed that untargeted incentive programs would uniformly increase LDCT screening uptake among all eligible people, regardless of their lung cancer mortality risk. For example, an untargeted incentive program that increased the LDCT screening rate to 10,000 per 100,000 eligible people would boost the total number of people screened by 416,000, from its baseline estimated value of 266,000 to 682,000. In this scenario, the number of people screened in each risk group would be identical—136,400.
Scenario 2: Risk-Targeted Incentive Programs:
To isolate the benefits attributable to risk targeting alone, we assumed that risk-targeted programs would likewise increase LDCT uptake but shift it toward higher-risk people. We explored three versions of this scenario. Our first version (“conservative”) assumed that 10 percent of the uptake gains would occur among people in the lowest-risk group, 15 percent in the second-lowest-risk group, 20 percent in the middle-risk group, 25 percent in the second-highest-risk group, and 30 percent in the highest-risk group. Our second version (“moderate”) assumed that 5 percent, 10 percent, 20 percent, 30 percent, and 35 percent of the uptake gains would occur in each of the five risk groups (from lowest to highest), respectively. Our last (“aggressive”) version assumed even greater skew, with 0 percent, 5 percent, 15 percent, 30 percent, and 50 percent of uptake occurring in each of the five risk groups, respectively. For example, as LDCT uptake increased to 10,000 per 100,000 eligible people, among 682,000 people screened, risk targeting under the “moderate” version of this scenario would achieve LDCT uptake among 238,700 people in the highest-risk group (35 percent of all screened people) and uptake among 34,100 people in the lowest-risk group (5 percent of all screened people).
Threshold Analysis For Implementation Costs
Because our hypothetical incentive programs (untargeted and risk-targeted) have unknown implementation costs, we conducted a threshold analysis to estimate upper-bound implementation costs that would cancel out the additional NMB of increasing LDCT screening uptake under such programs. If incentive program implementation costs were to exceed these estimated NMBs, investment in such programs would be inefficient.
Limitations
Our study had several limitations. First, we assessed hypothetical programs (targeted and untargeted) because no specific incentive programs have been designed to promote LDCT screening. Rather than specifying policy program details, such as addressing the important role of patient preferences in the form of patient-centered incentive programs,30,31 we investigated potential gains based on assumed program performance—that is, assumptions about how much the incentive program would increase LDCT screening in each of the five risk groups. Our results can shed light on the general implications of risk-targeted incentive programs, but application to real-world programs would require data on how those programs influence actual behavior.
Second, as with any trial-based analysis, the generalizability of our findings is limited. For example, the National Lung Screening Trial sample is younger on average than the real population of smokers. Hence, our estimated life expectancy gains are likely to overstate gains that might be observed in the actual population. On the other hand, there is no reason to believe that the trial results overstated heterogeneity in the real population, and it is heterogeneity that contributes to the benefit of risk-targeted screening and hence risk-targeted benefit design. Indeed, heterogeneity in the real population may be greater than in the trial population, which suggests that we may have understated the benefits of this strategy. In any case, our aim was to use lung cancer screening to illustrate this strategy and not to estimate the actual benefits of this approach for lung cancer screening.
Study Results
Consequences Of Low-Dose Computed Tomography Screening Across Five Risk Groups
The incremental life-year gains of LDCT over chest x-ray screening were positively associated with baseline lung cancer mortality risk (exhibit 2). Among people in the highest-risk group, life-year gains were 2.9 times greater than the corresponding gains among people in the lowest-risk group over a lifetime (4,900 versus 1,700 life-years gained per 100,000 people screened).
Exhibit 2. Life-years gained per 100,000 eligible adults screened by low-dose computed tomography (LDCT) instead of chest x-ray, by risk of lung cancer death.
