Abstract
Background and Aims:
Bile diversion to the ileum (GB-IL) has strikingly similar metabolic and satiating effects to Roux-en-Y gastric bypass (RYGB) in rodent obesity models. The metabolic benefits of these procedures are thought to be mediated by increased bile acids, though parallel changes in body weight and other confounding variables limits this interpretation.
Methods:
Global G protein-coupled bile acid receptor-1 null (Tgr5−/−) and intestinal-specific farnesoid X receptor null (FxrΔ/E) mice on high-fat diet as well as wild-type C57BL/6 and glucagon-like polypeptide 1 receptor deficient (Glp-1r−/−) mice on chow diet were characterized following bile diversion to the ileum (GB-IL).
Results:
GB-IL induced weight loss and improved oral glucose tolerance in HFD-fed Tgr5−/−, but not FxrΔ/E mice, suggesting a role for intestinal Fxr. GB-IL in wild-type, chow-fed mice prompted weight-independent improvements in glycemia and glucose tolerance secondary to augmented insulin responsiveness. Improvements were concomitant with increased levels of lymphatic GLP-1 in the fasted state and increased levels of intestinal Akkermansia muciniphila. Improvements in fasting glycemia after GB-IL were mitigated with Ex-9, a GLP-1 receptor antagonist, or cholestyramine, a bile acid sequestrant. The glucoregulatory effects of GB-IL were lost in whole body Glp-1r−/− mice.
Conclusions:
Bile diversion to the ileum improves glucose homeostasis via an intestinal Fxr-Glp-1 axis. Altered intestinal bile acid availability, independent of weight loss, and intestinal Akkermansia muciniphila appear to mediate the metabolic changes observed after bariatric surgery and might be manipulated for treatment of obesity and diabetes.
Keywords: metabolic surgery, gut microbiome, lymph fistula, glucagon-like polypeptide 1 (Glp-1)
Graphical Abstract
Introduction:
Obesity and its related metabolic comorbidities, including insulin resistance and type 2 diabetes (T2D), pose major healthcare threats worldwide1. Bariatric surgery is not only the most effective obesity treatment, but is also potential treatment for T2D2 in those with a BMI<35 kg/m2. Substantial evidence demonstrates that bariatric surgery, commonly Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG), is associated with more robust and durable weight loss and T2D resolution compared to medical and/or lifestyle interventions3, 4.
Even though surgical treatment of obesity is becoming widely accepted, the mechanisms of how these operations mediate their beneficial effects remain elusive. Surgery causes significant weight loss that improves insulin sensitivity and overall health, and it is not surprising that long-term T2D resolution correlates strongest with weight loss5. From clinical practice, however, we commonly observe many patients – even some requiring exogenous insulin – discontinuing most, if not all, diabetes medications within a few days postoperatively. These marked effects precede significant weight loss, suggesting the existence of weight-independent mechanisms.
Previous observations from this laboratory and others showed that RYGB and VSG are associated with elevated bile acids in both rodents6–8 and humans9–12. Bile acids have dual functions as detergents involved in lipid absorption and as hormones influencing metabolic processes via receptors such as Takeda G-protein coupled receptor 5 (Tgr5) and farnesoid X receptor (Fxr)13, 14. While bile acid signaling appears necessary for the weight loss and glucose tolerance effects of VSG in mice15, 16, a complete enumeration of the signaling events after bariatric surgery leading to improved glucose tolerance remains to be described.
To better understand the potential role of bile acids in the metabolic improvements after bariatric surgery we devised a mouse model, connecting the gall bladder (GB) to specific segments of the small intestine, without intestinal rerouting6. We showed that redirecting bile acids to the distal small intestine elicited metabolic changes collectively recapitulating all of the metabolic and physiologic improvements observed with RYGB. GB to ileum (GB-IL) anastomosis in obese, high-fat fed mice results in increased circulating bile acids, weight loss, fat malabsorption, improved glucose tolerance, increased energy expenditure and altered gut microbiota. While it is tempting to attribute the improved glucose tolerance to elevations in bile acids alone, the multiple roles of bile acid receptors and the concurrent reductions in body weight after surgery confound such interpretations.
Hence, in an attempt to dissect the independent role of bile acids, we performed bile diversion studies in high fat diet- (HFD-) fed Tgr5 null (Tgr5−/−; TGR5 KO) and intestinal epithelium Fxr null (FxrΔ/E) mice. Here again, we observed that GB-IL in globally-deficient Tgr5−/− mice improved glucose tolerance and also lead to decreased body weight. In contrast, FxrΔ/E mice did not display improvement in glucose tolerance and had minimal effect on body weight reduction. To control for changes in body weight, we performed studies in lean chow-fed mice, and found that bile diversion to the ileum resulted in similar improvements in oral glucose tolerance compared to those observed with RYGB and with GB-IL in HFD-fed mice. The results in the lean chow-fed mice mimicked those we previously observed with HFD-fed mice but were independent of weight loss or of any one of the other confounding variables observed with HFD-fed mice. Additionally, they demonstrate that lymphatic Glp-1 and the Glp-1 receptor (GLP-1r) mediate these bile acid-induced glucoregulatory effects6.
Methods
Animals & Surgical Operations
Male C57BL/6J mice at eight weeks of age were purchased from Jackson Laboratory (Bar Harbor, ME) and Glp-1R−/− mice obtained from Dr. Daniel Drucker (Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Canada)17. Both strains of mice were given ad libitum access to chow (4.5% fat, Purina). Intestine-specific Fxr-null (FxrΔ/E) mice were generated by crossing Fxrfl/fl18 with villin-creERT2 19 and genotyped as described. FxrΔ/E mice were injected daily for 5 consecutive days with tamoxifen (10 mg/ml in ethanol:peanut oil [1:10]; 50µl per injection) 2 weeks prior to surgery. Tgr5−/−20 (obtained from Dr. Galya Vassileva, Merck, Kenilworth, New Jersey) and FxrΔ/E mice had ad libitum access to a high-fat diet (F-3282, 60% fat by weight, Bio-Serv) for 12 weeks before and after surgery.
