Abstract
In this work, we are proposing the green synthesis of gold nanoparticles (AuNPs) using aqueous extracts of A. triphylla and evaluating their antibacterial and catalytic properties. Characterization was performed by UV–Vis and FT-IR spectroscopies, X-ray diffraction, and transmission electron microscopy (TEM). Antibacterial activity of AuNPs was analyzed using E. coli and S. Aureus and catalytic activity was determined by the degradation of methylene blue and congo red. UV–Vis analysis showed an increase in AuNPs concentration by increasing the extract concentration, volume extract, and precursor salt concentration. The crystalline nature of AuNPs was corroborated by X-ray diffraction. TEM analysis showed nanoparticles with spherical morphology (mostly) and size between 40 and 60 nm. These results are novel because they showed a homogeneous morphology and a narrow size distribution which is difficult to obtain in green synthesis processes. Results of antibacterial activity showed inhibition zones of 11.3 mm and 10.6 mm for S. Aureus and E. coli, respectively, indicating the bactericidal capacity of the nanoparticles. The degradation periods for methylene blue and congo red were 5 and 11 min, respectively, which are very short compared with previous reports. These results are of great significance for catalytic applications. Therefore, A. triphylla extracts made possible AuNPs synthesis and the nanoparticles obtained can be used as catalytic and antibacterial materials for water remediation.
Keywords: Green synthesis, Gold nanoparticles, Catalytic activity, Antibacterial, Bioremediation, Characterization, Aloysia triphylla
Introduction
Gold nanoparticles (AuNPs) have been widely studied because of their unique physical and chemical properties and their potential use in environmental and medical areas (Dhamecha et al. 2016; Shanker et al. 2016), for example, the use of AuNPs in remediation processes and wastewater treatment. Wastewater could contain organic dyes and toxic substances from textile, cosmetic and paper processing industries, which use them to color their products (Rasheed et al. 2018; Salem et al. 2018; Zhou and Srinivasan 2015). These industrial effluents could reach rivers and lakes, leading to an enormous risk to flora, fauna and human health. Treatment for the effluents could include absorption, filtration, and precipitation (Bhatt et al. 2017) that could have low efficiency and be expensive if the absorption materials are not selected properly (Fathima et al. 2018; Wang et al. 2018). Gold nanoparticles could be used as part of efficient absorption and remediation materials. Another areas for using nanoparticles require studying the antibacterial and the catalytic activity of AuNPs synthesized by green methods (Rajkumari et al. 2017; Yuan et al. 2016).
Considering the numerous applications of the AuNPS, different synthesis methods had been proposed. Physical methods are expensive and require a significant amount of energy (Shanker et al. 2016). Chemical methods are the most frequently used; however, they involve using toxic materials that are difficult to handle (Singh et al. 2017). It is also difficult to separate these substances from synthesized nanoparticles. Biological or green methods involve using organisms such as fungi (Manjunath et al. 2016), bacteria (Singh et al. 2016), algae (Parial et al. 2012), seaweeds (Deepak et al. 2017), and plants (Teimouri et al. 2018). Green methods are inexpensive and non-toxic techniques (alias Antonysamy et al. 2017). Nanoparticles synthesized by these techniques have been used in sensors, biomedicine, and water remediation (Nadeem et al. 2017). Plants are the organism most frequently used, because the process is simpler and cheaper than the processes based on other organisms (Vijayaraghavan and Ashokkumar 2017). A wide variety of plants had been used for the synthesis of AuNPs; for example, Pterocarpus santalinus (Keshavamurthy et al. 2018), Hypericum perforatum (Pasca et al. 2014), Eucalyptus oleosa (Pourmortazavi et al. 2017), Aloe arborescens (Altaf and Jaganyi 2016), and Buddleja globosa (Carmona et al. 2017). The extracts of the plant provide the reducing agents and stabilizers for the synthesis. Moreover, Aloysia triphylla (A. triphylla) is a medicinal plant with a high content of flavonoids and acid phenolics (Zamorano-Ponce et al. 2006). The A. triphylla extracts are used in the treatment of digestive disorders and have high antioxidant activity (Carnat et al. 1999). Therefore, A. triphylla is an excellent candidate for the green synthesis of gold nanoparticles due to its properties. To our knowledge, this plant only has been used before for silver nanoparticles in a previous report (López-Miranda et al. 2016). In this work, we are proposing the synthesis of AuNPs using aqueous extracts of A. triphylla and evaluating the gold nanoparticles antibacterial and catalytic properties for medical and environmental applications.
Materials and methods
Materials
The precursor salt, HAuCl4 analytical grade, was purchased from Sigma-Aldrich. The fresh A.triphylla leaves were collected from Zacapu Michoacán, México. Methylene blue (MB) and congo red (CR), both reactive grades, were purchased from Sigma-Aldrich. Distilled water was used for extracts and solutions.
Extract preparation
A.triphylla leaves were washed several times with distilled water. Subsequently, they were dried and kept in the dark for 10 days. 0.5, 1, and 2 g of leaves were mixed separately with 50 mL of distilled water each for analyzing concentration changes in the extracts. Mixtures were kept under magnetic stirring for 30 min at 50 °C. Samples were filtered using filter paper No. 41. Extracts (liquid phase) were cooled and stored at 4 °C for further use.
Synthesis of gold nanoparticles (AuNPs)
Precursor salt (HAuCl4) solutions of 1, 3, 5, 7, and 10 mM were prepared and 5 mL of a sample extract was mixed with 10 mL of each solution. Then, HAuCl4 5 mM solution was selected for the synthesis. Each extract sample was then mixed with 10 mL of HAuCl4 5 mM solution. Extract volume was varied from 1 to 5 mL for each set of experiments.
Characterization of AuNPs
UV–Vis spectroscopy (Metash UV6000) was performed to detect AuNPs and to determinate qualitatively the size, concentration, and distribution. X-ray diffraction (Bruker D8) was used to evaluate the crystal structure. The Debye–Scherrer equation was used to determine the crystal size. Morphology, size, and size distribution were obtained by transmission electron microscopy (Philips Tecnai F20). Finally, the organic compounds involved in the synthesis were determined by FT-IR spectroscopy (Bruker Vector 33).
