Abstract
Background & Aims:
The neuropathophysiology of fecal incontinence (FI) is incompletely understood. We examined the efferent brain-anorectal and spino-anorectal motor-evoked potentials (MEP) to characterize the locus of neuronal injury in patients with FI.
Methods:
We performed bilateral transcranial, translumbar, and transsacral magnetic stimulations in 27 patients with FI (19 female) and 31 healthy individuals (controls, 20 female) from 2015 through 2017. MEPs were recorded simultaneously from the rectum and anus using 4 ring electrodes. The difference in MEP latencies between the transcranial (TMS) and translumbar transsacral magnetic stimulations was calculated as cortico-spinal conduction time. MEP data were compared between patients with FI and controls. Patients filled out questionnaires that assessed the severity and effects of FI.
Results:
The MEP latencies with TMS were significantly longer in patients with FI than controls at most sites, and on both sides (P<.05). Almost all translumbar and transsacral MEP latencies were significantly prolonged in patients with FI vs controls (P < .01). The cortico-spinal conduction time were similar, on both sides, between patients with FI and controls. Ninety-three percent of patients had 1 or more abnormal translumbar and transsacral latencies, but neuropathy was patchy and variable, and not associated with sex or anal sphincter function or defects.
Conclusion:
Patients with FI have significant neuropathy that affects the cortico-anorectal and spino-anorectal efferent pathways. The primary loci are the lumbo-rectal, lumbo-anal, sacro-rectal, and sacro-anal nerves; the cortico-spinal segment appears intact. Peripheral spino-anal and spino-rectal neuropathy might therefore contribute to the pathogenesis of FI.
Keywords: anorectal disease, pathophysiology, mechanism, neuromodulation therapy
Introduction
Fecal incontinence (FI) affects 7% to 15% of the western population.1 Its pathophysiology is complex and multifactorial. 1 Previous studies using anal electromyography (EMG) 2, 3 and pudendal nerve terminal motor latency (PNTML) assessment 4–7 have suggested that neuropathy may play a role in causing FI.
Previously, we showed that rectal and anal motor evoked potential (MEP) latencies translumbar were significantly prolonged in FI patients. 8 This finding indicates that the nerve conduction between the lumbosacral spinal cord and both the rectum and anus are significantly abnormal and indicative of neuropathy in patients with FI. Recently, a study using transcranial magnetic stimulation (TMS) 9 showed that 23% of patients with FI without known central neurologic disease or anal sphincter lesion had abnormal anal evoked pressure curves (EPC). This suggests that the abnormal conduction of efferent signals from the brain to the anus may also be abnormal in FI. However, whether the efferent conductivity between the brain and rectum is also abnormal in patients with FI is unknown. Also, the locus of neurophysiological dysfunction in patients with FI, i.e., whether the neuropathy is confined to the peripheral nervous system or involves the brain-rectoanal, spino-rectoanal, or brain-spinal nerve pathways is unknown. A clear delineation of the locus of neuropathy may facilitate the use of targeted neuromodulation therapies.
Previously, we showed the feasibility of performing simultaneous TMS and translumbar magnetic stimulation (TLMS) and transsacral magnetic stimulation (TSMS) in the same individual. 8, 10 Such an analysis could provide comprehensive information regarding the descending brain-anorectal axis, and help to dissect out the precise loci of neuropathy in patients with FI, i.e., if it affects the central or peripheral nervous system or both.
In this study, we tested the hypothesis that the neural conduction between the brain and anorectum is deranged in patients with FI. Our aim was to examine the locus of neuropathy in patients with FI by performing bilateral TMS, TLMS and TSMS simultaneously and evaluating the rectal and anal MEPs and to compare these findings with healthy controls.
Materials and methods
Subjects
Patients with FI with at least 1 episode of formed or liquid stool leakage per week, as demonstrated on a prospective stool diary, and no previous history of back injury, spinal surgery, or neurological disease were enrolled in this study. Healthy subjects were screened with Mayo questionnaire, and those without previous surgery or medication use (except oral contraceptives, aspirin, or multivitamins) and a normal physical examination were recruited. The study protocol was approved by the institutional review boards at the Augusta University (No. 619411–17) and University of Iowa (No. Pro 00000245), and were registered at Clinical trial. gov (NCT02556151).
Experimental design
Each subject underwent magnetic stimulations at 3 different sites, transcranial (TMS), translumbar (TLMS) and transsacral (TSMS), in a random order after a rest period of 30 minutes. The motor evoked potentials (MEPs) were recorded simultaneously from the rectum and anal region using an anal probe. Each test took about 30 minutes to complete. Subsequently, all subjects underwent high resolution anorectal manometry (HRARM). Additionally, patients with FI filled out questionnaires that assessed the severity and impact of FI including the FI severity Index (FISI)11 and the FI Severity Score (FISS). 12 The FISI score assessed the frequency of different types of stool leakage (gas/ mucus/ liquid stool/ solid stool) with scores ranging from 4 to 24 (no leakage). FISS culls the information on the composition, frequency, amount and the patients’ feelings about the stool leakage. The severity scores range from 5 (mild) to 20 (severe leakage). Overall severity of FI was assessed on a visual analog scale, 0 (absent) to 10 (very severe).