Experimental procedures were performed according to protocols approved by the Vanderbilt University Medical Center Institutional Animal Care and Use Committee. Mice were monitored under the care of the Division of Animal Care in compliance with NIH guidelines. Mice were housed at 23°C on a 0700–1900 light/dark cycle for the duration of all experiments. At the end of all acute or chronic experiments (8 weeks), animals were quickly anesthetized following a 5h period of food-restriction to allow for blood, tissues, and feces/cecal contents to be collected for further assay.
Bile diversion to the ileum (GB-IL) was performed as previously described6. Bile flow was diverted from the gallbladder to the ileum (4 cm proximal to the ileocecal valve, GB-IL). GB-IL does not alter flow of exocrine pancreatic secretions, as care was taken to ligate the bile duct above the confluence of the pancreatic and common bile ducts using 9–0 nylon (Fig 1A). Animals were not food-restricted in the perioperative period, nor were antibiotics given for surgical prophylaxis. Early mortality (<1 week postop) was approximately 15%, being exclusively due to leakage of the gallbladder-to-bowel anastomosis.
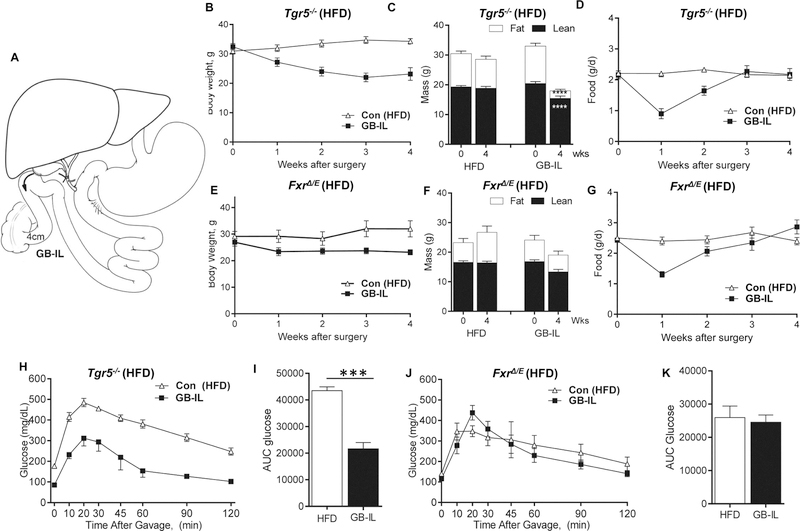
Figure 1. Effects of bile diversion to the ileum in HFD-fed, obese global Tgr5−/− and intestinal Fxr deficient (FxrΔ/E) mice.
(A) Surgical schematic of bile diversion operations6. Mice underwent surgical bile diversion to the ileum (GB-IL) or no operation (Chow). Average body weight (B, E), interval body composition assessments (C, F), daily food intake (D, G), oral glucose excursions (H and J) and glucose area under the curve (AUC; I and K) in high-fat diet- (HFD-) fed obese Tgr5−/− and FxrΔ/E mice after bile diversion to the ileum (GB-IL). (Tgr5−/−: n=14 Con HFD, 5 GB-IL; FxrΔ/E n=5 Con HFD, 7 GB-IL). Data are represented as the mean ± SEM. Statistical analysis using Student’s t-test. ***P<0.001; ****P<0.0001.
Additional methods can be found in the Supplementary Materials.
Results
Effects of GB-IL in HFD-fed Tgr5−/− and Fxr Δ/E mice
To isolate the role of bile acids in improved glucose tolerance after bariatric procedures, we subjected obese, high-fat diet- (HFD-) fed Tgr5−/− mice and mice disrupted of intestinal epithelium-specific Fxr (Fxr Δ/E) to GB-IL surgery (Fig 1A). In line with data obtained in obese wild-type C57BL/6J mice6, GB-IL surgery durably reduced body weight (Fig 1B) and adiposity (Fig 1C) in globally-deficient Tgr5−/− HFD fed mice. Early post-operative declines in food intake returned to chow levels by 4 wks (Fig 1D). In contrast, Fxr Δ/E HFD-fed mice displayed only minimal reductions in body weight (Fig 1E) and body mass (Fig 1F) and similar to GB-IL in Tgr5−/− mice, food intake at 4 wks was equivalent to controls (Fig 1G). The reductions in body weight observed in GB-IL Tgr5−/− mice were associated with significantly improved oral glucose excursions (Fig 1H) and improved oral glucose tolerance (Fig 1I). In contrast, GB- IL imparted no improvements in oral glucose tolerance in Fxr Δ/E mice (Fig 1J and K).
Anthropometric Measurements, Food Intake & Fecal Analyses of GB-IL in Chow-fed Mice
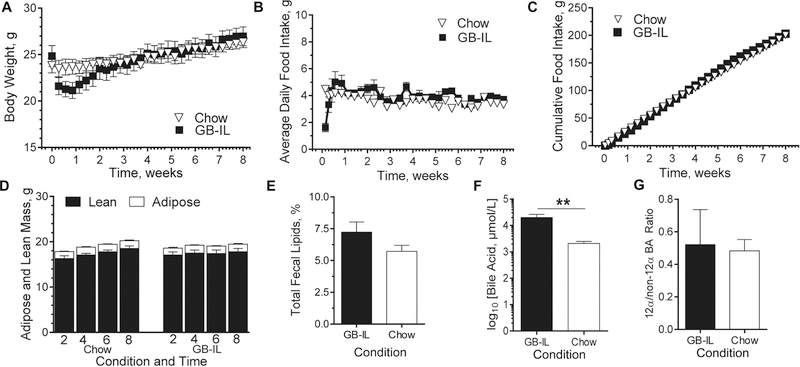
To control for the metabolic effects of reduced body weight that were evident in HFD-fed GB-IL mice, we performed a battery of studies in lean, low-fat (4.5% fat) chow mice after GB-IL surgery. The effects of a sham GB-D surgery were also investigated (data not shown but results were used for statistical testing). Body weight gains in C57BL/6J mice (Fig 2A) did not differ over the study period. Postoperative food intake was transiently decreased for a few days, consistent with the effects of surgery. After recovery, however, food intake was similar among all groups regardless of being expressed as either average daily (Fig 2B) or cumulative (Fig 2C) intake. In addition, body composition (Fig 2D) was also similar without any differences in fat or muscle mass (Supp Fig 1). Moreover, no groups showed evidence of anemia that can commonly confound surgical studies (Supp Fig 2). Unlike previous high fat feeding studies with fecal malabsorption6, total fecal lipid content (Fig 2E) was not significantly affected by GB-IL.