Antibacterial analysis
AuNPs synthesized with A. triphylla extracts were evaluated as an antibacterial agent. The analysis was performed through the disc diffusion method. The bacteria evaluated were E. coli (gram-negative) and S. Aureus (gram-positive). The AuNPs concentration used for the analysis was 50 µg/ml. Ampicillin and A. triphylla extract were used as the control agents. The concentration of ampicillin was 1 mg/mL, such as previous reports (Krishna et al. 2016; Mythili et al. 2018). A paper disc, permeated with 20 µl of AuNPs, was placed in the plate containing the particular bacterium. A similar procedure was conducted evaluating the antibiotic and A. triphylla extract. The plates were incubated for 24 h at 36 °C. Finally, the halos of the inhibition zone were measured. All the experiments were performed in triplicate for the statistical analysis.
Evaluation of catalytic activity
The catalytic properties of AuNPs were analyzed through the degradation of methylene blue (5 ppm) and congo red (15 ppm). 1 ml of dye was mixed with 50 µl of NaBH4 (0.1 M) and 100 µl of the solution containing the gold nanoparticles. Then, the sample was monitored by UV–Vis with 30 s time intervals, until the disappearance of the typical bands of methylene blue and congo red, respectively. Two control experiments were carried out for each organic dye. In the first experiment, solution only contained NaBH4 and in the second experiment, solution had AuNPs only.
Results and discussion
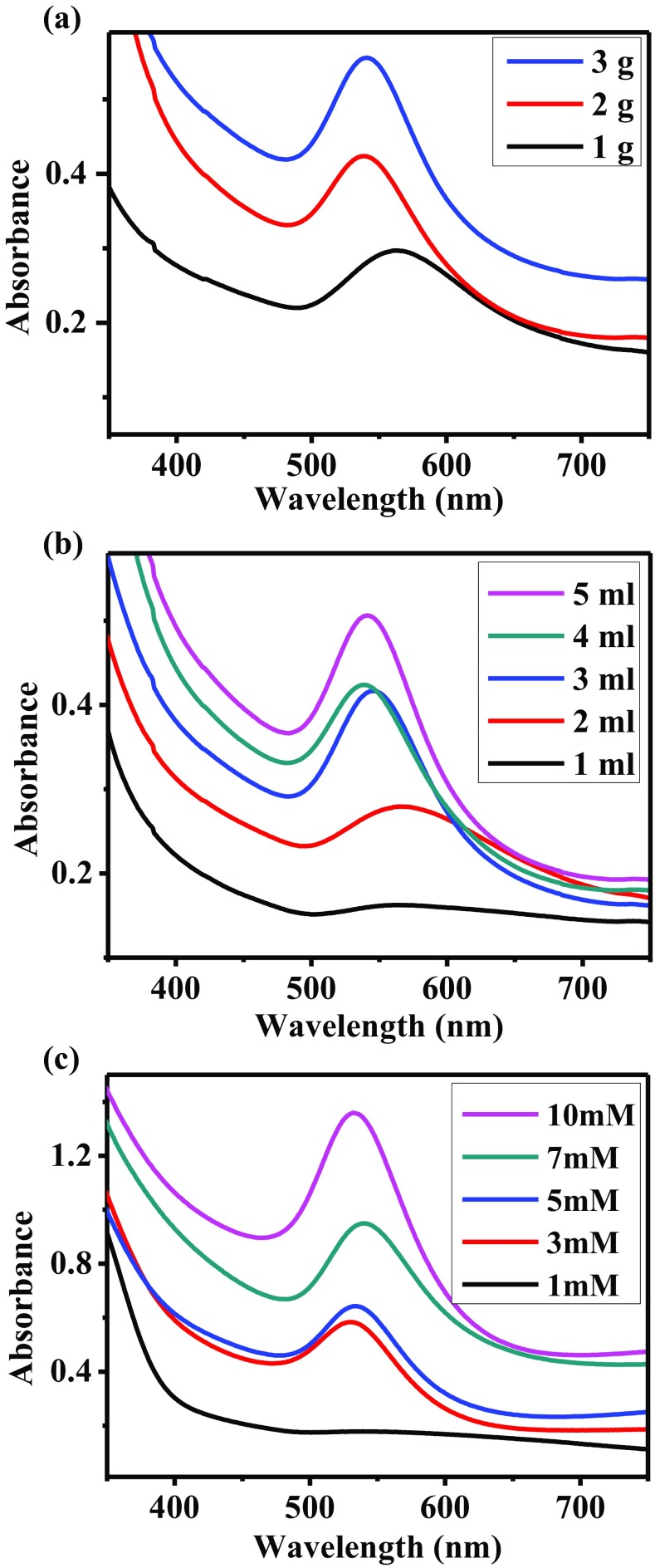
Figure 1a shows the effect of the extract concentration in the synthesis of AuNPs, varying the initial leaves weight. As the concentration increases, the surface plasmon resonance (SPR) or absorbance band of the AuNPs is more intense. This means that the concentration of nanoparticles synthesized increased. Moreover, the position of the band changes significantly. When 1 g of leaves was used, the SPR is located at 560 nm, while for 2 and 3 g the bands are centered at 530 nm. According to several investigations, the SPR position depends on the average size of the nanoparticles (Gangapuram et al. 2018; Lopes and Courrol 2018; Sana and Dogiparthi 2018). That is, as the size of the nanoparticles decreases, the absorption band shifts to the left. Finally, the width of the plasmon is the smallest for 3 g of A. triphylla compared with the other two samples, meaning a narrow size distribution. Therefore, the best results were obtained using the most concentrated extract. In this condition, there is a higher concentration of reducing agents and stabilizers involved in the AuNPs synthesis. The extract volume used in the synthesis reaction was also evaluated. The UV–Vis spectra recorded are shown in Fig. 1b. As can be observed, the characteristics of the SPR are improved with the increase of the A. triphylla extract volume. For 1 ml of extract, there is no evidence of an absorption band suggesting the presence of AuNPs. The spectrum corresponding to 2 ml of extract shows a low-intensity band, whose average width is significantly large. When 3 and 4 ml of A. triphylla extract were used, the absorption bands recorded have similar intensity. However, the second one is located at a shorter wavelength. Finally, the best result was obtained using 5 ml of extract. In this spectrum, the absorption band, centered at 530 nm, is the most intense and has the smallest average width. Therefore, it can be deduced that the concentration of reducing agents and stabilizer compounds increases by increasing the volume of A. triphylla extract. These results are consistent with those obtained in other investigations (Emmanuel et al. 2014; Umamaheswari et al. 2018). Figure 1c shows the UV–Vis spectra of the AuNPs synthesized with 5 ml of A. triphylla extract and varying the concentration of the precursor salt. As can be seen, for 1 mM HAuCl4 solution there is no evidence of AuNPs. When the concentration was increased to 3 mM, a band located at 540 nm appears which corresponds to the AuNPs synthesized. For the subsequent conditions, as the concentration of HAuCl4 increased, the SPR intensity also increased significantly. In this way, the highest quantity of nanoparticles was obtained using a 10 mM solution of HAuCl4. Moreover, this last spectrum shows the absorption band centered at 520 nm. The shift of the band suggests the synthesis of the smallest nanoparticles. According to LaMer theory (LaMer and Dinegar 1950), as the concentration of precursor salt increases, the ions are rapidly reduced and stabilized, resulting in the synthesis of a high density of nanoparticles, with a small size.