TMS, TLMS and TSMS
With the subject lying in the left lateral position, a probe containing 2 pairs of bipolar steel ring electrodes (Gaeltec, Gaeltec Devices Ltd., Dunvegan, Scotland) was placed such that 1 pair was located in the anal canal at 2 to 3cm from the anal verge and the other pair was located in the rectum at 7 to 9 cm from the anal verge. The probe was fixed using latex-free tape (3M, St Paul, Minnesota), and electrically grounded by placing a surface electrode on the gluteal muscle. Also, 3 surface electrodes were placed on anterior tibialis muscle. The magnetic stimulations were performed using the Magstim Rapid2 stimulator (The Magstim Company Limited, Whiteland, Wales, U.K.). TMS was performed with a basket shaped coil and TLMS and TSMS was performed with a 90mm circular coil (The Magstim Company Limited, Whiteland, Wales, U.K.). Subjects were semi recumbent (30-degree elevation) for the TMS study and prone for the TLMS and TSMS studies.
The cortico-rectal and cortico-anal MEPs were evoked by stimulating the brain bilaterally around the supermedial motor cortex (Brodmann area 4). 10, 13, 14 For the TLMS, the coil was applied on each side at the L3 level, about 4 cm lateral to the midline. For the TSMS the coil was applied on each side at the S3 level, about 4 cm lateral to the midline. 8 Typically, the magnetic stimulations began at 50% and thereafter in increments of 5% up to 90% until an adequate and reproducible MEP response, defined as an anal and rectal MEP response of 10 microvolts and an anterior tibialis MEP of 20 microvolts with 50% of trials was obtained. At least five MEP responses were recorded at each site.
Definition of terms and data analysis
Motor evoked potentials
The MEP data were analyzed manually using the Neuropack ® (Nihon Kohden, Tokyo, Japan) software. The MEP latency was defined as the interval between the onset of stimulus and the onset of the individual rectal or anal MEP waveform8 and was expressed in milliseconds (Figure 1). The mean value of the 5 MEP responses per site was calculated as the MEP response for that site. An abnormal MEP latency was defined as a value higher than the 95% confidence interval of healthy controls.
Figure 1.
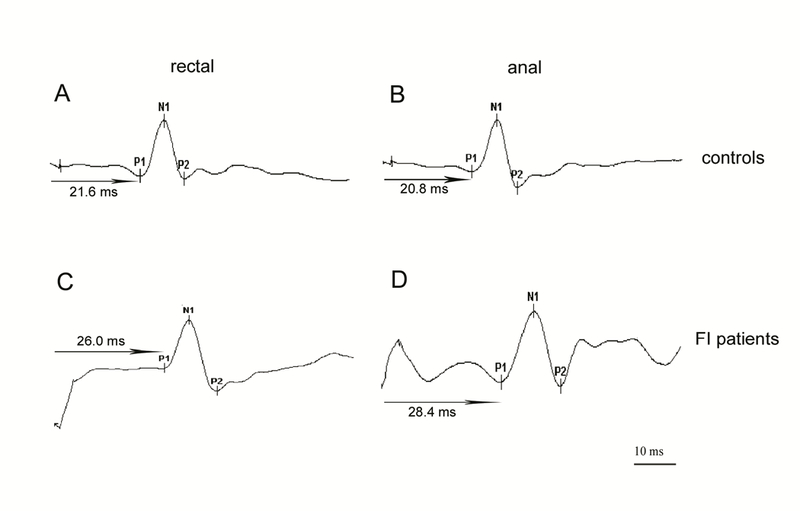
Typical rectal and anal MEP waveform patterns after TMS
Panels A and B show rectal and anal MEPs after TMS in healthy controls. Panels C and D show rectal and anal MEPs after TMS in FI patients. P1 is the onset of the MEP, N1 the peak of the MEP and P2 is the end of the MEP. The time interval between the magnetic stimulation and the onset of P1 is measured as the latency of the MEP response (arrow)
TMS, transcranial magnetic stimulation; MEP, motor evoked potential; FI, fecal incontinence
Cortico-lumbar and cortico-sacral spinal cord conduction
The brain-spinal cord conduction time was calculated from the MEP measurements obtained for the TMS, TLMS and TSMS. The difference between the MEP latencies TMS and TLMS on the same side (left or right), and from the same site (anal or rectal) was calculated as the cortico-spinal conduction time (CSCT). Likewise the difference between the TMS and TSMS were calculated. For example, left cortico-lumbar conduction time (anal) was the difference in the anal MEP latency between the cortico-anal MEP (TMS) and the left lumbo-anal MEP (TLMS), likewise for all the other 4 sites (lumbar and sacral) and for the anal and rectal locations.