Figure 2. Whole body and tissue specific responses to chronic bile diversion in lean chow-fed mice.
C57BL/6J mice underwent surgical bile diversion to the ileum (GB-IL), or no operation (Chow). (A) Body weight, (B) average daily and (C) cumulative food intake were measured for the entirety of the study (only every other day are shown in the graphs above for clarity). (D) Non-invasive body composition was measured at 2, 4, 6 and 8 weeks. (E) Fecal lipids were quantified from total feces over a 6-hour time period 4 weeks postop. (F) Total plasma bile acids (BAs). (G) The 12α/non-12α-hydroxylated bile acid ratio. Data are represented as the mean ± SEM. Kruskal-Wallis test of Chow, GB- D (data not shown), GB-IL, adjusted for multiplicity, was used on sample sizes of (A-C) n=10 GB-IL, 12 Chow; (D, F and G) 9 GB-IL, 12 Chow, (E) 10 GB-IL, 12 Chow. **P<0.01.
Bile Acid Metabolism
Several studies have attributed the effects of bariatric surgery, at least in part, to significant increases in circulating bile acids. In the current study we observed similar increases in circulating bile acids in all animal groups studied irrespective of genotype or dietary intervention (Fig 2F and Supp Table 1). The increases were due to robust elevations in primary and secondary conjugated bile acids (Supp Fig 3A-B). Conversely, neither primary nor secondary unconjugated bile acids, nor the 12α-hydroxylated/non-12α hydroxylated BA ratio (Fig 2G) significantly differed among groups.
We examined the livers of Chow and bile diverted mice and found no differences in the abundance of proteins that coordinate hepatic bile acid signaling except for increased Cyp7a1 expression (Supp Fig 4A) similar to previous studies3,16. Ileum mRNA levels of the basolateral and apical bile acid transporters (Supp Fig 4B) and the bile acid receptors Fxr and Tgr5 (Supp Fig 4C) did not significantly change after GB-IL.
Insulin Sensitivity and Glucose Tolerance
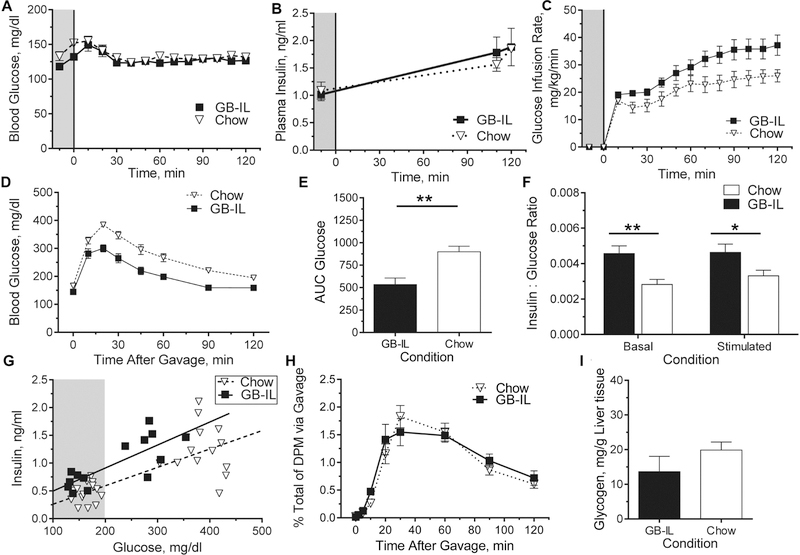
We examined whether the observed increases in circulating bile acids were associated with detectable effects on glucose metabolism and insulin sensitivity using a low dose hyperinsulinemic-euglycemic clamp. GB-IL mice had significantly lower fasting blood glucose (Fig 3A) compared to Chow (132±4.9 vs. 152±5.2 mg/dl, P<0.05), yet all groups had similar basal insulin levels, as well as similar rises in steady-state insulin concentrations during the clamp (Fig 3B). However, GB-IL required significantly more intravenous glucose compared to Chow to maintain euglycemia (Fig 3C). Nevertheless, the ratio of glucose infusion rates to steady-state insulin concentrations, a surrogate for peripheral insulin sensitivity (Supp Fig 4A), were not different among the groups (P=0.07). In contrast, hepatic insulin sensitivity showed a small, but significant improvement in GB-IL compared to Chow, as measured by insulin-mediated suppression of hepatic glucose output (Supp Fig 5B).
Figure 3. Glucose metabolism in lean, chronic bile diversion mice.
Chronic bile diversion mice underwent clamp studies at 4 weeks postoperative for whole body and tissue-specific assessment of insulin sensitivity and glucose kinetics. (A) Blood glucose was clamped at 5h fasted levels, with a (B) constant insulin infusion to elevate plasma insulin concentrations over food-restricted levels and a (C) variable glucose infusion to maintain euglycemia among the groups. (D) Chow and GB-IL mice also underwent standard oral glucose tolerance tests (OGTT; 2 mg/kg body weigh) at 4 weeks postoperatively; and (E) the corresponding area under curve (AUC0–120) glucose was calculated. (F) Ratios of insulin to glucose at baseline (t = 0 min) and following glucose gavage (t = 20 min) were assessed. (G) Regression lines obtained when comparing the glucose and insulin concentrations at basal fasting (shaded gray) and 20 min after glucose stimulation during an OGTT. Slopes among all lines are not significantly different, while the intercept for GB-IL compared to Chow is higher (P<0.01). (H) 3-[3H]-O-methylglucose (3-OMG) counts in peripheral circulation after administering glucose supplemented with 3-OMG tracer during OGTT. (I) Liver glycogen in 5h fasted GB-IL and Chow controls. Data are represented as the mean ± SEM. Kruskal-Wallis test of Chow, GB-D (data not shown), GB-IL, adjusted for multiplicity, was used on sample sizes of (A-C) 6 GB-IL, 8 Chow. (D-E) 13 GB-IL, 16 Chow; (F, G) 7 GB-IL, 14 Chow; (H) 6 GB-IL, 7 Chow; (I) 9 GB-IL, 12 Chow. *P<0.05, **P<0.01.
