Abstract
Background & Aims:
Increasing rates of young-onset colorectal cancer (CRC) have attracted substantial research and media attention, but we know little about racial disparities among younger adults with CRC. We examined racial disparities in young-onset CRC by comparing CRC incidence and relative survival among younger (age <50 years) adults, in 2 time periods.
Methods:
Using data from the Surveillance, Epidemiology, and End Results program of cancer registries, we estimated CRC incidence rates (per 100,000 persons ages 20 – 54 years) from 1992 through 2014, for different time periods (1992–1996 vs 2010–2014) and races (white vs black). Relative survival was calculated as the ratio of observed survival to expected survival in a comparable, cancer-free population.
Results:
From 1992–1996 to 2010–2014, CRC incidence increased from 7.5/100,000 to 11.0/100,000 in white individuals and from 11.7/100,000 to 12.7/100,000 in black individuals. The increase in rectal cancer was larger in whites (from 2.7/100,000 to 4.5/100,000) than in blacks (from 3.4/100,000 to 4.0/100,000); in the 2010–2014 time period, black and whites had similar rates of rectal cancer. Compared to whites, blacks had smaller increases in relative survival with proximal colon cancer but larger increases in survival with rectal cancer (from 55.3% to 70.8%).
Conclusion:
In an analysis of the Surveillance, Epidemiology, and End Results database, we found racial disparities in incidence of young-onset CRC and patient survival for cancer of the colon, but minimal difference for rectal cancer. Well-documented and recent increases in young-onset CRC have been largely due to increases in rectal cancer, especially in whites.
Keywords: SEER database, neoplasm, young adult, African American
Graphical Abstract
From 1992–1996 [ ] to 2010–2014 [
] to 2010–2014 [ ], increases in young-onset colorectal cancer have been largely due to increases in rectal cancer, especially in whites. In the most time recent period, blacks and whites have similar incidence and survival of rectal cancer, a sharp contrast to the striking disparities in colon cancer.
], increases in young-onset colorectal cancer have been largely due to increases in rectal cancer, especially in whites. In the most time recent period, blacks and whites have similar incidence and survival of rectal cancer, a sharp contrast to the striking disparities in colon cancer.
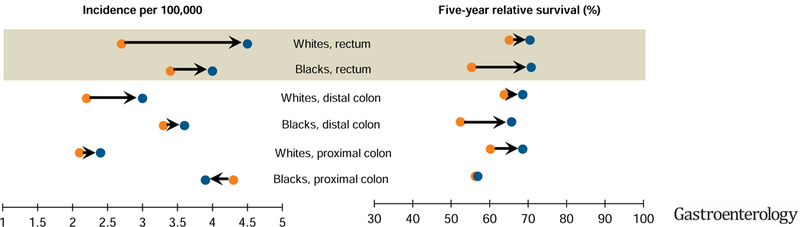
Lay Summary
The incidence of colorectal cancer is increasing in younger adults (age <50 years). The factor that has made the largest contribution to the increase in CRC among younger adults has been increasing rates of rectal cancer, which is more prominent among whites. Our findings provide clues for understanding reasons why incidence has increased.
Introduction
Despite overall reductions in colorectal cancer (CRC) incidence and mortality in the U.S.,1 blacks continue to experience a greater CRC burden than whites.2 CRC disproportionately affects blacks, and racial disparities persist across several important outcomes: higher incidence3 and mortality4 and worse CRC survival.5
Well-known, recent increases in CRC incidence have occurred in younger (ages <50 years) adults.3,6–8 Starting in the early 1990s, incidence rates have increased among younger adults from 8.6 per 100,000 in 1992 to 12.5 per 100,000 in 2015, an overall 45% increase.9 Increasing rates of young-onset CRC have attracted substantial research and media attention, but we know little about racial disparities in this population. Few studies6,10–12 have examined racial differences in incidence by anatomic subsite (colon vs. rectum), or the corresponding implications for survival, which may mask important differences in disease burden and etiology between whites and blacks. Examining racial disparities in both incidence and survival provides important insight concerning differences in risk factors, access to care, and treatment effectiveness.
To better understand CRC disparities between younger whites vs. blacks, we examined racial differences in CRC incidence in younger (age <50 years) adults during 1992 – 2014. We also examined differences in relative survival.
Methods
We derived CRC incidence using Surveillance, Epidemiology, and End Results (SEER) program of cancer registries during 1992 – 2014. The SEER 13 registries cover approximately 15% of the U.S. population and include Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterrey, and Alaska Native Tumor Registry. Age-adjusted incidence (using the 2000 standard population) were obtained using SEER*Stat version 8.3.5 as rates per 100,000 persons. Corresponding 95% confidence intervals were calculated as modified gamma intervals.13
We estimated incidence across two time periods (1992–96 vs. 2010–14) and by race (black vs. white), anatomic subsite, and stage at diagnosis. We selected the time period 1992–96 for a baseline comparison because this was when increases in CRC incidence were first observed in younger populations.3 Anatomic subsite included proximal colon (cecum, ascending colon, hepatic flexure, and transverse colon), distal colon (splenic flexure, descending colon, and sigmoid colon), and rectum (rectosigmoid junction and rectum). SEER defines stage at diagnosis using historic summary staging: local disease is confined to the large bowel, regional is limited to nearby lymph nodes or other organs, and distant is systemic metastasis.