HRARM and rectal sensory assessment
HRARM was performed by placing into the anorectum a 12-sensor circumferential solid-state probe (ManoScan AR Catheter, Medtronics, MN) with a 4 cm long balloon. Details of the manometric methods have been described previously. 15, 16 Anal sphincter pressures at rest, during squeeze and when pushing were measured. The sensory thresholds for first sensation, desire to defecate, urge to defecate, and the maximum tolerable volume were recorded. 17 Rectal compliance was evaluated with the rectal pressures at different volumes of distention. 17
Correlation of MEP latency with demographic and anorectal physiology and structure
To assess these associations, we measured the relationship between age, gender, ethnicity, history of anorectal injuries, and anal sphincter function and anal sphincter defects, FISI and FISS scores and the rectal and anal MEP latencies.
Statistical Analysis
Data were expressed as mean ± standard error of the mean. Mann-Whitney U test was used to compare the differences of age, FISI and FISS scores, MEP latencies and the number of abnormal latency sites at the rectal and anal sites between patients with and without rectal and anal trauma (surgery and obstetric), of different gender and ethnicity. Kruskal-Wallis test was used to compare the differences of age, FISI and FISS scores, MEP latencies and the number of abnormal latency sites at the rectal and anal sites among patients with different types of FI. Analysis of covariance (ANCOVA) test was used for the comparison of MEP latencies between FI patients and controls to adjust the effect of age and BMI. Multiple linear regression analysis was used to assess the association between the anal sphincter pressures and MEP latencies (the effect of age and BMI was adjusted). Logistic regression analysis was used to assess the association between the occurrence of IAS and EAS defect and the MEP latencies (the effect of age and BMI was adjusted). A p value of <0.05 was considered statistically significant.
Result
Subjects
Twenty-seven patients with FI (F/M = 19/8, mean age 59.6 ± 2.5 years, mean BMI 29.8 ± 1.3 kg/m2 ) were enrolled. The patients’ data were compared with those of 31 healthy controls (F/M= 20/11, mean age 39.4 ± 2.3 years, mean BMI 25.2 ± 0.5 kg/m2). The FI patients were older (p<0.001) and heavier (p=0.001) than controls. The gender distribution was similar (p=0.636).
Demographics
Predisposing and other demographic and key manometric and morphologic features are summarized in Supplemental Table 1. The median number of pregnancies in the patients with FI was 2 (range 1–5) while the median number of pregnancies in controls was 1 (range 1–4) (p<0.05). The obstetric history of FI patients is summarized in Supplemental Table 1. All controls had vaginal deliveries, and 2 also had C-section. Five control subjects reported vaginal tears and 2 had difficult labor that required forceps-assisted delivery. Based on the predominant type of FI, 25.9% (7/27) patients were categorized as passive FI, 55.6% (15/27) patients as urge FI and 18.5%(5/27) as both passive and urge FI.
Anorectal manometry
FI patients had significantly weak resting anal sphincter pressure compared to healthy controls (mean (95% CI):58 (48–67) vs 83 (78–87) mmHg) (p <0.001) (Supplemental Table 1). Likewise, FI patients had weak maximal squeeze pressure compared to healthy controls (120 (97,145) vs 233 (218, 249) mmHg) (p<0.001) and weak sustained squeeze pressure compared to healthy controls (69 (57, 82) vs 124(105,194) mmHg) (p<0.001). Four patients (14.8%) had rectal hyposensitivity, 21(77.8%) had rectal hypersensitivity, and 2 (7.4%) had normal rectal sensitivity. For those patients who had rectal hypersensitivity, the rectal compliance was either normal or decreased.
Transcranial motor evoked potentials (TMS-MEP)
Typical profiles of the MEP response in a patient with FI and a healthy control subject are shown in Figure 1. Abnormal MEP responses were seen in 22 FI patients (81.5%). Seven patients (25.9%) had abnormal MEPs at all sites, and on both sides (Figure 3).
Figure 3.
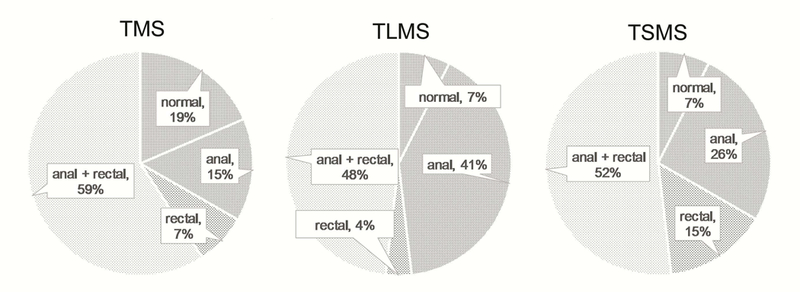
Distribution of the relationship between anal and rectal abnormal MEP latencies after TMS, TLMS and TSMS in FI patients
Only anal = the patient only had anal abnormal MEP (the rectal MEP was normal); Only rectal = the patient only had rectal abnormal MEP (the anal MEP was normal); Anal and rectal = both the anal and rectal MEPs were abnormal.