To investigate potential changes in peripheral insulin sensitivity or tissue glucose uptake driven by increased bile acids in GB-IL, we administered intravenous non-metabolizable 14C-deoxyglucose tracer under euglycemic conditions (Supp Table 2). We observed no differences in insulin-mediated tissue glucose uptake in skeletal muscle or in either visceral or subcutaneous white adipose as well as brown adipose tissue.
Previous data suggest that fasting intestinal glucose uptake may be increased after RYGB21. Thus, to examine this we harvested from the same mice duodenal, mid-jejunal and distal ileal segments, and found glucose uptake to be similar among groups (Supp Table 2).
Given the apparent lack of differences on tissue insulin sensitivity with a submaximal insulin dose, we investigated whether there may be altered insulin signaling in skeletal muscle in response to a maximal insulin dose. We did not observe any altered insulin signaling estimated by the ratio of phosphorylated-Akt473 to total-Akt, either in the basal state or under maximal insulin stimulation (Supp Fig 6).
We next examined whether the elevated plasma bile acids observed in GB-IL altered hepatic glucose handling using oral glucose tolerance tests (OGTT). Fasting glucose (mg/dl) was significantly lower in GB-IL (144.5±3.7) compared to Chow (166.2±4.5, P<0.0). Post-prandial blood glucose excursion and AUC (Fig 3D-E) were decreased in GB-IL mice compared to Chow. Interestingly, baseline fasting and peak plasma insulin concentrations were higher for the GB-IL mice resulting in significantly increased insulin to glucose ratios (Fig 3F). The improvement in oral glucose tolerance was durable, and even more robust, when tested by OGTT at eight weeks postoperative (Supp Fig 7). Regression modeling to compare the glucose and corresponding insulin responses at 4 weeks postoperative demonstrated similar slopes for the insulin responses among the groups (Fig 3G). However, the intercept of the GB-IL response line was significantly higher (P=0.013) compared to the chow-fed mice indicating that the GB-IL insulin values were significantly elevated for any given concentration of blood glucose compared to Chow.
To investigate the alternate possibility that bile diversion may be retarding intestinal glucose absorption and leading to a perceived but not actual improved glucose tolerance, we conducted OGTT using a glucose load supplemented with trace amounts of 3-[3H]-O-methylglucose (3-OMG) that verified similar intestinal glucose uptake in both groups (Fig 3H; P=0.57). Hepatic glycogen content was also not significantly different among groups (Fig 3I; P=0.27). Collectively, these experiments provide evidence that the bile acid-mediated improved glucose tolerance was not due to alterations in whole body or in skeletal muscle insulin sensitivity in GB-IL mice, but rather to a direct bile acid mediated effect on hepatic glucose handling.
Lymphatic Incretin Responses
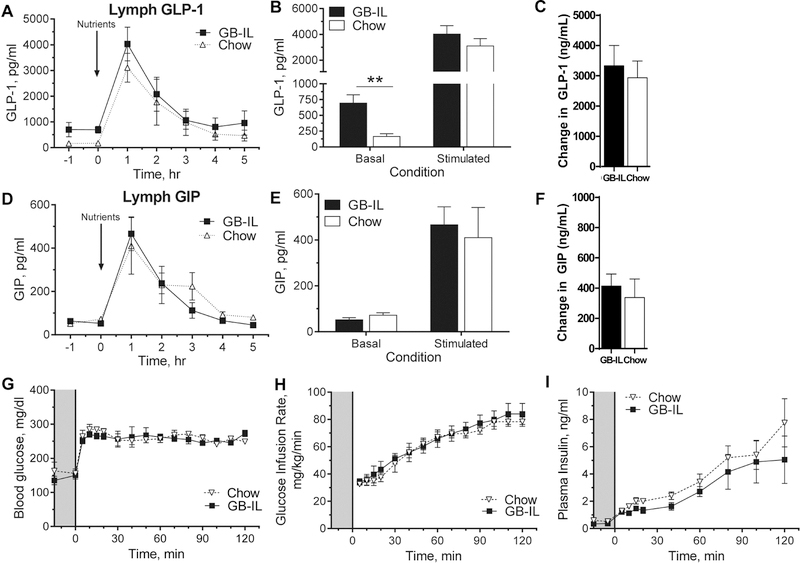
We attempted to measure peripheral GLP-1 concentrations, the secretion of which is stimulated by bile acids in the distal ileum22, as a possible mediator for the improved oral glucose tolerance in GB-IL mice. GLP-1 levels were below the lower limit of detection in these lean, healthy animals. We therefore proceeded with interrogation of basal and nutrient-stimulated incretin responses (Fig 4A-F) in mesenteric lymph as previously described and modified in our lab for conscious mice23. Basal lymph GLP-1 (Figs 4A-B) was significantly elevated (~4.5-fold) in GB-IL compared to chow controls. Following nutrient delivery, both groups showed comparable increases in GLP-1 compared to baseline (Fig 4C). However, the AUC response in GB-IL was over 70% greater than seen with the chow fed mice. Similarly, there were also detectable increases in nutrient-stimulated GIP (Fig 4D-F). However, there were no differences in GIP (Fig 4E and F) under basal or nutrient-stimulated conditions between groups.
Figure 4. Improvement in oral glucose tolerance after bile diversion to the ileum enhance lymphatic GLP-1 tone.
Bile diversion or control mice underwent mesenteric lymphatic cannulation at four weeks postop. Intestinal lymph samples were collected hourly before and after a nutrient bolus delivered at the indicated time as described in the ‘Methods’. Lymph concentrations of (A) GLP-1 and (D) GIP from the mesenteric lymph over time, as well as (B, E) basal (t = 0) and nutrient stimulated (t = 1h) and (C, F) 0 to 1 h changes are shown above. Bile diversion mice underwent hyperglycemic clamp studies at 4 weeks postoperative for assessments of glucose-stimulated insulin secretion independent of the gastrointestinal tract. (G) Blood glucose was clamped at 250 mg/dl in 5 h fasted mice, with a (H) variable glucose infusion to assess (I) insulin release. Data are represented as the mean ± SEM. Kruskal-Wallis test of Chow, GB-D (data not shown), GB-IL, adjusted for multiplicity, was used on sample sizes of (A-F) 7 GB-IL, 8 Chow; (G-I) 6 GB-IL, 4 Chow. **P<0.01.