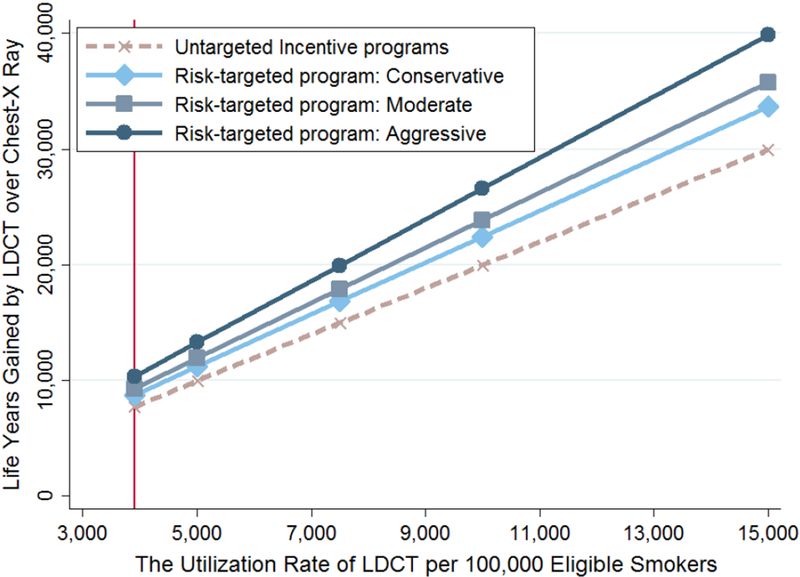
Source/Notes: SOURCE Authors’ analysis. NOTES The average is for all risk groups (explained in the text). In 2015, 6.82 million US adults were eligible for annual LDCT screening, as explained in the text. Whiskers indicate 95% confidence intervals. The interval bounds correspond to the people in a group at the 2.5 and 97.5 percentiles. For example, for people in the highest risk group over a lifetime horizon, the person at the 2.5 percentile gains 0.032 life-years (3,200 life-years per 100,000 people), whereas the person at the 97.5 percentile gains 0.0915 life-years (9,150 life years per 100,000 people).
Assuming that a year of life is worth $100,000, the NMB for LDCT screening exceeded the NMB for chest x-ray screening for all five risk groups because of the substantial value of the additional life-years saved as a result of the averted lung cancer deaths. However, the short-term (seven-year) analysis found the NMB for LDCT screening to be less than the NMB for chest x-ray screening for the three lowest-risk groups. Appendix exhibit C provides greater detail.11
Population Health And Economic Gains Under Different Policy Scenarios
We also estimated incremental population health gains across alternative uptake scenarios (exhibit 3). Appendix exhibit D details economic gains expressed in terms of NMB.11
Exhibit 3. Life-years gained per 100,000 eligible adults screened by low-dose computed tomography (LDCT) instead of chest x-ray, by aggressiveness of risk targeting in incentive programs.
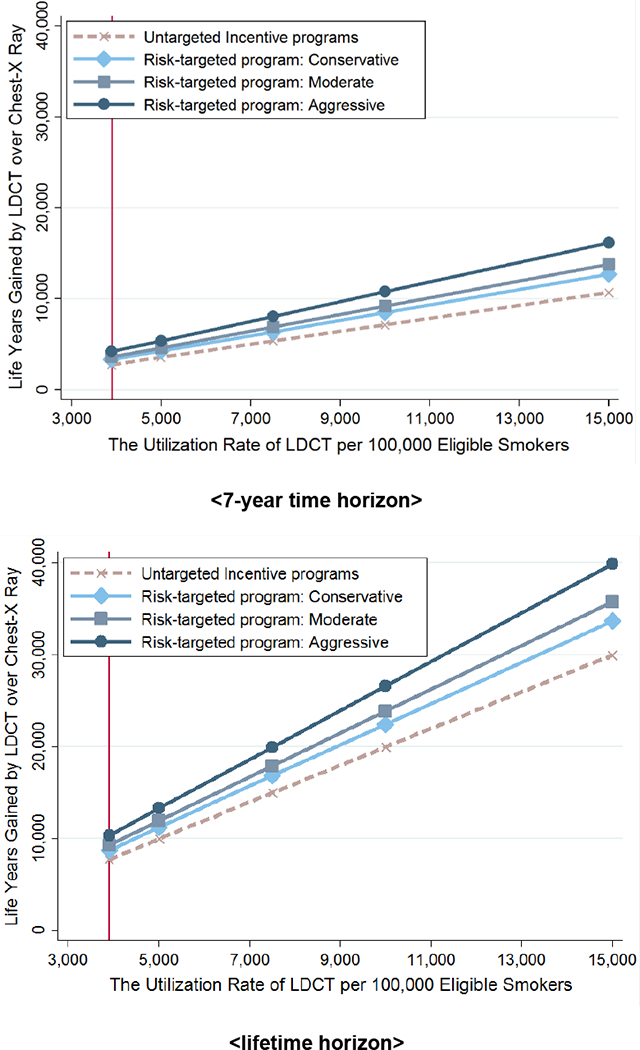
Source/Notes: SOURCE Authors’ analysis. NOTES In 2015, 6.82 million US adults were eligible for annual LDCT screening, as explained in the text. The current baseline estimate is that 3,900 out of every 100,000 eligible adults receive LDCT screening. Conservative, moderate, and aggressive risk targeting are explained in the text.