Four separate MEP responses were obtained for each subject, and the mean TMS data for both the FI patients and controls are summarized in Table 1. The MEP latencies at both the anal and rectal recording sites, and at both the left and the right sides were significantly prolonged in patients with FI compared to controls (p <0.05) except right anal MEP. Only 5 patients (18.5%) with FI had normal MEP responses at both sites and on both sides.
Table 1.
Differences in the MEP latencies obtained using TMS as well as the cortico-spinal conduction time (CSCT) between controls and patients with fecal Incontinence.
|
Controls n = 31 |
Fecal Incontinence n = 27 |
P Value before Adjusting for Age and BMI |
P Value after Adjusting for Age and BMI |
||
|---|---|---|---|---|---|
| Left anal (ms) | 22.0±0.58 | 24.59±1.08 | <0.001 | 0.041 | |
| TMS | Right anal (ms) | 21.78±0.52 | 24.74±1.11 | 0.084 | 0.090 |
| Left rectal (ms) | 21.13±0.39 | 23.59±0.84 | 0.047 | 0.139 | |
| Right rectal (ms) | 20.75±0.41 | 23.08±0.77 | 0.047 | 0.087 | |
| Left CL (anal) (ms) | 18.56±0.66 | 18.79±1.02 | 0.724 | 0.346 | |
| Right CL (anal) (ms) | 18.12±0.51 | 18.54±1.17 | 0.652 | 0.998 | |
| Left CS (anal) (ms) | 18.91±0.64 | 19.38±1.08 | 0.974 | 0.265 | |
| CSCT | Right CS (anal) (ms) | 18.47±0.59 | 18.54±1.07 | 0.501 | 0.924 |
| Left CL (rectal) (ms) | 18.25±0.44 | 19.74±0.90 | 0.277 | 0.339 | |
| Right CL (rectal) (ms) | 17.59±0.40 | 19.05±0.77 | 0.533 | 0.473 | |
| Left CS (rectal) (ms) | 18.12±0.44 | 19.16±1.02 | 0.494 | 0.739 | |
| Right CS (rectal) (ms) | 17.57±0.41 | 18.31±0.75 | 0.703 | 0.896 |
Data are expressed as mean±SEM. TMS, transcranial magnetic stimulation; MEP, motor evoked potential; CSCT, cortico-spinal condution time; CL, cortico-lumbar conduction time; CS, cortico-sacral conduction time; FI, fecal incontinence
Translumbar/ transsacral motor evoked potentials (TLMS-MEP, TSMS-MEP)
Eight separate MEP responses were obtained. Figure 2 shows typical MEP profiles of the TLMS and TSMS responses in a patient with FI and a healthy control. Table 2 summarizes the comparison of the MEP latencies between patients with FI and healthy controls. Overall, the MEP latencies obtained with both the TLMS and TSMS were significantly prolonged (most p<0.01) in patients with FI. Only one patient had normal MEP response for both the TLMS and TSMS. Most patients had abnormal MEP responses at both the anal and rectal sites (Figure 3).
Figure 2.
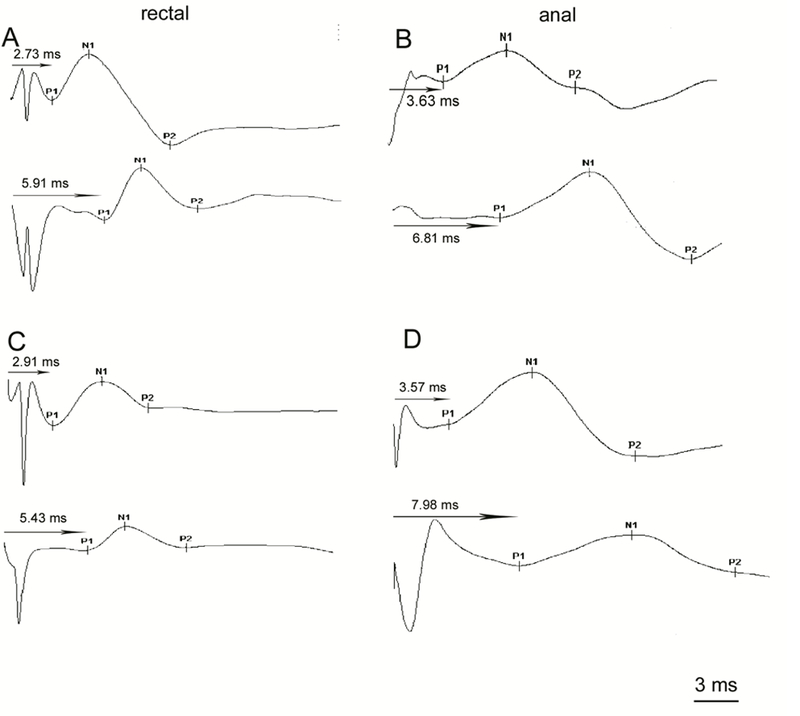
Typical rectal and anal MEP waveform patterns after TLMS and TSMS
Panel A shows the rectal MEPs after TLMS in a healthy control (upper) and a FI patient (lower). Panel B shows the anal MEPs after TSMS in a healthy control (upper) and a FI patient (lower). Panel C shows the rectal MEPs after TSMS in a healthy control (upper) and a FI patient (lower). Panel D shows the anal MEPs after TSMS in a healthy control (upper) and a FI patient (lower).