Given that the effects of GB-IL on glucose tolerance were dependent on enteral glucose and potentially GLP-1 signaling, we examined the effect of bile acids on insulin secretion using standard intravenous hyperglycemic (~250 mg/dL) clamps (Fig 4G-I)24. Despite similar hyperglycemia and glucose infusion rates, peak plasma insulin levels in GB-IL mice were not significantly different.
Pharmacologic and Genetic Blockade of GLP-1R Blunt the Glucoregulatory Effects of Elevated Bile Acids after GB-IL
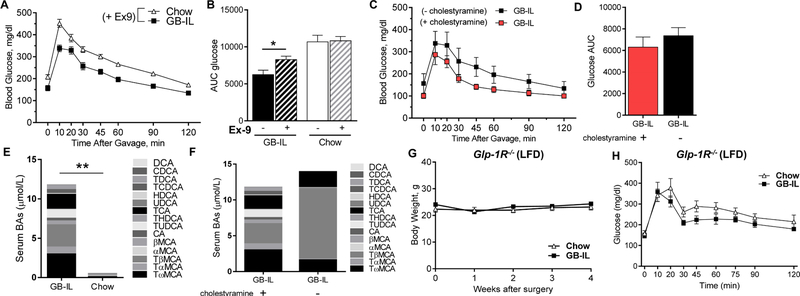
We tested directly whether GLP-1 signaling played a role in the improved glucose tolerance observed in GB-IL mice by treating animals with the GLP-1R antagonist, exendin-9 (Ex-9). Ex-9 treatment to GB-IL, but not Chow, mice prior to OGTT (Fig 5A) significantly worsened post-prandial blood glucose excursions and glucose AUC (Fig 5B).
Figure 5. Pharmacologic and genetic blockade of GLP-1R blunt the glucoregulatory effects of elevated bile acids after GB-IL.
Bile diversion to the ileum (GB-IL) and Chow mice underwent oral glucose tolerance testing. (A) Plasma glucose during OGTT after 50 µg exendin-9 (Ex-9) was administered by intraperitoneal injection into 5h fasted mice. (B) Glucose AUC0–120 in mice with and without Ex-9 pretreatment. Post-prandial blood glucose excursion (C) and glucose AUC0–120 (D) in GB-IL mice after pre-treatment with the bile acid sequestrant, cholestyramine (500 mg/kg p.o., daily for 3 days). Serum bile acid profiles in GB-IL and Chow mice after cholestyramine treatment (E). Serum bile acid profiles in GB-IL mice with and without cholestyramine treatment (F). Effects of GB-IL in mice lacking the Glp-1 receptor (Glp-1R−/−). Average body weight (G), and (H) oral glucose excursions in low-fat diet-(LFD-) fed, lean Glp-1R−/− mice after GB-IL. (Glp-1R−/−; n=7 Chow, 8 GB-IL). Data are presented the mean ± SEM. Statistical analysis using Student’s t-test. *P<0.05, **P<0.01.
To determine whether luminal bile acids were mediating these improvements, we performed OGTT in C57BL6/J GB-IL mice with and without the administration of the bile acid sequestrant, cholestyramine (500 mg/kg daily for 3 days; Fig 5C-D). Cholestyramine pretreatment significantly lowered fasting plasma glucose with similar blood glucose excursions in GB-IL mice, such that there were no differences in blood glucose AUC (Fig 5D). Measurements of total and fractional serum bile acid concentrations in cholestyramine-treated mice revealed that serum bile acids were still significantly elevated in GB-IL mice (Fig 5E); however, bile acid composition changed from a bile acid profile dominated by tauro-β-muricholic acid (TβMCA) to one with a more diversified bile acid complement where TβMCA was much less abundant (Fig 5F).
Additional experiments in mice globally deficient for GLP-1 receptor (Glp-1r−/−) were also undertaken. Body weight gains in GB-IL Glp-1r−/− mice (Fig 5G) and Glp-1r−/− mice fed a normal chow diet (Chow) did not differ over the study period. Oral glucose tolerance tests in Glp-1r−/− mice were largely equivalent, with Chow and GB-IL mice exhibiting similar fasting plasma glucose and similar glucose excursion (Fig 5H). Our findings are indicative of the importance of GLP-1 receptor signaling in mediating the improved metabolic effects of bile acids.