Untargeted Incentive Programs Versus Status Quo:
The untargeted program that increased LDCT uptake from 3,900 to 10,000 per 100,000 eligible people across all risk groups gained 12,300 life-years based on the lifetime analysis, yielding a NMB of $771M. Projected gains for the seven-year analysis amounted to 4,400 life-years, corresponding to a NMB of $51M.
Risk-Targeted Incentive Programs Versus Status Quo:
Holding overall LDCT uptake to baseline levels but using incentives to shift uptake toward higher-risk people yielded life expectancy gains. Our lifetime analysis projected that risk targeting would confer 1,060 (conservative) to 2,670 (aggressive) additional life-years, corresponding to a NMB gain of $88 million (conservative) to $225 million (aggressive) over the status quo. The seven-year analysis projected that risk targeting would confer gains of 560 (conservative) to 1,450 (aggressive) life-years and NMBs of $41 million (conservative) to $111 million (aggressive).
Risk-Targeted Versus Untargeted Incentive Programs:
Assuming the average uptake increased to 10,000 per 100,000 individuals, compared to the untargeted incentive program, the additional lifetime health gains associated with our three risk-shifting scenarios ranged from 2,470 (conservative scenario) to 6,600 (aggressive scenario) life-years gained, while the corresponding lifetime NMB gains ranged from $210 million to $560 million. In the seven-year analysis, the additional life-year gains ranged from 1,340 (conservative scenario) to 3,630 (aggressive scenario), with corresponding NMBs of $104 million to $282 million.
Upper-Bound Implementation Costs Consistent With Positive Net Benefits
Based on the seven-year projection (which is potentially more relevant for payers), an untargeted incentive program that increased LDCT screening from 3,900 to 10,000 per 100,000 eligible people would have a negative NMB if its costs exceeded $51 million. A risk-targeted program that also increased LDCT utilization from 3,900 to 10,000 people would have a negative NMB only if program costs exceeded $155 million (conservative uptake distribution) to $334 million (aggressive uptake distribution). Exhibit 4 shows the maximum program implementation costs under various scenarios that would be consistent with a positive NMB.
Exhibit 4:
Maximum thresholds for implementation costs under various scenarios (millions of dollars)
| 7-year projection | Lifetime projection | |||||||
|---|---|---|---|---|---|---|---|---|
| Projected LDCT screening rates (per 100,000) | Untargeted programs | Risk-targeted programs | Untargeted programs | Risk-targeted programs | ||||
| Conservative | Moderate | Aggressive | Conservative | Moderate | Aggressive | |||
| 3,900 (Baseline) | $0.0 | $41.0 | $63.8 | $110.6 | $0.0 | $88.0 | $134.6 | $225.0 |
| 5,000 | 9.6 | 61.6 | 90.8 | 150.8 | 144.0 | 249.1 | 308.9 | 424.7 |
| 7,500 | 30.5 | 108.5 | 152.4 | 242.3 | 457.5 | 615.2 | 704.9 | 878.6 |
| 10,000 | 51.4 | 155.4 | 213.9 | 333.8 | 771.1 | 981.3 | 1,100.9 | 1,332.5 |
| 15,000 | 93.1 | 249.2 | 336.9 | 516.8 | 1,398.2 | 1,713.6 | 1,892.9 | 2,240.4 |
SOURCE Authors’ analysis. NOTES The thresholds represent the maximum costs associated with implementing an incentive program to cancel out the additional net monetary benefits of increasing low-dose computed tomography (LDCT) screening under the program. We applied a valuation of $100,000 per life-year to calculate the net of monetized additional life-years gained and health care costs.