MEP, motor evoked potential; TLMS, translumbar magnetic stimulation; TSMS, transsacral magnetic stimulation; FI, fecal incontinence
Table 2.
Differences in the anal and rectal MEP latencies between controls and patients with FI after bilateral translumbar and transsacral magnetic stimulations
| Controls n = 31 |
Fecal Incontinence n = 27 |
P Value before Adjusting for Age and BMI |
P Value after Adjusting for Age and BMI |
|
|---|---|---|---|---|
| Left lumbar anal | 3.29±0.77 | 5.79±2.30 | <0.001 | 0.004 |
| Right lumbar anal | 3.39±0.68 | 6.20±2.40 | <0.001 | <0.001 |
| Left sacral anal | 2.99±0.68 | 5.20±1.86 | <0.001 | 0.002 |
| Right sacral anal | 3.14±0.74 | 6.20±2.17 | <0.001 | <0.001 |
| Left lumbar rectal | 2.89±0.68 | 3.84±1.54 | 0.007 | 0.067 |
| Right lumbar rectal | 3.19±0.74 | 4.02±1.60 | 0.006 | 0.067 |
| Left sacral rectal | 2.92±0.84 | 4.58±1.86 | <0.001 | 0.004 |
| Right sacral rectal | 2.97±0.77 | 4.77±2.22 | <0.001 | 0.007 |
Data were expressed as mean±SEM. TLSMS, translumbosacral magnetic stimulation; MEP, motor evoked potential; BMI, body mass index; FI, fecal incontinence
Cortico-lumbar and cortico-sacral conduction time
Eight separate cortico-lumbar and cortico-sacral conduction times were obtained. There was no difference in the cortico-lumbar spinal and cortico-sacral spinal conduction time between the FI patients and controls, and on both sides (Table 1).
Correlations of MEP latencies with age, gender, ethnicity, type of FI, and anorectal surgery, FISI and FISS scores, anorectal manometry and anal sphincter defects
We found that age, FISI and FISS scores and the MEP latencies at the rectal and anal sites were similar between blacks and whites, men and women, and whether patients had urge or passive type of FI. (Table 3) There was a trend towards higher MEP latencies in women, but only left lumborectal MEP was significantly prolonged (p=0.034), and the overall number of abnormal sites were comparable. Patients with a history of anorectal trauma (surgery, obsteric injury) had significantly more severe leakage (higher FISS score) (p=0.001) (Table 3). There was no association between the anal resting and squeeze pressure and bilateral MEPs (p>0.05). Also, there was no significant correlation between the external and internal anal sphincter defects and bilateral MEP latencies (p>0.05).
Table 3.
Relationships between the age, severity of leakage scores, MEP latencies, and number of sites with abnormal lumbar and sacral MEP latencies between patients with a history of anorectal trauma, type of FI, gender and ethnicity
| History of anorectal trauma/surgery |
FI type | Gender | Ethnicity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| yes | no | p | passive | urge | combined | p | male | female | p | black | white | P | |
| Age (years) |
61.9±2.9 | 57.8±3.8 | 0.393 | 64.1±4.6 | 58.7±3.2 | 56.0±7.3 | 0.612 | 62.3±3.5 | 58.5±3.2 | 0.690 | 58.5±3.9 | 60.1±3.2 | 0.690 |
| FISI | 11.9±1.0 | 14.4±1.0 | 0.070 | 12.7±1.5 | 14.3±1.0 | 11.0±1.5 | 0.238 | 14.8±1.4 | 12.7±0.9 | 0.201 | 12.4±1.5 | 13.7±0.9 | 0.440 |
| FISS | 16.5±0.7 | 12.6±0.7 | 0.001 | 14.4±1.1 | 14.2±0.9 | 14.8±1.9 | 0.931 | 12.1±1.1 | 15.3±0.6 | 0.023 | 14.3±1.2 | 14.4±0.7 | 0.873 |
| No. of sites with abnormal MEP latency |
5.1±0.7 | 4.2±0.6 | 0.226 | 4.6±1.0 | 4.7±0.6 | 4.4±0.9 | 0.929 | 3.9±0.9 | 4.9±0.5 | 0.259 | 3.9±0.8 | 4.9±0.5 | 0.320 |
| Left lumbar anal (s) |
6.0±0.5 | 5.6±0.7 | 0.407 | 5.7±1.0 | 5.9±0.6 | 5.7±0.8 | 0.852 | 5.3±0.7 | 6.0±0.6 | 0.750 | 5.3±0.6 | 6.0±0.6 | 0.595 |
| Right lumbar anal (s) |
6.0±0.8 | 6.3±0.6 | 0.464 | 5.2±0.7 | 7.1±0.7 | 4.8±0.3 | 0.054 | 5.4±0.4 | 6.5±0.6 | 0.633 | 6.3±0.9 | 6.2±0.6 | 0.710 |
| Left sacral anal (s) |
5.7±0.5 | 4.8±0.5 | 0.180 | 4.5±0.6 | 5.7±0.5 | 4.7±1.0 | 0.232 | 4.6±0.4 | 5.5±0.5 | 0.353 | 5.2±0.9 | 5.2±0.4 | 0.614 |