We calculated relative survival using the Ederer II method14 as the ratio of observed survival to expected survival in a comparable, cancer-free population. Relative survival provides a measure of excess mortality experienced by cancer patients without requiring cause of death information.15 Expected survival was calculated from population life tables of the U.S. general population and adjusted across strata of age (15–44, 45–54 years), sex, and race. To illustrate trends, we estimated five-year relative survival in whites and blacks (all ages 20–49 years), contrasting time period (1992–96 vs. 2010–14), anatomic subsite, and stage at diagnosis.
Results
Among whites and blacks ages 20 – 49 years, 31,859 incident cases of CRC were diagnosed in whites, and 5,203 in blacks during 1992 – 2014. Overall incidence was 9.2 per 100,000 and 12.2 per 100,000 among whites and blacks, respectively.
Incidence
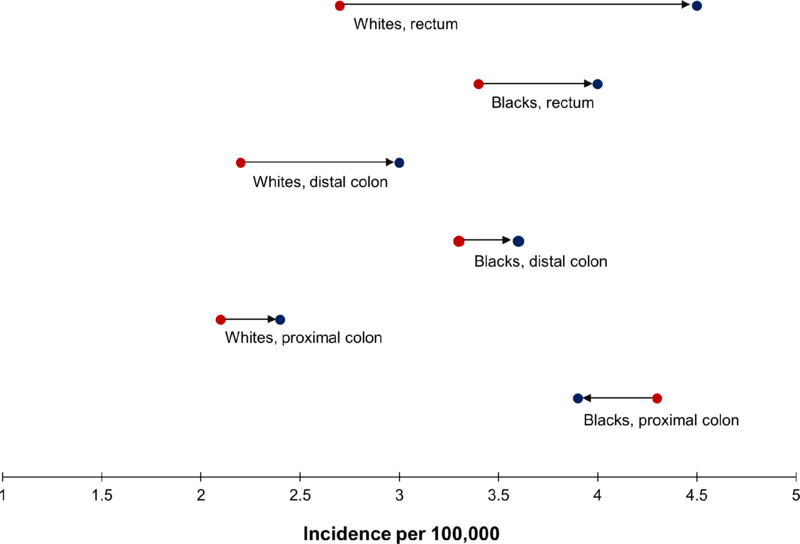
From 1992–96 to 2010–14, there were marked differences in incidence trends between whites and blacks. In whites, we observed a large increase in CRC incidence (from 7.5 to 11.0 per 100,000 or 47% relative increase), but in blacks, the increase was small (from 11.7 to 12.7 per 100,000) (Table 1). The incidence of proximal and distal colon cancer increased in whites but remained stable or decreased in blacks (Figure 1). Whites and blacks both experienced increases in rates of rectal cancer; however, this was more prominent in whites. Of the 3.5 per 100,000 absolute increase in CRC incidence among whites, more than half was from increases in rectal cancer. By 2010–14, whites had higher rates of rectal cancer than blacks.
Table 1.
Age-adjusted1 incidence (rate per 100,000) of colorectal cancer (age 20–49 years) by race and time period (1992–96 vs. 2010–14), overall and by anatomic subsite and stage at diagnosis, SEER 13, 1992—2014
| White | Black | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1992 – 96 | 2010 – 14 | Rate Diff.2 | 1992 – 96 | 2010 – 14 | Rate Diff. | 1992 – 96 | 2010 – 14 | Rate Diff. | |
| Overall | 7.5 ± 0.1 | 11.0 ± 0.1 | 3.5 | 11.7 ± 0.4 | 12.7 ± 0.4 | 1.0 | 7.9 ± 0.1 | 11.2 ± 0.1 | 3.3 |
| Anatomic subsite | |||||||||
| Proximal colon | 2.1 ± 0.1 | 2.4 ± 0.1 | 0.3 | 4.3 ± 0.2 | 3.9 ± 0.2 | −0.4 | 2.4 ± 0.1 | 2.6 ± 0.1 | 0.2 |
