Abstract
Background:
Perceived stress may be a modifiable risk factor for mild cognitive impairment (MCI) and ultimately dementia but studies on this topic from low- and middle-income countries (LMICs) are lacking.
Objective:
We assessed the association of perceived stress and MCI in six LMICs (China, Ghana, India, Mexico, Russia, South Africa) using nationally representative data.
Methods:
Cross-sectional, community-based data on individuals aged ≥50 years from the World Health Organization’s Study on Global Ageing and Adult Health were analyzed. The definition of MCI was based on the National Institute on Ageing-Alzheimer’s Association criteria. A perceived stress score [range 0 (lowest stress) −10 (highest stress)] was computed based on two questions from the Perceived Stress Scale. Multivariable logistic regression analysis was conducted to assess the association between perceived stress and MCI.
Results:
The mean (SD) age of the 32,715 participants was 62.1 (15.6) years and 51.7% were females. After adjustment for potential confounders including depression, in the overall sample, a one-unit increase in the perceived stress score was associated with a 1.14 (95%CI=1.11–1.18) times higher odds for MCI. The association was similar among those aged 50–64 and ≥65 years. Country-wise analysis showed that there is a moderate level of between-country heterogeneity in this association (I2=59.4%) with the strongest association observed in Russia (OR=1.33; 95%CI=1.15–1.55).
Conclusions:
If our study results are confirmed in prospective studies, addressing perceived stress may have an impact in reducing the risk for MCI and subsequent dementia in LMICs.
Keywords: Cognition, Perceived stress, Risk factor
1. Introduction
Globally, dementia is one of the major causes of disability and impaired autonomy in the older adult population, and is a source of significant burden to economic and social systems [1]. Current estimates project that 46 million people may be living with this condition worldwide, and as the consequence of global aging, this figure is projected to nearly triple by 2050 [2]. However, at present, there are no truly disease-modifying treatments for dementia. Thus, there is an increasing emphasis on the identification of modifiable risk factors for conditions known to predict the ultimate emergence of dementia as a means to develop interventions aiming to prevent or delay the onset of dementia. Specifically, mild cognitive impairment (MCI) is considered to be a preclinical transitional state of dementia [3],with a high conversion rate to dementia (12%, 20%, and 50% at 1, 3, and 5 years, respectively [4]), for which targeted interventions may be possible.
Currently, there is growing interest in the association between perceived stress and subsequent cognitive decline [5]. Perceived stress is defined as a consequence of events or demands that exceed an individual’s perceived ability to cope [6], and could be a modifiable risk factor for MCI/dementia [5]. For example, a longitudinal population-based study conducted in the U.S. showed that baseline perceived stress is associated with a faster rate of cognitive decline [7]. Some researchers have suggested that the high prevalence of dementia among older African Americans may be attributable to high levels of stress related with low socioeconomic status and discrimination throughout the life course [8]. Another small U.S. study found that stressful life events are associated with higher risk for conversion from MCI to dementia [9]. It has been hypothesized that stress may lead to cognitive decline through mechanisms such as dysregulation of hormones (e.g., cortisol) and increased production of pro-inflammatory cytokines which can impair the neural structure and function implicated in cognitive performance [5]. Alternatively, the Cognitive Health and Environment Life Course Model (CHELM) posits that environmental, demographic, lifestyle, and genetic factors are moderated by diseases risk factors such as stress and cardiometabolic risk factors, which in turn are associated with medical conditions (e.g., depression, diabetes, and cardiovascular diseases) that can increase risk for cognitive decline [10]. However, there is a glaring paucity of studies on the association between perceived stress and MCI. To the best of our knowledge, there is only one U.S. study which specifically focused on the association of perceived stress and MCI [11]. This community-based prospective study found that higher levels of stress (per 5-point increase in the Perceived Stress Scale) are associated with a 1.30 (95%CI=1.08–1.58) times higher risk for incident amnestic MCI among individuals aged ≥70 years (N=507). Although this study provided insight into the stress-MCI relationship, it was conducted in only one location, while the sample was derived from a single high-income country. Thus, it remains unclear whether the results from this study could be generalizable to other age groups or populations, including the general population of low- and middle-income countries (LMICs).
Examining the potential modifiable risk factor for MCI/dementia is particularly important in LMICs as the speed of aging in LMICs is superseding that of high-income countries [12], and among people with dementia, the proportion of those residing in LMICs are expected to increase from current rates of 58% to 68% by 2050 [2]. Furthermore, the rapid increase in cardiovascular diseases, obesity, diabetes, and hypertension observed in this setting may give rise to a parallel increase in the incidence and prevalence of dementia as these conditions have been reported to increase risk for dementia [2].
Perceived stress is an important risk factor to investigate in this setting as poverty is widespread in LMICs, and it is possible that people in LMICs are experiencing higher levels of stress or different types of stress compared to high-income countries for the multitude of distress linked to poverty and social inequality (e.g., food insecurity, adverse working conditions, financial strain). Furthermore, the rapid and drastic changes in economic and social landscape occurring across many LMICs including globalization and urbanization may also be contributing to increasing levels of perceived stress among people in this setting.
Thus, the aim of the current study was to assess whether perceived stress is associated with MCI independent of known risk factors for MCI among adults aged ≥50 years using nationally representative data from six LMICs included in the WHO Global Ageing and Adult Health study (SAGE).
2. Methods
2.1. The survey
Data from the SAGE were analyzed. These data are publically available through http://www.who.int/healthinfo/sage/en/. This survey was undertaken in China, Ghana, India, Mexico, Russia, and South Africa between 2007 and 2010. These countries broadly represent different geographical locations and levels of socio-economic and demographic transition. Based on the World Bank classification at the time of the survey, Ghana was the only low-income country, and China and India were lower middle-income countries although China became an upper middle-income country in 2010. The remaining countries were upper middle-income countries.