Discussion
Because an intervention’s clinical benefits depend on who receives it, targeting the people most likely to benefit is key to improving health care efficiency. Targeting high-risk people has been applied in many clinical areas, including routine eye examinations for patients with diabetes,32 screening for colorectal cancer starting at ages between fifty and seventy-five years,33 and pneumococcal vaccination for the elderly and those with specified chronic conditions.34 However, the application of incentive-based approaches to promote these interventions targeted to high- or higher-risk people has not been explored.
In the context of lung cancer screening using LDCT, our findings suggest that risk-targeted incentive programs could improve population health and yield greater economic benefits than untargeted incentive programs could. Although the lifetime net benefits of LDCT might be positive for all risk groups, the value of the health gains are greater for higher-risk groups. Hence, if resources are insufficient to achieve uptake among all eligible people, targeting the highest-risk people will be more efficient. Consequently, risk targeting can save more lives than untargeted programs.
However, the magnitude of health and economic gains largely depends on the extent of the risk targeting. In empirical evaluations of conventional incentive programs, the changes in utilization rates in response to untargeted incentive programs ranged from 5 percent to 9 percent for preventive services, such as cervical cancer screening35 and vaccination.6 Yet when baseline performance was poor, as is the case with LDCT screening, the incentive program resulted in the largest improvements of the process-of-care outcomes,5 which suggests the potential benefits of risk-targeted incentive programs.
Having established the potential benefit of risk targeting, our study raises the question of how to design programs to promote LDCT screening in the higher-risk group, especially in primary care settings. We explored other clinical areas and incentive programs that might offer insights.
First, provider-level financial incentives are important, because lung cancer screening relies on participation from health care providers—mainly primary care physicians. The lack of knowledge among physicians about reimbursement and clinical guidelines for lung cancer screening has been identified as a key barrier to the uptake of LDCT screening.14,36–38 A risk-targeted pay-for-performance program could be created to tie reimbursement to the attainment of certain LDCT referral goals among people at higher risk for lung cancer mortality.
Specifically, physicians might receive financial bonuses from payers, such as Medicare, depending on the proportion of their higher-risk patients they referred to lung cancer screening within a specified period. For example, primary care reforms in Ontario, Canada, introduced provider-level incentives tied to the proportion of patients who received a Pap smear (for example, $220 for 60 percent, $660 for 70 percent, and $2,200 for 80 percent of patients). The incentive program was associated with higher cervical cancer screening rates, compared to a traditional fee-for-service model.35
Second, health plans or employers could provide financial incentives that directly targeted people at higher risk to encourage annual LDCT screening. A risk-targeted incentive program might take various forms, including programs offering premium discounts, which would be analogous to insurance premium discounts offered to people who complete annual biometric screening;39,40 reward-based programs that offered cash bonuses to people who voluntarily underwent annual LDCT screening; and programs with deposit contracts that put participants at risk of losing a deposit if they failed to undergo annual screening.41
A pragmatic randomized controlled trial showed that financial incentives to participants are the most effective means of achieving smoking cessation.19 Also, recent studies on diabetes control and lipid lowering indicate that awarding financial incentives to both providers and patients can improve outcomes.20,42
Third, financial-based targeted incentive programs could be combined with other initiatives, such as smoking cessation or individual counseling programs. Smoking cessation is known to reduce the risk of lung cancer mortality by more than 50 percent.21 Since both smoking cessation and lung cancer screening can serve as “teachable moments,” the combined programs could increase use of LDCT screening, prevent more lung cancer mortality, and improve systemwide efficiency.43
Based on some of the real-life policy examples, we have shared some thoughts on designing incentive programs. However, without specific incentive programs in place, it is not possible to determine what benefits such programs might actually produce. Hence, our results should be viewed as a starting point.