| Distal colon | 2.2 ± 0.1 | 3.0 ± 0.1 | 0.8 | 3.3 ± 0.2 | 3.6 ± 0.2 | 0.3 | 2.3 ± 0.1 | 3.1 ± 0.1 | 0.8 |
| Rectum | 2.7 ± 0.1 | 4.5 ± 0.1 | 1.8 | 3.4 ± 0.2 | 4.0 ± 0.2 | 0.6 | 2.7 ± 0.1 | 4.4 ± 0.1 | 1.7 |
| Appendix/unspecified | 0.5 ± 0.0 | 1.1 ± 0.0 | 0.6 | 0.7 ± 0.1 | 1.1 ± 0.1 | 0.4 | 0.5 ± 0.0 | 1.1 ± 0.1 | 0.6 |
| Stage at diagnosis | |||||||||
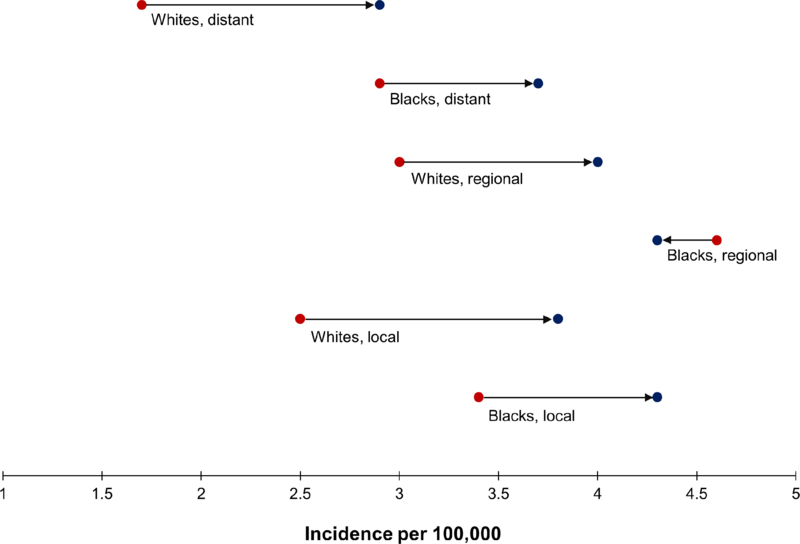
| Local | 2.5 ± 0.1 | 3.8 ± 0.1 | 1.3 | 3.4 ± 0.2 | 4.3 ± 0.2 | 1.1 | 2.6 ± 0.1 | 3.8 ± 0.1 | 1.2 |
| Regional | 3.0 ± 0.1 | 4.0 ± 0.1 | 1.0 | 4.6 ± 0.2 | 4.3 ± 0.2 | −0.3 | 3.2 ± 0.1 | 4.0 ± 0.1 | 0.8 |
| Distant | 1.7 ± 0.1 | 2.9 ± 0.1 | 1.2 | 2.9 ± 0.2 | 3.7 ± 0.2 | 0.8 | 1.8 ± 0.1 | 3.0 ± 0.1 | 1.2 |
| Unstaged | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.0 | 0.7 ± 0.1 | 0.5 ± 0.1 | −0.2 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.0 |
Age-adjusted to the 2000. U.S. standard population
Rate differences correspond to the incidence rate in 1992–96 subtracted from the incidence rate in 2010–14; a negative rate difference indicates decreasing incidence.
Figure 1.
Incidence (rate per 100,000) of colorectal cancer (ages 20–54 years) by anatomic subsite and race, SEER 13, shown as the rate over the period 1992–96 [●] and over the period 2010–14 [●] : 1992–96 ●→● 2010–14
For whites and blacks, incidence rates of local and distant disease increased similarly between 1992–96 and 2010–14 (Figure 2). For example, among whites, the rates of local disease increased from 2.5 to 3.8 per 100,000, accounting for nearly half of the overall increase in CRC incidence (Table 1). Rates of regional disease increased in whites but slightly decreased (from 4.6 to 4.3 per 100,000) in blacks. Despite larger increases in whites over the study period, the absolute incidence of CRC remained consistently higher across all stages in blacks, primarily driven by higher incidence of proximal colon cancer.
Figure 2.
Incidence (rate per 100,000) of colorectal cancer (ages 20–54 years) by stage at diagnosis and race, SEER 13, shown as the rate over the period 1992–96 [●] and over the period 2010–14 [●] : 1992–96 ●→● 2010–14
We observed a similar pattern in analyses stratified by sex, though women generally had lower incidence than men (results not shown).