Details of the survey methodology have been published elsewhere [13]. In brief, in order to obtain nationally representative samples, a multistage clustered sampling design method was used. The sample consisted of adults aged ≥18 years with oversampling of those aged ≥50 years. Trained interviewers conducted face-to-face interviews using a standard questionnaire. Standard translation procedures were undertaken to ensure comparability between countries. If a respondent was unable to undertake the interview because of limited cognitive function, then a separate questionnaire was administered to a proxy respondent. These individuals were not included in the current study. The survey response rates were: China 93%; Ghana 81%; India 68%; Mexico 53%; Russia 83%; and South Africa 75%. Sampling weights were constructed to adjust for the population structure as reported by the United Nations Statistical Division. Ethical approval was obtained from the WHO Ethical Review Committee and local ethics research review boards. Written informed consent was obtained from all participants.
2.2. Mild cognitive impairment (MCI) (Outcome)
MCI was ascertained based on the recommendations of the National Institute on Aging-Alzheimer’s Association [14]. We applied the identical algorithms used in previous publications using a dataset with the same survey questions to identify MCI [15,16]. Briefly, individuals fulfilling all of the following conditions were considered to have MCI:
(a). Concern about a change in cognition:
Individuals who replied ‘bad’ or ‘very bad’ to the question “How would you best describe your memory at present?” and/or those who answered ‘worse’ to the question “Compared to 12 months ago, would you say your memory is now better, the same or worse than it was then?” were considered to have this condition.
(b). Objective evidence of impairment in one or more cognitive domains:
was based on a <−1 SD cut-off after adjustment for level of education, age, and country. Cognitive function was assessed through the following performance tests: word list immediate and delayed verbal recall from the Consortium to Establish a Registry for Alzheimer’s Disease [17], which assessed learning and episodic memory; digit span forward and backwards from the Weschler Adult Intelligence Scale [18], that evaluated attention and working memory; and the animal naming task [17], which assessed verbal fluency.
(c). Preservation of independence in functional abilities:
It has been stated that people with MCI may have mild problems in performing complex tasks such as paying bills or shopping but they generally maintain their independence of function in life with minimal aid or assistance [14]. Thus, this was assessed by questions on self-reported difficulties with basic activities of daily living (ADL) in the past 30 days [19]. Specific questions were: “How much difficulty did you have in getting dressed?” and “How much difficulty did you have with eating (including cutting up your food)?” The answer options were none, mild, moderate, severe, and extreme (cannot do). Those who answered either none, mild, or moderate to both of these questions were considered to have preservation of independence in functional activities. All other individuals were deleted from the analysis (935 individuals aged ≥50 years).
(d). No dementia:
Individuals with a level of cognitive impairment severe enough to preclude the possibility to undertake the survey were not included in the current study. Specifically, the decision for non-inclusion was based on the IQ Code [20].
2.3. Perceived stress (Exposure)
In line with previous publications [21,22], we assessed perceived stress in the last month with the use of two questions which were taken from the Perceived Stress Scale [23]. This validated scale has been widely used to measure perceived stress worldwide. The questions asked were: “How often have you felt that you were unable to control the important things in your life?”; and “How often have you found that you could not cope with all the things that you had to do?” The answer options to these questions were: never (score=1), almost never (score=2), sometimes (score=3), fairly often (score=4), very often (score=5). As in a previous study which used the identical questions to measure perceived stress [22], we conducted factor analysis with polychoric correlations to incorporate the covariance structure of the answers provided for individual questions measuring a similar construct. The principal component method was used for factor extraction, while factor scores were obtained using the regression scoring method. These factor scores were later converted to scores ranging from 0–10 with higher values indicating higher levels of perceived stress. We also used the two individual questions with the original five answer options in some analyses.
2.4. Control variables
The analysis adjusted for a number of potential confounders which have been reported to be linked with both MCI and perceived stress [11,16,22]. These included sex, age (years), years of education, wealth quintiles based on country-specific income, depression, and number of chronic conditions. Questions based on the World Mental Health Survey version of the Composite International Diagnostic Interview were used for the endorsement of DSM-IV depression (See Appendix eTable 1). The number of chronic physical conditions was based on ten conditions (angina, arthritis, asthma, cataract, chronic lung disease, diabetes, edentulism, hearing problems, hypertension, stroke), assessed by self-report of diagnosis, symptoms, interviewer observation, or blood pressure measurement (See Appendix eTable 2).
2.5. Statistical analysis
The statistical analysis was performed with Stata 14.1 (Stata Corp LP, College station, Texas). The analysis was restricted to those aged ≥50 years. We included the middle-aged in this analysis as assessment of cognitive function and its risk factors at earlier ages is important for prevention of dementia since cognitive dysfunction appears up to 10 years before the actual dementia diagnosis [24], and there is burgeoning evidence base that intervening in mid-life is crucial [25]. We conducted multivariable logistic regression analysis to assess the association between perceived stress (exposure) and MCI (outcome). Using the continuous variable on perceived stress ranging from 0 to 10 as the exposure variable, we conducted hierarchical analysis that examined the effect of including different covariates in the model using the overall sample (i.e., age ≥50 years). Specifically, we constructed a total of five models: Model 1 - adjusted for sex, age and country; Model 2 - adjusted for factors in Model 1 and education; Model 3 - adjusted for factors in Model 2 and wealth; Model 4 - adjusted for factors in Model 3 and depression; and Model 5 - adjusted for factors in Model 4 and chronic conditions.