Although our simulation examined scenarios that increased overall utilization of LDCT screening across all eligible people, preferentially targeting higher-risk people might have the unintended consequence of reducing screening among lower-risk people. Given limited resources, it would be more efficient to target higher-risk people—a primary goal of risk-targeted incentive programs—but such programs should not deter lower-risk groups from seeking lung cancer screening.
Policy Implications
The Affordable Care Act mandates that health plans on the insurance Marketplaces cover, without cost sharing, annual lung cancer screening with LDCT as part of the benefits package. However, even without financial barriers, the uptake of LDCT screening remains disappointingly low.13,44 Clinically, with the routine use of electronic health records and validated risk-prediction models that are readily available online, there is a growing demand to use individualized risk information to identify people who should be screened for lung cancer, rather than using simple at-risk group inclusion criteria.45,46 Incentive programs that target higher-risk people could offer a way to increase utilization and efficiently maximize benefits in light of these challenges.
To ensure the successful implementation of risk-targeted incentive programs, however, policy makers need to identify clinical areas (such as lung cancer screening) where such programs might be particularly useful, as well as to balance trade-offs between the costs of implementing such programs and potential net benefits. For example, a risk-targeted incentive program with $10 monthly premium discounts for the highest-risk people (the top quintile in our study) would cost $84 million annually, assuming 50 percent enrollment among the 1.4 million (20 percent of the 6.8 eligible people) highest-risk people.
Conclusion
The value of health care depends on who receives the intervention. With growing information on which health care services work best for different people, designing incentive programs to target people who can gain the most has the potential to increase the uptake of LDCT screening and enhance population health and economic efficiency.
Supplementary Material
Acknowledgment
This research was funded by a grant from the National Institutes of Health (Grant No. U01NS086294). The authors thank Vaibhav Kumar, of the Tufts Medical Center, and David van Klaveren, of the Leiden University Medical Center, for their contribution to the development of the original risk-prediction model.
Bios for 2018–05148_Kim
Bio 1: David D. Kim (DKim3@tuftsmedicalcenter.org) is an assistant professor of medicine in the School of Medicine, Tufts University, and an investigator in the Center for the Evaluation of Value and Risk in Health, Tufts Medical Center, in Boston, Massachusetts.
Bio 2: Joshua T. Cohen is a research associate professor of medicine in the School of Medicine, Tufts University, and deputy director of the Center for the Evaluation of Value and Risk in Health, Tufts Medical Center.
Bio 3: John B. Wong is a professor of medicine in the School of Medicine, Tufts University and chief of the Division of Clinical Decision Making, Tufts Medical Center.
Bio 4: Babak Mohit is a postdoctoral research fellow in the Center for the Evaluation of Value and Risk in Health, Tufts Medical Center.
Bio 5: A. Mark Fendrick is a professor in the Department of Internal Medicine, University of Michigan, in Ann Arbor.
Bio 6: David M. Kent is a professor of medicine in the School of Medicine, Tufts University and director of the Predictive Analystics and Comparative Effectiveness Center, Tufts Medical Center.