Relative survival
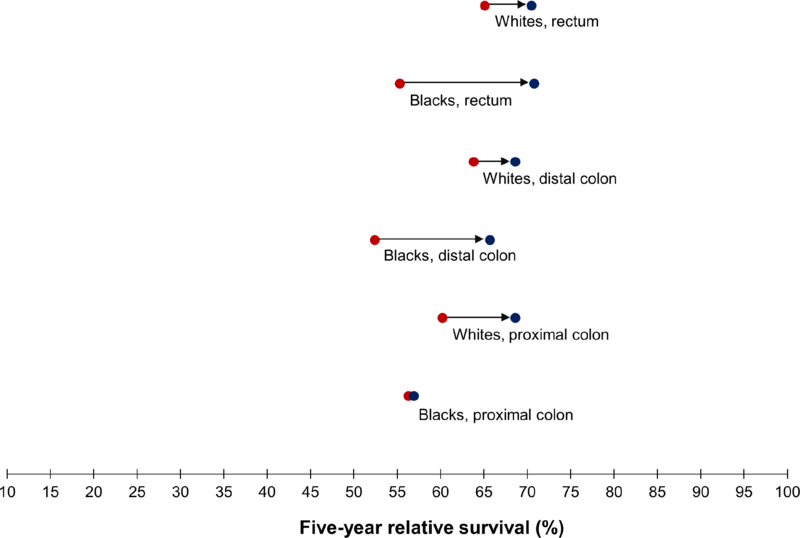
For both racial groups combined, five-year relative survival improved from 61.5% in 1992–96 to 67.7% in 2010–14 (Table 2). However, similar to incidence trends, relative survival differed for whites and blacks in the colon versus rectum. Compared to whites, blacks experienced smaller increases in relative survival of proximal colon cancer and much greater improvements in relative survival of rectal cancer (from 55.3 to 70.8%). As a result, by 2010–14, the survival disparity between blacks and whites had increased for cancers in the proximal colon but essentially disappeared for rectal cancer (Figure 3).
Table 2.
Five-year relative survival (including standard errors) of colorectal cancer (age 20 – 49 years) by race/ethnicity and time period (1992–96 vs. 2010–14), overall and by anatomic subsite and stage at diagnosis, SEER 13, 1992—2014
| White | Black | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1992 – 96 | 2010 – 14 | Diff.1 | 1992 – 96 | 2010 – 14 | Diff. | 1992 – 95 | 2010 – 14 | Diff. | |
| Overall | 63.0 ± 0.8 | 68.6 ± 0.8 | 5.6 | 54.3 ± 1.4 | 62.8 ± 1.9 | 8.5 | 61.5 ± 0.7 | 67.7 ± 0.8 | 6.2 |
| Anatomic subsite | |||||||||
| Proximal colon | 60.2 ± 1.4 | 68.6 ± 1.5 | 8.4 | 56.3 ± 2.8 | 56.9 ± 3.2 | 0.6 | 60.2 ± 1.2 | 66.3 ± 1.4 | 6.1 |
| Distal colon | 63.8 ± 1.4 | 68.6 ± 1.5 | 4.8 | 52.4 ± 3.3 | 65.7 ± 3.4 | 13.3 | 62.0 ± 1.3 | 68.2 ± 1.4 | 6.2 |
| Rectum | 65.1 ± 1.3 | 70.5 ± 1.3 | 5.4 | 55.3 ± 3.2 | 70.8 ± 3.3 | 15.5 | 63.7 ± 1.2 | 70.5 ± 1.2 | 6.8 |
| Appendix/unspecified | 60.6 ± 3.1 | 67.8 ± 2.7 | 7.2 | 46.1 ± 7.1 | 46.1 ± 7.7 | 0.0 | 58.2 ± 2.9 | 64.9 ± 2.6 | 6.7 |
| Stage at diagnosis | |||||||||
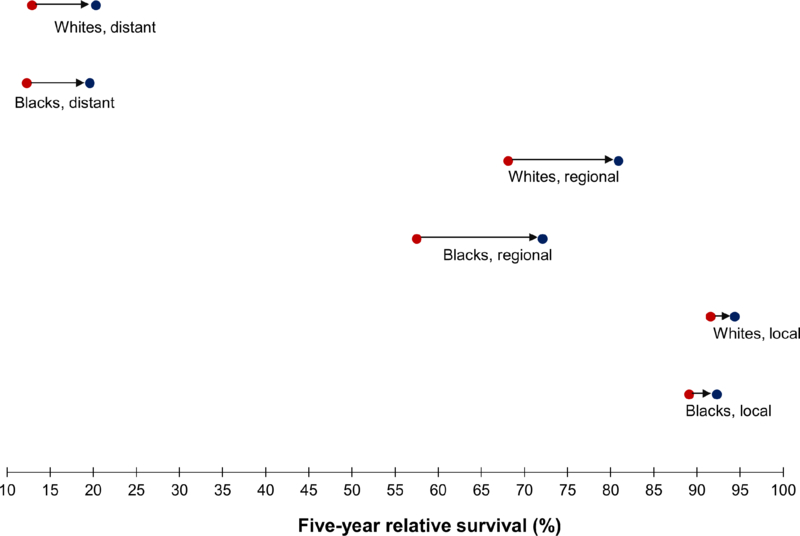
| Local | 91.6 ± 0.8 | 94.4 ± 0.8 | 2.8 | 89.1 ± 2.2 | 92.3 ± 2.2 | 3.2 | 91.3 ± 0.8 | 94.1 ± 0.8 | 2.8 |
| Regional | 68.1 ± 1.2 | 80.9 ± 1.2 | 12.8 | 57.5 ± 2.8 | 72.1 ± 3.2 | 14.6 | 66.3 ± 1.1 | 79.7 ± 1.1 | 13.4 |
| Distant | 12.9 ± 1.1 | 20.3 ± 1.6 | 7.4 | 12.3 ± 2.2 | 19.6 ± 2.8 | 7.3 | 12.8 ± 1.0 | 20.4 ± 1.4 | 7.6 |
| Unstaged | 68.6 ± 3.8 | 56.9 ± 5.0 | −11.7 | 49.2 ± 7.4 | 68.1 ± 9.5 | 18.9 | 64.1 ± 3.4 | 59.0 ± 4.4 | −5.1 |
Difference corresponds to relative survival in 1992–96 subtracted from related survival in 2010–14
Figure 3.