| Right Sacral anal (s) |
6.5±0.6 | 6.0±0.6 | 0.495 | 5.1±0.5 | 6.8±0.6 | 6.0±0.8 | 0.362 | 5.4±0.6 | 6.5±0.5 | 0.203 | 6.2±0.5 | 6.2±0.6 | 0.750 |
| Left lumbar rectal (s) |
4.2±0.2 | 3.6±0.5 | 0.102 | 3.7±0.7 | 4.1±0.4 | 3.3±0.5 | 0.633 | 2.9±0.4 | 4.2±0.4 | 0.034* | 3.9±0.3 | 3.8±0.4 | 0.595 |
| Right lumbar rectal (s) |
4.2±0.3 | 3.9±0.5 | 0.092 | 4.1±0.2 | 4.1±0.5 | 3.6±0.6 | 0.415 | 3.6±0.3 | 4.2±0.4 | 0.381 | 3.6±0.4 | 4.2±0.4 | 0.253 |
| Left Sacral rectal (s) |
4.8±0.6 | 4.4±0.5 | 0.643 | 3.8±0.6 | 5.1±0.5 | 3.9±0.5 | 0.343 | 4.5±0.8 | 4.6±0.4 | 0.751 | 4.2±0.6 | 4.8±0.4 | 0.267 |
| Right sacral rectal (s) |
5.2±0.7 | 4.5±0.5 | 0.435 | 4.4±1.2 | 5.0±0.5 | 4.6±0.7 | 0.262 | 3.8±0.4 | 5.2±0.6 | 0.067 | 4.6±0.5 | 4.8±0.6 | 0.770 |
Data are expressed as mean±SEM. FI= fecal incontinence; FISI= FI severity Index; FISS= the FI Severity Assessment; MEP= motor evoked potentials.
Discussion
In this comprehensive investigation of the efferent brain, spinal cord and pelvic floor nerve conduction using motor evoked potentials, we found that a majority of patients with FI exhibit anorectal neuropathy that affects both the cortico-anorectal and spino-anorectal efferent pathways. Further analysis revealed that the cortico-spinal segment was mostly intact as there was no difference in the spinal conduction time between the patients with FI and controls, whereas, the neuropathy primarily affected the lumbo-rectal, lumbo-anal, sacro-rectal and sacro-anal nerves. We also found that the neuropathy involves both the rectum and anus regions in about one half of patients. This finding underscores the importance of evaluating neuronal function in patients with FI, and that lumbar and sacral neuropathy could play an important role alongside other factors in the pathogenesis of FI.
TMS is routinely used for neurophysiological evaluation 18, 19 Recently, it has been used for the assessment of anal sphincter 9, 20, 21 and rectum. 21 These studies were mostly exploratory and involved healthy controls, and used electromyography and evoked pressure techniques. To our knowledge, there has been only one other study of motor response of the anal sphincter in patients with FI using TMS. 9 They found that 23% of patients with FI had abnormal evoked pressure latencies, and had significantly lower voluntary contraction amplitudes, and significantly higher rectal sensory thresholds than healthy controls. However, there was no statistical difference between the evoked pressure curves of healthy subjects and patients with FI. Moreover, they did not assess the rectal or spinal evoked responses. Unlike their study, we used the MEP, which is a direct and reproducible measurement of the neuroelectrical signal, that not only detects neuropathy but also quantifies it objectively.10 In contrast, evoked anal pressure curve is an indirect and less precise measure of nerve and muscle function.
Both the TMS and TLMS and TSMS tests were well tolerated with no adverse effect during the study. Our data show that there is no difference in the neural signal conduction time between the cortex and the lumbar or sacral spinal cord. Thus, the abnormal conduction time from the lumbar and sacral spinal cord to the rectum and anus is the main reason for the prolonged conduction time between the cortex to the rectum and/or the anus. Our findings not only corroborated previous studies 8, 23 but further extended those observations that although there is evidence of cortico-anorectal neuropathy, the primary locus for the delayed conduction is the peripheral spino-anorectal pathway and not central. This peripheral spino-anorectal neuropathy most likely stems from nerve damage due to the obstetrical injury, occult neural injury (twisting of pelvis/spine), diabetes, and other back and pelvic floor surgeries.