Fig. 1.
UV–Vis analysis of AuNPs synthesized with A. triphylla varying: (a) extract concentration, (b) extract volume, and (c) concentration of HAuCl4 precursor salt
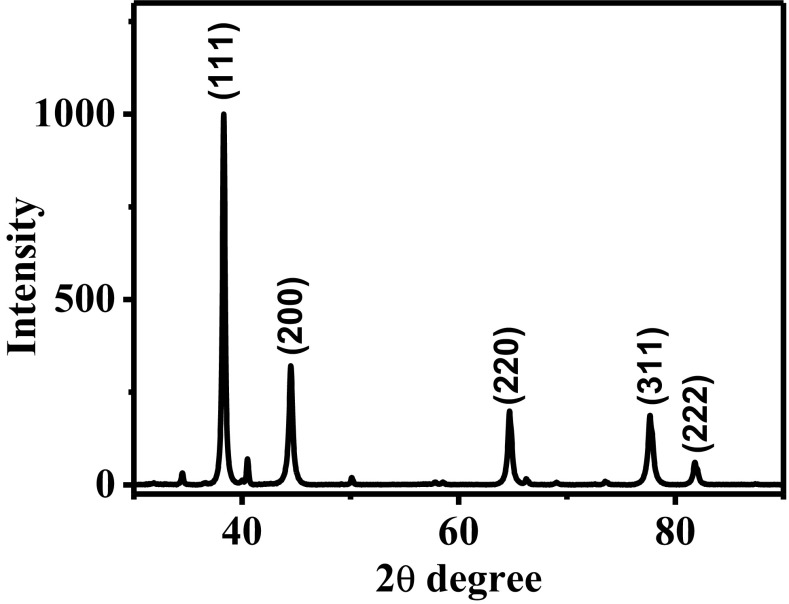
Figure 2 shows the X-ray diffraction (XRD) pattern of the nanoparticles synthesized with 5 ml of extract, based on 3 g of leaves, and 10 ml of HAuCl4 solution (10 mM). The five reflections corresponding to the gold phase with an FCC structure were observed. These reflections are located at 37°, 45°, 65°, 77° and 82° corresponding to (111), (200), (220), (311), and (222) crystallographic planes, respectively. The average crystal size was determined using Debye–Scherrer equation, obtaining a crystal size of 47 nm. These results corroborate the presence of metallic gold nanoparticles. On the other hand, some reflections are observed in the X-ray diffraction pattern, corresponding to organic compounds from the extract, acting as stabilizing agents.
Fig. 2.
X-ray diffraction pattern of AuNPs synthesized with A. triphylla extract
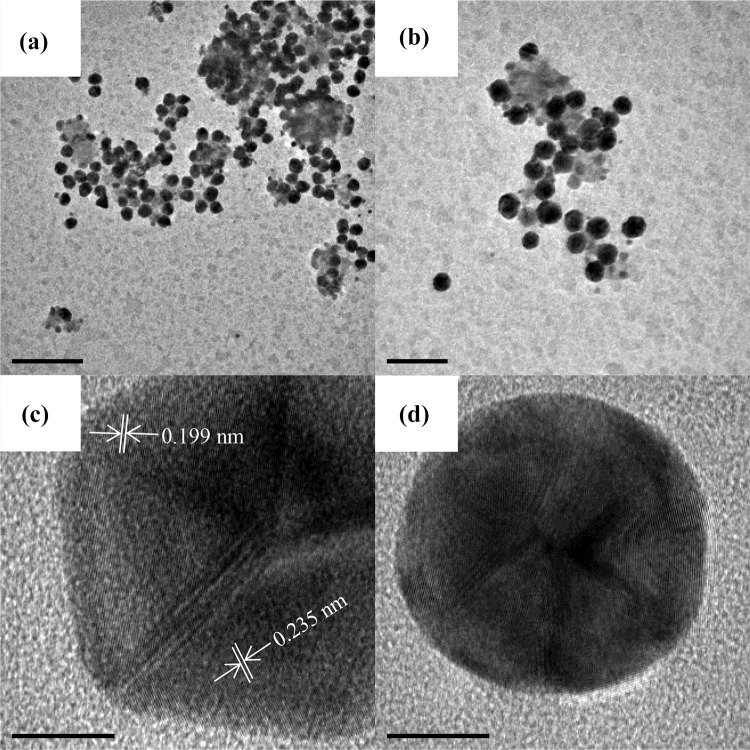
Figure 3 shows the transmission electron microscopy (TEM) analysis of AuNPs synthesized with 5 ml of A. triphylla extract. Figure 3a, b shows two low magnification TEM micrographs of AuNPs. A large density of nanoparticles is observed; also a fraction of nanoparticles seem to be confined in the organic matrix. The nanoparticles morphology is mostly spherical, and the particle size is between 40 and 60 nm. This result is coherent with the measurement obtained by X-ray diffraction. However, some triangular particles are observed which is typical of AuNPs synthesized by the green synthesis method (Ahmad et al. 2017). On the other hand, the Fig. 3c, d shows high-resolution TEM (HR-TEM) micrographs of the AuNPs. The interplanar distance was measured (0.235 nm), corresponding to (111) crystallographic planes of gold with FCC structure. These results demonstrate the ability of the A. triphylla extract as reducing agent for AuNPs synthesis. The presence of different morphologies is due to the intervention of different organic compounds whose reduction and stabilization rates are different.