Five-year relative survival of colorectal cancer (ages 20–54 years) by anatomic subsite and race, SEER 13, shown as the rate over the period 1992–96 [●] and over the period 2010–14 [●] : 1992–96 ●→● 2010–14
Differences in relative survival by stage remained approximately the same over time, and blacks had worse stage-specific survival than whites throughout the study period (Table 2). For example, relative survival of regional stage disease increased from 68.1 to 80.9% among whites, compared to an increase from 57.5 to 72.1% among blacks (Figure 4).
Figure 4.
Five-year relative survival of colorectal cancer (ages 20–54 years) by stage at diagnosis and race, SEER 13, shown as survival over the period 1992–96 [●] and over the period 2010–14 [●] : 1992–96 ●→● 2010–14
This pattern persisted in analyses stratified by sex, and women had slightly higher relative survival (results not shown).
Discussion
Well-documented, recent increases in young-onset CRC have largely manifested as increases in rectal cancer, particularly among whites. Increases in colon cancer were small by comparison – and seen only in whites. Thus, the dominant factor driving increases in CRC among younger adults has been increasing rates of rectal cancer, more prominent among whites. Similarly, survival increased for both blacks and whites, but this was more evident for rectal cancer. Blacks and whites now have similar rectal cancer incidence and relative survival, a sharp contrast to the marked disparities in proximal and distal colon cancer. We also observed larger increases in the incidence of local and distant (vs. regional) disease in both blacks and whites, with particularly notable increases in local disease among whites.
Our findings point to differences in the etiology of rectal (vs. colon) cancer that may explain the increase in incidence among younger adults. Factors more strongly associated with rectal cancer and similar in prevalence by race/ethnicity likely play a role. For example, dysbiosis-related factors, such as antibiotic use16–18 and periodontal disease,19 seem to be associated with higher risk of rectal cancer, mediated by their effect on microbial diversity and composition. Differences in pH levels across the entire colorectum may differentially influence susceptibility to environmental risk factors and create more favorable conditions for bacterial dysbiosis.20 Another possibility is the influence of tobacco and heavy alcohol use – these risk factors are differentially associated with colon vs. rectal cancer.21–25 Familial risk (both family history and genetic syndromes),20 obesity,26–28 and physical activity27,29 also appear less influential in development of rectal cancer. Growing evidence supports etiologic differences in colon and rectal cancer, but researchers have not yet examined whether these risk factors also act differently in blacks and whites.
Prior studies have generally focused on the increasing number of late-stage diagnoses among younger adults,6,8 but our results show a larger increase in local as opposed to distant disease. The increases in local disease suggest two possible underlying phenomena: 1) diagnostic factors,7 for example, screening with colonoscopy at age 40 or 45, which may facilitate earlier detection of small tumors; and 2) slow-growing tumors, which may be more amenable to detection at an early stage and more common in younger adults than previously thought. Although we know little about indications for colonoscopy in younger adults, or differences in receipt by race, our prior work shows increases in colonoscopy use that parallel incidence of young-onset CRC,7 which may explain increasing rates of local disease. Meanwhile, others have suggested unrecognized symptoms,30 such as rectal bleeding,31 contribute to delays in presentation and increasing rates of late-stage disease.