Overall, we found that one or more of the 8 MEP latencies we measured in each patient were abnormal in 93% of FI patients, and is similar to a prevalence of 87% reported previously. 8 Also, we found that the abnormal MEP latencies obtained with both the TMS, TLMS and TSMS at a single site (anal or rectal) in patients with FI ranges from 20%−40%, and at two sites (anal and rectal) from about 50%−60%. These results suggest that neuropathy is patchy and affects different nerve pathways in different patients. This finding is important and emphasizes that in routine clinical practice, TLMS and TSMS is quite sensitive and can provide an objective and comprehensive information regarding the underlying neuropathy.
Also, we found that the MEP latencies and the prevalence of abnormal MEP test in patients with FI were not associated with age, gender, ethnicity (blacks vs whites), or type of FI (urge or passive), the anal resting or squeeze sphincter pressures or sphincter defects. About 30% of patients in this study were men, and although the precise reason for their neuropathy is unclear, almost all had weak anal sphincters, 4 had sphincter defects, and 7/8 had sensory dysfunction (rectal hyper or hyposensitivity). In contrast, female patients with a history of anorectal trauma (obsteric or surgery) had more severe leakage as measured by FISS. These findings suggest that anorectal neuropathy is an independent risk factor in the pathogenesis of FI, but this requires further validation.
FI is a multifactorial dysfunction that involves anal sphincter dysfunction, puborectalis dysfunction, rectal hyper or hyposensitivity, impaired rectal compliance, diarrhea with irritants such as bile salts and neuropathy among others. Also, some factors are related, for example if the rectal compliance is impaired, then the rectal hypersensitivity is secondary to impaired rectal compliance. In our study, patients designated as hypersensitive had normal rectal compliance. Also, we found that although neuropathy could overlap with manometric or sensory dysfunctions, it may not correlate with other abnormalities and therefore is an independent risk factor. Identifying neuropathy may facilitate novel therapies such as translumbosacral neuromodulation therapy (TNT) for treatment of FI. Rrecently, we showed that TNT at 1 Hz frequency significantly reverses/improves lumbosacral neuropathy and decreases FI episodes possibly through neuromodulation and neuroplasticity. 24
The limitations of our study include the smaller sample size of patients with FI, who were older, with higher BMI than our controls. Also, our patients were evaluated in a tertiary care center, and possibly had more advanced FI than seen in community practice. However, the methodology test has been validated, and the data is similar to a previous study8 confirming these observations.
In conclusion, a majority of patients with FI demonstrate neuropathy that primarily affects the peripheral nervous system, chiefly the lumbar and sacral nerves that innervate the anus and rectum, with an intact cortico-spinal segment. Translumbosacral, anorectal magnetic stimulation (TAMS) is a useful, noninvasive and comprehensive test for detecting neuropathy in FI and reveals segmental neuropathophysiological dysfunction in FI. Delineation of the specific neuronal pathways and sites that are abnormal could pave the way for the development of targeted treatments using biofeedback, sacral nerve stimulation, repetitive magnetic stimulation and TNT.
What You Need to now.
Background
Fecal incontinence (FI) is a common clinical problem with a multifactorial etiology. Peripheral spino-anorectal neuronal dysfunction may predispose patients to fecal incontinence. We performed simultaneous examination of the bilateral, efferent cortico-anorectal, and spino-anorectal motor evoked potentials (MEPs) to evaluate the presence of and locus of neuronal injury in patients with FI.
Findings
Neuronal conduction was normal between the cortex and spinal cord, but it was abnormal and prolonged between the lumbar spinal nerve roots and the rectum and anus bilaterally, and likewise between the sacral spinal nerve roots and the rectum and anus, in 93% of measurements from patients with FI.
Implications for patient care
Peripheral lumbosacral neuropathy is common and could be an important pathophysiological dysfunction in FI patients. Trans-lumbosacral anorectal magnetic stimulation (TAMS) is a non-invasive and safe test for the evaluation and objective confirmation of an underlying neuropathy. It is important to validate these findings, which could have implications for management of patients with FI—especially use of neuromodulation therapies.
Acknowledgement
This study was supported by NIH grant 1U01DK082344–01 and grant R21DK104127. We thank Mrs. Helen Smith for excellent secretarial support.
Abbreviations:
- FI
fecal incontinence
- MEP
motor evoked potential
- TMS
transcranial magnetic stimulation
- TLMS
translumbar magnetic stimulation
- TSMS
transsacral magnetic stimulation
- CSCT
cortico-spinal conduction time
- EMG
electromyography
- PNTML
pudendal nerve terminal motor latency
- HRARM
high resolution anorectal manometry
- FISI
FI severity index
- FISS
FI severity score
Footnotes
Conflict of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest. All authors have approved the final version being submitted.