Fig. 3.
TEM analysis of AuNPs synthesized with A. triphylla extract. a, b Low magnification TEM micrographs showing the size and morphology. c, d HR-TEM micrographs showing the structure of the nanoparticles
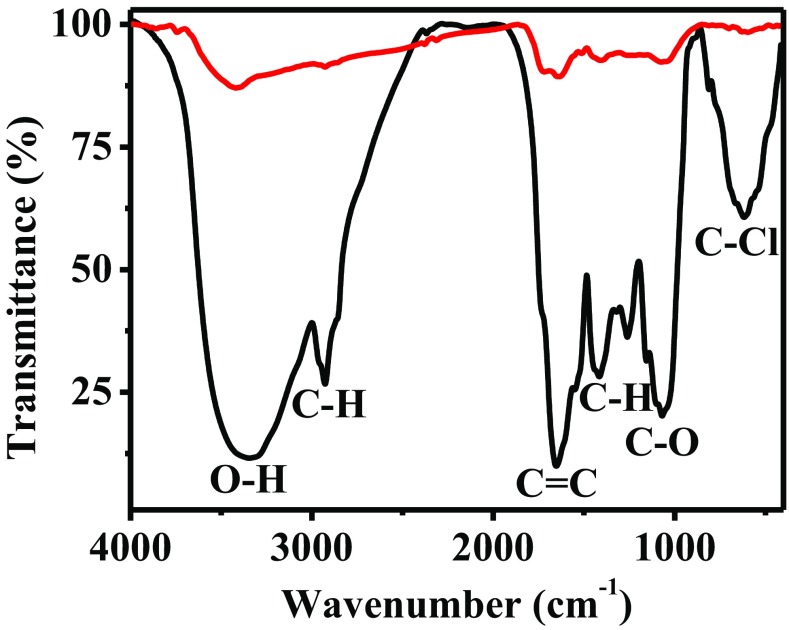
The FT-IR analysis, shown in Fig. 4, was performed to determine the organic compounds involved in the synthesis of AuNPs. The spectrum corresponding to the A. triphylla extract shows several characteristics bands (black line). The signal located at 3400 cm− 1 corresponds to the O–H bonds. The band at 2900 cm− 1 belongs to C–H stretching vibration. The signals from the aromatic rings are located between 1000 and 1600 cm− 1 and correspond to the C = C, C–H, and C–O vibrations. Finally, a strong signal at 950 cm− 1 is observed that corresponds to the C–Cl bond. Once the nanoparticles were synthesized and washed, the FT-IR spectrum was performed (red line). As can be seen, the intensity of the bands of the corresponding spectrum decreased significantly. Only the signals corresponding to the O–H, C = C, and C–O bonds are present, which are centered at 3200, 1600 and 1100 cm− 1, respectively. These results confirm that the flavonoids are responsible for the reduction of gold ions and stabilization of the nanoparticles synthesized.
Fig. 4.
FT-IR spectra of A. triphylla extract (black line) and AuNPs synthesized (red line)
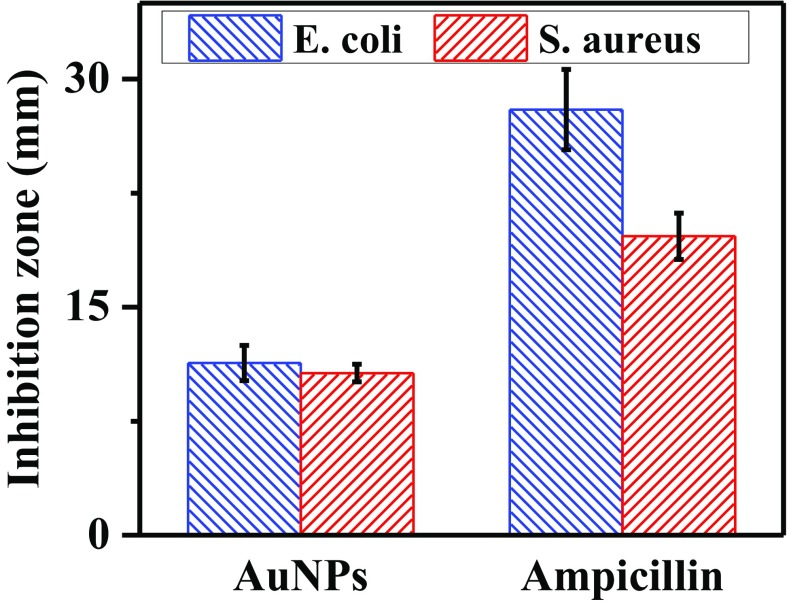
Figure 5 shows the results of AuNPs antibacterial activity against E. coli (gram-negative) and S. Aureus (gram-positive). For both bacteria, AuNPs produced inhibition zones. The halos measured were 11.3 mm for E. coli and 10.6 mm for S. Aureus. The inhibition diameters observed with ampicillin were 28 mm and 19 mm for E. coli and S. Aureus, respectively. Furthermore, A. triphylla extract did not show the formation of an inhibition zone that suggests antibacterial activity. Therefore, the results demonstrate the antibacterial activity of AuNPs, against Gram-negative and Gram-positive bacteria, which can be used for water bioremediation and biomedical applications. Several works have reported the antibacterial activity of AuNPs (Dhayalan et al. 2018; Hamelian et al. 2018; Mishra et al. 2014; Patra et al. 2016). However, the mechanism by which the nanoparticles interact with bacteria to cause their death is not yet clear. The bactericidal behavior can be attributed to the ability of AuNPs to damage the wall cell. The interaction between AuNPs and cells surface results in the alteration of some vital functions like permeability and respiration, leading to bacteria death (Suganya et al. 2015). Furthermore, Rajan et al. suggested that gold ions can be released causing damage to the bacteria cells and, therefore, increasing the antibacterial ability (Rajan et al. 2017). Moreover, the concentration and size of nanoparticles play an important role in these processes. The antibacterial capacity of AuNPs is a result of their high active surface area. Therefore, the increase of nanoparticles concentration leads to the improvement of bactericidal behavior (Hamelian et al. 2018; Mythili et al. 2018). On the other hand, the decrease of nanoparticles size leads to surface area increase. Moreover, the small nanoparticles are easily anchored to the cell, resulting in the enhancement of antibacterial activity (Geethalakshmi and Sarada 2013).