Cecal Microbiome
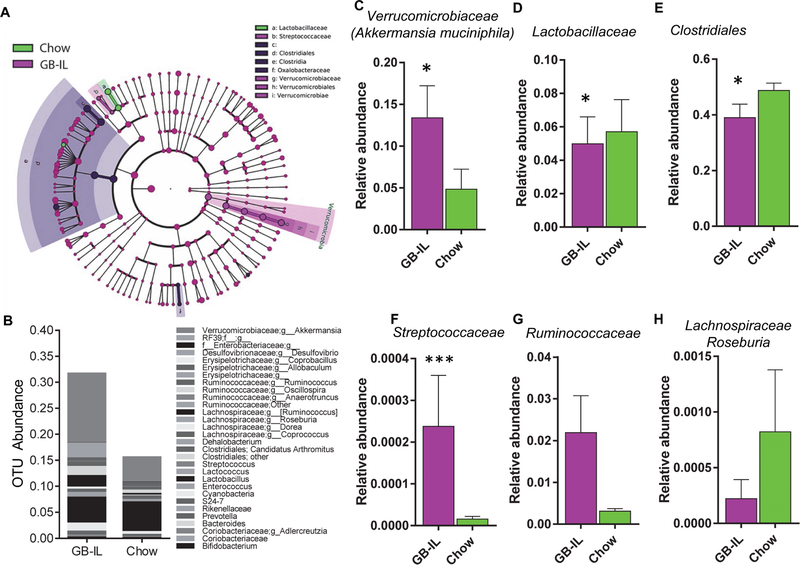
Previous studies from our group6 and others25, 26 demonstrated that gut microbial species change in response to surgical-induced weight loss, implicating the gut microbiome in the metabolic effects of bariatric surgery. Given the antimicrobial effects of bile acids and altered biliary flow by bile diversion, we examined the cecal microbiome to determine whether bile diversion might alter gut microbial content even in the absence of previous confounders following bariatric surgery. Compared to our previous studies of high-fat fed mice, GB-IL in chow-fed mice had grossly fewer changes in microbial taxa. Rarefaction analysis and Chao1 estimates (Supp Fig 8A and 8B) did not show any significant differences in microbial diversity, nor did principal component analysis show any separation of groups based on relative variance (Supp Fig 8C). Nonetheless, several taxa showed significant differences in abundance (Fig 6A, 6B and Supp Table 3). The marked increase in Verrucomicrobia corresponds to Akkermansia mucinophila, the only cultured representative of the taxon Verrucomicrobia (Fig 6C). GB-IL mice had relatively lower Lactobacillaceae relative to chow mice (Fig 6D), but were enriched in Clostridiales (Fig 6E) and Oxalobacteraceae. GB-IL mice also showed significant increases in two particular taxa relative to Chow, namely Streptococcaceae (Fig 6F) and Ruminococcaceae (Fig 6G). Lachnospiraceae members, including Roseburia species, were reduced relative to Chow controls in GB-IL mice (Fig 6H). Overall, alteration of bile flow by bile diversion, in GB-IL, was concordant with changes in several gut microbiome taxa that are associated with lean phenotypes27, 28.
Figure 6. Bile diversion to the ileum is associated with increased cecal Akkermansia muciniphila content.
(A) LeFSe cladrogram illustrating the taxa with the greatest differences in abundance among GB-IL and Chow mice. Control-enriched taxa (green) and GB-IL enriched taxa (magenta) are indicated by color. Various shades of background highlighting indicate changes at several taxonomic levels. Individual colored circles represent single OTUs and the size of each circle is proportional to the abundance of that given OTU as implemented in the LeFSe software. (B) Stacked bar graph illustrating relative OTU abundance by intervention of the thirty most highly abundant taxa among the groups (6 GB-IL, 6 Chow). Bar graphs illustrating OTU abundance differences, at the family level, in fecal (C) Verrucomibociaceae, of which Akkermansia muciniphila is the only member, (D) Lactobacillaceae, (E) Clostridiales, (F) Streptococcaceae, (G) Ruminococcaceae and (H) Lachnospiraceae (Roseburia sp.).
Discussion
It is well established that the metabolic improvements observed with bariatric procedures such as VSG, RYGB and bilio-pancreatic diversion can be attributed to both weight-dependent (late effects) as well as to weight-independent variables (early effects). Our application of a bariatric surgery model, namely gallbladder bile diversion to the ileum (GB-IL), to HFD-fed, obese, genetic models with bile acid receptor deficiencies (e.g. Tgr5−/− and intestinal epithelium-specific Fxr Δ/E) and to normal-chow fed lean models (wild-type C57BL6/J and Glp-1r−/−) allowed for direct interrogation of bile acid mediated phenotypes. Earlier studies from our laboratory6 in HFD-fed, wild-type mice subjected to the same selective bile diversion procedure resulted in significant increases in circulating bile acids, namely tauro-β-muricholic acid, an FXR antagonist29, associated with improved glucose homeostasis30. We attributed these improvements to a combination of enhanced signaling via a hepatic Fxr-Fgf15 axis, as well as to the associated significant weight loss that in many respects was identical to what we observed with another commonly used bariatric procedure, namely RYGB6. The data from the current study show that enhancing bile acid delivery to the terminal ileum improves oral glucose tolerance in an intestinal Fxr-dependent manner. The improvements after bile diversion occurred in the absence of Tgr5 and were coincident with enhanced basal GLP-1 tone in a weight independent manner.
Several hypotheses have been put forth31 for the metabolic improvements following bariatric procedures, but work from numerous laboratories, including our own6, 9, 32 attributed a significant component of the weight-independent as well as weight-dependent variables to favorable changes in bile acid signaling, which occur mostly via Fxr and TGR533. Ryan et al.16, using global Fxr-deficient mice, observed significant weight loss following VSG in wild-type but not global Fxr knockout mice on a high fat diet. The data from the current study extend these findings and show that the absence of intestinal Fxr (Fig 1D) but not Tgr5 (Fig 1B) was protective of weight loss in obese GB-IL mice. The mechanism for such a protective effect is not yet established, but it is clear that this is not related to alterations in daily or cumulative food intake (Figs 1D and1G). While the effect of intestinal Fxr activity on energy expenditure was not investigated in the current study, higher fasting plasma GLP-1 levels have been associated with higher rates of energy expenditure and fat oxidation34 possibly mediated by GLP-1 activating the autonomic nervous system35.
In an attempt to interrogate the bile acid-mediated phenotypes independent of weight loss, we carried out studies in lean chow-fed mice subjected to GB-IL diversion. Our results in lean mice (Fig 3D) showed similar improvements in glucose tolerance (Fig 1H) that were independent of changes in body weight (Fig 2A), body habitus (Fig 2B and2C) or food intake. GB-IL in the lean mice resulted in increased in total bile acids without any changes in intestinal bile acid transporters or with altered expression of Tgr5 or Fxr. The data also show that the enhancement in glucose tolerance is primarily due to an effect of bile acids on improved hepatic insulin sensitivity, independent of changes in hepatic glycogen content (Fig 3I) or intestinal glucose uptake (Fig. 3G). However, the increased circulating bile acids were associated with enhanced insulin secretion that are mediated by enhanced GLP-1 secretion.