Potential competing interests: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol 2015;110:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharucha AE, Daube J, Litchy W, et al. Anal sphincteric neurogenic injury in asymptomatic nulliparous women and fecal incontinence. Am J Physiol Gastrointest Liver Physiol 2012;303:G256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nowakowski M, Tomaszewski KA, Machura L, et al. Sensitivity and specificity of multichannel surface electromyography in diagnosing fecal incontinence. Folia Med Cracov 2017;57:29–38. [PubMed] [Google Scholar]
- 4.Fitzpatrick M, O’Brien C, O’Connell P R, et al. Patterns of abnormal pudendal nerve function that are associated with postpartum fecal incontinence. Am J Obstet Gynecol 2003;189:730–735. [DOI] [PubMed] [Google Scholar]
- 5.Gooneratne ML, Scott SM, Lunniss PJ. Unilateral pudendal neuropathy is common in patients with fecal incontinence. Dis Colon Rectum 2007;50:449–458. [DOI] [PubMed] [Google Scholar]
- 6.Suilleabhain CB, Horgan AF, McEnroe L, et al. The relationship of pudendal nerve terminal motor latency to squeeze pressure in patients with idiopathic fecal incontinence. Dis Colon Rectum 2001;44:666–671. [DOI] [PubMed] [Google Scholar]
- 7.Vernava AM 3rd, Longo WE, Daniel GL. Pudendal neuropathy and the importance of EMG evaluation of fecal incontinence. Dis Colon Rectum 1993;36:23–27. [DOI] [PubMed] [Google Scholar]
- 8.Rao SS, Coss-Adame E, Tantiphlachiva K, et al. Translumbar and transsacral magnetic neurostimulation for the assessment of neuropathy in fecal incontinence. Dis Colon Rectum 2014;57:645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paris G, Chastan N, Gourcerol G, et al. Evoked pressure curves from the external anal sphincter following transcranial magnetic stimulation in healthy volunteers and patients with faecal incontinence. Colorectal Dis 2013;15:e732–740. [DOI] [PubMed] [Google Scholar]
- 10.Remes-Troche JM, Tantiphlachiva K, Attaluri A, et al. A bi-directional assessment of the human brain-anorectal axis. Neurogastroenterol Motil 2011;23:240–8, e117–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockwood TH, Church JM, Fleshman JW, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum 1999;42:1525–1532. [DOI] [PubMed] [Google Scholar]
- 12.Noelting J , Zinsmeister AR and Bharucha AE Validating endpoints for therapeutic trials in fecal incontinence. Neurogastroenterol. Motil 2016;28: 1148–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbull GK, Hamdy SE, Aziz Q, et al. The cortical topography of human anorectal musculature Gastroenterology 1999;117:32–39. [DOI] [PubMed] [Google Scholar]
- 14.Herdmann J, Bielefeldt K, Enck P. Quantification of motor pathways to the pelvic floor in humans. Am J Physiol 1991;260:G720–723. [DOI] [PubMed] [Google Scholar]
- 15.Rao SS, Meduri K. What is necessary to diagnose constipation? Best Pract Res Clin Gastroenterol 2011;25:127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones MP, Post J, Crowell MD. High-resolution manometry in the evaluation of anorectal disorders: a simultaneous comparison with water-perfused manometry. Am J Gastroenterol 2007;102:850–855. [DOI] [PubMed] [Google Scholar]
- 17.Rao SSC, Hatfield R, Soffer E, et al. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol 1999;94:773–783. [DOI] [PubMed] [Google Scholar]
- 18.Galloway GM, Dias BR, Brown JL, et al. Transcranial magnetic stimulation--may be useful as a preoperative screen of motor tract function. J Clin Neurophysiol 2013;30:386–389. [DOI] [PubMed] [Google Scholar]
- 19.Niyazov DM, Butler AJ, Kadah YM, et al. Functional magnetic resonance imaging and transcranial magnetic stimulation: effects of motor imagery, movement and coil orientation. Clin Neurophysiol 2005;116:1601–1610. [DOI] [PubMed] [Google Scholar]
- 20.Pelliccioni G, Scarpino O, Piloni V. Motor evoked potentials recorded from external anal sphincter by cortical and lumbo-sacral magnetic stimulation: normative data. J Neurol Sci 1997;149:69–72. [DOI] [PubMed] [Google Scholar]
- 21.Turnbull GK, Hamdy S, Aziz Q, et al. The cortical topography of human anorectal musculature. Gastroenterology 1999;117:32–39. [DOI] [PubMed] [Google Scholar]
- 22.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 1998;108:1–16. [DOI] [PubMed] [Google Scholar]
- 23.Rao SS. Advances in diagnostic assessment of fecal incontinence and dyssynergic defecation. Clin Gastroenterol Hepatol 2010;8:910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang X, Sharma A, Patcharatrakul T, et al. Translumbar and Transsacral Magnetic Stimulation Therapy for the Treatment of Fecal Incontinence: Interim Analysis of a Dose Ranging Study. Gastroenterology 2018;154:S540–S541. [Google Scholar]