Fig. 5.
Antibacterial activity of AuNPs synthesized with A. triphylla extract
The catalytic activity of AuNPs was evaluated through the biodegradation of organic dyes. Figure 6 shows the analysis of the degradation of MB. The spectra were recorded every 30 s. This substance has two absorption bands located at 614 nm and 654 nm. Figure 6a shows the UV–Vis spectra of MB with AuNPs in the absence of NaBH4. The bands corresponding to MB remain without apparent changes during 15 min. Similar results were observed when MB was mixed with NaBH4 in absence of AuNPs (Fig. 6b). In this case, a slight decrease in the intensity bands of MB can be seen after 15 min; however, the change is negligible. Therefore, it is suggested that there is no degradation reaction of dye for both previous conditions. Figure 6c shows the UV–Vis spectra of MB in the presence of AuNPs and sodium borohydride. As observed, the intensity of the bands corresponding to MB decreases until it disappears. Figure 6d shows the plots of ln(A/A0) and biodegradation percentage of MB. A short induction period of 30 s can be noted in which the value of A/A0 is similar. Moreover, the rate constant of biodegradation, calculated from the linear part, was 12.73 × 10− 3 s− 1. From the biodegradation plot, it can be seen that, after the induction time, the rate increases significantly during the next 3.5 min, reaching 98% of degradation. Finally, the rate becomes slow, obtaining a value of 99.99%. The time to degrade MB is shorter than those reported in several works (Jyoti and Singh 2016; Nadaf and Kanase 2016). According to previous research, the rate and efficiency of biodegradation depend on the concentration, size, and shape of the nanoparticles (Bhatt et al. 2017; Vidhu and Philip 2014).
Fig. 6.
Biodegradation analysis of MB using: (a) AuNPs in the absence of NaBH4, (b) NaBH4 in the absence of AuNPs, (c) AuNPs in the presence of NaBH4, and (d) plots of ln(A/A0) and biodegradation percentage as a function of time
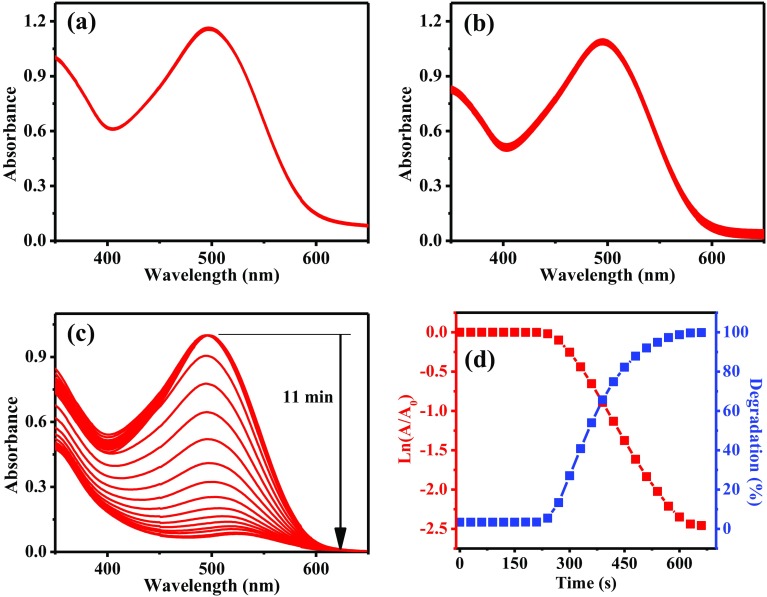
CR was also evaluated to determine the catalytic capacity of AuNPs. This substance has an SPR located at 497 nm. Figure 7 presents the monitoring by UV–Vis of the biodegradation of CR. Figure 7a shows the UV–Vis spectra of CR with AuNPs in the absence of NaBH4. The analysis was carried out for 15 min and no changes in CR signal were observed. Figure 7b corresponds to degradation analysis of CR with NaBH4 in the absence of AuNPs. The signal of CR decreased slightly after 15 min. Therefore, the results obtained using only AuNPs or NaBH4 indicate the low efficiency of the reaction. On the other hand, as can be seen in Fig. 7c, in the first six spectra the plasmon band corresponding to CR has the same absorbance. This behavior is due to an activation period which has been previously reported (Sengan et al. 2018). Then, the absorbance of SPR begins to decrease significantly until close to zero. The maximum biodegradation is reached in 11 min. After that, no relevant changes were observed. However, a weak band centered at approximately 530 nm is observed. This signal corresponds to AuNPs present in the sample. Figure 7d shows the evolution of ln(A/A0) and the CR biodegradation. The induction period was evident because no changes in A/A0 values are observed. After that time, the plot shows a linear behavior, from which the rate constant was determined (7.82 × 10− 3 s− 1). From the biodegradation plot, the induction time of 3.5 min is corroborated. Then, a rapid biodegradation is observed for 3 min, reaching 92% of degradation. Finally, the passivation is observed in the last 2.5 min. The total degradation of CR was 99.8% in 11 min. This degradation time was shorter than that reported by Nadaf et al., who obtained a similar biodegradation percentage in periods of 20 min (Nadaf and Kanase 2016).