We also conducted age-stratified analysis (50–64 and ≥65 years) as the risk factors of MCI may differ between mid-life and late-life [16]. Since a previous study showed that there may be differences in the hypothalamic-pituitary-adrenal (HPA) axis response to stress between males and females [26], we also tested whether sex is a moderator variable in the association between perceived stress and MCI by including a product term (sex X perceived stress) in the model using the overall sample. We also conducted country-wise analyses using the sample including all individuals aged ≥50 years. In order to assess the between-country heterogeneity that may exist in the association between perceived stress and MCI, we calculated the Higgins’ I2 based on estimates for each country. The Higgins’ I2 represents the degree of heterogeneity that is not explained by sampling error with a value of <40% often considered as negligible and 40–60% as moderate heterogeneity [27]. A pooled estimate was obtained by random-effect meta-analysis based on country-wise estimates. Finally, we also assessed the association between the two individual questions on perceived stress with the original five answer options and MCI using the overall sample with multivariable logistic regression.
The regression analyses were all adjusted for sex, age, years of education, wealth, depression, and number of chronic physical conditions apart from Model 1 to 4 in the hierarchical analysis. The analyses apart from the country-wise analysis were also adjusted for country using fixed effects models by including dummy variables for each country. All variables were included in the models as categorical variables with the exception of age, years of education, number of chronic physical conditions, and the perceived stress scale ranging from 0 to 10 (continuous variables). Less than 3.5% of the data were missing for the variables used in the analysis. Complete-case analysis was done. The sample weighting and the complex study design were taken into account in the analyses. Results from the regression analyses are presented as odds ratios (ORs) with 95% confidence intervals (CIs). The level of statistical significance was set at P<0.05.
3. Results
The final analytical sample comprised 32,715 individuals aged ≥50 years with preservation in functional abilities. The overall prevalence (95%CI) of MCI was 15.3% (95%CI=14.4%−16.3%). Sample characteristics are presented in Table 1. Overall, the mean (SD) age was 62.1 (15.6) years and 51.7% were females. Russia had the highest proportion of females, years of education, and number of chronic conditions. The prevalence of MCI increased linearly with increasing levels of perceived stress with the prevalence ranging from 11.3% to 30.9% between those with the lowest (score 0-<2) and highest (score 8–10) levels of stress (Figure 1). After adjustment for age, sex, and country, a one-unit increase in the level of perceived stress (range 0–10) was associated with a significant 1.18 times higher odds for MCI. The sequential inclusion of potential confounders slightly attenuated the association between perceived stress and MCI but this association remained significant even after adjustment for all potential confounders (OR=1.14; 95%CI=1.11–1.18) (Model 5) (Table 2). This association was the same in both age groups (i.e., 50–64 and ≥65 years) (Table 3). A significant interaction by sex was not observed in the overall sample. There was a moderate level of between-country heterogeneity in this association (Higgin’s I2=59.4%), with the strongest association being observed in Russia (OR=1.33; 95%CI=1.15–1.55) (Figure 2). Finally, analyses based on the two individual questions on perceived stress showed that greater frequency of perceived stress is associated with higher odds for MCI (Appendix eTable 3). Specifically, the highest frequency (very often) of perceived stress was associated with 3.66 or 3.85 times higher odds for MCI compared to the lowest frequency (never).
Table 1.
Sample characteristics
| Characteristic | Category | Overall (N=32,715) | China (N=12,815) | Ghana (N=4201) | India (N=6191) | Mexico (N=2070) | Russia (N=3766) | S. Africa (N=3672) |
|---|---|---|---|---|---|---|---|---|
| Sex | Female | 51.7 | 50.2 | 47.4 | 48.2 | 52.8 | 60.6 | 56.0 |
| Age (years) | Mean (SD) | 62.1 (15.6) | 62.4 (16.3) | 64.2 (19.7) | 61.1 (13.2) | 62.3 (17.4) | 63.4(14.8) | 61.4 (18.3) |
| Education (years) | Mean (SD) | 6.1 (8.9) | 5.6 (8.1) | 4.2 (9.9) | 3.8 (7.4) | 5.1 (7.9) | 11.2(5.1) | 6.0(10.1) |
| Wealth | Poorest | 16.9 | 16.0 | 18.4 | 18.2 | 14.4 | 15.6 | 20.7 |
| Poorer | 18.9 | 18.0 | 19.1 | 19.4 | 25.2 | 19.7 | 19.9 | |
| Middle | 19.4 | 20.4 | 20.3 | 18.5 | 16.7 | 19.0 | 18.5 | |
| Richer | 21.5 | 23.6 | 20.8 | 19.8 | 16.5 | 20.8 | 19.8 | |
| Richest | 23.3 | 22.1 | 21.4 | 24.2 | 27.2 | 24.9 | 21.1 | |
| Depression | Yes | 5.5 | 1.0 | 7.0 | 11.9 | 10.2 | 3.2 | 2.9 |
| No. of chronic physical conditions | Mean (SD) | 1.8 (2.5) | 1.5 (2.4) | 1.4 (2.0) | 1.8 (2.2) | 1.7 (2.2) | 2.3 (2.5) | 1.7(2.5) |
| Mild cognitive impairment | Yes | 15.3 | 24.3 | 7.4 | 9.7 | 17.6 | 9.6 | 8.5 |
| Perceived stress scorea | Mean (SD) | 4.0 (4.0) | 3.3 (4.0) | 5.1 (3.9) | 4.4 (3.9) | 2.4 (3.8) | 4.2 (2.9) | 4.6(5.3) |
Abbreviation: S. Africa South Africa; SD Standard deviation
Data are weighted column percentage unless otherwise stated.
Perceived stress was based on a scale ranging from 0–10 with higher scores corresponding to higher levels of stress.
FIGURE 1.