Prior studies have also shown racial differences in colon cancer survival are concentrated in younger (vs. older) adults,5,32–35 even after adjusting for confounders, such as treatment receipt.5,32–36 Because screening has not been recommended in this younger population, the racial disparities in relative survival that we and others have observed are unlikely to be the result of differential uptake of screening. Consequently, our findings raise a number of questions concerning the contribution of treatment37,38 and therapeutic response39,40 to racial differences in relative survival, which may also differ for colon and rectal cancers. We observed no difference in relative survival between blacks and whites with rectal cancer diagnosed in 2010–14, suggesting that equal receipt of guideline-recommended therapies41 has played a prominent role in achieving health equity for rectal cancer outcomes. Multimodality therapy (i.e., neoadjuvant chemoradiation followed by surgery) for locally advanced rectal cancer reduces risk of recurrence and improves survival42 and became standard in the early 2000s. Others have similarly reported improvements in overall and disease-free survival for rectal cancer patients between 2001 and 2012, noting that this is likely due to preoperative radiation therapy and chemotherapy.43
We also found that improvements in proximal colon cancer survival in blacks lagged far behind whites. The fact that survival disparities by race persist only in colon cancer may reflect differences in tumor biology and treatment response. Blacks are more frequently diagnosed with microsatellite stable tumors in the proximal colon, and patients with proximal colon cancer tend to have higher mortality and poorer outcomes.44 This is particularly true for those exhibiting low microsatellite instability45 or with advanced disease.46–49 Further, growing evidence suggests combination chemotherapies and targeted therapies (e.g., anti-VEGF or anti-EGFR) are less effective in treating proximal colon cancer,50,51 and tumors in blacks respond poorly to treatment – even when administered at similar rates as whites.39,40 Clinical characteristics (e.g., obesity, diabetes),52–55 mutation profiles,56,57 or differences in immune response may modify therapeutic effectiveness and contribute to poorer outcomes among blacks. Compared to whites, blacks have a greater burden of prognostic factors associated with inflammation, which may contribute to treatment resistance and tumor progression.58,59 Better understanding factors driving treatment response may reveal additional insight concerning racial disparities in colon cancer survival.
We acknowledge limitations inherent in cancer registry data. These data may not be uniformly accurate for all population subgroups, including racial/ethnic minorities, or geographic regions, and we had incomplete data on stage and anatomic subsite. However, prior studies on the quality of SEER data show near complete case ascertainment (98%),60 no differences in case ascertainment by race,61 and excellent agreement between SEER records and self-reported race.62 Sparse data precluded relative survival estimates by 5-year age groups. Relative survival ignores cause of death information, and instead, differences in relative survival reflect differences in cancer rather competing causes of death.15 Finally, although our findings raise the possibility that racial disparities in relative survival may be due to receipt of treatment and tumor biology, cancer registries do not systematically collect molecular characteristics or chemotherapy-based treatment regimens.
In summary, our findings emphasize the importance of distinguishing rectal vs. colon cancer while studying the increasing incidence of young-onset CRC and racial disparities in this age group. Although others have reported racial differences in mortality rates of young-onset CRC,11 mortality reflects both incidence and case fatality. We examined incidence and relative survival as markers of population burden, treatment receipt and effectiveness, and tumor biology. Moving forward, identifying etiologic differences in colon and rectal cancer, and how risk factors may act differently in black and whites, will advance our understanding of young-onset CRC.
What You Need to Know
Background: We investigated racial disparities (black vs white patients) in incidence of young-onset CRC and survival for different time periods (1992–1996 vs 2010–2014).
Findings: In an analysis of the Surveillance, Epidemiology, and End Results database, we found that the incidence of CRC increased from 7.5/100,000 to 11.0/100,000 in white individuals and from 11.7/100,000 to 12.7/100,000 in black individuals. In the 2010–2014 time period, the incidence of rectal cancer incidence did not differ significantly between races.
Implications for patient care: There are racial disparities in incidence of young-onset colon cancer and patient survival, but no significant differences between races in incidence of rectal cancer or survival
Acknowledgments
Grant support: National Center for Advancing Translational Sciences (KL2TR001103) and National Cancer Institute (P30CA142543) at the National Institutes of Health.
Abbreviations:
- CRC
colorectal cancer
- SEER
Surveillance, Epidemiology and End Results
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare no conflicts of interest or financial disclosures.
References
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA: a cancer journal for clinicians 2017;67(3):177–193. [DOI] [PubMed] [Google Scholar]
- 2.Kupfer SS, Carr RM, Carethers JM. Reducing colorectal cancer risk among African Americans. Gastroenterology 2015;149(6):1302–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy CC, Sandler RS, Sanoff HK, Yang YC, Lund JL, Baron JA. Decrease in Incidence of Colorectal Cancer Among Individuals 50 Years or Older After Recommendations for Population-based Screening. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2017;15(6):903–909. e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol 2012;30(4):401–405. [DOI] [PubMed] [Google Scholar]
- 5.Wallace K, Hill EG, Lewin DN, et al. Racial disparities in advanced-stage colorectal cancer survival. Cancer causes & control : CCC 2013;24(3):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. Journal of the National Cancer Institute 2017;109(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy CC, Lund JL, Sandler RS. Young-Onset Colorectal Cancer: Earlier Diagnoses or Increasing Disease Burden? Gastroenterology 2017;152(8):1809–1812. e1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA surgery 2015;150(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stat S.