The incretin GLP-1 contributes to enteral glucose tolerance by enhancing insulin secretion36. Previous studies showed that enhanced postprandial GLP-1 secretion after bile diversion in the rat7, 8, and dual Fxr and TGR5 activation stimulates GLP-1 secretion, resulting in enhanced hepatic insulin signaling, improved glucose tolerance, and improved insulin secretion in β cells37. The results from our study confirm these observations and show that bile acid signaling via intestinal Fxr leads to increased intestinal GLP-1 production, in the fasted but not in the post-prandial state (Fig 4A). The specificity of this effect of intestinal Fxr is demonstrated by additional treatments with either the GLP-1 receptor antagonist, Ex-9 (Fig 5A and5B), or the bile acid sequestrant, cholestyramine (Fig 5C and5D), both affecting only fasting plasma glucose, suggesting a physiological role for intestinal Fxr in regulating fasting GLP-1 tone. Our findings are consistent with the recent observations of Trabelsi et al., who demonstrated in cultured cells and global FXR knockout mice that Fxr deficiency improved GLP-1-mediated glucose disposal and bile acid sequestrant-stimulated GLP-1 production. Consistent with these findings, global deletion of GLP- 1R (Glp-1r −/−) abolished the glucoregulatory improvements observed with GB-IL (Fig 5G-H).
Bile diversion in lean Chow-fed animals resulted in similar microbiome profiles, as we previously observed with HFD obese mice, suggesting that the bile acid mediated phenotypes persist in a weight loss-independent manner. Thus, it is likely that the observed metabolic improvement in glucose tolerance may involve the gut microbiome. It is noteworthy that GB-IL is associated with significant elevations of Fxr antagonist tauro-β-muricholate6. Li et. al30, targeted the gut microbiome using Tempol, an antioxidant, to increase intestinal levels of tauro-β-muricholate and achieved significant weight loss. Additionally, Akkermansia muciniphila was also enriched in GB-IL in our mice, and in both rodents26 and humans39 following RYGB, given its strong associations with intestinal health40, as well as its strong inverse correlations with intestinal inflammation41,42.
The current study shares numerous physiological similarities with other bariatric procedures such as the endoluminal sleeve and the biliopancreatic diversion or the long-limb RYGB, all of which result in significant weight loss and resolution of obesity-associated comorbidities43, 44, 45–47. The exact mechanisms for these improvements are not well understood but have been attributed, in part, to increased circulating bile acids48. However, these clinically-based studies are extremely challenging to conduct and are frequently underpowered making translation of the findings difficult. Even though bile diversion through a gallbladder to intestinal anastomosis has not been directly examined in humans, our results shed light on physiologic pathways that may be exploited for future studies and novel therapies.
Mechanistically, it is not known whether or not bariatric operations have metabolic effects in otherwise lean subjects in the absence of weight loss. Bariatric surgery has anti-diabetic efficacy on ethnic groups that are predisposed to diabetes at lower BMI49–52. The findings of our present study could lend credence to the use of bile acid sequestrants for improving insulin sensitivity in both obese (and lean) subjects with type 2 diabetes, as they have been shown to have beneficial effects on glucose tolerance and incretin secretion53, 54,55. Additionally, the use of targeted delivery of bile acids to the distal intestine could also result in improved glucose tolerance. Zhang et al., demonstrated that taurocholate infusion to the mid-jejunum in healthy men augmented GLP-1 and insulin secretion28. These observations are consistent with effects mediated by increased distal intestinal bile acid delivery as observed in the current study.
Supplementary Material
Acknowledgements
We would like to thank Phil Williams and Jamie Adcock of the S. Rudolph Light Surgical Research Laboratories at Vanderbilt University Medical Center for their constructive feedback and assistance with these labor intensive and technically challenging studies. We thank Dr. Frank Gonzalez, Laboratory of Metabolism, Center for Cancer Research, National Cancer Institute, and National Institutes of Health for Fxrfl/fl mice. We thank Dr. Pierre Chambon for vil-Cre-ERT2 mice. We thank Dr. Galya Vassileva, Merck Research Lab, Kenilworth, New Jersey for Tgr5−/− mice. We thank Dr. Daniel Drucker, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Canada for Glp-1r−/− mice. Additionally, the studies described herein would not be possible without the impeccable technical efforts of Teri Stevenson, Vanderbilt University School of Medicine, Nashville, TN. This work was supported in part through a grant from the Society of American Gastrointestinal and Endoscopic Surgeons (VLA). The National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health supported this research, specifically by NIH grants DK059637 (Vanderbilt Mouse Metabolic Phenotyping Center), DK020593 (Vanderbilt Diabetes Research and Training Center), P30 CA68485 (Cancer Center Support Grant), DK058404 (Vanderbilt Digestive Disease Research Center), F32 DK103474 (VLA), U24 DK076169 MicroMouse sub-project 30835–22 (VLA), R01 DK105847 (NNA and CRF).
Grant Support: The National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health supported this work, specifically grants DK059637 (Vanderbilt Mouse Metabolic Phenotyping Center), DK020593 (Vanderbilt Diabetes Research and Training Center), DK058404 (Vanderbilt Digestive Disease Research Center), F32 DK103474 (VLA), U24 DK076169 MicroMouse sub-project 30835–22 (VLA), P30 CA68485 (Cancer Center Support Grant), R01 DK105847 (NNA and CRF). This work was also supported by a Research Grant from the Society of American Gastrointestinal and Endoscopic Surgeons (VLA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors declare no competing financial interests or other conflicts of interest. The study sponsors had no role in the collection, analysis, and interpretation of data.