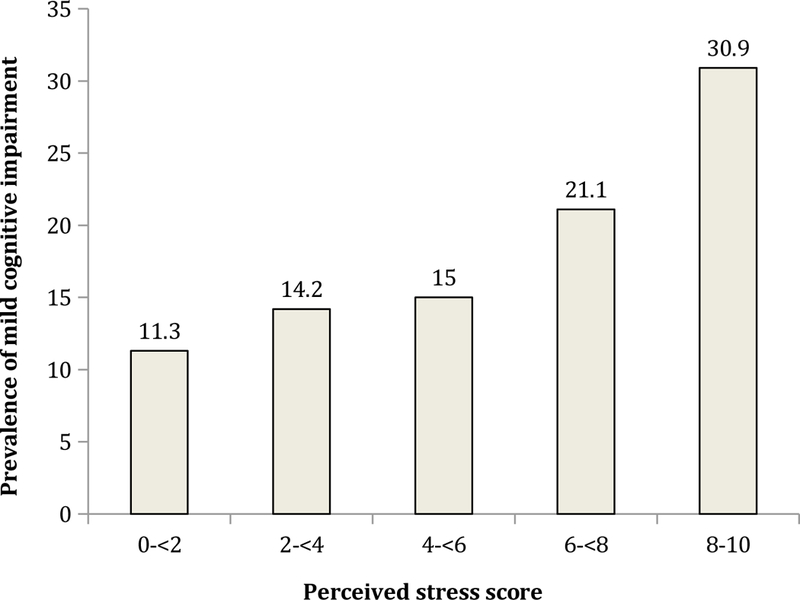
Prevalence of mild cognitive impairment by perceived stress score Perceived stress was based on a scale ranging from 0–10 with higher scores corresponding to higher levels of stress.
Table 2.
Association of perceived stress and other covariates with mild cognitive impairment (outcome) estimated by multivariable logistic regression among adults aged ≥50 years
| Characteristic | Category | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|---|
| Perceived stressa | Per unit increase | 1.18*** [1.15,1.22] |
1.17*** [1.14,1.21] |
1.15*** [1.12,1.19] |
1.15*** [1.12,1.19] |
1.14*** [1.11,1.18] |
| Sex | Male vs. Female | 0.89 [0.79,1.00] |
0.98 [0.87,1.10] |
0.94 [0.84,1.06] |
0.94 [0.83,1.06] |
0.97 [0.86,1.09] |
| Age (years) | Per unit increase | 1.03*** [1.02,1.03] |
1.02*** [1.02,1.03] |
1.02*** [1.02,1.03] |
1.02*** [1.02,1.03] |
1.02*** [1.01,1.02] |
| Education (years) | Per unit increase | 0.96*** [0.94,0.97] |
0.98** [0.96,0.99] |
0.98** [0.96,0.99] |
0.98** [0.96,0.99] |
|
| Wealth | Poorest | 1.00 | 1.00 | 1.00 | ||
| Poorer | 0.99 [0.83,1.18] |
0.99 [0.83,1.17] |
0.97 [0.82,1.16] |
|||
| Middle | 1.07 [0.89,1.30] |
1.07 [0.89,1.30] |
1.05 [0.87,1.27] |
|||
| Richer | 0.74** [0.61,0.90] |
0.74** [0.61,0.90] |
0.74** [0.61,0.89] |
|||
| Richest | 0.48*** [0.39,0.59] |
0.48*** [0.39,0.59] |
0.47*** [0.38,0.59] |
|||
| Depression | Yes vs. No | 1.04 [0.78,1.38] |
0.89 [0.67,1.19] |
|||
| No. of chronic conditions | Per unit increase | 1.19*** [1.14,1.23] |
Data are odds ratio [95% confidence interval].
Models are mutually adjusted for all variables in the respective columns and country.
Perceived stress was based on a scale ranging from 0–10 with higher scores corresponding to higher levels of stress.
p<0.01
p<0.001
Table 3.
Association of perceived stress and other covariates with mild cognitive impairment (outcome) by age groups estimated by multivariable logistic regression
| Characteristic | Category | 50–64 years | ≥65 years | ||
|---|---|---|---|---|---|
| Perceived stressa | Per unit increase | 1.14*** | [1.10,1.18] | 1.14*** | [1.10,1.19] |
| Sex | Male vs. Female | 0.85* | [0.73,0.98] | 1.13 | [0.94,1.36] |
| Age (years) | Per unit increase | 1.02 | [1.00,1.04] | 1.05*** | [1.04,1.06] |
| Education (years) | Per unit increase | 0.97** | [0.95,0.99] | 0.98 | [0.96,1.01] |
| Wealth | Poorest | 1.00 | 1.00 | ||
| Poorer | 0.95 | [0.76,1.19] | 0.94 | [0.72,1.22] | |
| Middle | 0.94 | [0.75,1.18] | 1.12 | [0.84,1.49] | |
| Richer | 0.74* | [0.57,0.95] | 0.64*** | [0.50,0.81] | |
| Richest | 0.39*** | [0.30,0.52] | 0.55*** | [0.40,0.76] | |
| Depression | Yes vs. No | 0.88 | [0.61,1.26] | 0.88 | [0.58,1.34] |
| No. of chronic conditions | Per unit increase | 1.23*** | [1.17,1.31] | 1.16*** | [1.10,1.23] |
Data are odds ratio [95% confidence interval].
Models are mutually adjusted for all variables in the Table and country.
Perceived stress was based on a scale ranging from 0–10 with higher scores corresponding to higher levels of stress.
p<0.05
p<0.01
p<0.001
FIGURE 2.