- 10.Araghi M, Fidler MM, Arnold M, Jemal A, Bray F, Soerjomataram I. The Future Burden of Colorectal Cancer Among US Blacks and Whites. Journal of the National Cancer Institute 2018. [DOI] [PubMed]
- 11.Siegel RL, Miller KD, Jemal A. Colorectal Cancer Mortality Rates in Adults Aged 20 to 54 Years in the United States, 1970–2014. JAMA : the journal of the American Medical Association 2017;318(6):572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis L, Abrahao R, McKinley M, et al. Colorectal Cancer Incidence Trends by Age, Stage, and Racial/Ethnic Group in California, 1990–2014. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2018. [DOI] [PubMed]
- 13.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Statistical methods in medical research 2006;15(6):547–569. [DOI] [PubMed] [Google Scholar]
- 14.Cho H, Howlader N, Mariotto AB, Cronin KA. Estimating relative survival for cancer patients from the SEER Program using expected rates based on Ederer I versus Ederer II method
- 15.Mariotto AB, Noone AM, Howlader N, et al. Cancer survival: an overview of measures, uses, and interpretation. Journal of the National Cancer Institute Monographs 2014;2014(49):145–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boursi B, Haynes K, Mamtani R, Yang YX. Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiology and drug safety 2015;24(5):534–542. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut 2018;67(4):672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dik VK, van Oijen MG, Smeets HM, Siersema PD. Frequent Use of Antibiotics Is Associated with Colorectal Cancer Risk: Results of a Nested Case-Control Study. Digestive diseases and sciences 2016;61(1):255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Momen-Heravi F, Babic A, Tworoger SS, et al. Periodontal disease, tooth loss and colorectal cancer risk: Results from the Nurses’ Health Study. International journal of cancer 2017;140(3):646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. International journal of cancer Journal international du cancer 2004;108(3):433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. International journal of cancer Journal international du cancer 2009;124(10):2406–2415. [DOI] [PubMed] [Google Scholar]
- 22.Hansen RD, Albieri V, Tjonneland A, Overvad K, Andersen KK, Raaschou-Nielsen O. Effects of smoking and antioxidant micronutrients on risk of colorectal cancer. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2013;11(4):406–415. e403. [DOI] [PubMed] [Google Scholar]
- 23.Tsoi KK, Pau CY, Wu WK, Chan FK, Griffiths S, Sung JJ. Cigarette smoking and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2009;7(6):682–688. e681–685. [DOI] [PubMed] [Google Scholar]
- 24.Nisa H, Kono S, Yin G, et al. Cigarette smoking, genetic polymorphisms and colorectal cancer risk: the Fukuoka Colorectal Cancer Study. BMC cancer 2010;10:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2011;22(9):1958–1972. [DOI] [PubMed] [Google Scholar]
- 26.Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obesity reviews : an official journal of the International Association for the Study of Obesity 2010;11(1):19–30. [DOI] [PubMed] [Google Scholar]
- 27.Robsahm TE, Aagnes B, Hjartaker A, Langseth H, Bray FI, Larsen IK. Body mass index, physical activity, and colorectal cancer by anatomical subsites: a systematic review and meta-analysis of cohort studies. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 2013;22(6):492–505. [DOI] [PubMed] [Google Scholar]
- 28.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. The American journal of clinical nutrition 2007;86(3):556–565. [DOI] [PubMed] [Google Scholar]
- 29.Cong YJ, Gan Y, Sun HL, et al. Association of sedentary behaviour with colon and rectal cancer: a meta-analysis of observational studies. Br J Cancer 2014;110(3):817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connell JB, Maggard MA, Livingston EH, Yo CK. Colorectal cancer in the young. Am J Surg 2004;187(3):343–348. [DOI] [PubMed] [Google Scholar]
- 31.Weingart SN, Stoffel EM, Chung DC, et al. Delayed Workup of Rectal Bleeding in Adult Primary Care: Examining Process-of-Care Failures. Joint Commission journal on quality and patient safety 2017;43(1):32–40. [DOI] [PubMed] [Google Scholar]
- 32.Andaya AA, Enewold L, Zahm SH, et al. Race and colon cancer survival in an equal-access health care system. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2013;22(6):1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon HH, Shi Q, Alberts SR, et al. Racial Differences in BRAF/KRAS Mutation Rates and Survival in Stage III Colon Cancer Patients. Journal of the National Cancer Institute 2015;107(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phatak UR, Kao LS, Millas SG, Wiatrek RL, Ko TC, Wray CJ. Interaction between age and race alters predicted survival in colorectal cancer. Annals of surgical oncology 2013;20(11):3363–3369. [DOI] [PubMed] [Google Scholar]
- 35.Holowatyj AN, Ruterbusch JJ, Rozek LS, Cote ML, Stoffel EM. Racial/Ethnic Disparities in Survival Among Patients With Young-Onset Colorectal Cancer. J Clin Oncol 2016;34(18):2148–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace K, DeToma A, Lewin DN, et al. Racial Differences in Stage IV Colorectal Cancer Survival in Younger and Older Patients. Clinical colorectal cancer 2017;16(3):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esnaola NF, Ford ME. Racial differences and disparities in cancer care and outcomes: where’s the rub? Surgical oncology clinics of North America 2012;21(3):417–437, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esnaola NF, Gebregziabher M, Finney C, Ford ME. Underuse of surgical resection in black patients with nonmetastatic colorectal cancer: location, location, location. Annals of surgery 2009;250(4):549–557. [DOI] [PubMed] [Google Scholar]
- 39.Polite BN, Sing A, Sargent DJ, et al. Exploring racial differences in outcome and treatment for metastatic colorectal cancer: results from a large prospective observational cohort study (BRiTE). Cancer 2012;118(4):1083–1090. [DOI] [PubMed] [Google Scholar]
- 40.Sanoff HK, Sargent DJ, Green EM, McLeod HL, Goldberg RM. Racial differences in advanced colorectal cancer outcomes and pharmacogenetics: a subgroup analysis of a large randomized clinical trial. J Clin Oncol 2009;27(25):4109–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy CC, Harlan LC, Lund JL, Lynch CF, Geiger AM. Patterns of Colorectal Cancer Care in the United States: 1990–2010. Journal of the National Cancer Institute 2015;107(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30(16):1926–1933. [DOI] [PubMed] [Google Scholar]
- 43.Heerva E, Carpelan A, Kurki S, et al. Trends in presentation, treatment and survival of 1777 patients with colorectal cancer over a decade: a Biobank study. Acta oncologica (Stockholm, Sweden) 2017:1–8. [DOI] [PubMed]
- 44.Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). In: American Society of Clinical Oncology; 2016.