Bio 7: Peter J. Neumann is a professor of medicine in the School of Medicine, Tufts University, and director of the Center for the Evaluation of Value and Risk in Health, Tufts Medical Center
Notes
- 1.CMS.gov. Medicare Advantage Value-Based Insurance Design Model [Internet]. Baltimore (MD): Centers for Medicare and Medicaid Services; [last updated 2018 Oct 23; cited 2018 Nov 16]. Available from: https://innovation.cms.gov/initiatives/VBID [Google Scholar]
- 2.Centers for Medicare and Medicaid Services. Roadmap for implementing value driven healthcare in the traditional Medicare fee-for-service program [Internet]. Baltimore (MD): CMS; [cited 2018 Nov 16]. Available from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/downloads/VBPRoadmap_OEA_1-16_508.pdf [Google Scholar]
- 3.Epstein AM, Lee TH, Hamel MB. Paying physicians for high-quality care. N Engl J Med. 2004;350(4):406–10 [DOI] [PubMed] [Google Scholar]
- 4.Chernew ME, Rosen AB, Fendrick AM. Value-based insurance design. Health Aff (Millwood). 2007;26(2):w195–203. DOI: 10.1377/hlthaff.26.2.w195 [DOI] [PubMed] [Google Scholar]
- 5.Mendelson A, Kondo K, Damberg C, Low A, Motúapuaka M, Freeman M, et al. The effects of pay-for-performance programs on health, health care use, and processes of care: a systematic review. Ann Intern Med. 2017;166(5):341–53 [DOI] [PubMed] [Google Scholar]
- 6.Houle SK, McAlister FA, Jackevicius CA, Chuck AW, Tsuyuki RT. Does performance-based remuneration for individual health care practitioners affect patient care? A systematic review. Ann Intern Med. 2012;157(12):889–99 [DOI] [PubMed] [Google Scholar]
- 7.Gibson TB, Wang S, Kelly E, Brown C, Turner C, Frech-Tamas F, et al. A value-based insurance design program at a large company boosted medication adherence for employees with chronic illnesses. Health Aff (Millwood). 2011;30(1):109–17 [DOI] [PubMed] [Google Scholar]
- 8.Frank MB, Fendrick AM, He Y, Zbrozek A, Holtz N, Leung S, et al. The effect of a large regional health plan’s value-based insurance design program on statin use. Med Care. 2012;50(11):934–9 [DOI] [PubMed] [Google Scholar]
- 9.Hirth RA, Cliff EQ, Gibson TB, McKellar MR, Fendrick AM. Connecticut’s value-based insurance plan increased the use of targeted services and medication adherence. Health Aff (Millwood). 2016;35(4):637–46 [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Gupta A, Fendrick AM. Value-based insurance design improves medication adherence without an increase in total health care spending. Health Aff (Millwood). 2018;37(7):1057–64 [DOI] [PubMed] [Google Scholar]
- 11.To access the appendix, click on the Details tab of the article online.
- 12.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jemal A, Fedewa SA. Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncol. 2017;3(9):1278–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ersek JL, Eberth JM, McDonnell KK, Strayer SM, Sercy E, Cartmell KB, et al. Knowledge of, attitudes toward, and use of low-dose computed tomography for lung cancer screening among family physicians. Cancer. 2016;122(15):2324–31 [DOI] [PubMed] [Google Scholar]
- 15.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–8 [DOI] [PubMed] [Google Scholar]
- 16.Ten Haaf K, Jeon J, Tammemägi MC, Han SS, Kong CY, Plevritis SK, et al. Risk prediction models for selection of lung cancer screening candidates: a retrospective validation study. PLoS Med. 2017;14(4):e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA. 2016;315(21):2300–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. N Engl J Med. 2018;378(24):2302–10 [DOI] [PubMed] [Google Scholar]
- 20.Asch DA, Troxel AB, Stewart WF, Sequist TD, Jones JB, Hirsch AG, et al. Effect of financial incentives to physicians, patients, or both on lipid levels: a randomized clinical trial. JAMA. 2015;314(18):1926–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321(7257):323–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar V, Cohen JT, van Klaveren D, Soeteman DI, Wong JB, Neumann PJ, et al. Risk-targeted lung cancer screening: a cost-effectiveness analysis. Ann Intern Med. 2018;168(3):161–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DD, Basu A. New metrics for economic evaluation in the presence of heterogeneity: focusing on evaluating policy alternatives rather than treatment alternatives. Med Decis Making. 2017;37(8):930–41 [DOI] [PubMed] [Google Scholar]
- 24.Fenwick E, Claxton K, Sculpher M. The value of implementation and the value of information: combined and uneven development. Med Decis Making. 2008;28(1):21–32 [DOI] [PubMed] [Google Scholar]