References:
- 1.Collaborators GBDO, Afshin A, Forouzanfar MH, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubino F, Nathan DM, Eckel RH, et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care 2016;39:861–77. [DOI] [PubMed] [Google Scholar]
- 3.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013;310:2416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med 2017;376:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadera BE, Lum K, Grant J, et al. Remission of type 2 diabetes after Roux-en-Y gastric bypass is associated with greater weight loss. Surg Obes Relat Dis 2009;5:305–9. [DOI] [PubMed] [Google Scholar]
- 6.Flynn CR, Albaugh VL, Cai S, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun 2015;6:7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohli R, Setchell KD, Kirby M, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology 2013;154:2341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 2012;153:3613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albaugh VL, Flynn CR, Cai S, et al. Early Increases in Bile Acids Post Roux-en-Y Gastric Bypass Are Driven by Insulin-Sensitizing, Secondary Bile Acids. J Clin Endocrinol Metab 2015;100:E1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 2014;22:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakatani H, Kasama K, Oshiro T, et al. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 2009;58:1400–7. [DOI] [PubMed] [Google Scholar]
- 12.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap--bile acids in metabolic control. Nat Rev Endocrinol 2014;10:488–98. [DOI] [PubMed] [Google Scholar]
- 14.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009;10:167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGavigan AK, Garibay D, Henseler ZM, et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut 2017;66:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014;509:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scrocchi LA, Brown TJ, MaClusky N, et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 1996;2:1254–8. [DOI] [PubMed] [Google Scholar]
- 18.Jiang C, Xie C, Li F, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest 2015;125:386–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.el Marjou F, Janssen KP, Chang BH, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 2004;39:186–93. [DOI] [PubMed] [Google Scholar]
- 20.Vassileva G, Golovko A, Markowitz L, et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J 2006;398:423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 2013;341:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brighton CA, Rievaj J, Kuhre RE, et al. Bile Acids Trigger GLP-1 Release Predominantly by Accessing Basolaterally Located G Protein-Coupled Bile Acid Receptors. Endocrinology 2015;156:3961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin X, Shen H, Liu M, et al. GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol Gastrointest Liver Physiol 2005;288:G943–9. [DOI] [PubMed] [Google Scholar]
- 24.Berglund ED, Li CY, Poffenberger G, et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes 2008;57:1790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujisaka S, Ussar S, Clish C, et al. Antibiotic effects on gut microbiota and metabolism are host dependent. J Clin Invest 2016;126:4430–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liou AP, Paziuk M, Luevano JM Jr., et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013;5:178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santacruz A, Collado MC, Garcia-Valdes L, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr 2010;104:83–92. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 2009;106:2365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sayin SI, Wahlstrom A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013;17:225–35. [DOI] [PubMed] [Google Scholar]
- 30.Li F, Jiang C, Krausz KW, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 2013;4:2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cummings DE, Rubino F. Metabolic surgery for the treatment of type 2 diabetes in obese individuals. Diabetologia 2018;61:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy IA, Smith NK, Erreger K, et al. Bile diversion, a bariatric surgery, and bile acid signaling reduce central cocaine reward. PLoS Biol 2018;16:e2006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavez-Talavera O, Tailleux A, Lefebvre P, et al. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017;152:1679–1694 e3. [DOI] [PubMed] [Google Scholar]
- 34.Pannacciulli N, Bunt JC, Koska J, et al. Higher fasting plasma concentrations of glucagon-like peptide 1 are associated with higher resting energy expenditure and fat oxidation rates in humans. Am J Clin Nutr 2006;84:556–60. [DOI] [PubMed] [Google Scholar]
- 35.Baggio LL, Ussher JR, McLean BA, et al. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol Metab 2017;6:1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breitman I, Isbell JM, Saliba J, et al. Effects of proximal gut bypass on glucose tolerance and insulin sensitivity in humans. Diabetes Care 2013;36:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pathak P, Xie C, Nichols RG, et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 2018. [DOI] [PMC free article] [PubMed]
- 38.Trabelsi MS, Daoudi M, Prawitt J, et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun 2015;6:7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palleja A, Kashani A, Allin KH, et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med 2016;8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016;65:426–36. [DOI] [PubMed] [Google Scholar]
- 41.Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013;110:9066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneeberger M, Everard A, Gomez-Valades AG, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep 2015;5:16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Habegger KM, Al-Massadi O, Heppner KM, et al. Duodenal nutrient exclusion improves metabolic syndrome and stimulates villus hyperplasia. Gut 2014;63:1238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim T, Holleman CL, Ptacek T, et al. Duodenal endoluminal barrier sleeve alters gut microbiota of ZDF rats. Int J Obes (Lond) 2017;41:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandler BJ, Rumbaut R, Swain CP, et al. One-year human experience with a novel endoluminal, endoscopic gastric bypass sleeve for morbid obesity. Surg Endosc 2015;29:3298–303. [DOI] [PubMed] [Google Scholar]
- 46.Brolin RE, LaMarca LB, Kenler HA, et al. Malabsorptive gastric bypass in patients with superobesity. J Gastrointest Surg 2002;6:195–203; discussion 204–5. [DOI] [PubMed] [Google Scholar]
- 47.Brolin RE. Long limb Roux en Y gastric bypass revisited. Surg Clin North Am 2005;85:807–17, vii. [DOI] [PubMed] [Google Scholar]
- 48.Werling M, Fandriks L, Olbers T, et al. Biliopancreatic Diversion is associated with greater increases in energy expenditure than Roux-en-Y Gastric Bypass. PLoS One 2018;13:e0194538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen R, Pinheiro JS, Correa JL, et al. Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m(2): a tailored approach. Surg Obes Relat Dis 2006;2:401–4, discussion 404. [DOI] [PubMed] [Google Scholar]
- 50.Di J, Zhang H, Yu H, et al. Effect of Roux-en-Y gastric bypass on the remission of type 2 diabetes: a 3-year study in Chinese patients with a BMI <30 kg/m(2). Surg Obes Relat Dis 2016;12:1357–1363. [DOI] [PubMed] [Google Scholar]
- 51.Dixon JB, Hur KY, Lee WJ, et al. Gastric bypass in Type 2 diabetes with BMI < 30: weight and weight loss have a major influence on outcomes. Diabet Med 2013;30:e127–34. [DOI] [PubMed] [Google Scholar]
- 52.Malapan K, Goel R, Tai CM, et al. Laparoscopic Roux-en-Y gastric bypass for nonobese type II diabetes mellitus in Asian patients. Surg Obes Relat Dis 2014;10:834–40. [DOI] [PubMed] [Google Scholar]
- 53.Marina AL, Utzschneider KM, Wright LA, et al. Colesevelam improves oral but not intravenous glucose tolerance by a mechanism independent of insulin sensitivity and beta-cell function. Diabetes Care 2012;35:1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonne DP, Hansen M, Knop FK. Bile acid sequestrants in type 2 diabetes: potential effects on GLP1 secretion. Eur J Endocrinol 2014;171:R47–65. [DOI] [PubMed] [Google Scholar]
- 55.Beysen C, Murphy EJ, Deines K, et al. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomised controlled study. Diabetologia 2012;55:432–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.