Fig. 7.
Biodegradation analysis of CR using: (a) AuNPs in the absence of NaBH4, (b) NaBH4 in the absence of AuNPs, (c) AuNPs in the presence of NaBH4, and (d) plots of ln(A/A0) and biodegradation percentage as a function of time
The degradation process is carried out by the electrons from NaBH4 reducing agent. However, in the absence of nanoparticles, the degradation rate is very slow because of the high reduction potential of organic dyes. The ability of AuNPs to enhance the degradation of organic dyes is attributed to their unique properties, i.e., the high surface area and reactivity (Saravanan et al. 2017). According to Desai, these properties lead to decreasing the reduction potential of organic dyes (Desai et al. 2018). Moreover, AuNPs act as a substrate adsorbing the NaBH4 and dye molecules. Therefore, AuNPs transfer the electrons from NaBH4 to organic dye, to carry out the reduction reaction (Choudhary et al. 2017; Umamaheswari et al. 2018). In this way, the complete degradation of dyes is performed in a short time.
Conclusions
It was possible to synthesize gold nanoparticles with the A.triphylla extracts. By increasing extract concentration and volume, the density of the nanoparticles with small size also increases. Increments on HAuCl4 concentration induced high absorbance. X-ray diffraction analysis indicates the presence of gold with FCC structure. The size of the nanoparticles obtained was 40–60 nm and most of them are spherical, as shown by the transmission electron microscopy. These results are novel and interesting because we obtained nanoparticles with homogeneous characteristics, and in green synthesis processes, it is very difficult to control those physical characteristics. On the other hand, the FT-IR results showed the involvement of flavonoids and phenolic compounds in the reduction and stabilization processes. AuNPs showed antibacterial activity against Gram-positive and Gram-negative bacteria; the inhibition zones for S. Aureus and E. coli were 11.3 mm and 10.6 mm, respectively. The catalytic activity of the AuNPs was evaluated through biodegradation of organic dyes. Methylene blue was biodegraded in 5 min and congo red in 11 min. These degradation periods (for both dyes) were very short. These results are of great significance for catalytic applications. Therefore, AuNPs obtained with A.triphylla extracts can be used as catalytic and antibacterial materials for water remediation.
Acknowledgements
The authors would like to acknowledge to the Laboratorio Nacional de Caracterización de Materiales (LaNCaM) at the CFATA–UNAM. Authors acknowledge to J. A. Cervantes-Chávez from Universidad Autónoma de Querétaro for the support in the antibacterial study reported in the present work. The authors are grateful to Ana L. Ramos-Jacques for her comments in proofreading the final manuscript.
Compliance with ethical standards
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
References
- Ahmad T, Bustam MA, Irfan M, Moniruzzaman M, Asghar HMA, Bhattacharjee S. Green synthesis of stabilized spherical shaped gold nanoparticles using novel aqueous Elaeis guineensis (Oil Palm) leaves extract. Extr J Mol Struct. 2017;1159:167–173. doi: 10.1016/j.molstruc.2017.11.095. [DOI] [Google Scholar]
- alias Antonysamy MJ, Santhanam A, Thangaiah S, Narayanan J. Green synthesis of silver nanoparticles using Cyathea nilgirensis Holttum and their cytotoxic and phytotoxic potentials. Part Sci Technol. 2017;36:578–582. doi: 10.1080/02726351.2016.1278292. [DOI] [Google Scholar]
- Altaf M, Jaganyi D. Characterization of triangular gold nanoparticles using Aloe arborescens leaf extract: a green synthesis approach. Synth React Inorg Met-Org Nano-Metal Chem. 2016;46:1332–1335. doi: 10.1080/15533174.2015.1068810. [DOI] [Google Scholar]
- Bhatt CS, Nagaraj B, Suresh AK. Nanoparticles-shape influenced high-efficient degradation of dyes: comparative evaluation of nano-cubes vs nano-rods vs nano-spheres. J Mol Liq. 2017;242:958–965. doi: 10.1016/j.molliq.2017.07.101. [DOI] [Google Scholar]
- Carmona ER, Benito N, Plaza T, Recio-Sánchez G. Green synthesis of silver nanoparticles by using leaf extracts from the endemic Buddleja globosa hope green chemistry. Lett Rev. 2017;10:250–256. doi: 10.1080/17518253.2017.1360400. [DOI] [Google Scholar]
- Carnat A, Carnat AP, Fraisse D, Lamaison JL. The aromatic and polyphenolic composition of lemon verbena. Tea Fitoter. 1999;70:44–49. doi: 10.1016/S0367-326X(98)00016-1. [DOI] [PubMed] [Google Scholar]
- Choudhary BC, Paul D, Gupta T, Tetgure SR, Garole VJ, Borse AU, Garole DJ. Photocatalytic reduction of organic pollutant under visible light by green route synthesized gold nanoparticles. J Environ Sci. 2017;55:236–246. doi: 10.1016/j.jes.2016.05.044. [DOI] [PubMed] [Google Scholar]
- Deepak P, Sowmiya R, Balasubramani G, Aiswarya D, Arul D, Josebin MPD, Perumal P. Mosquito-larvicidal efficacy of gold nanoparticles synthesized from the seaweed, Turbinaria ornata (Turner) J. Agardh 1848. Part Sci Technol. 2017;36:974–980. doi: 10.1080/02726351.2017.1331286. [DOI] [PubMed] [Google Scholar]
- Desai MP, Sangaokar GM, Pawar KD. Kokum fruit mediated biogenic gold nanoparticles with photoluminescent, photocatalytic and antioxidant activities. Process Biochem. 2018;70:188–197. doi: 10.1016/j.procbio.2018.03.027. [DOI] [Google Scholar]