- 45.Phipps AI, Lindor NM, Jenkins MA, et al. Colon and Rectal Cancer Survival by Tumor Location and Microsatellite Instability: The Colon Cancer Family Registry. Diseases of the colon and rectum 2013;56(8):937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Diseases of the colon and rectum 2010;53(1):57–64. [DOI] [PubMed] [Google Scholar]
- 47.Suttie SA, Shaikh I, Mullen R, Amin AI, Daniel T, Yalamarthi S. Outcome of right- and left-sided colonic and rectal cancer following surgical resection. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland 2011;13(8):884–889. [DOI] [PubMed] [Google Scholar]
- 48.Wray CM, Ziogas A, Hinojosa MW, Le H, Stamos MJ, Zell JA. Tumor subsite location within the colon is prognostic for survival after colon cancer diagnosis. Diseases of the colon and rectum 2009;52(8):1359–1366. [DOI] [PubMed] [Google Scholar]
- 49.Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Annals of surgical oncology 2008;15(9):2388–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boeckx N, Janssens K, Van Camp G, et al. The predictive value of primary tumor location in patients with metastatic colorectal cancer: A systematic review. Crit Rev Oncol Hematol 2018;121:1–10. [DOI] [PubMed] [Google Scholar]
- 51.Boeckx N, Koukakis R, Op de Beeck K, et al. Effect of Primary Tumor Location on Second- or Later-line Treatment Outcomes in Patients With RAS Wild-type Metastatic Colorectal Cancer and All Treatment Lines in Patients With RAS Mutations in Four Randomized Panitumumab Studies. Clinical colorectal cancer 2018. [DOI] [PubMed]
- 52.Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. Journal of the National Cancer Institute 2006;98(22):1647–1654. [DOI] [PubMed] [Google Scholar]
- 53.Ottaiano A, Nappi A, Tafuto S, et al. Diabetes and Body Mass Index Are Associated with Neuropathy and Prognosis in Colon Cancer Patients Treated with Capecitabine and Oxaliplatin Adjuvant Chemotherapy. Oncology 2016;90(1):36–42. [DOI] [PubMed] [Google Scholar]
- 54.Sinicrope FA, Foster NR, Yothers G, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer 2013;119(8):1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phipps AI, Shi Q, Newcomb PA, et al. Associations between cigarette smoking status and colon cancer prognosis among participants in North Central Cancer Treatment Group Phase III Trial N0147. Journal of Clinical Oncology 2013;31(16):2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phipps AI, Limburg PJ, Baron JA, et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology 2015;148(1):77–87. e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. New England Journal of Medicine 2000;342(2):69–77. [DOI] [PubMed] [Google Scholar]
- 58.Limagne E, Euvrard R, Thibaudin M, et al. Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer research 2016;76(18):5241–5252. [DOI] [PubMed] [Google Scholar]
- 59.Lotti F, Jarrar AM, Pai RK, et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. The Journal of experimental medicine 2013;210(13):2851–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer 1995;76(11):2343–2350. [DOI] [PubMed] [Google Scholar]
- 61.Thoburn KK, German RR, Lewis M, Nichols PJ, Ahmed F, Jackson-Thompson J. Case completeness and data accuracy in the Centers for Disease Control and Prevention’s National Program of Cancer Registries. Cancer 2007;109(8):1607–1616. [DOI] [PubMed] [Google Scholar]
- 62.Clegg LX, Reichman ME, Hankey BF, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes & Control 2007;18(2):177–187. [DOI] [PubMed] [Google Scholar]