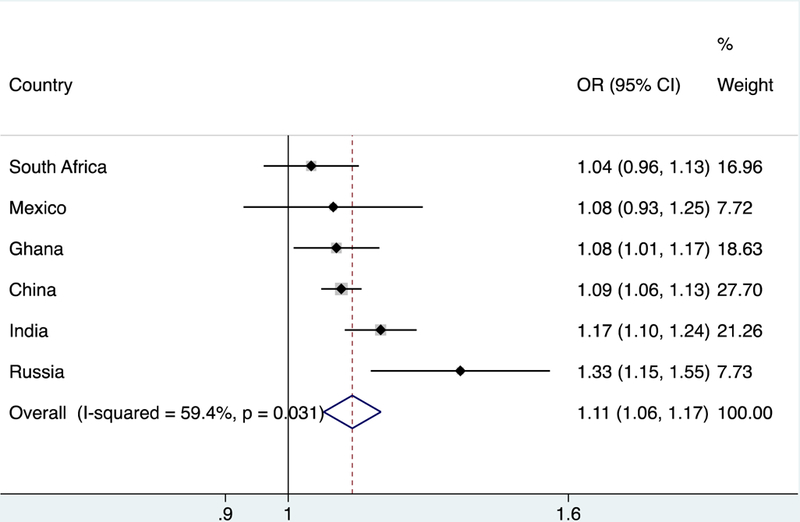
Country-wise association between perceived stress and mild cognitive impairment (outcome)
Abbreviation: OR Odds ratio; CI Confidence interval.
Perceived stress was based on a scale ranging from 0–10 with higher scores corresponding to higher levels of stress.
Estimates are adjusted for age, sex, education, wealth, depression, and number of chronic physical conditions.
The overall estimate was obtained by meta-analysis with random effects.
4. Discussion
In our study, we found that perceived stress is associated with MCI after adjustment for various potential confounders including depression in six LMICs which collectively comprise nearly half of the worldwide population [13]. Perceived stress was similarly associated with MCI in both the middle-aged and the older population. A moderate level of between-country heterogeneity in the association was observed with the association being most pronounced in Russia. The strength of the study includes the large sample size and the use of nationally representative samples from six countries. Furthermore, to the best of our knowledge, our study is the first to examine the association between perceived stress and MCI in LMICs, while it is also the first multicountry study on this topic, as well as the first study to focus on middle-aged individuals for whom the importance of targeted interventions are increasingly being recognized. Given that MCI is often considered as a prodromal stage of Alzheimer’s dementia, and that perceived stress may be a modifiable risk factor, investigating this association is important to provide information that can serve as a basis for preventive interventions.
Our study results are in line with the only study to date which specifically reported a heightened risk for MCI among the elderly with higher levels of perceived stress in the U.S. [11]. Perceived stress has been associated with poor cognitive performance in other studies but these were not specifically on MCI [7,28]. Furthermore, while not specifically on perceived stress or global life stress, related factors such as childhood adversities [29], work-related stress [30], and stressful life events [31] have been related with increased risk for cognitive decline or dementia.
The fact that the potential confounders assessed in our study including wealth, depression, and chronic physical diseases had very little influence in the association between perceived stress and MCI, and that perceived stress remained strongly associated with MCI even after adjustment for all potential confounders point to the possibility that the association may be partly explained by physiological factors. Although our study lacked data on physiological factors, hypothetically, stress may exert its negative effects on cognition through several physiological pathways pertaining to the central nervous, neuroendocrine, and immune systems. In terms of the neuroendocrine pathway, prolonged elevation of cortisol, which is a HPA axis response to chronic stress, may increase risk for stress-related cognitive decline. This response can lead to alterations in brain structure and function in the prefrontal cortex, hippocampus, and amygdala [32]. The hippocampus in particular is fundamental for memory and is viewed as the initial site of the neuropathology of Alzheimer’s disease [33]. Stress can potentially also increase the production of pro-inflammatory cytokines [34], which may increase vulnerability to neuropsychiatric disorders including depression and dementia [35,36]. Finally, animal studies have shown that stress may directly cause neuropathology associated with dementia such as synaptic loss [37], and increased levels of β-amyloid and phosphorylated tau [38].
The reason why a moderate level of between-country heterogeneity in the association between perceived stress and MCI was found is unknown. However, the variation may be attributable to the socio-historical conditions that are unique to each country. For example, the particularly strong association in Russia may be related with high levels of societal distress due to the collapse of the Soviet Union and subsequent high levels of smoking, alcohol consumption, depression, and deterioration of the health care system [39]. Future studies should assess how environmental and social-related factors may affect the stress-MCI relationship.
Interventions to relieve stress at the population or community level are generally lacking, particularly in LMICs. However, some individual level interventions have been developed, and have largely focused on cultivating mindfulness [40], though a few interventions have adopted a more cognitive approach [41]. Apart from psychotherapeutic interventions, other methods of stress reduction may entail the promotion of physical exercise [42], and possibly engagement in certain types of leisure activities [43]. Structural changes at the macro-level to alleviate poverty, address inequality, neighborhood safety or unemployment may also help reduce stress in LMICs.