- Dhamecha D, Jalalpure S, Jadhav K, Sajjan D. Green synthesis of gold nanoparticles using Pterocarpus marsupium: characterization and biocompatibility studies Particulate. Sci Technol. 2016;34:156–164. doi: 10.1080/02726351.2015.1054972. [DOI] [Google Scholar]
- Dhayalan M, Denison MIJ, Ayyar M, Gandhi NN, Krishnan K, Abdulhadi B. Biogenic synthesis, characterization of gold and silver nanoparticles from Coleus forskohlii and their clinical importance. J Photochem Photobiol B. 2018;183:251–257. doi: 10.1016/j.jphotobiol.2018.04.042. [DOI] [PubMed] [Google Scholar]
- Emmanuel R, Karuppiah C, Chen S-M, Palanisamy S, Padmavathy S, Prakash P. Green synthesis of gold nanoparticles for trace level detection of a hazardous pollutant (nitrobenzene) causing Methemoglobinaemia. J Hazard Mater. 2014;279:117–124. doi: 10.1016/j.jhazmat.2014.06.066. [DOI] [PubMed] [Google Scholar]
- Fathima JB, Pugazhendhi A, Oves M, Venis R. Synthesis of eco-friendly copper nanoparticles for augmentation of catalytic degradation of organic dyes. J Mol Liq. 2018;260:1–8. doi: 10.1016/j.molliq.2018.03.033. [DOI] [Google Scholar]
- Gangapuram BR, Bandi R, Madhusudhan A, Dadigala R, Kotu GM, Guttena V. Microwave assisted rapid green synthesis of gold nanoparticles using Annona squamosa L peel extract for the efficient catalytic reduction of organic pollutants. J Mol Struct. 2018;1167:305–315. doi: 10.1016/j.molstruc.2018.05.004. [DOI] [Google Scholar]
- Geethalakshmi R, Sarada D. Characterization and antimicrobial activity of gold and silver nanoparticles synthesized using saponin isolated from Trianthema decandra L. Ind Crops Prod. 2013;51:107–115. doi: 10.1016/j.indcrop.2013.08.055. [DOI] [Google Scholar]
- Hamelian M, Varmira K, Veisi H. Green synthesis and characterizations of gold nanoparticles using thyme and survey cytotoxic effect, antibacterial and antioxidant potential. J Photochem Photobiol B. 2018;184:71–79. doi: 10.1016/j.jphotobiol.2018.05.016. [DOI] [PubMed] [Google Scholar]
- Jyoti K, Singh A. Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. Genet Eng Biotechnol. 2016;14:311–317. doi: 10.1016/j.jgeb.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavamurthy M, Srinath BS, Rai VR. Phytochemicals-mediated green synthesis of gold nanoparticles using Pterocarpus santalinus L. (Red Sanders) bark extract and their antimicrobial properties Part. Sci Technol. 2018;36:785–790. doi: 10.1080/02726351.2017.1302533. [DOI] [Google Scholar]
- Krishna IM, Reddy GB, Veerabhadram G, Madhusudhan A. Eco-friendly green synthesis of silver nanoparticles using Salmalia malabarica: synthesis, characterization, antimicrobial, and catalytic activity studies. Appl Nanosci. 2016;6:681–689. doi: 10.1007/s13204-015-0479-6. [DOI] [Google Scholar]
- LaMer VK, Dinegar RH. Theory, production and mechanism of formation of monodispersed hydrosols. J Am Chem Soc. 1950;72:4847–4854. doi: 10.1021/ja01167a001. [DOI] [Google Scholar]
- Lopes C, Courrol L. Green synthesis of silver nanoparticles with extract of Mimusops coriacea and light. J Lumin. 2018;199:183–187. doi: 10.1016/j.jlumin.2018.03.030. [DOI] [Google Scholar]
- López-Miranda JL, Borjas-Garcia S, Esparza R, Rosas G. Synthesis and catalytic evaluation of silver nanoparticles synthesized with Aloysia triphylla leaf extract. J Cluster Sci. 2016;27:1989–1999. doi: 10.1007/s10876-016-1062-3. [DOI] [Google Scholar]
- Manjunath HM, Joshi CG, Raju NG. Biofabrication of gold nanoparticles using marine endophytic fungus–Penicillium citrinum. IET nanobiotechnol. 2016;11:40–44. doi: 10.1049/iet-nbt.2016.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Kumari M, Pandey S, Chaudhry V, Gupta K, Nautiyal C. Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp. Bioresour Technol. 2014;166:235–242. doi: 10.1016/j.biortech.2014.04.085. [DOI] [PubMed] [Google Scholar]
- Mythili R, et al. Biogenic synthesis, characterization and antibacterial activity of gold nanoparticles synthesised from vegetable waste. J Mol Liq. 2018;262:318–321. doi: 10.1016/j.molliq.2018.04.087. [DOI] [Google Scholar]
- Nadaf NY, Kanase SS. Biosynthesis of gold nanoparticles by Bacillus marisflavi and its potential in catalytic dye degradation. Arab J Chem. 2016 doi: 10.1016/j.arabjc.2016.09.020. [DOI] [Google Scholar]
- Nadeem M, Abbasi BH, Younas M, Ahmad W, Khan T. A review of the green syntheses and anti-microbial applications of gold nanoparticles. Green Chem Lett Rev. 2017;10:216–227. doi: 10.1080/17518253.2017.1349192. [DOI] [Google Scholar]
- Parial D, Patra HK, Dasgupta AKR, Pal R. Screening of different algae for green synthesis of gold nanoparticles European. J Phycol. 2012;47:22–29. doi: 10.1080/09670262.2011.653406. [DOI] [Google Scholar]
- Pasca R-D, Mocanu A, Cobzac S-C, Petean I, Horovitz O, Tomoaia-Cotisel M. Biogenic syntheses of gold nanoparticles using plant extracts particulate. Sci Technol. 2014;32:131–137. doi: 10.1080/02726351.2013.839589. [DOI] [Google Scholar]
- Patra JK, Kwon Y, Baek K-H. Green biosynthesis of gold nanoparticles by onion peel extract: Synthesis, characterization and biological activities. Adv Powder Technol. 2016;27:2204–2213. doi: 10.1016/j.apt.2016.08.005. [DOI] [Google Scholar]