- 25.Kim DD, Wilkinson CL, Pope EF, Chambers JD, Cohen JT, Neumann PJ. The influence of time horizon on results of cost-effectiveness analyses. Expert Rev Pharmacoecon Outcomes Res. 2017;17(6):615–23 [DOI] [PubMed] [Google Scholar]
- 26.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7 [DOI] [PubMed] [Google Scholar]
- 27.Ryen L, Svensson M. The willingness to pay for a quality adjusted life year: a review of the empirical literature. Health Econ. 2015;24(10):1289–301 [DOI] [PubMed] [Google Scholar]
- 28.Nimdet K, Chaiyakunapruk N, Vichansavakul K, Ngorsuraches S. A systematic review of studies eliciting willingness-to-pay per quality-adjusted life year: does it justify CE threshold? PLoS One. 2015;10(4):e0122760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20(3):332–42 [DOI] [PubMed] [Google Scholar]
- 30.Kerr EA, Hayward RA. Patient-centered performance management: enhancing value for patients and health care systems. JAMA. 2013;310(2):137–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caverly TJ, Cao P, Hayward RA, Meza R. Identifying patients for whom lung cancer screening is preference-sensitive: a microsimulation study. Ann Intern Med. 2018;169(1):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Diabetes Association. Standards of Medical Care in Diabetes—2018 abridged for primary care providers. Clin Diabetes. 2018;36(1):14–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knudsen AB, Zauber AG, Rutter CM, Naber SK, Doria-Rose VP, Pabiniak C, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315(23):2595–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Vaccines and preventable diseases: pneumococcal vaccination [Internet]. Atlanta (GA): CDC; [last updated 2017 Dec 6; cited 2018 Nov 16]. Available from: https://www.cdc.gov/vaccines/vpd/pneumo/index.html [Google Scholar]
- 35.Pendrith C, Thind A, Zaric GS, Sarma S. Financial incentives and cervical cancer screening participation in Ontario’s primary care practice models. Healthc Policy. 2016;12(1):116–28 [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffman RM, Sussman AL, Getrich CM, Rhyne RL, Crowell RE, Taylor KL, et al. Attitudes and beliefs of primary care providers in New Mexico about lung cancer screening using low-dose computed tomography. Prev Chronic Dis. 2015;12:E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis JA, Petty WJ, Tooze JA, Miller DP, Chiles C, Miller AA, et al. Low-dose CT lung cancer screening practices and attitudes among primary care providers at an academic medical center. Cancer Epidemiol Biomarkers Prev. 2015;24(4):664–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klabunde CN, Marcus PM, Silvestri GA, Han PK, Richards TB, Yuan G, et al. U.S. primary care physicians’ lung cancer screening beliefs and recommendations. Am J Prev Med. 2010;39(5):411–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattke S, Kapinos K, Caloyeras JP, Taylor EA, Batorsky B, Liu H, et al. Workplace wellness programs: services offered, participation, and incentives. Rand Health Q. 2015;5(2):7. [PMC free article] [PubMed] [Google Scholar]
- 40.Maeng DD, Geng Z, Marshall WM, Hess AL, Tomcavage JF. An analysis of a biometric screening and premium incentive–based employee wellness program: enrollment patterns, cost, and outcome. Popul Health Manag. 2018;21(4):303–8 [DOI] [PubMed] [Google Scholar]
- 41.Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain-McGovern J, et al. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372(22):2108–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorincz IS, Lawson BC, Long JA. Provider and patient directed financial incentives to improve care and outcomes for patients with diabetes. Curr Diab Rep. 2013;13(2):188–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One. 2013;8(8):e71379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinsinger LS, Anderson C, Kim J, Larson M, Chan SH, King HA, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399–406 [DOI] [PubMed] [Google Scholar]
- 45.Tammemägi MC. Improving implementation of lung cancer screening with risk prediction models. Ann Intern Med. 2018;169(1):54–5 [DOI] [PubMed] [Google Scholar]
- 46.Katki HA, Kovalchik SA, Petito LC, Cheung LC, Jacobs E, Jemal A, et al. Implications of nine risk prediction models for selecting ever-smokers for computed tomography lung cancer screening. Ann Intern Med. 2018;169(1):10–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