The study results should be interpreted in the light of several limitations. First, some individuals with mild dementia may have been included in our analytical sample owing to the fact that the study was not designed to make clinical diagnoses of dementia. However, it is reassuring that the prevalence of MCI in our study was within previously reported figures Second, there is currently no consensus in terms of the acceptable level of functional impairment that individuals with MCI could present. We used a conservative definition for preservation of independence in functional abilities, which has been used in previous publications [15,16], so as not to exclude MCI cases with disability not related with their cognitive ability. It is possible that the results may differ slightly depending on the definition used. Third, due to data availability, we were only able to use an abridged version of the perceived stress scale which may differ in terms of validity and reliability compared to the original scale. Furthermore, although the original perceived stress scale was designed to be sensitive to chronic stress [23], it is possible that our abridged version does not necessarily reflect stress of a chronic nature. Given that chronic stress is considered to be important in the etiology of dementia, future studies should consider measures of perceived stress that take chronicity into account or assess how cumulative stress across the lifespan is associated with MCI/dementia. At least our analysis on the frequency of stress, which may correlate with chronicity to a certain extent, showed that greater frequency of stress is associated with MCI in a dose-dependent fashion. We were unable to assess the influence of poor health care and some lifestyle factors (e.g., alcohol consumption) in the association between MCI and perceived stress due to lack of data or availability of only crude measures. Finally, because this was a cross-sectional study, causality cannot be inferred. It is possible that eroding cognitive acuity in MCI may lead to higher levels of perceived stress. However, a previous longitudinal study showed that MCI is unlikely to precede perceived stress [11]. Specifically, this longitudinal study found that increasing levels of perceived stress at baseline was found to increase the risk of future onset of amnestic MCI in individuals without amnestic MCI or dementia at baseline. Furthermore, in this same study, the authors assessed whether the association between perceived stress would be greatest for times closest to the assessment of perceived stress. This was done as under the reverse causality hypothesis, it would be expected that the association between amnestic MCI and perceived stress would be strongest for times closest to the assessment of perceived stress. However, a complete opposite result was found where the association was stronger after 3 years of follow-up. Furthermore, under this hypothesis, Perceived Stress Scale scores should increase prior to onset of MCI but the overall change in the linear slope for the Perceived Stress Scale score was not significantly different from zero, while there were no differences in the Perceived Stress Scale score slopes between those who did and did not develop MCI.
In conclusion, our study results suggest that perceived stress might be a modifiable risk factor for MCI and subsequent dementia in LMICs. Our cross-sectional study can serve as a platform for future longitudinal studies to assess temporal associations. If confirmed in longitudinal and intervention studies, low-cost interventions designed to alter responses to stress might be a viable strategy to reduce the onset of cognitive decline and subsequent dementia in LMICs.
Acknowledgments
Funding
This work was supported by the Miguel Servet contract financed by the CP13/00150 and PI15/00862 projects, integrated into the National R + D + I and funded by the ISCIII - General Branch Evaluation and Promotion of Health Research - and the European Regional Development Fund (ERDF-FEDER) to [AK]; National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London to [BS]; and the National Institute on Alcohol Abuse and Alcoholism (NIAAA) of the National Institutes of Health (grant number T32AA014125) to [HO].
APPENDIX
eTable 1.
Algorithm for the diagnosis of depression
| 1. At least one of the two following symptoms in the last 12 months: |
| (a) A period, lasting several days, of feeling sad, empty or depressed. |
| (b) A period lasting several days with a loss of interest in most things the participant usually enjoys such as personal relationships, work or hobbies/recreation. |
| AND |
| 2. The period of sadness/loss of interest/low energy lasted for more than two weeks and was most of the day and nearly every day. |
| AND |
| 3. Five or more of the following symptoms: |
| (a) Loss of appetite |
| (b) Insomnia (problems falling asleep or waking up too early) |
| (c) Decreased energy or tiredness all the time |
| (d) Slowing down in moving around or restless/jittery. |
| (e) Negative feelings/loss of confidence or frequent feelings of hopelessness. |
| (f) Slowed thinking or difficulties concentrating (e.g., listening to others, working, watching TV, listening to the radio). |
| (g) Thoughts of death, wishes of own death or suicide attempt. |
| (h) Feelings of sadness, emptiness or depression lasting several days. |
| (i) Anhedonia: loss of interest in things the participant usually enjoys. |
eTable 2.
Details on the diagnosis of chronic physical conditions
| Condition | (a) Self-reported diagnosis | (b) Symptom-based algorithm or other method of diagnosisa |
|---|---|---|
| Angina | Have you ever been diagnosed with angina or angina pectoris (a heart disease)? | Rose questionnaire1 |
| Arthritis | Have you ever been diagnosed with/told you have arthritis (a disease of the joints, or by other names rheumatism or osteoarthritis)? | Affirmative answers to all four of the following: |
| 1. During the last 12 months, have you experienced pain, aching, stiffness or swelling in or around the joints (e.g., in arms, hands, legs or feet) which were not related to an injury and lasted for more than a month? | ||
| 2. During the last 12 months, have you experienced stiffness in the joint in the morning after getting up from bed, or after a long rest of the joint without ` movement? | ||
| 3. Did this stiffness last for less than 30 minutes? | ||
| 4. Did this stiffness go away after exercise or movement in the joint? | ||
| Asthma | Have you ever been diagnosed with asthma (an allergic respiratory disease)? | 1. During the last 12 months, have you experienced attacks of wheezing or whistling breathing? (Yes) |
| AND | ||
| 2. “Yes” to at least one of the following (past 12 months): | ||
| (a) Have you experienced an attack of wheezing that came on after you stopped exercising or some other physical activity? | ||
| (b) Have you had a feeling of tightness in your chest? | ||
| (c) Have you woken up with a feeling of tightness in your chest in the morning or any other time? | ||
| (d) Have you had an attack of shortness of breath that came on without an obvious cause when you were not exercising or doing some physical activity? | ||
| Cataract | In the last 5 years, were you diagnosed with a cataract in one or both of your eyes (a cloudiness in the lens of the eye)? | 1. In the last 12 months, have you experienced cloudy or blurry vision? (Yes) |
| AND | ||
| 2. In the last 12 months, have you experienced vision problems with light, such as glare from bright lights, or halos around lights? (Yes) | ||
| Chronic lung disease | Have you ever been diagnosed with chronic lung disease (emphysema, bronchitis, COPD)? | 1. During the last 12 months, have you experienced any shortness of breath at rest (while awake)? (Yes) |
| OR | ||
| 2. “Yes” to both of the following (past 12 months): | ||
| (a) Have you experienced any coughing or wheezing for 10 minutes or more at a time? | ||
| (b) Have you experienced any coughing up of sputum or phlegm on most days of the month for at least 3 months? | ||
| Diabetes | Have you ever been diagnosed with diabetes (high blood sugar)? (not including diabetes associated with a pregnancy) | NA |
| Edentulism | “Have you lost all of your natural teeth?” | NA |
| Hearing problem | NA | Interviewer observation |
| Hypertension | Have you ever been diagnosed with high blood pressure (hypertension)? | Blood pressure was measured three times with a one-minute interval with the use of a wrist blood pressure monitor (Medistar Wrist Blood Pressure Model S) and the mean value of the three measurements was calculated. Hypertension was defined as having at least one of the following: systolic blood pressure ≥140 mmHg; diastolic blood pressure ≥90 mmHg. |
| Stroke | Have you ever been told by a health professional that you have had a stroke? | NA |
For all chronic physical conditions, we assumed that the individual had the condition if they fulfilled at least one of the following: (a) affirmative answer to self-reported diagnosis or (b) symptom-based algorithm or other method of diagnosis.