- Pourmortazavi SM, Taghdiri M, Makari V, Rahimi-Nasrabadi M, Batooli H. Reducing power of Eucalyptus oleosa leaf extracts and green synthesis of gold nanoparticles using the extract international. J Food Prop. 2017;20:1097–1103. doi: 10.1080/10942912.2016.1203334. [DOI] [Google Scholar]
- Rajan A, Rajan AR, Philip D. Elettaria cardamomum seed mediated rapid synthesis of gold nanoparticles and its biological activities. OpenNano. 2017;2:1–8. doi: 10.1016/j.onano.2016.11.002. [DOI] [Google Scholar]
- Rajkumari J, Meena H, Gangatharan M, Busi S. Green synthesis of anisotropic gold nanoparticles using hordenine and their antibiofilm efficacy against Pseudomonas aeruginosa IET. Nanobiotechnology. 2017;11:987–994. doi: 10.1049/iet-nbt.2017.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed T, Bilal M, Li C, Nabeel F, Khalid M, Iqbal HM. Catalytic potential of bio-synthesized silver nanoparticles using Convolvulus arvensis extract for the degradation of environmental pollutants. J Photochem Photobiol B. 2018;181:44–52. doi: 10.1016/j.jphotobiol.2018.02.024. [DOI] [PubMed] [Google Scholar]
- Salem MA, Bakr EA, El-Attar HG. Pt@ Ag and Pd@ Ag core/shell nanoparticles for catalytic degradation of Congo red in aqueous solution. Spectrochim Acta Part A Mol Biomol Spectrosc. 2018;188:155–163. doi: 10.1016/j.saa.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Sana SS, Dogiparthi LK. Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity. Mater Lett. 2018;226:47–51. doi: 10.1016/j.matlet.2018.05.009. [DOI] [Google Scholar]
- Saravanan C, Rajesh R, Kaviarasan T, Muthukumar K, Kavitake D, Shetty PH. Synthesis of silver nanoparticles using bacterial exopolysaccharide and its application for degradation of azo-dyes. Biotechnol Rep. 2017;15:33–40. doi: 10.1016/j.btre.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengan M, Veeramuthu D, Veerappan A. Photosynthesis of silver nanoparticles using Durio zibethinus aqueous extract and its application in catalytic reduction of nitroaromatics, degradation of hazardous dyes and selective colorimetric sensing of mercury ions. Mater Res Bull. 2018;100:386–393. doi: 10.1016/j.materresbull.2017.12.038. [DOI] [Google Scholar]
- Shanker U, Jassal V, Rani M, Kaith BS. Towards green synthesis of nanoparticles: from bio-assisted sources to benign solvents. Rev Int J Environ Anal Chem. 2016;96:801–835. doi: 10.1080/03067319.2016.1209663. [DOI] [Google Scholar]
- Singh P, Kim Y-J, Zhang D, Yang D-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34:588–599. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Singh J, Kukkar P, Sammi H, Rawat M, Singh G, Kukkar D (2017) Enhanced catalytic reduction of 4-nitrophenol and congo red dye By silver nanoparticles prepared from Azadirachta indica leaf extract under direct sunlight exposure. Part Sci Technol:1–10. 10.1080/02726351.2017.1390512
- Suganya KU, Govindaraju K, Kumar VG, Dhas TS, Karthick V, Singaravelu G, Elanchezhiyan M. Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against gram positive organisms. Mater Sci Eng C. 2015;47:351–356. doi: 10.1016/j.msec.2014.11.043. [DOI] [PubMed] [Google Scholar]
- Teimouri M, Khosravi-Nejad F, Attar F, Saboury AA, Kostova I, Benelli G, Falahati M. Gold nanoparticles fabrication by plant extracts: synthesis, characterization, degradation of 4-nitrophenol from industrial wastewater, and insecticidal activity—a review. J Clean Prod. 2018;184:740–753. doi: 10.1016/j.jclepro.2018.02.268. [DOI] [Google Scholar]
- Umamaheswari C, Lakshmanan A, Nagarajan N. Green synthesis, characterization and catalytic degradation studies of gold nanoparticles against congo red and methyl orange. J Photochem Photobiol B. 2018;178:33–39. doi: 10.1016/j.jphotobiol.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Vidhu V, Philip D. Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron. 2014;56:54–62. doi: 10.1016/j.micron.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan K, Ashokkumar T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis. Charact Tech Appl J Environ Chem Eng. 2017;5:4866–4883. doi: 10.1016/j.jece.2017.09.026. [DOI] [Google Scholar]
- Wang L, Lu F, Liu Y, Wu Y, Wu Z. Photocatalytic degradation of organic dyes and antimicrobial activity of silver nanoparticles fast synthesized by flavonoids fraction of Psidium guajava L. leaves. J Mol Liq. 2018;263:187–192. doi: 10.1016/j.molliq.2018.04.151. [DOI] [Google Scholar]
- Yuan C-G, Huo C, Gui B, Cao W-P. Green synthesis of gold nanoparticles using Citrus maxima peel extract and their catalytic/antibacterial activities. IET Nanobiotechnol. 2016;11:523–530. doi: 10.1049/iet-nbt.2016.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamorano-Ponce E, et al. Anti-genotoxic effect of Aloysia triphylla infusion against acrylamide-induced DNA damage as shown by the comet assay technique. Mutat Res/Genet Toxicol Environ Mutagenesis. 2006;603:145–150. doi: 10.1016/j.mrgentox.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Zhou R, Srinivasan M. Photocatalysis in a packed bed: degradation of organic dyes by immobilized silver nanoparticles. J Environ Chem Eng. 2015;3:609–616. doi: 10.1016/j.jece.2015.02.004. [DOI] [Google Scholar]