These algorithms have been used in previous publications 2, 3 and those of arthritis, asthma, and chronic lung disease have been validated.2, 4
Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ.1962;27: 645–658.
Arokiasamy P, Uttamacharya, Kowal P, et al. Chronic Noncommunicable Diseases in 6 Low- and Middle-Income Countries: Findings From Wave 1 of the World Health Organization’s Study on Global Ageing and Adult Health (SAGE). Am J Epidemiol. 2017;185: 414–428.
Garin N, Koyanagi A, Chatterji S, et al. Global Multimorbidity Patterns: A Cross-Sectional, Population-Based, Multi-Country Study. J Gerontol A Biol Sci Med Sci. 2016;71: 205–214.
Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370: 851–858.
eTable 3.
Association of individual items of the perceived stress scale and other covariates with mild cognitive impairment (outcome) estimated by multivariable logistic regression
| In the past 30 days, how often… | |||||
|---|---|---|---|---|---|
| have you felt that you were unable to control the important things in your life? | have you found that you could not cope with all the things that you had to do? | ||||
| Characteristic | Category | OR | 95%CI | OR | 95%CI |
| Perceived stress | Never | 1.00 | 1.00 | ||
| Almost never | 0.96 | [0.79,1.17] | 1.02 | [0.87,1.20] | |
| Sometimes | 1.24* | [1.02,1.51] | 1.39*** | [1.17,1.65] | |
| Fairly often | 2.15*** | [1.67,2.78] | 2.56*** | [1.99,3.29] | |
| Very often | 3.66*** | [2.58,5.20] | 3.85*** | [2.74,5.42] | |
| Sex | Female | 1.00 | 1.00 | ||
| Male | 0.97 | [0.86,1.09] | 0.97 | [0.86,1.10] | |
| Age (years) | 1.02*** | [1.01,1.02] | 1.01*** | [1.01,1.02] | |
| Education (years) | 0.98** | [0.96,0.99] | 0.98* | [0.96,1.00] | |
| Wealth | Poorest | 1.00 | 1.00 | ||
| Poorer | 0.97 | [0.82,1.15] | 0.97 | [0.82,1.16] | |
| Middle | 1.05 | [0.86,1.27] | 1.04 | [0.86,1.26] | |
| Richer | 0.73*** | [0.61,0.88] | 0.74** | [0.61,0.89] | |
| Richest | 0.45*** | [0.37,0.56] | 0.46*** | [0.37,0.57] | |
| Depression | Yes vs. No | 0.88 | [0.65,1.17] | 0.86 | [0.65,1.15] |
| No. of chronic conditions | Per unit increase | 1.19*** | [1.14,1.23] | 1.19*** | [1.14,1.23] |
Abbreviation: OR Odds ratio; CI Confidence interval
Models are mutually adjusted for all variables in the Table and country.
p<0.05
p<0.01
p<0.001
Footnotes
Conflicts of interest
None.
References
- 1.Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, Jonsson L, Liu Z, Prince M: The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 2017;13:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Disease International: World Alzheimer Report, 2015,
- 3.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E: Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 4.Solfrizzi V, Panza F, Colacicco AM, D’Introno A, Capurso C, Torres F, Grigoletto F, Maggi S, Del Parigi A, Reiman EM, Caselli RJ, Scafato E, Farchi G, Capurso A: Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology 2004;63:1882–1891. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg MS, Tanev K, Marin MF, Pitman RK: Stress, PTSD, and dementia. Alzheimers Dement 2014;10:S155–165. [DOI] [PubMed] [Google Scholar]
- 6.Lazarus RS: Stress and emotion: A new synthesis New York, Springer, 2006. [Google Scholar]
- 7.Aggarwal NT, Wilson RS, Beck TL, Rajan KB, Mendes de Leon CF, Evans DA, Everson-Rose SA: Perceived stress and change in cognitive function among adults 65 years and older. Psychosom Med 2014;76:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forrester S: Perceived Stress: A Mechanism for Cognitive Decline Among Minorities? Am J Geriatr Psychiatry 2017;25:35–36. [DOI] [PubMed] [Google Scholar]
- 9.Peavy GM, Jacobson MW, Salmon DP, Gamst AC, Patterson TL, Goldman S, Mills PJ, Khandrika S, Galasko D: The influence of chronic stress on dementia-related diagnostic change in older adults. Alzheimer Dis Assoc Disord 2012;26:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anstey KJ: Optimizing cognitive development over the life course and preventing cognitive decline: Introducing the Cognitive Health Environment Life Course Model (CHELM). International Journal of Behavioral Development 2013;38:1–10. [Google Scholar]
- 11.Katz MJ, Derby CA, Wang C, Sliwinski MJ, Ezzati A, Zimmerman ME, Zwerling JL, Lipton RB: Influence of Perceived Stress on Incident Amnestic Mild Cognitive Impairment: Results From the Einstein Aging Study. Alzheimer Dis Assoc Disord 2016;30:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization: Global health and ageing, 2011,
- 13.Kowal P, Chatterji S, Naidoo N, Biritwum R, Fan W, Lopez Ridaura R, Maximova T, Arokiasamy P, Phaswana-Mafuya N, Williams S, Snodgrass JJ, Minicuci N, D’Este C, Peltzer K, Boerma JT: Data resource profile: the World Health Organization Study on global AGEing and adult health (SAGE). Int J Epidemiol 2012;41:1639–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH: The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lara E, Koyanagi A, Domenech-Abella J, Miret M, Ayuso-Mateos JL, Haro JM: The Impact of Depression on the Development of Mild Cognitive Impairment over 3 Years of Follow-Up: A Population-Based Study. Dement Geriatr Cogn Disord 2017;43:155–169. [DOI] [PubMed] [Google Scholar]
- 16.Lara E, Koyanagi A, Olaya B, Lobo A, Miret M, Tyrovolas S, Ayuso-Mateos JL, Haro JM: Mild cognitive impairment in a Spanish representative sample: prevalence and associated factors. Int J Geriatr Psychiatry 2016;31:858–867. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989;39:1159–1165. [DOI] [PubMed] [Google Scholar]
- 18.The Psychological Corporation: The Psychological Corporation: The WAIS III-WMS III Updated Technical Manual San Antonio, 2002, [Google Scholar]
- 19.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW: Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 20.Jorm AF, Jacomb PA: The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med 1989;19:1015–1022. [DOI] [PubMed] [Google Scholar]
- 21.DeVylder JE, Koyanagi A, Unick J, Oh H, Nam B, Stickley A: Stress Sensitivity and Psychotic Experiences in 39 Low- and Middle-Income Countries. Schizophr Bull 2016;42:1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vancampfort D, Koyanagi A, Ward P, Veronese N, Carvalho AF, Solmi M, Mugisha J, Rosenbaum S, De Hert M, Stubbs B: Perceived Stress and its Relationship with Chronic Conditions and Multimorbidity Among 229,293 Community-Dwelling Adults in 44 Low-and Middle-Income Countries. Am J Epidemiol 2017; 186(8):979–989. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S, Kamarck T, Mermelstein R: A global measure of perceived stress. J Health Soc Behav 1983;24:385–396. [PubMed] [Google Scholar]
- 24.Amieva H, Jacqmin-Gadda H, Orgogozo JM, Le Carret N, Helmer C, Letenneur L, Barberger-Gateau P, Fabrigoule C, Dartigues JF: The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain 2005;128:1093–1101. [DOI] [PubMed] [Google Scholar]
- 25.Alzheimer’s Disease International: World Alzheimer Report 2014
- 26.Kudielka BM, Kirschbaum C: Sex differences in HPA axis responses to stress: a review. Biol Psychol 2005;69:113–132. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG: Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 28.Munoz E, Sliwinski MJ, Scott SB, Hofer S: Global perceived stress predicts cognitive change among older adults. Psychol Aging 2015;30:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naismith SL, Parker RM: Childhood Stress and Adversity is Associated with Late-Life Dementia in Aboriginal Australians. Am J Geriatr Psychiatry 2017 [DOI] [PubMed]
- 30.Andel R, Crowe M, Hahn EA, Mortimer JA, Pedersen NL, Fratiglioni L, Johansson B, Gatz M: Work-related stress may increase the risk of vascular dementia. J Am Geriatr Soc 2012;60:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tschanz JT, Pfister R, Wanzek J, Corcoran C, Smith K, Tschanz BT, Steffens DC, Ostbye T, Welsh-Bohmer KA, Norton MC: Stressful life events and cognitive decline in late life: moderation by education and age. The Cache County Study. Int J Geriatr Psychiatry 2013;28:821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Dagher A, Lupien SJ: Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology 2010;35:179–191. [DOI] [PubMed] [Google Scholar]
- 33.Braak H, Braak E: Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 34.Leonard BE: The HPA and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry 2005;20 Suppl 3:S302–306. [DOI] [PubMed] [Google Scholar]
- 35.Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctot KL, Carvalho AF: Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 2017;135:373–387. [DOI] [PubMed] [Google Scholar]
- 36.Lai KSP, Liu CS, Rau A, Lanctot KL, Kohler CA, Pakosh M, Carvalho AF, Herrmann N: Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry 2017 [DOI] [PubMed]
- 37.Tata DA, Marciano VA, Anderson BJ: Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol 2006;498:363–374. [DOI] [PubMed] [Google Scholar]
- 38.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM: Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci 2006;26:9047–9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leon DA, Shkolnikov VM: Social stress and the Russian mortality crisis. JAMA 1998;279:790–791. [DOI] [PubMed] [Google Scholar]
- 40.Grossman P, Niemann L, Schmidt S, Walach H: Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res 2004;57:35–43. [DOI] [PubMed] [Google Scholar]
- 41.Hains A, Szyjakowski M: A Cognitive Stress-Reduction Intervention Program for Adolescents. Journal of Counseling Psychology 1990;37:79–84. [Google Scholar]
- 42.Stubbs B, Vancampfort D, Rosenbaum S, Firth J, Cosco T, Veronese N, Salum GA, Schuch FB: An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: A meta-analysis. Psychiatry Res 2017;249:102–108. [DOI] [PubMed] [Google Scholar]
- 43.Iwasaki Y, Mannell RC, Smale BJ, Butcher J: Contributions of leisure participation in predicting stress coping and health among police and emergency response services workers. J Health Psychol 2005;10:79–99. [DOI] [PubMed] [Google Scholar]
- 44.Petersen RC: Mild Cognitive Impairment. Continuum (Minneap Minn) 2016;22:404–418 [DOI] [PMC free article] [PubMed] [Google Scholar]


